Abstract
We hypothesized that the distinct maturational processes take place across different cortical areas from middle fetal stage to normal time of birth and these maturational processes are altered in late third trimester. Fractional anisotropies (FA) from diffusion tensor imaging (DTI) infer the microstructures of the early developing cortical plate. High-resolution DTI of 11 fetal brain specimens at postmenstrual age of 20 weeks (or simplified as 20 weeks), 19 in vivo brains at 35 weeks, and 17 in vivo brains at normal time of birth at term (40 weeks) were acquired. Population-averaged age-specific DTI templates were established with large deformation diffeomorphic metric mapping for subject groups at 20, 35, and 40 weeks. To alleviate partial volume effects, skeletonized FA values were used for mapping averaged cortical FA to the cortical surface and measuring FA at 12 functionally distinctive cortical regions. Significant and heterogeneous FA decreases take place in distinct cortical areas from 20 to 35 weeks and from 35 to 40 weeks, suggesting differentiated cortical development patterns. Temporally nonuniform FA decrease patterns during 35–40 weeks compared with those during 20–35 weeks were observed in higher-order association cortex. Measured skeletonized FA suggested dissociated changes between cerebral cortex and white matter during 35–40 weeks.
Keywords: cortical microstructure, DTI, fetal brain, heterogeneous, population-averaged template
Introduction
During fetal development, cortical neurons are generated in the ventricular and subventricular zone and migrate toward the cortical plate along the radial glial scaffold (Rakic 1972, 1995; Sidman and Rakic 1973). In the cortical plate, migrating neurons interact with each other or with existing neurons of the cortical plate through synaptic formation, dendritic arborization, and axonal growth (e.g., Sidman and Rakic 1973; Kostović and Jovanov-Milosević 2006; Bystron et al. 2008). The predominantly radially organized structures in the cortical plate are disrupted by these complicated yet well-organized processes (e.g., Rakic 1972, 1995, 1988; Sidman and Rakic 1982; Kostović et al. 2002), from mid-fetal (around postmenstrual age of 20 or 20 weeks) (see Engle et al. 2004 for definition of postmenstrual age) to normal time of birth (40 weeks). The fetal and preterm brain ages in week (week) hereafter are all postmenstrual ages.
High-resolution diffusion tensor imaging (DTI) (Basser et al. 1994), a type of magnetic resonance imaging (MRI) method, has been applied as an effective noninvasive approach to reveal the structural changes of early developing cerebral cortex. DTI-derived fractional anisotropy (FA) is highly sensitive to the disruption of the radial glial scaffold and can be an effective quantitative probe to imply cortical maturation processes. Specifically, in the premature cortical palate, the water molecules diffuse preferably along the direction perpendicular to the cortical surface through radially arranged scaffolding of glial cells where high cortical FA values are usually observed. The cortical maturation processes including dendritic arborization, synaptic formation, and neuronal differentiation disrupt the radial organization and result in FA decrease. Such FA decreases have been documented in a number of studies on developing human (McKinstry et al. 2002; Mukherjee et al. 2002; Neil et al. 2002; Maas et al. 2004; Deipolyi et al. 2005; Huang et al. 2006; Huang et al. 2009; Ball et al. 2013; Huang et al. 2013) and animal cerebral cortex (Thornton et al. 1997; Mori et al. 2001; Kroenke et al. 2007, 2009; Sizonenko et al. 2007; Huang et al. 2008; Takahashi et al. 2011).
The cortical FA measurements of the fetal brain during second trimester suggest distinct cortical structural changes at different cortical areas during 13–21 weeks of gestation (Huang et al. 2013). We hypothesize that the distinct maturational processes of the human brain cortical plate from middle fetal stage (20 weeks) to normal time of birth (40 weeks) continue to take place across the cortical areas, and these maturational processes are altered in late third trimester. 20, 35, and 40 weeks are 3 characteristic time points during human fetal development. Twenty weeks is right at the middle time point of fetal development. Thirty-five weeks is in the middle of third trimester, during which rapid human brain maturational processes take place in preparation for birth. Forty weeks marks the end of fetal brain development. Human brain structures are highly variable across different individuals. Population-averaged brain structures at a certain time point can better represent the age-specific brain structures than individual ones with less bias. In this study, we aimed to access not only the spatiotemporal pattern of cortical maturation patterns during 20–35 and 35–40 weeks but also find out if there are any dissociated changes between cerebral cortex and white matter (WM) during the late third trimester, that is, 35–40 weeks. High-resolution DTI of 11 fetal brain specimens at 20 weeks, 19 in vivo brains at 35 weeks, and 17 in vivo brains at 40 weeks were acquired. Age-specific population-averaged FA maps were established with large deformation diffeomorphic metric mapping (LDDMM) (Miller et al. 2002) for intragroup registration at 20, 35, and 40 weeks, respectively. To alleviate partial volume effects, skeletonized FA values were used for mapping averaged cortical FA to the cortical surface and measuring cortical FA at 12 functionally distinct regions of interests (ROIs). The temporal course of entire cortical FA was also accessed and compared with entire WM FA changes.
Materials and Methods
In Vivo Preterm Subjects and Ex Vivo Fetal Specimens
Data from 36 normal subjects and 11 ex vivo specimens were acquired. They were divided into 3 groups based on the postmenstrual age, with the first group including 11 ex vivo fetal brains in the middle of second trimester (age range 19–20 weeks, 19.5 ± 0.52 weeks), second group including 19 perinatal brains in the middle of third trimester (age range at scan: 34.3–35.8 weeks, 35.1 ± 0.55 weeks; age range at birth: 31–34.8 weeks, 33.1 ± 1.23 weeks), and third group including 17 perinatal brains around term (age range at scan 40–41.7 weeks, 40.7 ± 0.55 weeks; age range at birth: 39.3–41.4 weeks, 40.2 ± 1.20 weeks). The ex vivo fetal brains were obtained from the University of Maryland Brain and Tissue Bank (BTB) for Developmental Disorder (NICHD contract no. N01-HD-9-0011). Normality of the ex vivo fetal brain at mid-fetal stage was ensured by the BTB. All in vivo preterm and term neonates were recruited from Parkland Hospital at Dallas. They were part of the cohort for studying normal prenatal and perinatal development and selected after rigorous screening procedures conducted by a neonatologist (L.C.) and an experienced pediatric radiologist (N.R.), based on subjects' ultrasound, clinical MRI, and subjects' and mothers' medical record. Exclusion criteria include mother's excessive drug or alcohol abuse during pregnancy; grade III–IV intraventricular hemorrhage; periventricular leukomalacia; hypoxic-ischemic encephalopathy; lung disease or brochopulmonary dysplasia; body or heart malformations; chromosomal abnormalities; necrotizing enterocolitis that requires intestinal resection or complex feeding/nutritional disorders; defects or anomalies of forebrain, brainstem, or cerebellum; brain tissue dys- or hypoplasias; abnormal meninges (for postmortem tissues); alterations in the pial or ventricular surface; or WM lesions. All subjects' parents gave informed written consent approved by the Institutional Review Board.
