Abstract
Out‐of‐pocket spending is increasingly recognized as an important barrier to accessing health care, particularly in low‐income and middle‐income countries (LMICs) where a large portion of health expenditure comes from out‐of‐pocket payments. Emerging universal healthcare policies prioritize reduction of poverty impact such as catastrophic and impoverishing healthcare expenditure. Poverty impact is therefore increasingly evaluated alongside and within economic evaluations to estimate the impact of specific health interventions on poverty. However, data collection for these metrics can be challenging in intervention‐based contexts in LMICs because of study design and practical limitations. Using a set of case studies, this letter identifies methodological challenges in collecting patient cost data in LMIC contexts. These components are presented in a framework to encourage researchers to consider the implications of differing approaches in data collection and to report their approach in a standardized and transparent way. © 2016 The Authors. Health Economics published by John Wiley & Sons Ltd.
Keywords: out‐of‐pocket payments, catastrophic expenditures, poverty, data collection methods, economic evaluation
1. Introduction
As universal access to health care becomes a greater international priority, interest has grown in reducing the level of financial catastrophe and impoverishment caused by health‐related expenditure (64th World Health Assembly, 2011). As a result, there is increased recognition that the impact of health interventions on poverty and equity should be incorporated into economic evaluations (Bill and Melinda Gates Foundation et al., 2014) – particularly in low‐income and middle‐income countries (LMICs) where out‐of‐pocket expenditures make up a large proportion of total health expenditure (World Health Organization, 2015). This is evidenced by the growing popularity of ‘extended’ economic evaluations, which incorporate assessments of the potential financial risk protection impact of an intervention or technology (Verguet et al., 2014). In the context of this growing importance of poverty impact metrics in health planning and decision making, there is need for high‐quality data to estimate the impact of health expenditures on poverty and vulnerability. To date, the majority of research reporting the poverty impact of health expenditures has drawn on data from large cross‐sectional surveys such as the Living Standards Measurement Survey or World Health Survey. While these datasets facilitate equity analyses evaluating the distribution of health impacts or financial pooling mechanisms across socioeconomic status analysis at the national level (Xu et al., 2003; Lu et al., 2009), they cannot be easily used to capture the impact of a specific health intervention on poverty and may not always include detail on indirect costs or income loss, which can be key aspects of the poverty impact of illness.
Collecting this type of data within a smaller‐scale study setting can substantially increase the time and cost of data collection. Many studies therefore avoid collecting data for a poverty impact analysis altogether. Where poverty impact data are collected as part of intervention evaluations, there are notable inconsistencies in data collection methods. Systematic reviews of existing patient cost studies in LMICs highlight a lack of standard approaches across cost ingredients, data sources, sampling methodologies, and recall periods, even where the same measure of poverty impact is used (Barter et al., 2012; Tanimura et al., 2014; Kankeu et al., 2013; Alam & Mahal, 2014; McIntyre et al., 2006). This can lead to challenges in assessing the comparability, quality, and accuracy of results. In part, this heterogeneity may stem from limited practical guidance or standards on collecting patient‐incurred cost data. Reporting guidelines for economic evaluations largely cover provider perspectives (Drummond & Jefferson, 1996; Husereau et al., 2013) and are neither updated to reflect information necessary for poverty impact metrics nor provide guidance when constraints in data collection require compromise, such as limiting the sample size or restricting the length of the questionnaire.
The aim of this letter is to highlight challenges faced in collecting data on patient costs within economic evaluation platforms in LMICs. We discuss practical issues around collecting patient‐incurred cost and household income data, including comprehensiveness of the survey instrument, timing of interviews, sampling, and survey administration. To illustrate these issues, we use four case studies from our own research as examples (Kufa et al., 2014; Foster et al., 2015; Ilboudo et al., 2014; Mfinanga et al., 2015) (Table 1). Finally, we present a framework of methodological choices in planning research on poverty impact metrics (Table 2) to encourage researchers to report their approach in a standardized and transparent way and to consider potential implications of varying approaches in data collection (Figure 1).
Table 1.
