SUMMARY
Background &aim
n-3 PUFA has been shown to decrease the risk of several components of the metabolic syndrome; however, the role of n-3 PUFA on glucose metabolism is not clear. Our aim was to systematically review the effect of n-3 PUFA on IS by conducting a meta-analysis of available RCTs.
Methods
We followed the guidelines of Cochrane’s review of systematic interventions. We searched MEDLINE, EMBASE, CENTRAL and clinicaltrials.gov from the beginning of each database until October 2010. Meta-analysis was performed using a random effects model to estimate a pooled SMD and the corresponding 95% CI.
Results
From 303 screened citations, 11 RCTs (n = 618) were eligible for inclusion in the analysis. In a pooled estimate, n-3 PUFA intervention had no effects on IS compared to placebo (SMD 0.08, 95% CI −0.11 −0.28). Similarly, n-3 PUFA had no effects on IS in sub-group analyses (Type 2 diabetes vs. other population; QUICKI and other test subgroups). In the HOMA subgroup, n-3 PUFA was associated with a statistically significant increase in IS (SMD 0.30, CI 0.03–0.58) when compared to placebo.
Conclusion
This meta-analysis is consistent with a lack of n-3 PUFA effects on IS.
Keywords: Insulin sensitivity, Diabetes, Omega-3 polyunsaturated fatty acids, Randomized controlled trials, Meta-analysis
Intake of omega-3 polyunsaturated fatty acids (n-3 PUFA) has been shown to decrease the risk of CVD1 as well as produce a favorable effect on many markers of the metabolic syndrome.2–5 These beneficial effect of omega-3 fatty acids have also been established for certain proinflammatory cytokines which are markers of both CVD and metabolic syndrome.6–8 Despite these known benefits, the role of omega-3 fatty acid on insulin resistance is still marred by controversy. Clinical parameters used to study the role of n-3 PUFA on glucose metabolism include fasting glucose, HbA1C, and insulin sensitivity (IS). While some studies have reported an unfavorable effect of n-3 PUFA on glucose metabolism9–13 other studies have reported no effects of n-3 PUFA on glucose metabolism.14–16
Insulin sensitivity (IS) is a measure of insulin responsiveness as well as insulin resistance, a disorder estimated to affect about 35% of US adults17 and it plays a significant role in the development of type 2 diabetes (T2D).18–20 Hence the effect of n-3 PUFA on IS will provide substantial information on how n-3 PUFA affects glucose-insulin homeostasis while further elucidating the role of n-3 PUFA on the risk of T2D. We therefore tested the hypothesis that n-3 PUFA is associated with a better Insulin sensitivity (IS) by conducting a meta-analysis of randomized controlled trials (RCT).
1. Methods
The present systematic review and meta-analysis was performed according to the guidelines published in the Cochrane handbook for systematic reviews of intervention.21
1.1. Inclusion/exclusion criteria
In order to minimize publication bias, our primary search identified all trials that measured and reported the effect of n-3 PUFA, irrespective of the type, on insulin sensitivity assessed either directly or indirectly. Studies were included if they were randomized control trials with fish oil or n-3 PUFA as the only active intervention. Cross-over trials were included if data at the end of the first intervention phase were available. Studies that measured insulin sensitivity as either the reciprocal of fasting insulin or glucose-insulin ratio were excluded as these methods of assessing insulin sensitivity leads to erroneous results in subjects with glucose intolerance or T2D.22
1.2. Search strategy
We performed a comprehensive electronic literature search of: MEDLINE using the Cochrane sensitivity- and precision-maximizing approach,23 Embase using the McMaster Hedges Team search filter for optimizing sensitivity and specificity,24 the Cochrane Central Register of Controlled Trials, and clinicaltrials.gov. All databases were searched from the beginning of each database until October 2010. Our comprehensive search was performed using the following key words in combination as both MeSH terms and text words: [omega-3 fatty acids or alpha-Linolenic Acid or Docosahexaenoic Acids or Eicosapentaenoic Acid] and [Avignon index or Stumvollindex or Matsuda index or HOMA or homeostasis model assessment or QUICKI or quantitative insulin sensitivity check index or modified insulin suppression test or IST or insulin suppression test or FSIVGTT or MINMOD or minimal model analysis or minimal model or glucose disposal rate or hyperinsulinemic euglycemia clamp or euglycemic clamp or glucose clamp or index of insulin sensitivity or insulin resistance or insulin sensitivity]. This was done with no language restriction. Study selection began through review of titles and abstracts. Full texts were pulled when the abstract could not be used to make sufficient judgment for inclusion. In addition, all studies that met our inclusion criteria had their references manually searched to locate additional studies. Conflicting opinions in making a decision was resolved through discussion amongst study authors. Full articles retrieved were examined independently by two investigators to identify relevant studies. For the purpose of this analysis we treated the data for the MUFA and SAFA diet subgroups in the KANWU study25 as independent data denoting them as Rosalba (MUFA diet) 2006 and Rosalba (SAFA diet) 2006 respectively (Table 1)
Table 1.
