Abstract
The homeoprotein Engrailed (Engrailed-1/Engrailed-2, collectively En1/2) is not only a survival factor for mesencephalic dopaminergic (mDA) neurons during development, but continues to exert neuroprotective and physiological functions in adult mDA neurons. Loss of one En1 allele in the mouse leads to progressive demise of mDA neurons in the ventral midbrain starting from 6 weeks of age. These mice also develop Parkinson disease-like motor and non-motor symptoms. The characterization of En1 heterozygous mice have revealed striking parallels to central mechanisms of Parkinson disease pathogenesis, mainly related to mitochondrial dysfunction and retrograde degeneration. Thanks to the ability of homeoproteins to transduce cells, En1/2 proteins have also been used to protect mDA neurons in various experimental models of Parkinson disease. This neuroprotection is partly linked to the ability of En1/2 to regulate the translation of certain nuclear-encoded mitochondrial mRNAs for complex I subunits. Other transcription factors that govern mDA neuron development (e.g. Foxa1/2, Lmx1a/b, Nurr1, Otx2, Pitx3) also continue to function for the survival and maintenance of mDA neurons in the adult and act through partially overlapping but also diverse mechanisms.
Keywords: Dopaminergic neurons, Homeoproteins, Parkinson disease, Engrailed, Mitochondria, Substantia nigra pars compacta, Retrograde degeneration
1. Introduction
Mesencephalic dopaminergic (mDA) neurons in the ventral midbrain are the main source of dopamine (DA) in the adult brain. Dysfunctions in the signaling of this neurotransmitter is involved in several neurological and psychiatric disorders [1–3]. In recent years, tremendous progress has been made in dissecting the genetic networks and signaling pathways that govern various steps of mDA neuron development such as patterning, induction and specification of mDA progenitors, and their subsequent differentiation into mature mDA neurons. These studies mainly performed using transgenic mouse models have revealed that mDA neuron ontogenesis is controlled by the concerted action of several transcription factors including Engrailed-1/Engrailed-2 (collectively Engrailed or En1/2), Foxa1/2, Lmx1a/b, Nurr1, Otx2 and Pitx3, and growth factors or morphogens such as Shh, Fgf8, Tgf-ß and Wnt1 [4–6]. It was shown for instance that En1/2 alleles are critically required for the survival and maintenance of mature mDA neurons during late embryonic life in a dose-dependent manner [7–10]. Other transcription factors such as Nurr1 and Pitx3 play key roles in the acquisition of a mature mDA neuron identity during development by regulating the expression of several mDA neuron-specific markers such as tyrosine hydroxylase (TH) and dopamine transporter (DAT) [5, 6].
Interestingly, a number of recent studies have shown that several transcription factors, which control mDA neuron development, display continued expression in adult mDA neurons and are required for the maintenance of these neurons throughout life [11, 12]. These findings are particularly interesting in the context of Parkinson disease (PD), since possible genetic links between some of these transcription factors (e.g. EN1/2, NURR1, PITX3, LMX1A/B) and PD have been reported [13–17], although these findings await further confirmations. Deciphering the role played by these transcription factors in mDA neurons during development and in the adult, and elucidating the underlying mechanisms are an emerging field in PD research.
This review is mainly focused on the homeoprotein Engrailed, which belongs to the class of DNA binding homeodomain containing transcription factors. The expression of En1/2 during development starts early at embryonic day E8 in a patch of cells in the anterior neuroepithelium in the midbrain hindbrain boundary region. The extension of brain expression of En1/2 changes during the course of development and then confines to midbrain and hindbrain structures in the adulthood [18]. In the midbrain, En1/2 expression in the adult becomes highly restricted to mDA neurons of the substantia nigra pars compacta (SNpc), which preferentially degenerate in PD, and of the ventral tegmental area (VTA) [7, 8]. Interestingly, recent work, using genetic and toxicological mouse models, support the idea that EN1/2 might be in the PD pathway [19–22]. We review here the lessons emerging from these studies, which bring important insights into mDA neuron physiology, our understanding of PD pathogenesis and which open new perspectives for PD therapeutics.