Case study characteristics
| MERGE (Kufa et al., 2014) | XTEND (Foster et al., 2015) | ECONPOP (Ilboudo et al., 2014) | REMSTART (Mfinanga et al., 2015) | |
|---|---|---|---|---|
| Country | South Africa | South Africa | Burkina Faso | Zambia and Tanzania |
| Aim of study | Implementation and evaluation of an optimized model for scaling up TB/HIV integration at primary care clinics | Evaluation of the implementation of a new TB diagnostic, XPert MTB/RIF | Multidisciplinary study to estimate costs and consequences of abortion | Trial assessing a complex intervention to reduce mortality in ART‐naive patients beginning ART |
| Study design | Cluster‐randomized trial | Cluster‐randomized trial | Cross‐sectional survey | Individually randomized control trial |
| Time frame | Cross‐sectional | Cohort | Cross‐sectional | Longitudinal |
| Sampling for cost data | Convenience sample at study facilities | Random subsample of study‐enrolled patients | Convenience sample at study facilities | All participants at study clinics. Clinics chosen for convenience |
| Location of interview | Facility | Facility | Facility | Facility |
| Sample size | 459 for costs | 351 for costs | 304 for economic study | 1375 for costs |
| 3,478 total for trial | 4,656 total for trial | 1,999 total for trial | ||
| Subgroups (n) | TB only (41) | No TB treatment (302) | Induced (37) | Intervention (684) |
| TB/HIV (119) | Control (691) | |||
| HIV only (299) | Started on treatment (49) | Spontaneous (267) | Tanzania (870) | |
| Zambia (505) | ||||
| OOP cost ingredients | Transport for individual and companion, medicines and consumables, diagnostics, consultation fees, special food/supplements, and inpatient accommodation | Transport for individual and companion, medicines and consumables, diagnostics, consultation fees, special food/supplements, and inpatient accommodation | Medicines and consumables, consultation fees, ultrasound, informal payments, pre‐referral costs, and hospitalization | Transport and ‘other’ costs |
| Recall period (costs) | The last visit to each provider (variable; max 5 months) | The last month | ~1 day (interviewed on discharge) | 1 day (cost of visit only) |
| Household/individual costs | Individual and guardian / caregiver | Individual and guardian / caregiver | Individual | Individual and guardian / caregiver |
| Average length of interview (min) | ~60 | ~45 | ~20 | ~25 |
| Diary/recall | Recall | Recall | Recall | Recall |
| Indirect cost measurement | Human capital approach and income loss | Income loss | None | Human capital approach |
| Additional health services costed | Pharmacy, GP, outpatient hospital, inpatient hospital, and traditional healer | Pharmacy, GP, outpatient hospital, inpatient hospital, and traditional healer | None | None |
| Source of income data | Annual individual income before and after diagnosis | Annual individual income before and after diagnosis | None (GDP per capita used as proxy) | Individual income in last month |
| Interviewers used | Research assistants | Nurses and research assistants | Trained female interviewers | Trained field workers |
| Medium of recording | Paper survey | Electronic survey | Paper survey | Paper survey |
| Average cost (95% CI) | Monthly OOP expenditures: $1.02 ($0.44 ‐ $1.60)Monthly travel costs: $2.31 ($1.75 – $2.87)Monthly food costs: $10.93 ($9.44 – $12.41)Monthly indirect costs: $15.67 ($11.88 – $19.46)Monthly income loss: $25.83 ($16.33 – $35.33)Monthly guardian costs: $6.43 ($4.59 – $8.26)Monthly carer costs: $4.63 ($1.60 – $7.65) | Total OOP expenditures: $111.83Total loan interest: $43.32Total income loss: $54.82Total guardian costs: $32.11Total carer costs: $81.99Total episode cost: $324.07 | Total OOP expenditures: $52.80 ($47.36–$58.24) | OOP expenditures for one visit to study facility: $1.96 ($1.80–$2.13) |
| Average annual income (95% CI) | $2,565 ($2,225 – $2,905) | $1,237 ($1,001 – $1,474) | Not measured (GDP per capita used as proxy) | Tanzania: $244 ($212 – 276) |
| Zambia: $219 ($199–239) | ||||
| National poverty line (USD) | $773 | $773 | $184 | Tanzania: $234 |
| Zambia: $266 | ||||
| GDP per capita (USD) | $6,618 | $6,618 | $531 | Tanzania: $695 |
| Zambia: $1,845 | ||||
| Frequency of catastrophic expenditure at 20% threshold (95% CI) | 40% (36–45%) | 59% (54–65%) | 10% (6–14%) | 4% (3–5%) |
| Minimum sample size required to estimate frequency of catastrophic expenditure with 95% CI | Error margin 5%: 2,282 | Error margin 5%: 1,057 | Error margin 5%: 13,689 | Error margin 5%: 36,504 |
| Error margin 10%: 570 | Error margin 10%: 264 | Error margin 10%: 3,422 | Error margin 10%: 9,126 | |
| Error margin 15%: 254 | Error margin 15%: 117 | Error margin 15%: 1,521 | Error margin 15%: 4,056 |
ART, antiretroviral therapy; OOP, out‐of‐pocket; GDP, gross domestic product; GP, general practitioner.