DTI Acquisition
DTI of Ex Vivo Mid-Fetal Brain Specimens at around 20 Weeks
Fetal brain samples around 20 weeks were kept immersed in the fixation solution until 48 h before the MR experiments. The samples were then transferred to PBS to wash out the fixative. The samples were immersed in PBS in a custom-made MR compatible chamber throughout the MR scanning. Three-dimensional multiple spin echo DTI was performed in a 4.7T Bruker scanner with a 70-mm-inner-diameter Bruker volume coil. A multiple echo (number of echoes = 8) sequence was adopted to improve the SNR. A set of diffusion-weighted images (DWIs) were acquired in 7 linearly independent directions with the following parameters: effective TE = 66 ms, TR = 0.8 s, FOV = 48–52/48–52/48–52 mm, imaging matrix = 128 × 72 × 72 (zero filled to data matrix = 128 × 128 × 128), b value = 1000 s/mm2. The resultant image resolution was isotropic 280–350 µm. The total imaging time was ∼20 h per brain with 2 signal averages.
DTI of in Vivo Preterm and Term Subjects around 35 and 40 Weeks
All neonates were well fed before scanning and kept asleep during scan. Besides earplugs and earphones, extra foam padding was applied to reduce the sound of the scanner while the neonates were asleep. DTI data were acquired from 3T Philips Achieva MR system (Cleveland) at Children's Medical Center. The DTI imaging parameters were as follows: TE = 78 ms, TR = 6850 ms, in-plane field of view = 168 × 168 mm2, in-plane imaging resolution = 1.5 × 1.5 mm2, slice thickness = 1.6 mm, slice number = 60, 30 independent diffusion gradient directions (Jones et al. 1999) uniformly distributed in space, b-value = 1000 s/mm2, and repetition = 2. The axial DWI image dimension was 256 × 256 after reconstruction. The total acquisition time was 11 min for DTI. With 30 DWI volumes and 2 repetitions, we accepted those scanned DWI datasets with <5 DWI volumes affected by motion more commonly seen in scanning of neonates and toddlers. Note that one DWI volume corresponds to one diffusion gradient direction. The affected volumes were replaced by the good volumes of another DTI repetition during postprocessing.
Data Analysis
Fitting of Diffusion Tensor and Generation of DTI-Derived Maps
DWIs acquired from all the subjects were processed offline using DTIStudio (mristudio.org; Jiang et al. 2006). DWIs for each subject were corrected for motion and eddy current by registering all the DWIs to the b0 image using a 12-parameter (affine) linear image registration with automated image registration algorithm (Woods et al. 1998). Six elements of the diffusion tensor were then fitted. FA maps, derived from diffusion tensor, were obtained for all subjects. DTI color-encoded map combines the information of FA and largest tensor eigenvector ν1, which can be used for indicating fiber orientation. In the colormap, red (R), green (G), and blue (B) colors are assigned to left–right, anterior–posterior, and superior–inferior orientations, respectively.
Establishment of Population-Averaged DTI Maps
A single-subject brain with median brain size and relatively straight medial longitudinal fissure was used as a single-subject template for intersubject registration at 20 weeks. The anterior commissure (AC) can be clearly identified in DTI color-encoded map (see, e.g., Huang et al. 2009) and was aligned with the upper edge of the rectum in the single-subject template at 20 weeks. Population-averaged FA maps and DTI color-encoded maps at 20 weeks were generated with affine (Woods et al. 1998) and LDDMM (Miller et al. 2002) transformation. For DTI at 35 and 40 weeks, the 6-step procedures used to generate the JHU-neonate-nonlinear atlas (Oishi et al. 2011) were adopted to generate the population-averaged templates, besides the generation of single-subject templates. Briefly, these 6 steps include 1 step of AC–PC (posterior commissure) alignment, 1 step of direct averaging of images, 3 consecutive steps of averaging of images after 3 affine transformations, respectively, and 1 last step of averaging after LDDMM transformation. For tensor transformation, the affine transformation matrix and LDDMM transformation matrix obtained from the scalar images were applied to the tensor field to create normalized tensor fields with details described in the literature (Xu et al. 2003). Such tensor transformations were conducted with DiffeoMap software (mristudio.org).
Skeletonization
Skeletonization of the averaged 20 and 35 weeks; brain was based on the averaged FA maps at these 2 time points. Note that high FA was observed not only at the WM but also cortical plate of averaged fetal brain FA at 20 and 35 weeks. Skeletonization function in TBSS of FSL (fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) was used to extract both the “core” cortical plate and “core” WM FA values from averaged FA map. Since cortical FA at 40-week brain is relatively low, applying the skeletonization function to the averaged FA map at 40 weeks could not provide skeletons for the cortical plate, but only skeletons of WM. We adopted a method (Ball et al. 2013) based on averaged cortical segmentation map to obtain the skeleton. Specifically, after cortical segmentation of each 40-week brain was obtained using FAST (“FMRIB's Automated Segmentation Tool,” Functional MRI of the Brain Centre), LDDMM was applied to nonlinearly transform the segmented cortical plate to the template space. Skeletonization function was applied to the averaged cortical segmentation map to obtain the skeleton of 40-week brain. To be consistent to the 20- and 35-week skeletonized cortical FA, highest FA value in the cortical plate and perpendicular to the cortical skeleton was kept as the skeletonized FA of the 40-week brain.
Mapping of the Cortical FA to the Surface and Measurement of Skeletonized Cortical and WM FA
Mapping of the Cortical FA
The FA values on the cortical surface of 20-, 35-, and 40-week brains were projected from the averaged skeletonized cortical FA at these 3 time points, respectively. The averaged diffusion-weighted images (aDWIs) of the single-subject template at each time point were used for three-dimensional surface reconstruction. Specifically, triangular meshes of the cortical surface were created by using Amira software (FEI) from the isosurface function after intensity thresholding aDWI of the template brain. On each triangular vertex, an FA value was assigned by reading the skeletonized FA value perpendicular to the surface. In rare cases when the line perpendicular to the surface did not cross the cortical skeletonized FA, a cubic box with 5 voxels on each side and centered at the surface vertex was established and the maximum skeletonized FA value of this cubic box ROI was assigned as the FA value of this triangular vertex.
