Abstract
Objective
To evaluate long‐term outcomes in psoriatic arthritis (PsA) patients who achieved or did not achieve minimal disease activity (MDA) through 5 years of golimumab treatment in the GO‐REVEAL trial.
Methods
The GO‐REVEAL trial was a phase III, randomized, double‐blind trial with placebo‐control through week 24 followed by an open‐label extension of golimumab 50/100 mg treatment up to 5 years. In these post‐hoc analyses, MDA was defined by the presence of ≥5 of 7 PsA outcome measures (≤1 swollen joint, ≤1 tender joint, Psoriasis Area and Severity Index [PASI] ≤1, patient pain score ≤15, patient global disease activity score ≤20 [range 0–100], Health Assessment Questionnaire disability index [HAQ DI] ≤0.5, and ≤1 tender enthesis point).
Results
Treatment with golimumab yielded significantly higher MDA response rates versus patients randomized to placebo at week 14 (23.5% versus 1.0%; P < 0.0001), week 24 (28.1% versus 7.7%; P < 0.0001), and week 52 (42.4% versus 30.2%; P = 0.037). MDA was achieved at least once by ∼50% of golimumab‐treated patients overall. Irrespective of treatment randomization, achievement of MDA at ≥3 and ≥4 consecutive visits was associated with significantly less radiographic progression and more improvement in MDA components allowing specific assessment of physical function (HAQ DI) and overall disease activity (patient global assessment of disease activity) at week 256 versus patients not achieving MDA. Logistic regression analyses indicated that a 1‐unit higher baseline HAQ DI score yielded a significantly lower likelihood of achieving MDA at ≥3 (odds ratio 0.514 [95% confidence interval 0.321–0.824]; P = 0.006) and ≥4 (odds ratio 0.480 [95% confidence interval 0.290–0.795]; P = 0.004) consecutive visits.
Conclusion
Among golimumab‐treated PsA patients, better long‐term functional improvement, patient global assessment, and radiographic outcomes were observed when patients achieved persistent MDA.
INTRODUCTION
With the advent of biologic agent therapy and the advancement of treat‐to‐target recommendations for the management of psoriatic arthritis (PsA), goals of therapy have become more aggressive, with minimal disease activity (MDA) being a feasible objective target 1, 2, 3. As defined by the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group, MDA is “that state of disease activity deemed a useful target of treatment by both the patient and physician, given current treatment possibilities and limitations,” and encompasses both remission and low disease activity 4. The PsA MDA criteria, a composite of 7 tools used to assess disease activity, have been preliminarily validated in both observational and interventional trial cohorts 5, 6.
Box 1. Significance & Innovations.
We retrospectively assessed the implications of achieving minimal disease activity (MDA) over a 5‐year time period, which is a more rigorous goal than typically evaluated in psoriatic arthritis (PsA) clinical trials.
Among the approximately 50% of golimumab‐treated patients who achieved MDA through 5 years, better long‐term functional improvement, patient global assessment, and radiographic outcomes were observed.
Radiographic benefit in patients with persistent MDA was more pronounced in patients using methotrexate at baseline.
Patients with a 1‐unit higher baseline Health Assessment Questionnaire disability index score had a significantly lower likelihood of achieving MDA at ≥3 and ≥4 consecutive study visits.
In PsA, achievement of MDA earlier in the disease course could potentially obviate the development of structural damage. As such, we evaluated long‐term radiographic progression and physical function in patients who either achieved or did not achieve MDA through 5 years of participation in the randomized, placebo‐controlled, GO‐REVEAL study of golimumab in PsA patients 7, 8, 9, 10.
PATIENTS AND METHODS
The GO‐REVEAL trial was conducted according to guidelines set forth by the Declaration of Helsinki and International Conference on Harmonization good clinical practices. Specifically, the institutional review board or ethics committee at each study site approved the protocol, and all patients provided written informed consent prior to the start of any study‐related procedures. Details of the GO‐REVEAL patient eligibility criteria and trial design have been previously published 7, 8, 9, 10. Despite treatment with disease‐modifying antirheumatic or nonsteroidal antiinflammatory drugs, patients had active PsA, defined by the presence of at least 3 swollen and 3 tender joints, and the presence of plaque psoriasis with a qualifying lesion at least 2 cm in diameter.