Table 2.
Framework for planning/reporting data collection
| Study planning component | Items for consideration |
|---|---|
| Comprehensiveness of survey design | • Which OOP expenditures are included? |
| • What is the level of disaggregation in cost ingredients and how long is the survey? | |
| • Are any context‐specific variables included? | |
| • How is income measured, and whose income is collected (i.e., personal or household income)? | |
| Time frame and recall | • What is the recall period for the survey? Is it appropriate to capture all economic outcomes? |
| • What is the complexity of the disease pathway? Is there resulting potential for recall bias? | |
| • Is there potential for cost truncation in the context of chronic disease and/or future complications? | |
| • Are coping strategies used to estimate the long‐term economic impact of health spending? | |
| • What is the recall period for income measurement (i.e., current vs. pre‐diagnosis)? | |
| Sample size and representativeness | • What is the confidence interval and margin of error deemed acceptable? |
| • If estimating impoverishing expenditures, what is the distribution of pre‐diagnosis income relative to the poverty line? | |
| • Are any adjustments to sample size required to account for clustering or non‐response? | |
| Data sources and survey administration | • Is a cost diary or recall used to capture expenditures? |
| • Are data supplemented with any additional data sources, such as retrospective records review or GIS data? | |
| • Where is the interview conducted and by whom? | |
| • What is the medium of collecting and recording data (i.e., electronic, paper, or telephone surveys)? |
GIS, geographic information system.
Figure 1.
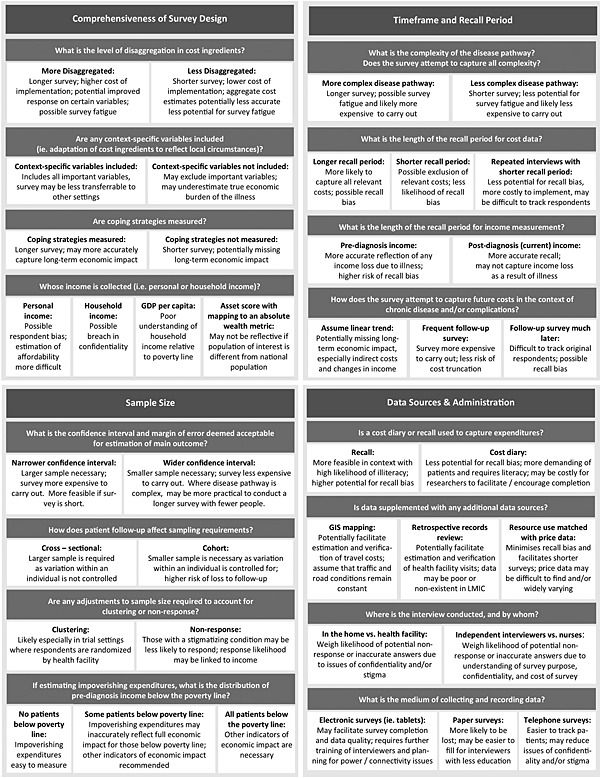
Potential advantages and limitations of alternative approaches in data collection
2. Comprehensiveness of Survey Design
There is a rich theoretical literature on the measurement of affordability in health care. The most common indicators of poverty impact are catastrophic expenditure (defined where health spending exceeds a threshold percentage of household income) and impoverishing expenditure (defined where health spending pushes a household below the poverty line) (Wagstaff, 2011; Wagstaff & van Doorslaer, 2014; Wagstaff & Eozenou, 2003). A number of theoretical challenges are associated with estimating the poverty impact of illness, which are not addressed in detail in this letter, including the appropriate denominator and thresholds for analysis and how to represent the long‐term impact of health spending (Xu et al., 2003; McIntyre et al., 2006; Russell, 1996; Flores et al., 2008; Niëns et al., 2010; Niëns & Brouwer, 2013; Pal, 2012; Wingfield et al., 2014; Onoka et al., 2011; Moreno‐Serra et al., 2011; Chuma et al., 2006; Sauerborn et al., 1996; Kruk et al., 2009). The data required are defined by the metric of poverty impact chosen but can include data on direct out‐of‐pocket expenditures for health care, any indirect costs of time associated with being ill or accessing care, and any further economic impact measures such as income loss or loan interest.
The main challenge in survey design is the representation of complex patient experiences within a manageable survey length. Survey length is of particular concern when a patient cost questionnaire follows a lengthy clinical investigation, as it increases the risk of survey fatigue and participation refusal and increases resources required to conduct the survey. Our four case studies had a range of survey durations; this is largely a function of the complexity of the patient pathways in question. MERGE and XTEND attempted to cover the overall costs of a complex illness episode over a range of different providers, whereas ECONPOP covered only a recent hospitalization and REMSTART covered only the current visit. Survey durations for each study are detailed in Table 1.