Measurement of Cortical FA at 12 Cortical ROIs
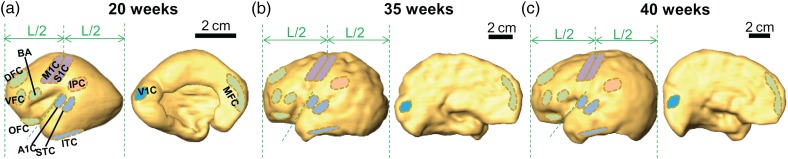
To obtain the surface ROIs, surface editing functions in Amira software were used to directly and manually delineate these ROIs from the three-dimensionally reconstructed cortical surface of 20-, 35-, and 40-week single-subject template brains. The placement of 12 ROIs followed previously published descriptions (Johnson et al. 2009; Kang et al. 2011) and protocol (Huang et al. 2013), since there is no digital cortical parcellation atlas for fetal brains at 20 and 35 weeks. The cortical surfaces of 35 and 40 weeks template were smoothed (as shown in Fig. 1) for convenient surface ROI delineation. These ROIs represented the Broca area (BA), orbital prefrontal cortex (OFC), dorsolateral prefrontal cortex (DFC), medial prefrontal cortex (MFC), ventrolateral prefrontal cortex (VFC), motor cortex (M1C), somatosensory cortex (S1C), posterior inferior parietal cortex (IPC), primary auditory cortex (A1C), posterior superior temporal cortex (STC), inferior temporal cortex (ITC), and primary visual (occipital) cortex (V1C). The delineated 12 cortical ROIs for 20-, 35-, and 40-week brain are demonstrated in Figure 1. The DFC, OFC, MFC, VFC, M1C, and BA are located at the frontal cortex. A1C, STC, and ITC are located at the temporal cortex. S1C and IPC are located at the parietal cortex. V1C is located at the occipital cortex. The detailed ROI placement procedure and reproducibility test results of manual placement of these ROIs can be found in Supplementary Material. Briefly, the intrarater reproducibility is almost perfect for all cortical ROI placements on brains at all 3 ages. The interrater agreement is either almost perfect or substantial except placement of IPC of 35-week brain. The procedure described in “mapping of the cortical FA” above to project skeletonized FA to the cortical surface was inversed to obtain the skeletonized FA values corresponding to a specific cortical ROI surface piece for each individual subject. The cortical FA values at the ROIs of each individual subject were measured in the template space.
Figure 1.
The 12 cortical ROIs (BA, DFC, VFC, MFC, OFC, M1C, S1C, IPC, A1C, STC, ITC, and V1C) on the lateral and medial view of the reconstructed cortical surface of single-subject template brain at 20 weeks (a), 35 weeks (b), and 40 weeks (c). The vertical dashed green lines define the most anterior and most posterior boundary of the cerebral hemisphere. L is the distance between the most anterior and most boundary. The oblique dashed green line along the upper edge of the temporal lobe is also shown as the reference line. BA, Broca area; DFC, dorsolateral prefrontal cortex (PFC); VFC, ventrolateral PFC; MFC, medial PFC; OFC, orbital PFC; M1C, motor cortex; S1C, somatosensory cortex; IPC, posterior inferior parietal cortex; A1C, primary auditory cortex; STC, posterior superior temporal cortex; ITC, inferior temporal cortex; V1C, primary visual (occipital) cortex.
Measurement of FA of Entire Cortical Surface and WM
The skeleton based on population-averaged FA maps at 20- and 35-week brains was manually segmented into cerebral cortex and WM. Note that the skeleton based on population-averaged FA map at 40-week brains was exclusively inside WM, and the skeleton of cortical plate of 40-week brains was obtained from averaged cortical segmentation map described in details earlier. In the template space, the cortical and WM skeletons were used as binary masks. Together with the FA map of individual subjects in the template space, averaged skeletonized FA values for entire cortical surface and WM of each individual were calculated.
Statistical Analysis
All statistical procedures were performed using R statistical software version 3.0.2 (http://www.r-project.org/). General linear model (GLM) as shown in the following equation was used to test the FA decrease rate differences among different regions during 20–35 or 35–40 weeks and FA decrease rate differences between the periods of 20–35 and 35–40 weeks.
| (1) |
where FAi,j,k was defined as averaged FA values in region Ri, at a specific time point τj, and of subject k; β0,i,j,k was the constant; β1,i,j,k, β2,i,j,k, and β3,i,j,k represent the parameters to be estimated for Ri, τj, and Riτj, respectively; ϵi,j,k was the error term, satisfying identical independent distribution (i.i.d.) with standard deviation; Ri was one of the cortical ROIs, namely, BA, OFC, DFC, MFC, VFC, M1C, S1C, IPC, A1C, STC, ITC, and V1C; τj was one of the time points, namely, 20, 35, and 40 weeks; i was from 1 to 12, indicating ith region; j was from 1 to 3, indicating jth time points; k was from 1 to 11 at 20 weeks, 1 to 19 at 35 weeks, and 1 to 17 at 40 weeks.
Test of Significant FA Decrease in 20–35 or 35–40 Weeks
The null hypothesis is that the FA rate is equal to 0 during the periods of 20–35 or 35–40 weeks. Rejection of the null hypothesis indicates significant FA decrease at a specific time period and region. The general linear model was reduced to include only the age variable τj with j = 1,2 for 20–35 weeks and j = 2,3 for 35–40 weeks as well as the error and constant term. Each time period's statistics were Bonferroni corrected (Bonferroni 1936) for multiple comparisons.
Test of Pairwise Interregion Differences of FA Decrease Rates during 20–35 or 35–40 Weeks
The null hypothesis is where the FA rates for a chosen pair of regions are equal. Rejection of the null hypothesis indicates significant FA rate differences between the 2 regions. Each pairwise FA subset was fitted with the full general linear model. The subset was also fitted to the reduced general linear model removing the interaction term. The 2 model fits were compared using ANOVA revealing if the pairwise regions have significant differences in FA rates. Statistical P-values were Bonferroni corrected for multiple comparisons.
Test of FA Decrease Rates between the Periods of 20–35 and 35–40 Weeks at the Same Region
The null hypothesis is where the FA rate from 20 to 35 weeks is equal to the FA rate from 35 to 40 weeks at the same region. Rejection of the null hypothesis indicates a significant acceleration or deceleration of the FA rate from 20–35 to 35–40 weeks. The general linear model was reduced to include only the age variable τj as well as the error and constant term. Rejection of the null hypothesis that β2 in 2 time periods are equal reveals a significant alteration of the FA rate between the 2 time periods and a temporally nonuniform FA decrease in 35–40 weeks compared with 20–35 weeks. Statistics were also Bonferroni corrected for multiple comparisons.
Results
Population-Averaged DTI Maps at 20, 35, and 40 Weeks
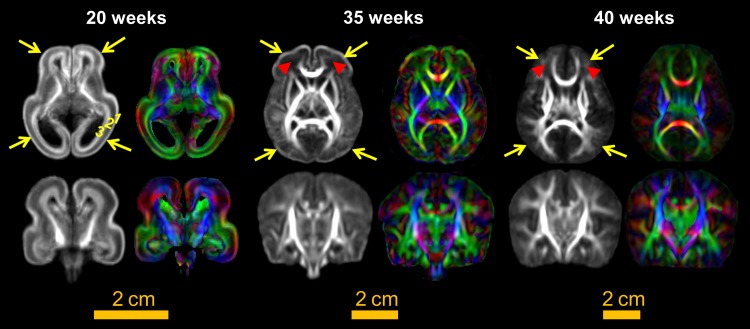
In Figure 2, the population-averaged FA and DTI color-encoded maps at 20, 35, and 40 weeks delineate cortical and WM structures at each time point with less individual biases. As early as 35 weeks, adult-like deep WM and subcortical nuclei can be appreciated. From 20 to 40 weeks, dramatic macrostructural changes of brain anatomy can be observed. These changes include that relative volumes of ventricles get smaller, relative volumes of WM become larger, gyrification becomes more prominent, and unique fetal cerebral wall gets reorganized. For population-averaged FA map at 20 weeks, the laminar structure of the cerebral wall can be appreciated with 3 layers clearly differentiated. These 3 layers include cortical plate, subplate, and an inner layer from outside to inside (Huang et al. 2013). The laminated organization disappears at 35 or 40 weeks. The subplate and peripheral unmyelinated WM at 35 weeks are located at apparent dark FA regions (indicated by red arrows) between high-FA cortical plate and deep WM. At 40 weeks, these dark regions with small FA at 35 weeks become brighter and are exclusively peripheral WM (also indicated by red arrows), whereas the subplate is no longer visible. As highlighted by the yellow arrows, the most prominent feature of the population-averaged FA maps in Figure 2 is the bright cortical FA band at 20 and 35 weeks and absence of such cortical FA band at 40 weeks. Population-averaged cortical FA appears brightest for 20-week brains among all ages with the bright cortical FA band shown across the entire cortical plate. The bright cortical FA band is more prominent at the frontal and temporal cortex at 35 weeks. It disappears in relatively short period of 5 weeks during 35–40 weeks, indicating a possible acceleration of FA decrease during these 5 weeks. In terms of FA contrasts between cortical plate and WM, averaged cortical FA looks as bright as WM FA at 20 weeks. As the brain matures, cortical FA decreases while WM FA increases. At 40 weeks, WM FA is predominantly higher than that of cortical FA.