2. Engrailed and PD connection
2.1. Hallmarks of PD
The main hallmark of PD, which represents the second most common neurodegenerative disorder after Alzheimer’s disease, is the slow and progressive loss of mDA neurons of the SNpc [1–3]. These neurons project to the dorsal striatum and form the nigrostriatal pathway, involved in the control of voluntary movements. The massive loss of these neurons ultimately results in severe striatal DA deficiency, which leads to classical PD-associated motor symptoms such as tremor, rigidity, bradykinesia and postural instability. Another hallmark of PD is the presence of intraneuronal inclusions mainly containing α-synuclein protein aggregates within the cell body (Lewy bodies) or processes (Lewy neurites). Age is the major risk factor for the development of PD [23], and the prevalence and incidence greatly increase after the age of 80 years. Exposure to environmental factors (i.e. pesticides) can also increase the risk to develop the disease [3]. Diagnosis of PD occurs with the onset of motor symptoms, but this can be preceded by a pre-motor or prodromal phase of 20 years or more [3]. It has been assumed for long time that the first motor symptoms appear when more than 80% of striatal DA is lost [1]. However, a reassessment of all available data suggests that the loss of SNpc mDA neurons remains asymptomatic until 30% of mDA neuron cell bodies and 50-60% of axonal terminals are lost [24]. In any case, adaptive changes in DA terminals and postsynaptic striatal neurons appear to compensate for significant losses of striatal DA to preserve motor behavior. It was recently shown that toxigenetic ablation of mDA neurons in the mouse following targeted expression of the diphtheria toxin A gene during development results in a depletion of more than 95% of striatal DA and this does not lead to any motor symptoms in young-adult or aged mutant mice, suggesting that the ability to compensate for severe DA deficiency might be higher in the mouse than in humans [25]. Thus, early diagnosis can be instrumental for disease management but this still remains a major hurdle. Currently available treatments provide only symptomatic relief and do not modify the course of the disease [26].
Genetic studies in the past 15 years have identified a number of genetic mutations (e.g. gene duplications/triplications or missense mutations) in certain familial autosomal dominant or recessive monogenic forms of PD (i.e. SNCA, Parkin, PINK1, LRRK2, DJ-1, VPS35, ATP13A2, GBA) [27, 28]. Recessively inherited Parkinsonism is frequently associated with early disease onset (before the age of 40 years). Although the majority (>90%) of PD forms are sporadic, the phenotypes of familial and sporadic forms are very similar, implying that they might arise from common underlying mechanisms [3, 29]. The study of the function of PD-linked genes has been instrumental to advance our understanding of the cellular processes involved in PD pathogenesis as discussed below. However, most of the genetically engineered mouse models generated through changes in PD-linked gene expression do not exhibit the cardinal feature of progressive loss of mDA neurons in the SNpc [30, 31]. To this respect, genetically engineered mice, in which expression of transcription factor genes controlling mDA neuron development are altered, have proven very useful animal models for PD research [11].
2.2. Progressive loss of mDA neurons in En1+/- mice
Previous loss of function studies demonstrated that En1/2 protect mature mDA neurons during late embryonic development against caspase-3-mediated apoptotic cell death in a dose-dependent manner [7–10]. Subsequently, several studies using various mouse models with targeted disruption of En1/2 alleles showed that En1/2 continues to be required for the survival of adult mDA neurons. The effects of a complete loss of En1 on adult mDA neuron survival could not be analyzed due to neonatal lethality of En1-/- mutants (OF1 background) [32]. However, the phenotype of mice heterozygous for En1 (En1+/-) has now been extensively characterized by several groups [19, 21, 22, 33, 34]. These mice present a normal number of mDA neurons in the SNpc until the age of 6 weeks after birth. After this age, these neurons start to die progressively and their number decreases by about 40% (as compared to wild-type mice) at 48 weeks of age when their number is stabilized. En1/2 gene dosage effect on survival was also seen in En1+/-; En2-/- mice (C57BL/6 background) which present a massive loss of mDA neurons in the SNpc of young adult mice. It was shown that En1/2 survival activity on mDA neurons in these mice could be mediated through both, the activation of Erk1/2 MAPK survival pathway and suppression of the pro-apoptotic activity of the pro-neurotrophin receptor p75NTR [10]. Finally, a recent study also analyzed En1-/- mice, which are viable on a C57BL/6 background, and reported a much more rapid and pronounced postnatal loss of mDA neurons [35]. All these observations support the idea that En1/2 continues to be required for the survival and/or maintenance of a subset of mDA neurons during adulthood.