Disaggregation of cost ingredients will also affect survey length, and researchers may need to prioritize certain aspects to cover in depth. However, it is known that major drivers for patient costs can vary by setting and across income quintiles (Tanimura et al., 2014; Saksena et al., 2010 2010), making it difficult to pre‐suppose any exclusions or the relative attention placed on each aspect of expenditure or income measured. Surveys should be adapted to accurately represent the setting of interest, and researchers must be clear about which ingredients they do include and how ingredients are disaggregated.
Another widely recognized challenge is measurement of permanent income in LMICs, where informal employment is common and income is often seasonal (Ferguson et al., 2003; Deaton, 1997). Income data are difficult to collect in a small survey setting; as interviews in an intervention evaluation are conducted individually, accurate estimation of household income is often impossible. Researchers will need to decide whether personal income is an appropriate proxy for household income in their study context and be clear about the limitations of such a decision. In the XTEND and MERGE case studies, respondents consistently reported themselves to be the primary breadwinners in the household; personal income was therefore collected, with the limitation that these analyses may have underestimated the economic burden on the family as they did not account for the fact that income is shared amongst household members. On the other hand, within the ECONPOP sample, respondents were often not the primary breadwinners and often could not estimate household income. The decision was therefore made to use an assumption of gross domestic product per capita as a proxy rather than risk breaking the confidentiality of the interview by asking family members. This decision has implications for the metrics used; in this case, we did not have a firm understanding of where households lay in relation to the poverty line at baseline and, therefore, would not have been able to report on impoverishing expenditures.
Where researchers are unable to collect income directly, asset indices may also be used as a proxy measure of household socioeconomic position. Information on assets can be simpler to collect than income or consumption but result in ordinal data (Ferguson et al., 2003). In order to convert an asset index into monetary terms, necessary for the denominator of threshold metrics such as catastrophic or impoverishing expenditures, these data need to be mapped to an absolute wealth metric (Hruschka & Hadley, 2015; Howe et al., 2012). This may pose issues if income diversity in the population of interest is substantially different from that of the national population.
3. Time Frame and Recall
Deciding on the appropriate timing for the survey may also be difficult in a study where survey timing is based primarily on outcome measurement. The clinical pathways for some types of illness (for example, TB) can be long and complex, making recall bias a significant concern. This is illustrated in the XTEND survey, where patients enrolled in the trial could only be interviewed at the end of a 6‐month follow‐up period. To accommodate this, an additional sample of those on TB treatment outside the trial enrollees was also surveyed to increase sample size and allow for shorter recall periods between interviews. When capturing income loss as a result of illness in the case of complex clinical pathways, researchers will also need to weigh the risks of recall bias against the anticipated benefit of soliciting information on income before the illness.
There is also the potential for cost truncation in chronic illness or conditions with complications. The long‐term economic impact of illness can be substantial (Ilboudo et al., 2013). This can be captured by following a cohort along the clinical pathway (as in the XTEND study) or with follow‐up surveys conducted later (Ilboudo et al., 2013). However, it is a particular problem for lifelong treatments such as antiretroviral therapy.
Finally, dissaving or other coping strategies can also be an important reflection of the long‐term impact of illness, and where possible, it may be helpful to include questions on coping strategies in the survey. Surveys may directly ask how households mobilized payment for healthcare services (Flores et al., 2008), or longitudinal surveys may be able to conduct repeated asset surveys, capturing any depletion of assets caused by illness (Ilboudo et al., 2013). This is only a partial measure of the economic impact of illness on households; however, it is a useful proxy where income measurement is impossible.
4. Sample Size and Representativeness
Sample size considerations are key in the planning stages of a study and will depend on the aims, nature, and scope of the study, and the degree of precision (confidence interval and margin of error) deemed appropriate (Lwanga & Lemeshow, 1991). Household surveys generally follow United Nations guidelines of a 5–10% margin of error at the 95% confidence interval, with further adjustment to account for clustering and non‐response (United Nations Statistical Division, 2008). However, this degree of precision may be difficult to achieve in an intervention‐based context, and researchers need to be pragmatic. Some trade‐off in error margin will likely need to be made in the interests of practicality of the survey; this is especially true for outcomes that are particularly rare in the population of interest, as illustrated in Table 1. This decision should also be taken within the context of the larger uncertainty associated with the survey – for example, spending more time in the interview to avoid recall bias may produce more reliable results than spending additional time interviewing a great many more patients.