Figure 2.
FA (left in each panel) and color-encoded orientation (right in each panel) maps of the population-averaged DTI templates at 20, 35, and 40 weeks. Axial and coronal views are shown in the upper and lower panels, respectively.
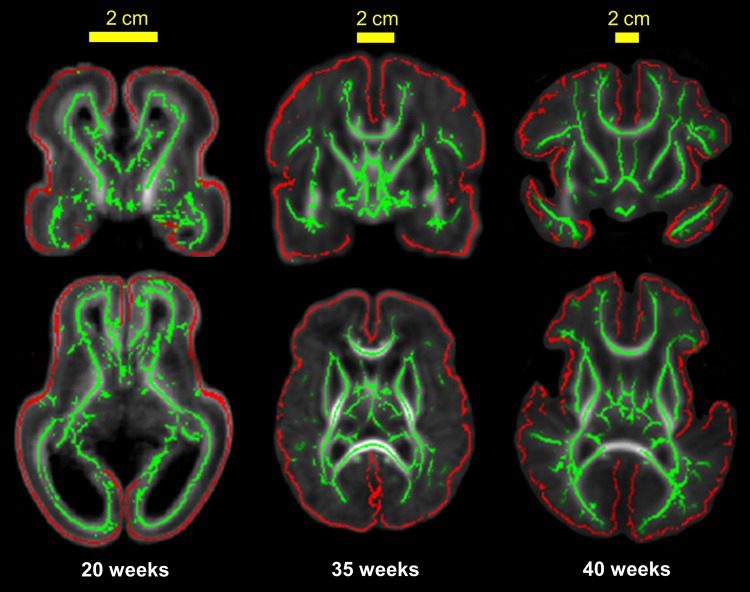
Figure 3 shows the cortical (red lines) and WM (green lines) skeletons overlaid on averaged FA maps of 20-, 35-, and 40-week brains. It is clear that the cortical and WM skeletons are well located at the center of the cortical plate and the “core” WM, respectively (Fig. 3).
Figure 3.
Skeletonized cerebral cortex (red) and WM (green) overlaid on the population-averaged FA map of 20-, 35-, and 40-week brains. Alignment of the red cortical skeleton to the center (along the direction of perpendicular to the surface) of cerebral cortex and green skeleton to the core WM can be clearly appreciated. Coronal and axial views are shown in the upper and lower panels, respectively.
Cortical FA Distribution at 20, 35, and 40 Weeks
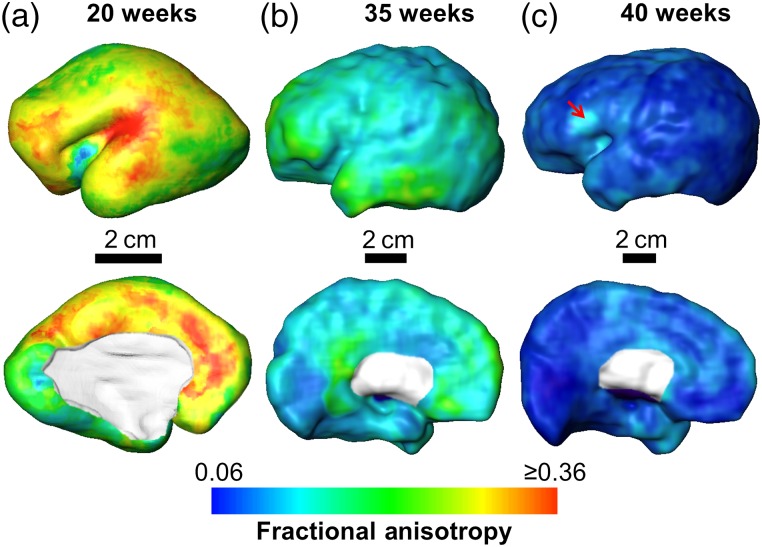
The characteristic averaged FA maps across the cortical surface at 20, 35, and 40 weeks are shown in Figure 4. The FA values of the overall cortical plate decrease significantly from mid-fetal stage to the time of normal birth, with the highest value around 0.36 to the lowest value around 0.06. In Figure 4, the overall cortical FA values are relatively high at 20 and 35 weeks, consistent to the bright cortical FA band shown in Figure 2. In addition, the cortical FA profiles are heterogeneous across the cortical surface at 20 and 35. At 20 weeks, the FA values appear high around the Sylvian fissure, medial frontal area, and primary motor and somatosensory areas around central gyrus; on the contrary, FA values at primary visual area appear low. At 35 weeks, higher cortical FA are shown in lateral and medial frontal regions whereas FA values at primary sensorimotor regions including primary somatosensory, motor, auditory, and visual are relatively low. At 40 weeks, the FA values are uniformly low across most of the cortical plate with only small isolated areas such as Broca area showing relatively higher FA (pointed by the red arrow).
Figure 4.
Mapping of averaged skeletonized cortical FA to the cortical surface of single-subject template brain at 20 weeks (a), 35 weeks (b), and 40 weeks (c). Lateral and medal view are shown in the upper and lower panels, respectively. Cortical FA values were encoded with the color bar on the bottom. Red arrow in c points to the Broca area where relatively high FA can be observed at 40 weeks.