Characteristics of eligible studies.
| Study | Mean Age (Range), Years | % Male | Participants | n-PUFA dose grams/day | Trial duration | Control oil | IS Measure | Mean Baseline IS | Dropout |
|---|---|---|---|---|---|---|---|---|---|
| Abete 200845 | 36 | 56.20% | Non-diabetic, obese subjects |
Experimental group had fish based diet 3 days a week, while control did not. | 8 weeks | NA | HOMA IR | 2.65 | E -20% C-20% |
| Ingrid LM 200626 | (40–75) | 50% | Type 2 Diabetic |
2.42 g per day | 9 weeks | Corn oil | Glucose utilization measured by hyperinsulinemic isoglycemic clamp | NA | E -7.7% C-0% |
| Ingrid T 199540 | 53.67 | 64% | Non-diabetic, untreated HTN |
4 g per day | 16 weeks | Corn oil | ISI -hyperglycemic clamps | 0.17 | E -9.5% C-4.8% |
| Josune 201036 | 75 | 20% | Required TEN |
0.138 g per day | 6 months | NA | HOMA IR, QUICKI | a1.14 b0.41 | E -47% C-39% |
| Maiken 201041 | 14.3 | 100% | Slightly over weight |
1.5 g per day | 16 weeks | Mixture of Palm, soy and rapseed oil | HOMA IR | 1.1 | 10.3% combined |
| Morvarid 200742 | 55 | 0% | Type 2 Diabetic |
1.8 g per day | 2 months | Paraffin oil | HOMA S | 69.1 | 10.3% overall |
| Spadaro 200843 | 50.73 | 52.80% | NAFLD |
2 g per day | 6 months | NA | HOMA IR | 3.75 | E -10% C-10% |
| Stefano 200935 | 29.9 | 50% | OPD |
2 g per day | 12 weeks | Olive oil | QUICKI | 0.35 | E -0% C-0% |
| Tracy 200744 | 38.49 | 20% | Abdominally obese | c11 g per day | 8 weeks | NA | QUICKI | 0.34 | E -0% C-3.6% |
| Woodman 200237 | 61.2 | 76% | Type 2 Diabetic with treated HTN d |
4 g per day | 6 weeks | Olive oil | ISI measured by low-dose insulin and glucose infusion test | 5.46 | 28.8% overall |
| Rosalba 200625 (MUFA and SAFA diet groups combined) | 48.74 | 53% | Healthy subjects, non-diabetic MUFA: SAFA: |
3.6 gper day | 3 months | Olive oil | ISI measured with MINMOD program | 4.44 | 3.7% overall |
– subjects in experimental group who completed the trials, – subjects in the control group who completed the trials, E - Intervention group, C – Control group, HTN – Hypertension, NA – Not Available, ISI – Insulin sensitivity index, OPD – Offspring of patients with type 2 diabetes, TEN – Total Enteral Nutrition, NAFLD – Non-alcoholic Fatty Liver Disease.
– HOMA IR score.