It is noteworthy that VTA mDA neurons, located in the vicinity of SNpc, are relatively spared in En1+/- mice (20% loss at 48 weeks) as is the case in human PD [36]. These neurons project to the nucleus accumbens, the amygdala, the hippocampus and the prefrontal cortex to form the mesolimbic and mesocortical pathways, involved in motivation, reward, addiction, cognition and memory. The molecular determinants of the selective vulnerability of mDA SNpc neurons as compared to the VTA are still not known. Some of these differences may arise from the fact that SNpc mDA neurons express elevated levels of a highly active glycosylated form of DAT (glyco-DAT) and inwardly rectifying potassium channel (GIRK2), whereas mDA neurons in the VTA express more of the calcium binding protein calbindin D28K (CALB1) [36]. More recent single cell gene expression profiling indicates that there might be a much greater heterogeneity in mDA neurons within the SNpc or VTA [37]. For instance, En1 expression appears to be higher in mDA neurons located in the lateral ventral part of the SNpc [37], which hosts neurons that preferentially die in En1+/- mice; mDA neurons in the ventral tier of the SNpc are also the most vulnerable in human PD.
Loss of SNpc mDA neurons in En1+/- mice leads to decreased striatal DA. These mice develop PD-like motor symptoms such as reduced locomotor activity (distance travelled, rearing), increased amphetamine-sensitization, and defective motor coordination (rotarod). These mice also present some non-motor phenotypic alterations, such as depressive-like behavior (forced swimming test), anhedonic-like behavior (saccharine preference) and poor social interaction indicating that the moderate loss of mDA neurons in the VTA could affect the mesolimbic system in En1+/- mice [19]. En1 haplodeficient mice thus represent a valuable animal model to gain insight into the mechanisms leading to mDA neuron degeneration in PD.
3. Insights from En1+/- mice into PD pathogenesis
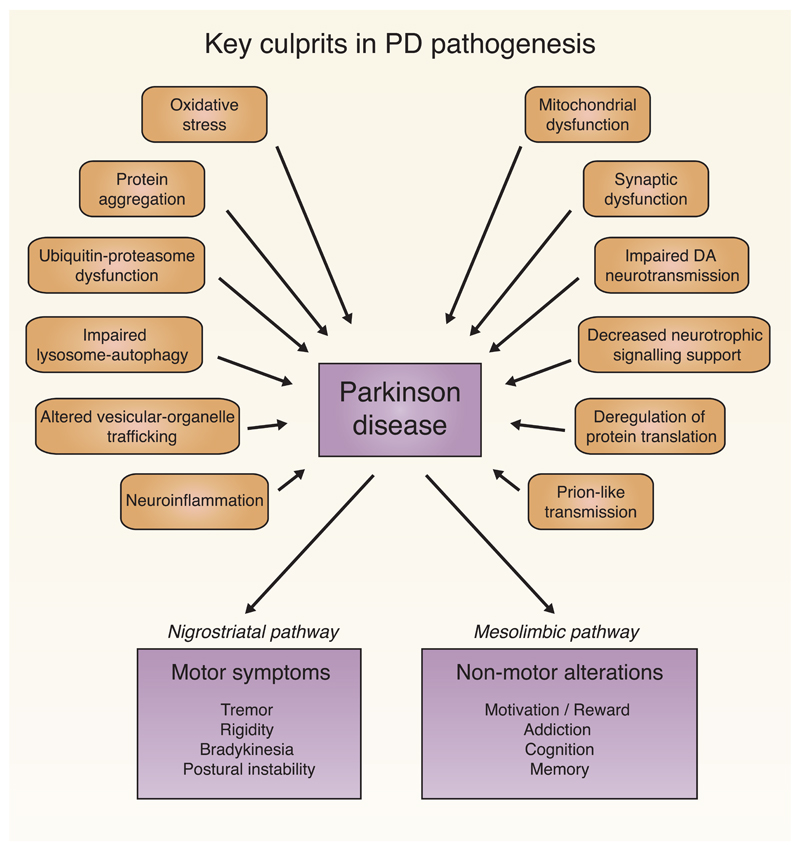
The molecular and cellular mechanisms involved in PD pathogenesis are still not entirely elucidated. However, the picture emerging from a number of studies is that PD pathogenesis involves dysregulation of several interconnected cellular processes and molecular pathways [1, 3, 29]. A major pathogenic pathway, which received particular attention, points towards mitochondrial dysfunction [38]. This is not only related to mitochondrial energy production, but also to many aspects of mitochondrial dynamics such as fusion-fission, trafficking, and quality control [39, 40]. Other pathogenic culprits include oxidative stress, accumulation and/or aggregation of misfolded proteins, defects in the ubiquitin-proteasome system, impairment in the lysosome-autophagy pathway, altered vesicular-organelle trafficking, defective synaptic function, neuro-inflammation, and prion-like cell-to-cell transmission of α-synuclein aggregates [1, 3, 29]. The major players contributing to PD pathogenesis are shown in Figure 1. Recent studies have also suggested that nucleolar stress, defective DNA damage/repair pathways and associated chromatin modifications might also play an important role in many neurodegenerative diseases including PD [41–45]. We briefly review here the contribution of En1+/- mouse model for dissecting the mechanisms of PD pathogenesis, particularly those related to mitochondrial activity and retrograde degeneration of mDA neurons involving synaptic dysfunction and autophagy.