Heterogeneous FA Decreases across the Cerebral Cortex during 20–35 and 35–40 Weeks
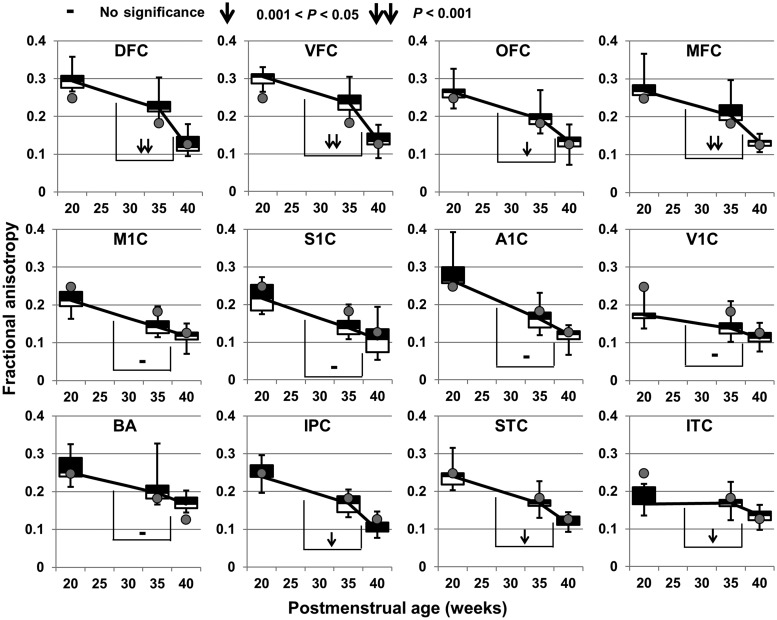
FA decreases significantly at all 12 cortical ROIs during both 20–35 and 35–40 weeks, except that FA at ITC during 20–35 weeks does not change significantly (Figure 5 and Table 1). Furthermore, these FA decreases vary significantly across the cortical plate at 12 cortical ROIs, as shown in Tables 2 and 3 and Figure 5. Specifically, the slope of FA decrease at any cortical ROI is significantly different from FA decrease slope at one or multiple other ROIs during 20–35 weeks (Table 2), and these slope differences are more widespread during 35–40 weeks (Table 3). During 20–35 weeks, FA decreases at A1C most sharply and there are little FA changes at ITC, as shown in Figure 5 and Table 2. These 2 regions demonstrate most different decrease patterns from mid-fetal to middle third trimester. During 35–40 weeks, FA decreases at frontal areas (e.g., OFC, DFC, VFC, and MFC) more sharply than primary sensorimotor areas (e.g., M1C, S1C, A1C, and V1C), as shown in Figure 5 and Table 3.
Figure 5.
Distinctive time courses of FA decreases at 12 cortical ROIs during 20–35 and 35–40 weeks. The FA values averaged across all cortical ROIs are shown as gray dots at each time point. FA measurements of cortical ROI from all subjects at a certain age are shown as boxplots. See the caption of Figure 1 for abbreviation of cortical ROIs. The symbols on the bridges connecting 2 FA descending lines during 20–35 and 35–40 weeks demonstrate the statistical significance of the descending slope differences, that is, the significance of acceleration of FA decrease at a certain cortical ROI in 35–40 weeks. All P-values were calculated after Bonferroni correction.
Table 1.
P-values for significant FA decreases in different cortical areas
| DFC | VFC | OFC | BA | M1C | S1C | IPC | A1C | STC | ITC | MFC | V1C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–35 weeks | <0.0001 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.3453 | <0.0001 | 0.0040 |
| 35–40 weeks | <0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0004 | 0.0014 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0015 |
Note: The P-values smaller than Bonferroni's-corrected threshold (0.05/12 = 0.0042) are in bold. See the caption of Figure 1 for the expansions of the abbreviations.
Table 2.
P-values of pairwise comparisons of FA decrease rates during 20–35 weeks between different cortical regions
| VFC | OFC | BA | M1C | S1C | IPC | A1C | STC | ITC | MFC | V1C | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFC | 0.7918 | 0.9604 | 0.5798 | 0.8659 | 0.5150 | 0.3860 | 0.0035 | 0.6693 | 0.0003 | 0.8252 | 0.0365 | 0.8935 |
| VFC | 0.8406 | 0.7362 | 0.9240 | 0.3551 | 0.2466 | 0.0015 | 0.4816 | 0.0005 | 0.9844 | 0.0571 | 0.9298 | |
| OFC | 0.6230 | 0.9117 | 0.5009 | 0.3793 | 0.0042 | 0.6465 | 0.0006 | 0.8683 | 0.0500 | 0.9293 | ||
| BA | 0.6781 | 0.2656 | 0.1897 | 0.0020 | 0.3540 | 0.0073 | 0.7416 | 0.1905 | 0.6822 | |||
| M1C | 0.4085 | 0.2918 | 0.0020 | 0.5456 | 0.0004 | 0.9466 | 0.0486 | 0.9925 | ||||
| S1C | 0.8531 | 0.0196 | 0.8127 | <0.0001 | 0.4089 | 0.0081 | 0.4774 | |||||
| IPC | 0.0243 | 0.6619 | <0.0001 | 0.3024 | 0.0035 | 0.3824 | ||||||
| A1C | 0.0099 | <0.0001 | 0.0032 | <0.0001 | 0.0024 | |||||||
| STC | 0.0001 | 0.5351 | 0.0126 | 0.6153 | ||||||||
| ITC | 0.0014 | 0.1074 | 0.0021 | |||||||||
| MFC | 0.0797 | 0.9441 | ||||||||||
| V1C | 0.0866 |
Note: The P-values smaller than Bonferroni's-corrected threshold (0.05/12 = 0.0042) are in bold. See the caption of Figure 1 for the expansions of the abbreviations.
Table 3.
P-values of pairwise comparisons of FA decrease rates during 35–40 weeks between different cortical regions
| VFC | OFC | BA | M1C | S1C | IPC | A1C | STC | ITC | MFC | V1C | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFC | 0.8220 | 0.0127 | <0.0001 | <0.0001 | <0.0001 | 0.0010 | <0.0001 | <0.0001 | <0.0001 | 0.1185 | <0.0001 | 0.0003 |
| VFC | 0.0211 | <0.0001 | <0.0001 | <0.0001 | 0.0019 | 0.0001 | 0.0001 | <0.0001 | 0.1783 | <0.0001 | 0.0007 | |
| OFC | 0.0154 | 0.0019 | 0.0193 | 0.5573 | 0.0790 | 0.1454 | 0.0123 | 0.2583 | 0.0016 | 0.3272 | ||
| BA | 0.6622 | 0.9892 | 0.0337 | 0.4005 | 0.1636 | 0.8340 | 0.0004 | 0.5728 | 0.0884 | |||
| M1C | 0.6623 | 0.0038 | 0.1583 | 0.0339 | 0.4598 | <0.0001 | 0.8758 | 0.0308 | ||||
| S1C | 0.0418 | 0.4238 | 0.1851 | 0.8514 | 0.0006 | 0.5754 | 0.0922 | |||||
| IPC | 0.1732 | 0.3245 | 0.0272 | 0.0584 | 0.0032 | 0.6720 | ||||||
| A1C | 0.6025 | 0.4670 | 0.0028 | 0.1295 | 0.4068 | |||||||
| STC | 0.1698 | 0.0044 | 0.0274 | 0.7077 | ||||||||
| ITC | 0.0001 | 0.3833 | 0.1310 | |||||||||
| MFC | <0.0001 | 0.0348 | ||||||||||
| V1C | 0.0215 |
Note: The P-values smaller than Bonferroni's-corrected threshold (0.05/12 = 0.0042) are in bold. See the caption of Figure 1 for the expansions of the abbreviations.