– QUICKI score.
– Flaxseed oil.
– Combined EPA and DHA groups.
1.3. Data extraction
Two investigators (A.O.A and J.S.N) independently extracted all data through the use of a standardized form; the validity of data extraction was assessed by comparing the independently abstracted results for concordance and any disagreement was resolved by reaching a consensus through discussion. Integrity of extracted data was evaluated by comparing data across studies. Information extracted from each trial included: author identification, year of publication, funding source, country, study design, inclusion/exclusion criteria, type of intervention and placebo, duration of intervention, dose/mode of administration of intervention, baseline characteristics (age, sex, total cholesterol, LDL cholesterol, HDL cholesterol, fasting glucose, fasting insulin, insulin sensitivity), after-intervention report for insulin sensitivity, method used to assess insulin sensitivity and directionality of scale used to measure insulin sensitivity. Dr. Ingrid L Mostad was contacted to provide the after-intervention data for the variables listed in Table 1 of his published study26 similarly Dr. Rosalba Giacco was contacted to provide the needed data for the variables listed in Fig. 1 of her study.25
1.4. Quality assessment
We used the Cochrane Collaboration’s tool for assessing risk of bias,27 which emphasizes a domain based approach using the domains of adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other risk of bias.
1.5. Missing data
Study authors were contacted for information on missing data and other relevant information needed to complete this review.
1.6. Statistical analysis
Meta-analysis was conducted to ascertain the effect of n-3 PUFA exposure on insulin sensitivity. Differences in post-intervention values between intervention and control groups were analyzed as standardized mean difference (SMD) and the corresponding 95% CI were computed using the DerSimonian-laird random effects model, which takes into account the assumption that individual studies are measuring different effects.28 When the standard deviation (SD) was not reported, this was calculated from the Standard error of mean (SEM), p values or the confidence intervals (CI). Where the intervention was given to more than one group, we combined the data for such group.29 To ensure uniformity in the direction of the varying metrics used to measure insulin sensitivity, we reversed this for some studies. The likelihood of statistical heterogeneity was tested for using the χ2 test. The amount and statistical significance of this were reported as I2 and P value. I2 > 50% was considered substantial amount of statistical heterogeneity. To evaluate publication bias, a funnel plot of the treatment effect versus SE was visually inspected. Further evaluation of publication bias was assessed with the Begg30 and Egger test.31
We sought to detect the source of statistical heterogeneity if any, by performing subgroup analysis. Subgroup analysis was performed for: (1) study with population setting of T2D and others; (2) measures of IS which we grouped according to: HOMA, QUICKI and other measurement of insulin sensitivity. Sensitivity analysis was performed for studies with low risk of bias in domains of randomization, blinding and incomplete outcome data reporting. Estimated SMD were re-expressed into a quantifiable IS metric by multiplying with the baseline SD of one of the included trials using a method suggested by Cochrane.32 We chose QUICKI for this purpose based on available literature suggesting the use of QUICKI in defining IS.33,34 The baseline IS measure in the trial by Rizza S et al35 was used to provide an estimate of the baseline variation for IS since this value was reported for their entire study population, moreover their trial had a relatively large sample size amongst included trials.