Fig. 1.
Alterations in various cellular processes and molecular pathways contribute to PD development and associated motor and non-motor symptoms.
3.1. Mitochondrial dysfunction and its link to PD
Historically, the impairment of complex I of the mitochondrial respiratory chain has long been considered as the key culprit in PD pathogenesis [38]. This concept was based on several findings: i) complex I inhibitors such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) or rotenone can cause PD-like symptoms in rodents, non-human primates or even humans after accidental intoxication, ii) complex I activity was found to be reduced in postmortem PD brain tissues, iii) mutations in complex I subunits such as the nuclear-encoded NDUFV2 were associated with PD, iv) large-scale deletions in mitochondrial DNA were reported specifically in the SNpc of patients with sporadic PD, and v) several proteins encoded by genes mutated in PD (i.e. PINK1, Parkin, DJ-1, LRRK2 or SNCA) were found to be associated with mitochondria [46]. The complex I hypothesis is quite attractive, as mDA neurons are the highest energy consuming cells in the brain and rely on a very active mitochondrial respiratory chain/oxidative phosphorylation (OxPhos) system for ATP production [47]. This is intricately coupled to elevated ROS (reactive oxygen species) generation, which, in turn, causes oxidative stress and cellular damage [39, 40, 46].
The idea that a failure to sustain the high-energetic demand of mDA neurons can be crucial in PD pathogenesis was demonstrated in the MitoPark mouse model, generated by targeted disruption of the mitochondrial transcription factor Tfam. These mice present respiratory chain deficiencies and develop several features of PD, including progressive degeneration of the nigrostriatal system [48]. These mice also present behavioral phenotypes, associated to striatal dysfunction, which precede the loss of mDA neurons in the SNpc [49], suggesting retrograde degeneration (see below). The hypothesis that bioenergetics defects might contribute to PD pathogenesis was also supported by the strong association of PD with genes encoding OxPhos subunits and enzymes of the glucose metabolism. These genes are all regulated by the transcription factor PGC-1α, a positive regulator of mitochondrial biogenesis and cellular antioxidant responses [50]. Interestingly the PD-linked gene Parkin appears to be a positive regulator of PGC-1α by constantly degrading PARIS (Parkin interacting substrate), a negative regulator of PGC-1α. Mutations in Parkin can increase PARIS protein levels leading to decreased PGC-1α and associated energetic deficits [51]. Finally, a recent study has shown that elevated mitochondrial bioenergetics and axonal arborization size could be key contributors to the vulnerability of SNpc mDA neurons [52].