In each of our case studies, the sampling for out‐of‐pocket expenditures was restricted to a subsample of participants because of practical considerations of the study; for MERGE, XTEND, and ECONPOP, a subsample of the study population was taken, while in REMSTART, the number of follow‐up visits was limited. Table 1 shows the sample size for each case study and the ideal sample sizes necessary for various specifications of relative precision to estimate catastrophic expenditure.
Sampling considerations pose particular issues for the estimation of impoverishing expenditures when most patients are already below the poverty line – for example, where targeting those already in poverty may be a desired feature of interventions or where investigating diseases such as HIV and TB, which disproportionately affect those below the poverty line (Bates et al., 2004). When this is the case, impoverishment becomes infrequent, making power to detect the true proportion of impoverishment very low; a different metric of poverty impact should be used in these cases. All three case studies estimating income had a large proportion of poor patients: 64% of XTEND patients, 45% of MERGE patients, and 70% of REMSTART patients had a pre‐diagnosis income below the national poverty lines (Chibuye, 2014; Statistics South Africa, 2014; OECD, 2013; Laokri et al., 2013).
5. Data Sources and Survey Administration
Finally, researchers will need to identify data sources and plan administration of the survey. Researchers from the Database of Instruments for Resource Use Measurement team working in a high‐income country setting (Ridyard et al., 2015) propose a taxonomy for methods of resource use measurement including the following: the source of data, who completes the resource use measurement, how it is administered, how it is recorded, and the medium of recording. Work in LMICs requires some additional consideration, as described subsequently.
Cost diaries are considered to be the gold standard in patient cost collection (Wiseman et al., 2005; Goossens et al., 2000), but they can be time and cost intensive for researchers, especially where there is high illiteracy; patient recall is more common in low‐income settings (Beegle et al., 2012). This can be supplemented with geographic information system or other mapping data to facilitate estimation and verification of travel costs where patients are unable to estimate distances (Siedner et al., 2013), and retrospective records review can also combat recall bias in the case of frequent health facility visits (Das et al., 2012). Information on resource use can also be matched with price data to minimize recall bias; however, in LMIC, there is much wider variation in price, and market prices may not accurately reflect the economic value of resources (Hutton & Baltussen, 2005).
There may also be a distinction in survey quality depending on the interviewer and where the interview takes place. Independent research assistants may be preferable to nurses if the subject material is sensitive. Individual income and spending can be sensitive, and patients may be inclined to under‐report or over‐report income if the purpose of the interview is not well understood (Morris et al., 2000). Using trained interviewers who understand the principles and rationale for collecting patient costs also substantially affects the quality of the data; for example, the MERGE study initially experienced poor data quality, which improved after retraining interviewers. Similarly, the location of the interview will affect data quality; perceived privacy will impact patient recall and willingness to disclose details on income and spending.
Finally, the medium of recording will require particular consideration in LMICs. Electronic or telephone surveys may facilitate survey completion (Walther et al., 2011) but will require some further training of interviewers in data entry and security, and planning for power and connectivity issues in fieldwork.
6. Discussion
Using the aforementioned four case studies, we have highlighted important considerations in measuring patient costs and income in order to estimate the impact of illness on economic vulnerability in intervention‐based contexts in LMICs.
Poverty impact metrics are currently data hungry and are therefore often excluded from study surveys because of time and budgetary constraints in a research study. Going forward in these settings, economists first and foremost have a responsibility to communicate data requirements in the study design phase and advocate for the collection of patient cost data as an essential part of the economic evaluation. Additional information on patient costs and the poverty impact of health spending is more costly to collect, but these forms of analysis are increasingly important to policy makers and program planners and therefore have a high value of information.
Inevitably, some degree of variation in methods will occur across studies where context and data availability vary. Economists therefore also must communicate with each other where different approaches are possible or where compromise as to the gold standard of data collection may be managed. Robust reporting of data collection methods can help other researchers understand and interpret findings and facilitate standardization of methods. Our recommendations for reporting data collection methods for patient costs are summarized in Table 2.
Finally, it may be possible to minimize the additional cost of collecting patient cost and poverty impact data, through restricting data needs and clarifying where alternative methods are acceptable. Several alternative methodological approaches are available, and researchers must weigh limitations of potential alternatives in their own setting. Some potential advantages and limitations of various methodological approaches are described in Figure 1. We advocate for further methodological work to investigate the means to minimize the impact of cost ingredient aggregation, cost truncation, and other forms of compromise when planning poverty impact studies in LMICs, and to investigate the external validity of results that parallel effect estimates particularly in clinical trials.