Where more than one method for measuring IS was used to assess IS in a trial, we chose the method(s) inputting all study subjects. Based on this, Josune 201036 had 2 different IS measurements that qualified to be used for our review which were treated separately during sub-group analysis of IS measurement. Woodman 2002 et al.37 had 2 different n-3 PUFA arms (EPA and DHA). Since EPA and DHA are alternative forms of n-3 PUFA38 we pooled the data for the DHA and EPA sub groups into one n-3 PUFA group using the approach suggested by Cochrane.23 The 1-study removed method was used to investigate the cause of heterogeneity. Significant level was set at 0.05 and all P values were two tailed (α = 0.05). All analysis were conducted using RevMan 5.39
2. Results
Search strategy and study selection are depicted in Fig. 1. A total of 11 RCT’s were included in these analyses.25,26,35–37,40–45 Table 1 highlights study details. IS was a direct focus in 5 of the trials.24,26,42,44,45 Most studies used 0.138–4 g/d of n-3 PUFA as intervention except in the study by Abete et al.45 where the intervention was fatty fish. When all 11 trials were pooled, n-3 PUFA had no effect on IS (SMD = 0.08, 95% CI –0.11 to 0.28, Fig. 2a). In a sensitivity analysis, these findings were not altered when analyses were restricted to studies with a low risk of bias (Fig. 2b), T2D population vs. other populations (Fig. 2b), and type of IS test. The only exception is the HOMA subgroup where we observed that compared to placebo, n-3 PUFA was associated with a statistical significant increase in IS (Fig. 2b). There was no evidence for heterogeneity for the effect of n-3 PUFA on IS (I2 = 32%, P = 0.14) nor publication bias as revealed by a symmetrical funnel plot (Fig. 3) and non-statistically significant Begg’s test (p = 0.78) and Egger’s test (p = 0.95).
Fig. 1.
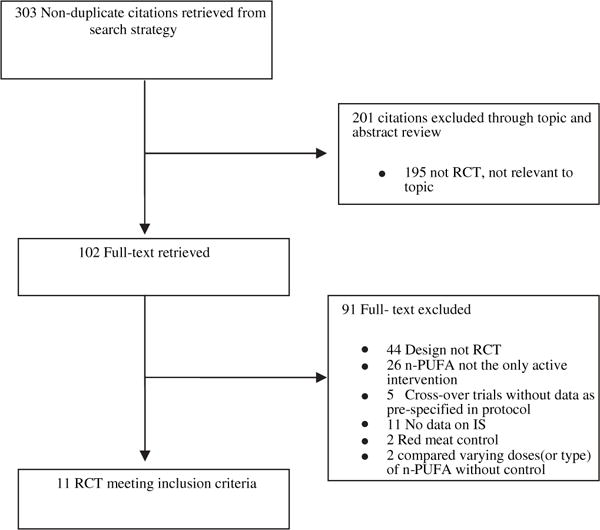
Flow diagram of study identification, inclusion and exclusion.
Fig. 2.
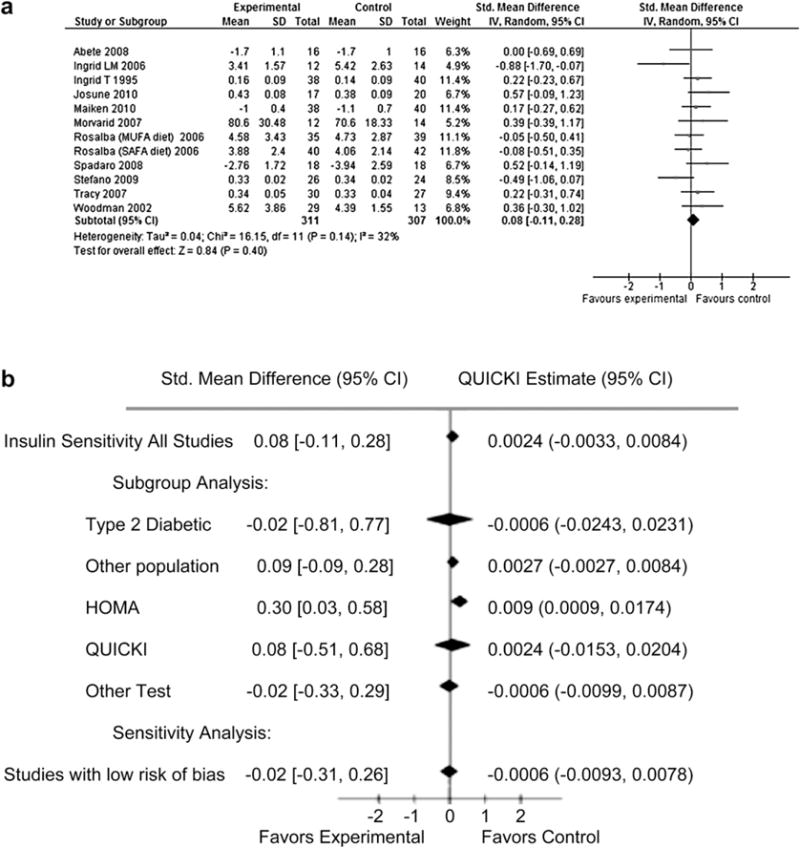
a: Forest plot showing all included trials. b: Subgroup and Sensitivity analysis.