The GO‐REVEAL trial was a phase III, randomized, double‐blind trial with placebo‐control through week 24 followed by an open‐label extension lasting up to 5 years. A total of 405 patients were randomized (1:1.3:1.3) to receive blinded subcutaneous injections of placebo, golimumab 50 mg, or golimumab 100 mg every 4 weeks. Randomization was stratified by methotrexate (MTX) use reported at baseline. Janssen Biotech supplied golimumab and placebo as sterile liquids for subcutaneous injection.
At week 16, patients with <10% improvement from baseline in their swollen and tender joint counts entered early escape, with dose escalation from placebo to golimumab 50 mg or from golimumab 50 mg to golimumab 100 mg. Patients randomized to the golimumab 100 mg group had no change in treatment based on week‐16 response. Beginning at week 24, all patients still receiving placebo crossed over to golimumab 50 mg. Thus, all patients received blinded golimumab 50 mg or 100 mg every 4 weeks between the week 24 and week 52 database locks. Following the week 52 database lock, patients received open‐label subcutaneous golimumab (50 or 100 mg) injections every 4 weeks, and patients receiving golimumab 50 mg or 100 mg could increase or decrease the dose to 100 mg or 50 mg, respectively, at the investigator's discretion.
For the purpose of these post‐hoc analyses, and as validated by Coates and Helliwell 5, MDA was defined as the presence of at least 5 of the following 7 PsA outcome measures: swollen joint count ≤1 of 66 evaluated; tender joint count ≤1 of 68 evaluated; Psoriasis Area and Severity Index (PASI) ≤1 (range 0–72)11; patient pain visual analog scale (VAS) score ≤15 (range 0–100); patient global assessment of disease activity VAS score of ≤20 (range 0–100); Health Assessment Questionnaire disability index (HAQ DI) score ≤0.5 (range 0–3)12, 13; and tender enthesis points ≤1.
These post‐hoc analyses of the GO‐REVEAL trial utilized observed data from randomized patients with nonmissing MDA and/or radiographic data at weeks 14, 24, 52, 104, 148, 196, and 256, time points at which data were available to analyze the MDA status. Analyses of variance based on van der Waerden normalized scores were used for 1) treatment comparisons of MDA achievement; 2) comparisons of MDA achievement categories (never, ≥3 consecutive time points, ≥4 consecutive time points) by randomized treatment group and also by changes from baseline to week 256 in the PsA‐modified Sharp/van der Heijde score (SHS) 14, 15, HAQ DI score, PASI score (among patients with ≥3% of body surface area with psoriasis involvement at baseline), and patient global assessment of disease activity; and 3) comparisons of MDA achievement categories (never, ≥3 consecutive time points, ≥4 consecutive time points) by MTX use at baseline (yes/no). The endpoints of MDA achievement at ≥3 and ≥4 consecutive time points (in this case representing a minimum of at least 38 and 90 weeks, respectively, with minimal disease) were chosen to provide a reasonable number of patients available for analysis per treatment group within the context of a clinically meaningful and sustained timeframe. Logistic regression analyses were conducted to assess the effect of baseline disease characteristics on achievement of MDA at ≥3 and ≥4 consecutive time points. Baseline characteristics assessed included HAQ DI score, MTX use, Disease Activity Score in 28 joints using the C‐reactive protein level (DAS28‐CRP) 16, 17, patient global assessment of disease activity, PsA duration, tender joint count, swollen joint count, and CRP level.
RESULTS
Patient disposition and baseline characteristics
As reported previously 10, 126 of 405 patients (31.1%) discontinued study treatment through week 252, with the most common reasons for study agent discontinuation being adverse events and unsatisfactory therapeutic response. Baseline disease characteristics, as well as the proportions of patients reporting MTX use at baseline, were generally consistent among randomized treatment groups (see Supplementary Table 1 available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22576/abstract) and were generally consistent between patients who continued and discontinued study agent (data not shown).
Achievement of MDA
Seven patients randomized to placebo and 3 patients randomized to golimumab treatment were excluded from the MDA analyses due to missing data. Thus, 395 of the 405 randomized patients (97.5%) were included in these analyses. As shown in Table 1, treatment with golimumab was associated with significantly larger proportions of patients who achieved MDA when compared with those receiving placebo during the placebo‐controlled period at week 14 (23.5% versus 1.0%; P < 0.0001) and week 24 (28.1% versus 7.7%; P < 0.0001), as well as following crossover to golimumab at week 52 (42.4% versus 30.2%; P = 0.037). Baseline MTX use did not affect the achievement of MDA (Table 1).