The possible link between En1/2 and mitochondria was recognized while studying the role of Engrailed in axon guidance. It is important to recall that many homeoproteins, including Engrailed, are endowed with the ability to be secreted and internalized by live cells. The sequences which allow En1/2 to transduce cells have been characterized and are located within the DNA binding homeodomain [53, 54]. Physiological roles of homeoprotein non-cell autonomous activity of homeoproteins have now been found in several system; in axon guidance for Engrailed, in the critical period and cortical plasticity for Otx2, and in eye development for Pax6 [18, 55–60]. Moreover, in addition to being a transcription factor, Engrailed can also regulate translation, thanks to its ability to bind the translation initiation factor eIF4E, another common feature of many homeoproteins [61, 62]. In the context of Engrailed and axon guidance, it was shown that Engrailed protein transduction can guide retinal axons and that Engrailed activity is dependent on its ability to function as a translation regulator through the mTOR pathway [55, 63]. The search for Engrailed translation targets showed that En1/2 protein transduction can stimulate the translation of nuclear-encoded mitochondrial mRNAs for certain complex I subunits (e.g. Ndufs1/Ndufs3), leading to increased ATP production in retinal growth cones or elevated complex I activity in midbrain synaptoneurosomes [20, 63]. In line with these observations, the level of Ndufs1 was decreased in mDA neurons of En1+/- mice which may reflect a bioenergetics problem underlying mDA neuron degeneration in these mice [20]. Finally, another En1/2 translation target, identified in Xenopus retinal axons, is Lamin B2, a major constituent of the nuclear envelope. Lamin B2 translation in axons regulates mitochondrial size, mitochondrial membrane potential and supports axon survival [64]. Mitochondria are critically required for long-term axonal survival and maintenance [65]; En1/2 might thus play an important role to sustain mitochondrial activity for axon maintenance throughout adulthood.
The importance of translation regulation for mitochondrial function was recently underscored in a study showing that the PD-linked genes PINK1 and Parkin control localized translation of nuclear-encoded respiratory chain component mRNAs on the mitochondrial outer membrane [66]. Some of the translationally repressed mRNAs, which localize on mitochondria in a PINK1/Tom20-dependent manner, are activated by PINK1/parkin through displacement of the attached repressors and enhanced binding of translational activators such as eIF4G [66]. More generally, it was recently hypothesized that deregulation of translation control might be a crucial factor in PD pathogenesis, which might have so far been overlooked [67]. The translation initiation factor eIF4G1 might be in the PD pathway [68, 69] possibly also through its ability to reprogram translation under stress conditions [70].
Mitochondria also appear to be targets of Nurr1, another developmental transcription factor critically required for mDA neuron maintenance in the adult. Indeed, several nuclear-encoded mitochondrial genes were identified as potential Nurr1 transcriptional targets [71]. Finally, gene expression profiling in the MN9D dopaminergic cell line also identified nuclear-encoded mitochondrial subunits of the respiratory chain as potential Lmx1a transcriptional targets, suggesting a possible link also between Lmx1a and mitochondria [72].
Although the complex I hypothesis has been central for PD pathogenesis for almost two decades, recent work suggest that other mitochondrial alterations could also be critical in PD pathogenesis [46]. Indeed, PD-linked genes such as PINK1 and Parkin that localize to mitochondria also critically regulate mitochondrial dynamics (e.g. control of mitochondrial fusion/fission, or mitochondrial trafficking and axonal transport, as well as mitochondrial quality control). The term ‘mitochondrial quality control’ encompasses the degradation of damaged mitochondrial proteins via autophagy, the export of misfolded and/or aggregated proteins through mitochondria-derived vesicles towards lysosomes/peroxisomes for degradation and the elimination of damaged mitochondria by mitophagy. Alterations in such processes may ultimately lead to energetic deficits [46, 73–75]. It will be interesting to examine whether mDA neurons in En1+/- mice present any defects in mitophagy since the autophagy/mitophagy marker LC3 was decreased in mDA neurons in these mice (see below) [21].