Fig. 3.
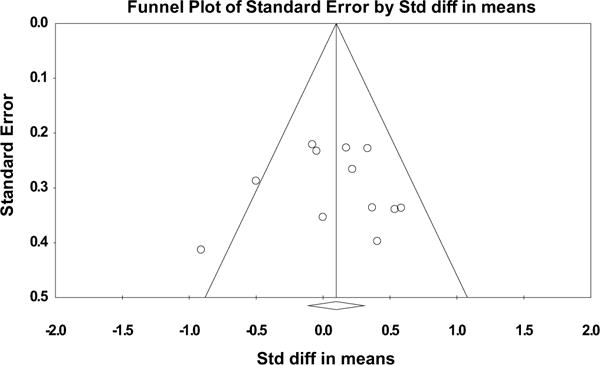
Funnel Plot to detect publication bias of included trials.
3. Discussion
This meta-analysis shows no overall association between intake of n-3 PUFA and IS. To the best of our knowledge, this is the first study to review systematically the effects of n-3 PUFA on IS in RCTs. Hartweg J et al.46 however conducted a meta-analysis for the effect of n-3 PUFA on fasting insulin release using a population of T2D. Under normal conditions, a comparison of their results to ours can be made on the basis of glucose-insulin homeostasis; rising blood glucose stimulates insulin release, the result of which is an insulin mediated action of enhanced glucose utilization in striated and adipose tissues as well as inhibition of hepatic glucose production.47 With IS being a measure of insulin responsiveness, we anticipate that a change in IS would lead to an adaptive change in insulin secretion. Hence a decrease in IS should reflect an increase in insulin secretion levels. This hypothesis is supported by published data.48–50 Hartweg J et al.46 reported that n-3 PUFA was associated with a statistically non-significant reduction in fasting insulin. However, their study population of T2D subjects makes a comparison to our data difficult since insulin levels could be erroneously high in T2D subjects.22
Importantly, since we found no overall association between n-3 PUFA and IS, this suggests that n-3 PUFA may not be a risk factor for disorders of glucose metabolism. Other authors have also suggested thesame.9–12 A meta-analysis by Montori et al.51 showing that fish oil supplementation in T2D had no significant effect on fasting glucose is consistent with our findings. Furthermore, the fact that n-3 PUFA consumption may not play a role in the development of glucose metabolism disorders is evident from two meta-analysis reporting that compared to control, n-3 PUFA was not associated with a significant change in glycated hemoglobin.46,51 Similar findings have been reported in related observational studies. In one instance, the ARIC study52 found no association between the intake of marine omega-3 fatty acids and the risk of T2D after a nine year follow-up; similar conclusion was reached by the investigators of the Kouopio study53 and Hodge et al.54 with both concluding that incident T2D was not associated with polyunsaturated fatty acids and dietary long-chain n-3 fatty acids respectively. Furthermore, a recent study by Djoussé et al.55 showed that after a median follow-up of 10.6 years, plasma phospholipids n-3 fatty acids were not associated with a higher incidence of T2D. These reports are in support of our result. Thus, n-3 PUFA may not perturb glucose-insulin homeostasis.
Conversely, there are varying results from published data. The Nurses’ Health Study56 found an increased risk of T2D when the highest to the lowest quintiles of long-chain omega-3 fatty acids were compared. Investigators from the Women’s Health Study57 also documented an increased risk of T2D with the intake of long-chain omega-3 fatty acids. Meanwhile, using data from the Cardiovascular Health Study, Djoussé et al.55 observed that individuals with the highest concentration of Plasma phospholipids n-3 fatty acids had a lower risk of T2D. Similarly, a recent publication from the Singapore Chinese Health Study58 showed that amongst 43,176 participants, increased intake of omega-3 fatty acids was associated with a decreased risk of T2D. We note however that our findings are contrary to this.