Table 1.
Achievement of minimal disease activity at each visit assessed over 5 years by randomized treatment and by baseline methotrexate (MTX) use in randomized patients included in post‐hoc analysesa
| Placebo→Golimumab 50 mg/100 mgb | Golimumab 50 mg/100 mgc | MTX use at baseline | No MTX use at baseline | |
|---|---|---|---|---|
| No. patients with available data | 106 | 289 | 194 | 201 |
| Week 14 | 1/104 (1.0) | 67/285 (23.5) | 34/192 (17.7) | 34/197 (17.3) |
| P | < 0.0001 | 0.91 | ||
| Week 24 | 8/104 (7.7) | 80/285 (28.1) | 40/192 (20.8) | 48/197 (24.4) |
| P | < 0.0001 | 0.41 | ||
| Week 52 | 29/96 (30.2) | 111/262 (42.4) | 70/183 (38.3) | 70/175 (40.0) |
| P | 0.037 | 0.73 | ||
| Week 104 | 32/87 (36.8) | 107/250 (42.8) | 69/177 (39.0) | 70/160 (43.8) |
| P | 0.33 | 0.37 | ||
| Week 148 | 41/84 (48.8) | 122/236 (51.7) | 79/167 (47.3) | 84/153 (54.9) |
| P | 0.65 | 0.17 | ||
| Week 196 | 37/82 (45.1) | 106/222 (47.7) | 74/161 (46.0) | 69/143 (48.3) |
| P | 0.68 | 0.69 | ||
| Week 256 | 34/77 (44.2) | 106/205 (51.7) | 70/150 (46.7) | 70/132 (53.0) |
| P | 0.26 | 0.29 |
Values are the numerator/denominator (%) of patients.
Group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock.
Group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock.
Through week 256, MDA was achieved at least once by approximately 50% of golimumab‐treated patients (Figure 1). In addition, larger proportions of golimumab‐randomized patients compared with placebo‐randomized patients achieved MDA at ≥5 (24.9% versus 12.3%; P = 0.007), ≥6 (16.6% versus 2.8%; P = 0.000), and ≥7 (11.4% versus 0.0%; P = 0.000) consecutive time points (Table 2).
Figure 1.
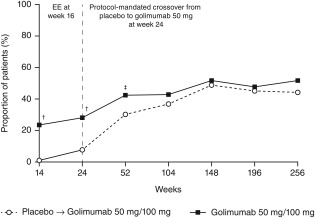
Proportions of patients who achieved minimal disease activity by randomized treatment and visit over 5 years. The placebo→golimumab 50 mg/100 mg group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. The golimumab 50 mg/100 mg group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. EE = early escape; † = P < 0.0001 vs. placebo; ‡ = P < 0.05 vs. placebo.
Table 2.
Achievement of minimal disease activity (MDA) over 5 years in randomized patients included in post‐hoc analysesa
| Placebo→golimumab 50 mg/100 mgb | Golimumab 50mg/100 mgc | P | |
|---|---|---|---|
| No. patients with available data | 106 | 289 | |
| Achievement of MDA | |||
| Never | 52 (49.1) | 122 (42.2) | 0.22 |
| ≥1 consecutive time point | 54 (50.9) | 167 (57.8) | 0.22 |
| ≥2 consecutive time points | 43 (40.6) | 129 (44.6) | 0.47 |
| ≥3 consecutive time points | 30 (28.3) | 104 (36.0) | 0.15 |
| ≥4 consecutive time points | 23 (21.7) | 84 (29.1) | 0.14 |
| ≥5 consecutive time points | 13 (12.3) | 72 (24.9) | 0.007 |
| ≥6 consecutive time points | 3 (2.8) | 48 (16.6) | 0.000 |
| ≥7 consecutive time points | 0 (0.0) | 33 (11.4) | 0.000 |
Values are the number (%) of patients unless indicated otherwise.
Group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock.
Group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock.