This supplement confirms the increasing implementation and sophistication of economic evaluation in LMICs (Vassall et al., 2016; Pitt et al., 2016). Going forward in these settings, evaluations need to tackle policy concerns around equity and poverty. Researchers should be challenged to address fundamental data gaps for measuring the impact of illness on economic vulnerability through stronger reporting of methods and further methodological work.
Conflict of Interest
The authors declare no conflicts of interest.
Original Publication
This work draws on experience from case studies, which have been previously published; however, all methodological recommendations and other work associated with this letter are original and have not previously been published.
Ethics Statement
No ethical approval was required for this work, as it draws only on secondary data. All case studies presented in this letter obtained ethical approval for data collection; ethics statements for these studies can be found in their corresponding research articles. All case studies gave approval for their findings to be included in this letter.
Acknowledgements
The authors are grateful to the study teams for the MERGE, XTEND, REMSTART, and ECONPOP trials for use of their data and for their insight on challenges encountered in data collection. The MERGE study team includes Tendesayi Kufa, Piotr Hippner, Salome Charalambous, Katherine Fielding, Alison Grant, and Gavin Churchyard. The ECONPOP study team includes Johanne Sundby, Katerini T. Storeng, Ouattara Fatoumata, Hanne Lichtwark, Drabo Seydou, Ramatou Ouédraogo, Patrick Ilboudo, and Torsvik Gaute. The XTEND study team includes Susan Cleary, Lucy Cunnama, Gavin Churchyard, and Edina Sinanovic. The REMSTART study team includes Sode Matiku, Bernard Ngowi, Duncan Chanda, Sokoine Lesikari, Christian Bottomley, Saidi Egwaga, Amos Kahwa, Peter Mwaba, Sayoki Mfinanga, and Shabbar Jaffar. In addition, the authors are grateful to Catherine Pitt and Ulla Griffiths for their comments on an early draft of this letter.
Sweeney, S. , Vassall, A. , Foster, N. , Simms, V. , Ilboudo, P. , Kimaro, G. , Mudzengi, D. , and Guinness, L. (2016) Methodological Issues to Consider When Collecting Data to Estimate Poverty Impact in Economic Evaluations in Low‐income and Middle‐income Countries. Health Econ., 25: 42–52. doi: 10.1002/hec.3304.
The copyright line for this article was changed on 21 January 2016 after original online publication.
References
- 64th World Health Assembly . Sustainable health financing structures and universal coverage 2011: 4–7.
- Alam K, Mahal A. 2014. Economic impacts of health shocks on households in low and middle income countries: a review of the literature. Global Health 10: 21 DOI:10.1186/1744-8603-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter DM, Agboola SO, Murray MB et al. 2012. Tuberculosis and poverty: the contribution of patient costs in sub‐Saharan Africa – a systematic review. BMC Public Health 12: 980 DOI:10.1186/1471-2458-12-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates I, Fenton C, Gruber J et al. 2004. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infectious Diseases 4: 267–77. [DOI] [PubMed] [Google Scholar]
- Beegle K, De Weerdt J, Friedman J et al. 2012. Methods of household consumption measurement through surveys: experimental results from Tanzania. Journal of Development Economics 98: 3–18. DOI:10.1016/j.jdeveco.2011.11.001. [Google Scholar]
- Bill and Melinda Gates Foundation , Health Intervention and Technology Assessment Programme , Centre for Health Economics , et al Bill and Melinda Gates Foundation Methods for Economic Evaluation Project (MEEP) Final Report. 2014.