The present meta-analysis has some limitations. First, we may not have identified unpublished reports; however, our comprehensive literature search and the absence of publication bias suggest that our meta-analysis results are not driven by a selective publication of positive findings. Second, we had a limited number of randomized clinical trials; hence, our analysis did not address issues relating to dose and duration of intervention. Third, the quality of methodological approach varied across randomized trials included; however sensitivity analysis of trials with high quality maintained the robustness of our data. Furthermore, we did not identify any statistical heterogeneity between included trials. Lastly, we note the relative non-comparability of the different IS measured used in disorders of glucose metabolism.22 Nevertheless, our statistical approach was based on computation of SMD’s using a random effects model, which is a valid method to pool studies with different metrics for the same outcome measure. The strengths of our analysis also deserve mention. As in other meta-analysis, we pooled several trials which provide strong statistical power to detect small differences. Our strict inclusion criteria as well as abstraction of data by two independent investigators are additional strengths of our analysis.
In conclusion, we found that n-3 PUFA consumption did not affect IS. Larger scale trials may provide more conclusive result of n-3 PUFA consumption and IS.
Acknowledgments
We thank Dr. Ingrid L Mostad and Dr. Rosalba Giacco for providing data on their respective study for inclusion in the current meta-analysis.
Abbreviations
- CAD
coronary artery disease
- CI
confidence interval
- CVD
cardiovascular disease
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- FSIVGTT
frequently sampled intravenous glucose tolerance
- HDL
high density lipoprotein
- HOMA
homeostasis model assessment
- IR
insulin resistance
- IS
insulin sensitivity
- IST
insulin suppression test
- LDL
low density lipoprotein
- Mesh
medical subject headings
- MINMOD
minimal modal analysis
- n-3 PUFA
Omega-3 polyunsaturated fatty acid
- NAFLD
non-alcoholic fatty liver disease
- QUICKI
quantitative insulin sensitivity check index
- RCTs
Randomized Controlled Trials
- SD
standard deviation
- SMD
standardized mean difference
- T2D
type 2 diabetes
Footnotes
Statement of authorship
The author’s responsibilities were as follows – AOA and LD were involved in designing the study; AOA and JSN carried out literature search and data analysis; AOA drafted the manuscript; AOA, JSN, JBM and LD critically appraised the manuscript; and LD supervised the study. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006 Aug 21;98(4A):3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [Epub 2006 May 30] [DOI] [PubMed] [Google Scholar]
- 2.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006 Nov;189(1):19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [Epub 2006 Mar 10] [DOI] [PubMed] [Google Scholar]
- 3.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(suppl):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 4.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002 Aug;20(8):1493–9. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Buckley JD, Howe PR. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009 Nov;10(6):648–59. doi: 10.1111/j.1467-789X.2009.00584.x. [Epub 2009 May 12] [DOI] [PubMed] [Google Scholar]
- 6.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000 Jan;71(1 Suppl):343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 7.Madsen T, Skou HA, Hansen VE, Fog L, Christensen JH, Toft E, et al. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am J Cardiol. 2001 Nov 15;88(10):1139–42. doi: 10.1016/s0002-9149(01)02049-5. [DOI] [PubMed] [Google Scholar]
- 8.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 9.Schectman G, Kaul S, Kissebah AH. Effect of fish oil concentrate on lipoprotein composition in NIDDM. Diabetes. 1988 Nov;37(11):1567–73. doi: 10.2337/diab.37.11.1567. [DOI] [PubMed] [Google Scholar]
- 10.Glauber H, Wallace P, Griver K, Brechtel G. Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1988 May;108(5):663–8. doi: 10.7326/0003-4819-108-5-663. [DOI] [PubMed] [Google Scholar]