Impact of MDA on patient outcomes through 5 years
Irrespective of treatment randomization, achievement of MDA at ≥3 and ≥4 consecutive time points was associated with significantly less radiographic progression (Figure 2) and significantly more improvement in components of the MDA criteria that allow specific assessment of physical function, i.e., HAQ DI (Figures 3A and B), and overall disease activity, i.e., patient global assessment of disease activity (Figures 3C and D), at week 256 when compared with patients who never achieved MDA. Consistent with absolute change in SHS, when assessed by the proportions of patients who achieved SHS change <0 from baseline to year 5, there was a trend toward less progression among patients who achieved MDA at ≥3 (41 of 116, 35.3%) and ≥4 (34 of 95, 35.8%) consecutive time points versus patients who never achieved MDA (22 of 95, 23.2%; P = 0.054 and P = 0.056, respectively). In addition, similar patterns of more improvement/less progression in SHS were observed for patients who achieved MDA based on 6 of 7 and all 7 of the MDA components, especially with longer durations of treatment when adequate numbers of patients could be included in the subset of patients who achieved MDA (see Supplementary Table 2 available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22576/abstract).
Figure 2.
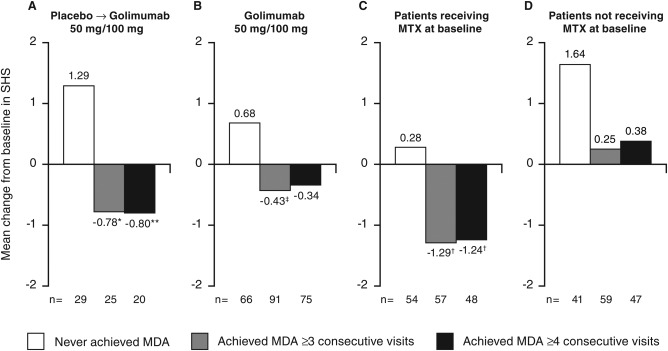
Mean change in psoriatic arthritis–modified Sharp/van der Heijde score (SHS) from baseline to week 256 by randomized treatment (A, B) and by baseline methotrexate (MTX) use (C, D). Decreases from baseline in SHS represent improvement. (See Supplementary Table 1 available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22576/abstract for baseline SHS values). The placebo→golimumab 50 mg/100 mg group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. The golimumab 50 mg/100 mg group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. MDA = minimal disease activity; ∗ = P < 0.005 vs. never achieved MDA; ‡ = P < 0.05 vs. never achieved MDA; ∗∗ = P < 0.01 vs. never achieved MDA; † = P < 0.0001 vs. never achieved MDA.
Figure 3.

Mean change in Health Assessment Questionnaire disability index (HAQ DI) (A, B) and patient global assessment of disease activity (PtGA) (C, D) from baseline to week 256 by randomized treatment. Decreases from baseline in HAQ DI represent improvement and increases in PtGA indicate worsening disease activity (See Supplementary Table 1 available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22576/abstract for baseline HAQ DI and PtGA values). The placebo→golimumab 50 mg/100 mg group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. The golimumab 50 mg/100 mg group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. MDA = minimal disease activity; ∗ = P < 0.005 vs. never achieved MDA; † = P < 0.0001 vs. never achieved MDA.
Achievement of MDA at ≥3 and ≥4 consecutive time points did not appear to affect changes in the skin symptoms of PsA, assessed by the PASI score, at week 256 when evaluated in patients with ≥3% of body surface area with psoriasis involvement at baseline (Figures 4A and B). Of note, patients who achieved persistent MDA and who reported MTX use at baseline demonstrated significantly less radiographic progression (Figures 2C and D) and significantly less improvement in PASI score (Figures 4C and D) at week 256 than patients with MDA not receiving MTX. Effects of MTX use were not observed for changes in HAQ DI or patient global assessment of disease activity (data not shown).
Figure 4.
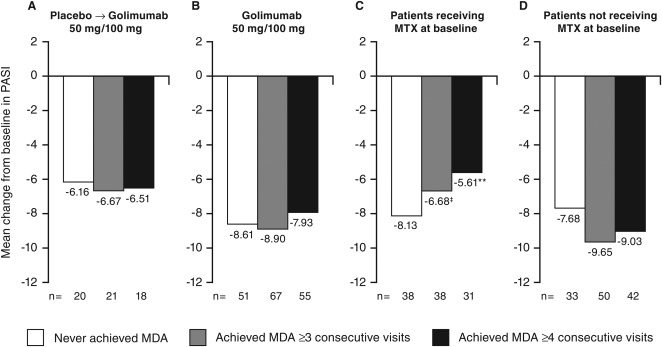
Mean change in Psoriasis Area and Severity Index (PASI) score from baseline to week 256 by randomized treatment (A, B) and by baseline methotrexate (MTX) use (C, D). Decreases from baseline indicate improved activity (See Supplementary Table 1 available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22576/abstract for baseline PASI values). The placebo→golimumab 50 mg/100 mg group includes patients randomized to placebo who early escaped/crossed over at week 16/24 to receive golimumab 50 mg, with the possibility to increase golimumab from 50 to 100 mg after the week‐52 database lock. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. The golimumab 50 mg/100 mg group includes patients randomized to receive golimumab 50 mg who early escaped at week 16 or dose escalated after the week‐52 database lock to receive golimumab 100 mg and also includes patients randomized to receive golimumab 100 mg. All patients could decrease the golimumab dose from 100 to 50 mg after the week‐52 database lock. MDA = minimal disease activity; ‡ = P < 0.05 vs. without MTX at baseline; ∗∗ = P < 0.01 vs. without MTX at baseline.