Temporally Nonuniform Cortical FA Decreases during 35–40 Weeks Compared with that during 20–35 Weeks
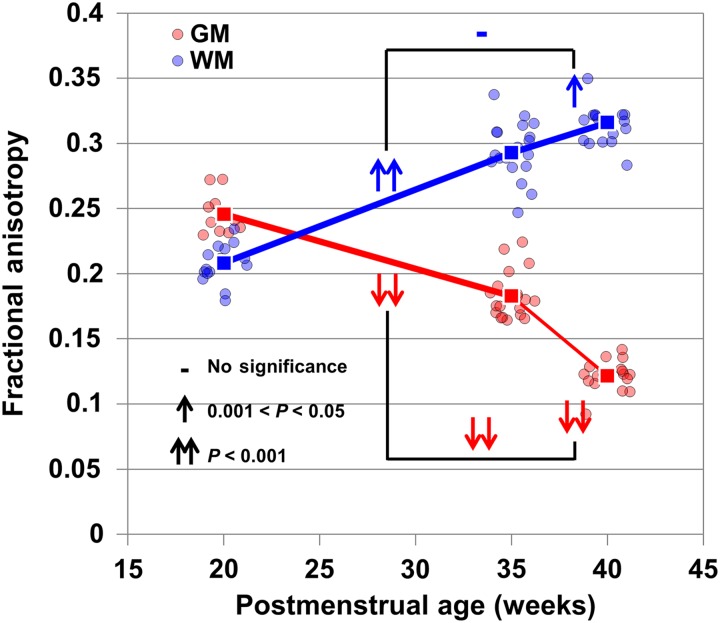
With the statistical comparisons of FA decrease rates between 2 developmental periods of 20–35 and 35–40 weeks, Figure 5 demonstrates that temporally nonuniform FA decreases (shown as single or double downwards arrow) take place in all frontal cortical ROIs (DFC, VFC, OFC, and MFC) and several other regions (IPC, STC, and ITC) whereas such nonuniform FA decreases do not occur at primary sensorimotor regions (M1C, S1C, A1C, and V1C). Figure 6 shows the averaged FA of entire cortical skeleton and WM skeleton of all subjects. The cortical FA changes are more dramatic during 35–40 weeks compared with 20–35 weeks, whereas the WM FA changes are more uniform across these 2 periods. In fact, WM FA increases are slower during 35–40 weeks compared with 20–35 weeks. Statistical analysis revealed that temporally nonuniform decreases can be found for the averaged FA of entire cortical plate whereas nonuniform changes cannot be found for the averaged FA of entire WM (Figure 6), suggesting disassociated WM and cortical FA changes from middle third trimester to the time of normal birth.
Figure 6.
Averaged FA values of all cortical skeleton voxels and all WM skeleton voxels of each subject are shown as red and blue dot, respectively. The red and blue lines connect the mean cortical FA and mean WM FA of all subjects at a certain age, shown as red and blue squares. Mean cortical and mean WM FA were averaged from all cortical and WM skeleton voxels, respectively. The symbols on the bridges connecting 2 FA descending lines during 20–35 and 35–40 weeks demonstrate the statistical significance of the descending slope differences, that is, the significance of acceleration of decrease of entire cortical skeleton FA in 35–40 weeks. There are no statistical ascending slope differences for entire WM skeleton FA increase in 35–40 weeks, compared to that in 20–35 weeks.
Discussion
20, 35, and 40 weeks are 3 characteristic time points in the second half of fetal development during which dramatic microstructural and macrostructural changes take place. Age-specific population-averaged templates were established for these characteristic time points with high-resolution DTI. Cortical and WM skeletons were obtained based on these population-averaged templates to effectively alleviate partial volume effects (Smith et al. 2006; Jeon et al. 2012) for DTI metric measurements. Inhomogeneous and characteristic cortical FA profiles at the 3 time points were visualized after mapping the averaged cortical FA to the surface. Distinctive FA decrease patterns of the cortical ROIs have been revealed during both 20–35 and 35–40 weeks with regional FA measurements at the 12 cortical ROIs. Temporally nonuniform FA decreases of frontal areas and other nonsensorimotor areas during 35–40 weeks compared with those of 20–35 weeks have been found, suggesting altered cortical differentiation in these regions during the late third trimester. Such temporally nonuniform FA change pattern was also found with the averaged FA of the entire cortical plate, but not with the averaged FA of the entire brain WM.
The population-averaged DTI templates from 11 specimens at 20 weeks, 19 subjects at 35 weeks, and 17 subjects at 40 weeks characterize the common anatomical features at these time points with less individual biases. The imaging resolution of the included DTI data is high, 0.283–0.353 mm3 for 20-week fetal brain and 1.5 × 1.5 × 1.6 mm3 for 35- and 40-week brain. It is very difficult and rare to collect the high-resolution DTI data from a group of subjects at 20 or 35 weeks. To the best of our knowledge, we have presented the first age-specific population-averaged high-resolution DTI templates at 20 and 35 weeks. These templates could serve as the anatomical references for brains at these developmental stages.
The cortical plate includes cytoarchitectonically (Economo and Koskinas 1925) and functionally unique cortical regions. In immature cortex, water molecules are more likely to diffuse along the radial glial scaffold (Rakic 1972, 1995; Sidman and Rakic 1973) instead of perpendicular to it, resulting in high cortical FA that is associated with well-organized radial glial scaffold (McKinstry et al. 2002). Cortical maturational processes including dendritic arborization and synaptic formation lead to FA decrease. The relationship of cortical FA and maturational processes has been observed in both human (e.g., McKinstry et al. 2002; Mukherjee et al. 2002; Neil et al. 2002; Maas et al. 2004; Deipolyi et al. 2005; Huang et al. 2006, 2009, 2013; Ball et al. 2013) and animal (e.g., Thornton et al. 1997; Mori et al. 2001; Kroenke et al. 2007; Sizonenko et al. 2007; Huang et al. 2008; Kroenke et al. 2009; Takahashi et al. 2011) studies of the early developing brains.
The formation of unique cortical regions is likely to be associated with distinctive regional dendritic arborization and synaptic formation that disrupt the well-organized radial glial scaffold (i.e., water diffusion barrier) and underlie maturational processes of the unique brain cortical regions. Spatiotemporal characterization of the heterogeneous cortical FA changes with high-resolution DTI thus offers an effective means to noninvasively access the maturational processes of different cortical regions. Heterogeneity of decreases in FA has been reported by Deipolyi et al. (2005) with DTI of preterm brains from 25 to 38 weeks. During a younger age range of second trimester, spatiotemporally distinctive cortical FA decrease of human fetal brain has also been recorded (Huang et al. 2013). With inclusion of more cortical ROIs and coverage of wider age range of 20–40 weeks, we hypothesized that such regionally distinctive FA decrease occurs in 20–35 and 35–40 weeks. Figure 4 demonstrated the heterogeneous cortical FA profile at 20, 35, and 40 weeks. Figure 5 and Table 2 quantitatively characterized and confirmed the distinctive cortical FA decreases in both 20–35 and 35–40 weeks at different functional cortical ROIs. The heterogeneity of the cortical development among various regions has been consistent to the findings with measurements from different aspects, including quantitative gyral scoring with MRI (van der Knaap et al. 1996), regional cerebral blood flow with PET (Chugani et al. 1987), and synaptic density with histology (Huttenlocher and Dabholkar 1997).