- 11.Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004 Oct;80(4):887–95. doi: 10.1093/ajcn/80.4.887. [DOI] [PubMed] [Google Scholar]
- 12.Risérus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002 Sep;25(9):1516–21. doi: 10.2337/diacare.25.9.1516. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care. 1998 Apr;21(4):494–500. doi: 10.2337/diacare.21.4.494. [DOI] [PubMed] [Google Scholar]
- 14.Puhakainen I, Ahola I, Yki-Järvinen H. Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1995 Jan;61(1):121–6. doi: 10.1093/ajcn/61.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Annuzzi G, Rivellese A, Capaldo B, Di Marino L, Iovine C, Marotta G, et al. A controlled study on the effects of n-3 fatty acids on lipid and glucose metabolism in non-insulin-dependent diabetic patients. Atherosclerosis. 1991 Mar;87(1):65–73. doi: 10.1016/0021-9150(91)90233-s. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi A, et al. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care. 1998 May;21(5):717–24. doi: 10.2337/diacare.21.5.717. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care. 2006 Nov;29(11):2396–402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 18.Moller DE, Flier JS. Insulin resistance-mechanisms, syndromes, and implications. N Engl J Med. 1991 Sep 26;325(13):938–48. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991 Mar;14(3):173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007 Jul 31;104(31):12587–94. doi: 10.1073/pnas.0705408104. [Epub 2007 Jul 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. Available from: www.cochrane-handbook.org. [accessed through 1.10.2010 and 6.12.2010] [Google Scholar]
- 22.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008 Jan;294(1):E15–26. doi: 10.1152/ajpendo.00645.2007. [Epub 2007 Oct 23] [DOI] [PubMed] [Google Scholar]
- 23.Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.2 (updated September 2009) The Cochrane Collaboration; 2009. Available from: www.cochrane-handbook.org. [accessed 6.10.2010] [Google Scholar]
- 24.Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Lib Assoc. 2006;94:41–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Giacco R, Cuomo V, Vessby B, Uusitupa M, Hermansen K, Meyer BJ, et al. KANWU Study Group Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr Metab Cardiovasc Dis. 2007 Oct;17(8):572–80. doi: 10.1016/j.numecd.2006.06.006. [Epub 2006 Nov 28] [DOI] [PubMed] [Google Scholar]
- 26.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr. 2006 Sep;84(3):540–50. doi: 10.1093/ajcn/84.3.540. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.1 (updated September 2008) The Cochrane Collaboration; 2008. Chapter 8: assessing risk of bias in included studies. Available from: www.cochrane-handbook.org. [accessed 15.11.2010] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Deeks JJ, Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.1 (updated September 2008) The Cochrane Collaboration; 2008. Chapter 7: selecting studies and collecting data. Available from: www.cochrane-handbook.org. [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 31.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.1 (updated September 2008) The Cochrane Collaboration; 2008. Available from: www.cochrane-handbook.org. [Google Scholar]
- 33.Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, et al. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006 Aug;21(4):695–700. doi: 10.3346/jkms.2006.21.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hrebícek J, Janout V, Malincíková J, Horáková D, Cízek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002 Jan;87(1):144–7. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]
- 35.Rizza S, Tesauro M, Cardillo C, Galli A, Iantorno M, Gigli F, et al. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 2009 Oct;206(2):569–74. doi: 10.1016/j.atherosclerosis.2009.03.006. [Epub 2009 Mar 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olza J, Mesa MD, Aguilera CM, Moreno-Torres R, Jiménez A, Pérez de la Cruz A, et al. Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin Nutr. 2010 Feb;29(1):31–7. doi: 10.1016/j.clnu.2009.06.003. [Epub 2009 Jul 1] [DOI] [PubMed] [Google Scholar]