3.2. Retrograde degeneration of mDA neurons
It was proposed several years ago that mDA neuron death in PD could be a « dying-back » degeneration process, considering that the DA transporter DAT was particularly enriched in striatal terminals rather than on SNpc mDA neuron cell bodies [76]. A recent assessment of available data further supports the idea that the loss of striatal dopaminergic axon terminals can indeed be an early and dominant feature of PD, as the extent of loss of striatal dopaminergic markers largely exceeds that of SNpc mDA neuron loss at the time of motor symptom onset [24, 77, 78]. The synaptic and axonal pathologies observed with the PD-linked genes SNCA and LRRK2 also suggest retrograde degeneration of mDA neurons in cellular or mouse models of PD. Several studies indicate that disease-related effects of α-synuclein first occur at pre-synaptic terminals where the protein is predominantly localized. It was shown for instance that exogenous α-synuclein fibrils can induce intraneuronal aggregates of endogenous α-synuclein, which initially form at pre-synaptic terminals and then propagate retrogradely to the neuronal soma [79]. Accumulation of pathologic α-synuclein leads to a selective decrease in synaptic proteins and reduced DA release, progressive alterations in neuronal excitability and connectivity and eventually to cell death. Similarly, the pathogenesis resulting from mutations in LRRK2, the most common genetic form of PD, may also initially involve axonal pathology. Transgenic mice expressing the disease-causing human LRRK2 mutation (R1441G) exhibit fragmented TH-positive axons associated with axonal spheroids and display dystrophic neurites [80], although there is no loss of mDA neurons in this model. As mentioned above, impaired mitochondrial dynamics can greatly contribute to PD pathogenesis. It was shown that conditional knockout of the mitofusin 2 (Mfn2) gene, encoding an essential factor for mitochondrial fusion, results in loss of dopaminergic terminals in the striatum, which occurs much earlier than the onset of mDA neuron loss [81]. Accumulation of fragmented mitochondria as well as reduced mitochondrial mass and transport resulting from Mfn2 deficiency may ultimately contribute to neuronal loss. Of note, mDA neuron death in the classical MPTP neurotoxin model of PD might as well initially involve degeneration of distal axons [78, 82]. In conclusion, all these observations point to a retrograde mode of mDA neuron degeneration.
The phenotype of En1 heterozygous mice strongly pleads for a retrograde degeneration of the nigrostriatal system. These mice display early signs of degeneration of mDA axons terminals in the striatum, which precede the loss of mDA neuron cell bodies [21]. Dopaminergic terminals become dystrophic and swollen, contain autophagic vacuoles and present deficits in DA release and uptake. Individual axons in the nigrostriatal pathway of En1+/- mice undergo fragmentation, supporting the idea that axonal transport failure might be an early feature of PD [83]. The nigral dopaminergic cell bodies also exhibit signs of decreased autophagy accompanied by an increase in mTOR activity and a decrease of the autophagic marker LC3B [21]. Impairment of autophagy is considered as an important culprit in PD pathogenesis [84–86]. Its relevance was recently assessed in a mouse model generated by the conditional deletion of the autophagy-related gene Atg7, which recapitulates many pathologic features of PD, including age-related loss of mDA neurons [87]. In conclusion, En1+/- mouse represents a valuable model for PD and Table 1 recapitulates the phenotypic alterations resulting from En1 haplodeficiency.
Table 1.