- Chibuye M. 2014. Interrogating urban poverty lines – the case of Zambia. Environment and Urbanization: 0956247813519047. [Google Scholar]
- Chuma JM, Thiede M, Molyneux CS. 2006. Rethinking the economic costs of malaria at the household level: evidence from applying a new analytical framework in rural Kenya. Malaria Journal 5: 76 DOI:10.1186/1475-2875-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Hammer J, Sánchez‐Paramo C. 2012. The impact of recall periods on reported morbidity and health seeking behavior. Journal of Development Economics 98: 76–88. DOI:10.1016/j.jdeveco.2011.07.001. [Google Scholar]
- Deaton A. 1997. The Analysis of Household Surveys: A Microeconometric Approach to Development Policy. Johns Hopkins University Press for the World Bank: Baltimore. [Google Scholar]
- OECD . 2013. Development Co‐operation Report 2013: Ending Poverty. OECD Publishing: Paris: http://dx.doi.org/10.1787/dcr-2013-en [Google Scholar]
- Drummond M, Jefferson TO. 1996. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 313, no. 7052: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BD, Tandon A, Gakidou E et al. 2003. Estimating permanent income using indicator variables. Health systems performance assessment: debates, methods and empiricism. World Health Organization: Geneva: 747–760. [Google Scholar]
- Flores G, Krishnakumar J, O'Donnell O et al. 2008. Coping with health‐care costs: implications for the measurement of catastrophic expenditures and poverty. Heal Econ 17: 1393–412. DOI:10.1002/hec.1338. [DOI] [PubMed] [Google Scholar]
- Foster N, Vassall A, Cleary S et al. 2015. The economic burden of TB diagnosis and treatment in South Africa. Social Science and Medicine 130: 42–50. DOI:10.1016/j.socscimed.2015.01.046 [DOI] [PubMed] [Google Scholar]
- Goossens MEJB, Mölken MPMHR Van, Vlaeyen JWS et al. 2000. The cost diary: a method to measure direct and indirect costs in cost‐effectiveness research. Journal of Clinical Epidemiology 53: 688–95. DOI:10.1016/S0895-4356(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Howe LD, Galobardes B, Matijasevich A et al. 2012. Measuring socio‐economic position for epidemiological studies in low‐ and middle‐income countries: a methods of measurement in epidemiology paper. International Journal of Epidemiology 41: 871–86. DOI:10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschka DJ, Hadley C. Estimating the absolute wealth of households. 2015. 483–90. [DOI] [PMC free article] [PubMed]
- Husereau D, Drummond M, Petrou S et al. 2013. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Heal Econ 14: 367–72. DOI:10.1007/s10198-013-0471-6. [DOI] [PubMed] [Google Scholar]
- Hutton G, Baltussen R. 2005. Cost valuation in resource‐poor settings. Heal Policy Plan 20: 252–9. DOI:10.1093/heapol/czi025. [DOI] [PubMed] [Google Scholar]
- Ilboudo PGC, Russell S, D'Exelle B. 2013. The Long Term Economic Impact of Severe Obstetric Complications for Women and Their Children in Burkina Faso. PLoS ONE 8: e80010 DOI:10.1371/journal.pone.0080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilboudo PGC, Greco G, Sundby J et al. 2014. Costs and consequences of abortions to women and their households: a cross‐sectional study in Ouagadougou, Burkina Faso. Health Policy and Planning 1–8. DOI:10.1093/heapol/czu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankeu HT, Saksena P, Xu K et al. 2013. The financial burden from non‐communicable diseases in low‐ and middle‐income countries: a literature review. Heal Res Policy Syst 11: 31 DOI:10.1186/1478-4505-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk ME, Goldmann E, Galea S. 2009. Borrowing and selling to pay for health care in low‐ and middle‐income countries. Health Aff (Millwood) 28: 1056–66. DOI:10.1377/hlthaff.28.4.1056. [DOI] [PubMed] [Google Scholar]
- Kufa T, Hippner P, Charalambous S et al. 2014. A cluster randomised trial to evaluate the effect of optimising TB/HIV integration on patient level outcomes: the ‘merge’ trial protocol. Contemporary Clinical Trials 39: 280–7. DOI:10.1016/j.cct.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Laokri S, Drabo MK, Weil O et al. 2013. Patients are paying too much for tuberculosis: a direct cost‐burden evaluation in Burkina Faso. PLoS One 8e56752.: . DOI:10.1371/journal.pone.0056752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Chin B, Li G et al. 2009. Limitations of methods for measuring out‐of‐pocket and catastrophic private health expenditures. Bulletin of the World Health Organization 87: 238–44. DOI:10.2471/BLT.08.054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwanga SK, Lemeshow S. 1991. Sample size determination in health studies: a practical manual. World Health Organization: Geneva, Switzerland. [Google Scholar]