Logistic regression analyses were conducted to assess the effect of baseline disease characteristics (HAQ DI score, MTX use, DAS28‐CRP, patient global assessment of disease activity, PsA duration, tender joint count, swollen joint count, and CRP level) on achievement of MDA at ≥3 and ≥4 consecutive time points. Analysis results revealed that patients with a 1‐unit higher baseline HAQ DI score had a significantly lower likelihood of achieving MDA at ≥3 (odds ratio [OR] 0.514 [95% confidence interval (95% CI) 0.321–0.824]; P = 0.006) and ≥4 (OR 0.480 [95% CI 0.290–0.795]; P = 0.004) consecutive time points.
DISCUSSION
The phase III, randomized, double‐blind GO‐REVEAL trial of 405 PsA patients treated with golimumab for up to 5 years 7, 8, 9, 10 provided an opportunity to retrospectively assess the implications of achieving a treatment goal, namely MDA, that is more rigorous than the typical objectives of therapeutic studies in PsA. The composite PsA MDA criteria have been preliminarily validated in both observational and interventional trial cohorts 5, 6. Also, it was previously found that MDA might be predicted after 3 months of anti‐tumor necrosis factor (TNF) therapy by factors such as baseline age, CRP level, and function (as assessed by the Bath Ankylosing Spondylitis Functional Index) in a smaller cohort (n = 146) of PsA patients 18. The implications of achieving MDA over a longer time period have not been explored to date. Based on patient data collected from the GO‐REVEAL study, treatment with golimumab resulted in achievement of MDA in approximately 50% of patients through 5 years. Importantly, better long‐term functional improvement, patient global assessment of disease activity, and radiographic, although not skin, outcomes were observed when patients achieved persistent MDA. Radiographic benefit in patients with persistent MDA was more pronounced in patients using MTX at baseline. Results of regression analyses identified baseline HAQ DI score to be a significant predictor of a patient's ability to achieve persistent MDA, whereby patients with a 1‐unit higher baseline HAQ DI score had a significantly lower likelihood of achieving MDA at ≥3 and ≥4 consecutive study visits.
Among patients who received placebo through the initial 16 or 24 weeks of the GO‐REVEAL study, a 4‐ to 6‐month delay in initiation of golimumab did not result in a penalty of the investigated clinical outcomes (HAQ DI, patient global assessment of disease activity). However, a delay in active treatment appeared to lead to approximately twice the amount of radiographic progression by year 5, although the overall amount of progression was still significantly limited in patients who started on placebo and switched to golimumab. It also appeared that delaying the start of golimumab may have affected long‐term skin improvement, although there is not much difference between MDA achievers and nonachievers, suggesting that the effect of TNF inhibitors on the skin might be more universal than the effect on other domains of patient response.
The analyses reported herein have several limitations. While some GO‐REVEAL patients achieved MDA at ≥5, ≥6, and ≥7 consecutive time points, the endpoints of MDA achievement at ≥3 and ≥4 consecutive time points were primarily employed in analyses. These endpoints were selected to provide ample patients available for analysis per treatment group within the context of a clinically meaningful sustained timeframe, in this case representing at least 38 and 90 weeks, respectively, of minimal disease. In addition, MDA is a composite response achieved by meeting 5 of 7 possible criteria, and we do not discern if any of the 7 criteria are more important in achieving MDA than others. Also, while the MDA is based on a possible total of 68 tender and 66 swollen joints, we utilized the DAS28‐CRP as a baseline variable in the regression analyses conducted, which could potentially underestimate the amount of arthritis. However, the DAS28 is more commonly employed in both clinical and research settings, and actual baseline joint counts were also incorporated into the regression model. Finally, approximately 30% of all GO‐REVEAL patients discontinued over the course of the 5‐year trial. The analyses reported herein, however, utilize observed data and derive from up to 395 of the 405 randomized patients. In conclusion, in patients with active PsA, aiming for MDA as part of a treat‐to‐target strategy may provide long‐term radiographic and functional benefit.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Kavanaugh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kavanaugh, van der Heijde, Beutler, Gladman, Mease, Krueger, McInnes, Coates.