Another interesting finding is the alteration of the FA decrease pattern at the cortical ROIs other than primary sensorimotor regions during late third trimester (35–40 weeks) compared with early developmental period from mid-fetal to middle third trimester (20–35 weeks), as shown in Figure 5. The alteration of the FA decrease pattern in late third trimester was also found across the entire cortical surface whereas entire brain WM FA increase pattern is statistically unchanged (Fig. 6). Sharp FA decease of the frontal cortex from preterm brains of 27 to 37 weeks has also been reported in a very recent cortical microstructure study (Ball et al. 2013). Relative high FA (Figs 4, and 5 and Deipolyi et al. 2005) at the frontal cortex compared with that of sensorimotor cortex suggests frontal cortex is immature, namely having less dendritic arborization, synaptic formation, or cellular differentiation. On the other hand, the macroscopic connectomic investigations (e.g., Jakab et al. 2014 and Thomason et al. 2014 with functional MRI of in-utero fetuses; Doria et al. 2010 with functional MRI of preterm infants) of fetal and perinatal brains revealed heterogeneity of emerging network and formation of adult-like architecture of the brain configuration at 40 weeks. Furthermore, it was suggested that full repertoire of functional brain networks of adult patterns emerged at term after rapid brain maturational process in the third trimester (Doria et al. 2010). In light of this macroscopic connectomic view of fetal and perinatal brain development, cortical microstructural changes and maturation of brain connectivity may be interacted during the brain network formations. Although further studies are warranted to delineate the relationship of cortical microstructural changes and macroscopic connectomic alterations, a possible interpretation is that frontal cortex has to go through an altered microstructural maturation in 35–40 weeks to catch up with the maturational status of primary sensorimotor areas for getting the brain ready for developmental events after normal time of birth, that is, 40 weeks.
Third trimester is featured with multiple active developmental events involving premyelinating oligodendrocytes, microglia, axons, subplate neurons, the proliferative cerebral dorsal subventricular zone, and ventral germinative epithelium of the ganglionic eminence, thalamus, cortex, and cerebellum (Volpe 2009). The present study added the aspect of cortical FA changes reflecting the rapid and heterogeneous cortical maturation in nonsensorimotor regions during late third trimester. This study was designed to make the interval between the birth and scan time as short as possible to limit the effects of exposure to the extrauterine environment. In addition, all in vivo subjects were carefully screened by a neonatologist and a pediatric neuroradiologist and born after 31 weeks with averaged 33.1 weeks and averaged 40.2 for 35- and 40-week populations, respectively, to ameliorate the preterm effects. With 2 and 0.5 weeks of differences between birth and scan time for the 35- and 40-week populations, respectively, the contribution of extrauterine exposure could not be completely avoided, but the effects are considered to be relatively trivial with the expectation of preterm effects taking place years after birth. Nonetheless, cautions needs to be taken when interpreting the altered FA change pattern during late third trimester. The concept of enhanced vulnerability of rapidly developing events during maturation was postulated decades ago (Dobbing et al. 1970; Dobbing 1974). For example, studies on psychiatric disorders such as autism have suggested fetal period is a critical window for neurodevelopmental vulnerability (Bauman and Kemper 2005; Walker et al. 2013). Frontal cortex plays a key role in various neuropsychiatric disorders (Paus et al. 2008). Taken together, potential link of vulnerability of brain development in late third trimester (to factors such as certain drugs, hormones, or undernutrition) because of rapid frontal cortical microstructural changes and later onset of neuropsychiatric disorders deserves further study.
Imaging resolution must be high for small and young brains of 20, 35, and 40 weeks to delineate the details of anatomy. The DTI sequences adapted to adult brain scans have been widely used in in vivo preterm DTI that has a typical resolution of 2 × 2 × 2 mm3. Our DTI sequence with 1.5 × 1.5 × 1.6 mm3 imaging resolution on a clinical scanner was tailored specifically for the preterm brains that have much smaller brain size compared with adult human brain. Revealing sufficient anatomical details of postmortem brains at mid-fetal stage (20 weeks) demands even higher resolution of 0.283–0.353 mm3. Despite significant imaging resolution differences, the number of voxels that a 20-week fetal brain includes and the number of voxels that a 40-week brain includes are similar both ∼119 000, suggesting that similar amount of anatomical details have been recorded at both ages. Even with the high resolution, the partial volume effects on FA measurements at both cerebral cortex and WM could be significant and cannot be ignored (Smith et al. 2006; Jeon et al. 2012). To alleviate the partial volume effects, we adopted a method to generate both cortical and WM skeletons, and only FA measurement at core WM and central (along the direction perpendicular to the cortical surface) cortical regions were measured. Skeletonized FA measurements (Smith et al. 2006) have been well published since this method has been proposed. Skeletonization of the cerebral cortex was recently applied based on the probabilistic segmentation map of the cerebral cortex (Ball et al. 2013). The cortical skeletonization of 20- and 35-week brain in this study required both high-resolution FA map in the native space and good quality of intersubject registration. Figure 3 clearly demonstrates the good alignment of cortical skeleton to the center of cerebral cortex and good alignment of WM skeleton to the core WM.
Previous studies comparing the FA of postmortem animal brain specimens with that of in vivo brain found that formalin fixation alters diffusivity magnitude but not anisotropy (Sun et al. 2003, 2005). That is why only FA was adopted for quantitative analysis, although other DTI-derived metrics such as mean, axial, and radial diffusivity offers important and complementary microstructural information. Several recent MRI studies of postmortem fetal brain specimens (e.g., Takahashi et al. 2012) also suggested that given good fixation, the structural barriers of the fetal brain tissues can be well preserved and sensitive to diffusion MRI. Ideally same MR imaging sequence and the same MR system should be used for consistency. However, the imaging resolution used for 35- or 40-week brain is not sufficient to capture anatomical details for the much younger and smaller fetal brain at 20 weeks. High magnetic strength and long scan time were applied to DTI scan of 20-week fetal brain to increase resolution. Automatic cortical labeling was not feasible as digital fetal and preterm brain atlases at 20 or 35 weeks do not exist. It is also very difficult to ensure consistency of the atlas labeling with the dramatic morphological change of the cortical surface from 20 to 40 weeks with any automatic labeling method. Instead, we adopted the protocol of manual cortical ROI placement used for regional transcriptome analysis (Johnson et al. 2009; Kang et al. 2011) and cortical FA measurement at second trimester (Huang et al. 2013). This protocol is well adapted to smooth cortical surface of human fetal brain and can effectively delineate the functionally distinct cortical regions.