- McIntyre D, Thiede M, Dahlgren G et al. 2006. What are the economic consequences for households of illness and of paying for health care in low‐ and middle‐income country contexts? Social Science and Medicine 62: 858–65. DOI:10.1016/j.socscimed.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Mfinanga S, Chanda D, Kivuyo SL et al. 2015. Cryptococcal meningitis screening and community‐based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open‐label, randomised controlled trial. Lancet 385: 2173–82. DOI:10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- Moreno‐Serra R, Millett C, Smith PC. 2011. Towards improved measurement of financial protection in health. PLoS Medicine 8e1001087.: . DOI:10.1371/journal.pmed.1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SS, Carletto C, Hoddinott J et al. 2000. Validity of rapid estimates of household wealth and income for health surveys in rural Africa. Journal of Epidemiology and Community Health 54: 381–7.http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1731675&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niëns LM, Brouwer WBF. 2013. Measuring the affordability of medicines: importance and challenges. Health Policy 112: 45–52. DOI:10.1016/j.healthpol.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Niëns LM, Cameron A, Van de Poel E et al. 2010. Quantifying the impoverishing effects of purchasing medicines: a cross‐country comparison of the affordability of medicines in the developing world. PLoS Medicine 7: DOI:10.1371/journal.pmed.1000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoka C a, Onwujekwe OE, Hanson K et al. 2011. Examining catastrophic health expenditures at variable thresholds using household consumption expenditure diaries. Trop Med Int Heal 16: 1334–41. DOI:10.1111/j.1365-3156.2011.02836.x. [DOI] [PubMed] [Google Scholar]
- Pal R. 2012. Measuring incidence of catastrophic out‐of‐pocket health expenditure: with application to India. International Journal of Health Care Finance and Economics 12: 63–85. DOI:10.1007/s10754-012-9103-4. [DOI] [PubMed] [Google Scholar]
- Pitt C, Goodman C, Hanson K. 2016. Economic evaluation in global perspective: A bibliometric analysis of the recent literature. Health Economics 25: S1. DOI: 10.1002/hec.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridyard CH, Hughes DA, Team DIRUM. 2015. Taxonomy for methods of resource use measurement. Health Economics 24: 372–8. DOI:10.1002/hec.3029. [DOI] [PubMed] [Google Scholar]
- Russell S. 1996. Ability to pay for health care: concepts and evidence. Health Policy and Planning 11: 219–37. DOI:10.1093/heapol/11.3.219. [DOI] [PubMed] [Google Scholar]
- Saksena P, Xu K, Durairaj V. 2010. The drivers of catastrophic expenditure: outpatient services, hospitalization or medicines. World Heal Rep 2010.
- Sauerborn R, Adams A, Hien M. 1996. Household strategies to cope with the economic costs of illness. Social Science and Medicine 43: 291–301. http://www.ncbi.nlm.nih.gov/pubmed/8844932 [DOI] [PubMed] [Google Scholar]
- Siedner MJ, Lankowski A, Tsai AC et al. 2013. GPS‐measured distance to clinic, but not self‐reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS 27: 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics South Africa. Poverty trends in South Africa: an examination of absolute poverty between 2006 and 2001. 2014. www.statssa.gov.za
- Tanimura T, Jaramillo E, Weil D et al. 2014. Financial burden for tuberculosis patients in low‐ and middle‐income countries: a systematic review. European Respiratory Journal 1–13. DOI:10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Statistical Division . 2008. Designing Household Survey Samples: Practical Guidelines, United Nations Publications 2008: New York. [Google Scholar]
- Vassall A, Mangham‐Jefferies L, Gomez G et al 2016. Incorporating demand and supply side constraints in economic evaluation in low- and middle-income countries. Health Economics 25: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguet S, Laxminarayan R, Jamison D. 2014. Universal public finance of tuberculosis treatment in India: an extended cost‐effectiveness analysis. Health Economics. 2014. [DOI] [PubMed]
- Wagstaff A. 2011. Measuring financial protection in health In Performance measurement for health system improvement, Smith PC, Mossialos E, Papanicolas I, Leatherman S. (eds). Cambridge University Press: Cambridge, 114–137. [Google Scholar]
- Wagstaff A, Eozenou PHV. 2003. CATA meets IMPOV: a unified approach to measuring financial protection in health. World Bank Policy Research Working Paper, 6861.
- Wagstaff A, van Doorslaer E. 2014. Catastrophe and impoverishment in paying for health care: with applications to Vietnam 1993–1998. Health Economics 12: 921–34. DOI:10.1002/hec.776. [DOI] [PubMed] [Google Scholar]
- Walther B, Hossin S, Townend J et al. 2011. Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data. PLoS One 6: e25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield T, Boccia D, Tovar M et al. 2014. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi‐drug resistance: a prospective cohort study, Peru. PLoS Medicine 11: doi:10.1371/journal.pmed.1001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman V, Conteh L, Matovu F. 2005. Using diaries to collect data in resource‐poor settings: questions on design and implementation. Health Policy and Planning 20: 394–404. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO National Health Accounts . http://www.who.int/health‐accounts/en/ (accessed 15 Mar 2015).
- Xu K, Evans DB, Kawabata K et al. 2003. Household catastrophic health expenditure: a multicountry analysis. Lancet 362: 111–7. DOI:10.1016/S0140-6736(03)13861-5. [DOI] [PubMed] [Google Scholar]