Acquisition of data
Kavanaugh, Gladman, Mease, McInnes.
Analysis and interpretation of data
Kavanaugh, Beutler, Mease, Krueger, McInnes, Helliwell, Coates, Xu.
ROLE OF THE STUDY SPONSOR
Janssen Research & Development and Merck/Schering‐Plough provided funding. The steering committee (Drs. Kavanaugh, Gladman, Mease, Krueger, and McInnes) supervised the study and assisted with study design. Data were collected by the investigators and entered into a Janssen database. Janssen statisticians and programmers conducted the analyses, and members of the steering committee, with medical writing assistance, prepared the manuscript. All authors reviewed and approved the manuscript content before submission and jointly agreed to submit the final version. Publication of these study data was not contingent upon approval from Janssen or Merck/Schering‐Plough.
Supporting information
Supplementary Table 1. Baseline disease characteristics, randomized patients
Supplementary Table 2. Mean improvement in PsA‐modified SHS by achievement of MDA based on 6 of 7 and 7 of 7 MDA components in randomized patients included in post‐hoc analyses
ACKNOWLEDGMENTS
The authors thank Katrien van Beneden (formerly of Janssen) for her role in conceptualizing the data analyses reported herein, Linda Tang of Janssen for statistical support, and Michelle L. Perate and Mary H. Whitman (Janssen Scientific Affairs) for assisting with manuscript preparation and submission. A complete list of study investigators was previously reported 7.
ClinicalTrials.gov identifier: NCT00265096.
REFERENCES
- 1. Olivieri I, D'Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 2014;10:531–42. [DOI] [PubMed] [Google Scholar]
- 2. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Braun J, Dougados M, Emery P, Fitzgerald O, Helliwell P, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wells GA, Boers M, Shea B, Brooks PM, Simon LS, Strand CV, et al. Minimal disease activity for rheumatoid arthritis: a preliminary definition. J Rheumatol 2005;32:2016–24. [PubMed] [Google Scholar]
- 5. Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken) 2010;62:965–9. [DOI] [PubMed] [Google Scholar]
- 6. Coates LC, Cook R, Lee KA, Chandran V, Gladman DD. Frequency, predictors, and prognosis of sustained minimal disease activity in an observational psoriatic arthritis cohort. Arthritis Care Res (Hoboken) 2010;62:970–6. [DOI] [PubMed] [Google Scholar]
- 7. Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez‐Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty‐four–week efficacy and safety results of a randomized, placebo‐controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 8. Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, et al. Golimumab in psoriatic arthritis: one‐year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo‐controlled trial. Arthritis Rheum 2012;64:2504–17. [DOI] [PubMed] [Google Scholar]
- 9. Kavanaugh A, McInnes IB, Mease PJ, Krueger GG, Gladman DD, van der Heijde D, et al. Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long‐term extension of the randomised, placebo‐controlled GO‐REVEAL study. Ann Rheum Dis 2013;72:1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavanaugh A, McInnes IB, Mease P, Krueger GG, Gladman D, van der Heijde D, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long‐term extension of a randomised, placebo‐controlled trial (the GO‐REVEAL study). Ann Rheum Dis 2014;73:1689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 12. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 13. Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- 14. Van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, van 't Hof MA, van Rijswijk MH, et al. Biannual radiographic assessments of hands and feet in a three‐year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35:26–34. [DOI] [PubMed] [Google Scholar]
- 15. Van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods [review]. Ann Rheum Dis 2005;64(Suppl II):ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 17. Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28‐joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C‐reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iervolino S, Di Minno MN, Peluso R, Lofrano M, Russolillo A, Di Minno G, et al. Predictors of early minimal disease activity in patients with psoriatic arthritis treated with tumor necrosis factor‐α blockers. J Rheumatol 2012;39:568–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Baseline disease characteristics, randomized patients
Supplementary Table 2. Mean improvement in PsA‐modified SHS by achievement of MDA based on 6 of 7 and 7 of 7 MDA components in randomized patients included in post‐hoc analyses