Conclusion
In conclusion, population-averaged DTI templates were generated at 3 characteristic time points of 20, 35, and 40 weeks. These templates could serve as anatomical references for brain development at these stages. Heterogeneous spatiotemporal FA across the cortical surface was revealed with the surface mapping of skeletonized cortical FA. Significant and heterogeneous FA decreases in both 20–35 and 35–40 weeks have been found with FA measurements at 12 functionally distinct cortical ROIs. Among these cortical ROIs, the altered cortical FA decreases were found at higher-order prefrontal cortical areas in the late third trimester, suggesting temporally nonuniform maturation in these cortical regions during 20–40 weeks. Temporally nonuniform decreases can be found for the averaged FA of entire cortical plate whereas averaged FA increase of entire WM is statistically unchanged, suggesting disassociated WM and cortical FA changes in late third trimester.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institute of Health (R01 MH092535 and R01 MH092535-S1 to H.H.).
Notes
Conflict of Interest: None declared.
Supplementary Material
References
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. 2013. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci USA. 110:9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Le Bihan D. 1994. MR diffusion tensor spectroscopy and imaging. Biophys J. 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. 2005. Neuroanatomical observation of the brain in autism: a review and future directions. Int J Dev Neurosci. 23:183–187. [DOI] [PubMed] [Google Scholar]
- Bonferroni CE. 1936. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Scienze Economiche E Commerciali di Firenze. 8:3–62. [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: boulder committee revisited. Nat Re Neurosci. 9:110–122. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. 1987. Positron emission tomography study of human brain functional development. Ann Neurol. 4:487–497. [DOI] [PubMed] [Google Scholar]
- Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM et al. 2005. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. NeuroImage. 27:579–586. [DOI] [PubMed] [Google Scholar]
- Dobbing J. 1974. The later growth of the brain and its vulnerability. Pediatrics. 53:2–6. [PubMed] [Google Scholar]
- Dobbing J, Hopewell JW, Lynch A, Sands J. 1970. Vulnerability of developing brain. I Some lasting effects of x-irradiation. Exp Neurol. 28:442–449. [DOI] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG et al. 2010. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo CV, Koskinas GN. 1925. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Wien und Berlin: Verlagvon Julius Springer. [Google Scholar]
- Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn. 2004. Age terminology during the perinatal period. Pediatrics. 114:1362–1364. [DOI] [PubMed] [Google Scholar]
- Huang H, Jeon T, Sedmak G, Pletikos M, Vasung L, Xu X, Yarowsky P, Richards LJ, Kostović I, Sestan N et al. 2013. Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cereb Cortex. 23:2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller M-I, Mori S. 2009. Anatomical characterization of human fetal brain development with diffusion tensor MRI. J Neurosci. 29:4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yamamoto A, Hossain MA, Younes L, Mori S. 2008. Quantitative cortical mapping of fractional anisotropy in developing rat brains. J Neurosci. 28:1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards L-J, Yarowsky P, Donohue P, Graham E, van Zijl P-C et al. 2006. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 33:27–38. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schopf V, Langs G. 2014. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci. 8:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon T, Mishra V, Uh J, Weiner M, Hatanpaa KJ, White CL 3rd, Zhao YD, Lu H, Diaz-Arrastia R, Huang H. 2012. Regional changes of cortical mean diffusivities with aging after correction of partial volume effects. Neuroimage. 62:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, van Ziji PC, Kim J, Pearison GD, Mori S. 2006. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 81:106–116. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. 2009. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 62:494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. 1999. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 42:515–525. [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G et al. 2011. Spatio-temporal transcriptome of the human brain. Nature. 478:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milosević N. 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 11:415–422. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judas M, Rados M, Hrabac P. 2002. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 12:536–544. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. 2009. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb Cortex. 19:2916–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. 2007. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 27:12506–12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Mukherjee P, Carballido-Gamio J, Veeraraghavan S, Miller SP, Partridge SC, Henry RG, Barkovich AJ, Vigneron DB. 2004. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage. 22:1134–1140. [DOI] [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, Almli CR, Shimony JS, Shiran SI, Neil JJ. 2002. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 12:1237–1243. [DOI] [PubMed] [Google Scholar]
- Miller MI, Trouve A, Younes L. 2002. On the metrics and euler-lagrange equations of computational anatomy. Annu Rev Biomed Eng. 4:375–405. [DOI] [PubMed] [Google Scholar]
- Mori S, Itoh R, Zhang J, Kaufmann WE, van Zijl PC, Solaiyappan M, Yarowsky P. 2001. Diffusion tensor imaging of the developing mouse brain. Magn Reson Med. 46:18–23. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo T-E, Neil JJ, McKinstry RC. 2002. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. Am J Neuroradiol. 23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Neil JJ, Miller J, Mukherjee P, Hüppi PS. 2002. Diffusion tensor imaging of normal and injured developing human brain-a technical review. NMR Biomed. 15:543–552. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Emst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI et al. 2011. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 56:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. 2008. Why do many psychiatric disorders emerge during adolescence. Nat Rev Neurosci. 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1972. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 145:61–83. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell—a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18:383–388. [DOI] [PubMed] [Google Scholar]
- Rakic P. 1988. Specification of cerebral cortical areas. Science. 241:170–176. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. 1982. Development of the human central nervous system. In: Haymaker W, Adams RD, editors. Histology and Histopathology of the Nervous System. (IL: ): Springfield; p. 3–145. [Google Scholar]
- Sidman RL, Rakic P. 1973. Neuronal migration, with specific reference to developing human brain: a review. Brain Res. 62:1–35. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Camm EJ, Garbow JR, Maier SE, Inder TE, Williams CE, Neil JJ, Hüppi PS. 2007. Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI. Cereb Cortex. 17:2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM et al. 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Liang HF, He YY, Schmidt RE, Hsu CY, Song SK. 2005. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infracted brain. Magn Reson Med. 53:1447–1451. [DOI] [PubMed] [Google Scholar]
- Sun SW, Neil JJ, Song SK. 2003. Relative indicies of water diffusion anisotropy are equivalent to live and formalin-fixed mouse brains. Magn Reson Med. 50:743–748. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Dai GP, Rosen GD, Wang RP, Ohki K, Folkerth RD, Galaburda AM, Wedeen VJ, Grant PE. 2011. Developing neocortex organization and connectivity in cats revealed by direct correlation of diffusion tractography and histology. Cereb Cortex. 21:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda AM, Grant PE. 2012. Emerging cerebral connectivity in the human fetal brain: An MR tractography study. Cereb Cortex. 22:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, Yeo L, Mody S, Berman S, Hassan SS et al. 2014. Intrinsic functional brain architecture derived from graph theoretical analysis hin the human fetus. PLoS one. 9:e94423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JS, Ordidge RJ, Penrice J, Cady EB, Amess PN, Punwani S, Clemence M, Wyatt JS. 1997. Anisotropic water diffusion in white and gray matter on the neonatal piglet brain before and after transient hypoxia ischemia. Magn Res Imaging. 15:433–440. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van Wezel-Meijler G, Barth PG, Barkhof F, Ader HJ, Valk J. 1996. Normal gyration and sulcation in preterm and term neonate: appearance on MR images. Radiology. 200:389–396. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 2009. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. 2013. Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biol Psychiatry. 74:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. 1998. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 22:139–152. [DOI] [PubMed] [Google Scholar]
- Xu D, Mori S, Shen D, van Ziji PC, Davatzikos C. 2003. Spatial normalization of diffusion tensor fields. Magn Reson Med. 50:175–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.