- 37.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002 Nov;76(5):1007–15. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 38.Calder PC, Yaqoob P. Understanding omega-3 polyunsaturated fatty acids. Postgrad Med. 2009 Nov;121(6):148–57. doi: 10.3810/pgm.2009.11.2083. [DOI] [PubMed] [Google Scholar]
- 39.Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2008. [Google Scholar]
- 40.Toft I, Bønaa KH, Ingebretsen OC, Nordøy A, Jenssen T. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med. 1995 Dec 15;123(12):911–8. doi: 10.7326/0003-4819-123-12-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen MH, Mølgaard C, Hellgren LI, Lauritzen L. Effects of fish oil supplementation on markers of the metabolic syndrome. Pediatr. 2010 Sep;157(3):395–400. 400.e1. doi: 10.1016/j.jpeds.2010.04.001. Epub 2010 May 15. [DOI] [PubMed] [Google Scholar]
- 42.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007 Dec;86(6):1670–9. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 43.Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008 Mar;40(3):194–9. doi: 10.1016/j.dld.2007.10.003. Epub 2007 Dec 4. [DOI] [PubMed] [Google Scholar]
- 44.Nelson TL, Stevens JR, Hickey MS. Adiponectin levels are reduced, independent of polymorphisms in the adiponectin gene, after supplementation with alpha-linolenic acid among healthy adults. Metabolism. 2007 Sep;56(9):1209–15. doi: 10.1016/j.metabol.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Abete I, Parra D, Crujeiras AB, Goyenechea E, Martinez JA. Specific insulin sensitivity and leptin responses to a nutritional treatment of obesity via a combination of energy restriction and fatty fish intake. J Hum Nutr Diet. 2008 Dec;21(6):591–600. doi: 10.1111/j.1365-277X.2008.00902.x. [Epub 2008 Aug 27] [DOI] [PubMed] [Google Scholar]
- 46.Hartweg J, Perera R, Montori VM, Dinneen SF, Neil AHAWN, Farmer AJ. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD003205.pub2. Art. No.: CD003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahn BB. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes. 1996 Nov;45(11):1644–54. doi: 10.2337/diab.45.11.1644. [DOI] [PubMed] [Google Scholar]
- 48.Pontiroli AE, Monti LD, Costa S, Sandoli PE, Pizzini A, Solerte SB, et al. In middle-aged siblings of patients with type 2 diabetes mellitus normal glucose tolerance is associated with insulin resistance and with increased insulin secretion. The SPIDER study. Eur J Endocrinol. 2000 Nov;143(5):681–6. doi: 10.1530/eje.0.1430681. [DOI] [PubMed] [Google Scholar]
- 49.Pimenta WP, Santos ML, Cruz NS, Aragon FF, Padovani CR, Gerich JE. Insulin secretion, insulin sensitivity, and hepatic insulin extraction in first-degree relatives of type 2 diabetic patients. Braz J Med Biol Res. 2003 Mar;36(3):301–8. doi: 10.1590/s0100-879x2003000300003. [Epub 2003 Mar 7] [DOI] [PubMed] [Google Scholar]
- 50.Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;95:690–8. doi: 10.1172/JCI117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000 Sep;23(9):1407–15. doi: 10.2337/diacare.23.9.1407. [Review] [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH, ARIC Study Investigators Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003 Jul;78(1):91–8. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 53.Laaksonen DE, Lakka TA, Lakka HM, Nyyssönen K, Rissanen T, Niskanen LK, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med. 2002 Jun;19(6):456–64. doi: 10.1046/j.1464-5491.2002.00707.x. [DOI] [PubMed] [Google Scholar]
- 54.Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007 Jul;86(1):189–97. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 55.Djoussé L, Biggs ML, Lemaitre RN, King IB, Song X, Ix JH, et al. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr. 2011 May 18; doi: 10.3945/ajcn.111.013334. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009 Sep;90(3):613–20. doi: 10.3945/ajcn.2008.27424. [Epub 2009 Jul 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Djoussé L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011 Jan;93(1):143–50. doi: 10.3945/ajcn.110.005603. [Epub 2010 Oct 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brostow DP, Odegaard AO, Koh WP, Duval S, Gross MD, Yuan JM, et al. Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2011 May 18; doi: 10.3945/ajcn.110.009357. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