Phenotypic alterations in En1+/- mice.
| At the cellular and molecular level |
| Preferential loss of SNpc mDA neurons |
| Impaired autophagy |
| Increased mTOR activation |
| Axonal fragmentation |
| Defective DA release and uptake |
| Decreased nuclear-encoded mitochondrial proteins |
| At the motor and non-motor behavioural level |
| Reduced locomotor activity (distance travelled, rearing) |
| Increased amphetamine-sensitization |
| Defective motor coordination (rotarod) |
| Depressive-like behaviour (forced simming test) |
| Anhedonic-like behaviour (saccharine preference) |
| Poor social interaction |
Degeneration of mDA neurons in a retrograde fashion has also been suggested from the study of conditional knockout mice for Lmx1a, in which mDA neurons are progressively lost both in the SNpc and the VTA [17]. Prior to the onset of mDA cell body loss, these mice present abnormally large striatal nerve terminals that are filled with autophagic and lysosomal vesicles, very much like En1+/- mice. In these mice, alteration of the autophagy/lysosomal pathway could be a consequence of increased mTOR activity in mDA neurons as in En1+/- mice [21]. Interestingly, rapamycin treatment normalizes the phenotypic alterations resulting from targeted Lmx1a disruption [17]. Finally, conditional inducible deletion of Nurr1 in mDA neurons in mice (5 weeks-old) results in a progressive pathology, associated with loss or reduced striatal DA content, impaired motor behavior, as well as dystrophic axons and fragmented dendrites containing varicosities [71], although no major loss of mDA neurons was reported in these mice.
4. Engrailed as a therapeutic protein for PD
As already mentioned above, several homeoproteins have the ability to transduce cells [53, 54] and this feature makes them particularly attractive for therapeutic purposes. Taking advantage of this property, it was demonstrated that En1/2 protein transduction provides efficient neuroprotection in various contexts. It was first shown that loss of mDA neurons in En1+/- mice can be halted by infusing recombinant En1/2 proteins (En1 and En2 are biochemically equivalent in the adult midbrain) in the SNpc [19]. More importantly, En1/2 protein transduction-mediated protection of mDA neurons was reported in several experimental models of PD, in vitro and in vivo, including the MPTP, rotenone, 6-OHDA (6-hydroxydopamine) and mutated α-synuclein (A30P) models [20]. Intriguingly, unilateral Engrailed infusion in naive mice increases striatal DA content on the ipsilateral (injected) side. This leads to an amphetamine-induced turning behavior contralateral to the side of infusion, indicating an activation of the nigrostriatal pathway following Engrailed infusion in the SNpc [20]. This observation is interesting from a therapeutic point of view since En1/2 is able not only to protect mDA neurons in several PD models, but also to enhance the physiological activity of mDA neurons on a naive background. From a mechanistic point of view, it was shown that Engrailed-mediated protection of mDA neurons against MPTP was partly linked to its ability to up-regulate the translation of certain nuclear-encoded mitochondrial complex I subunits (e.g. Ndufs1/Ndufs3), thus enhancing complex I activity and ATP synthesis [20, 63].
In the context of homeoproteins and neuroprotection, it is also noteworthy to describe some of the work related to Otx2, an important regulator of mDA neuron subtype identity during development [88]. In the adult, Otx2 continues to be expressed in a subset of mDA neurons in the central and medio-lateral area of the VTA [89]. Conditional knockout of Otx2 in adult mice results in selective loss of the axonal projections from VTA mDA neurons [90, 91]. Otx2 acts as a negative regulator of DAT and an inverse correlation between Otx2 expression and glyco-DAT (highly active form of DAT) levels in mDA neurons has been reported [89]. It was shown that Otx2 gain of function in SNpc mDA neurons reduces glyco-DAT levels, and protects them from MPTP toxicity [89]. Finally, it was reported that forced expression of Otx2 in mDA neurons in the SNpc of En1+/- mice also prevents the progressive loss of mDA neurons caused by En1 haploinsufficiency [33]. It is likely that Otx2 and En1/2 share some common neuroprotective mechanisms. Also, Otx2 expression in the VTA mDA neurons might explain that these neurons are relatively spared in En1+/- mice. Otx2 protein transduction could thus also be of potential therapeutic interest for neuroprotection in PD. It is noteworthy that Otx2 protein transduction has previously been reported to save retinal ganglion cells (RGCs) from NMDA (N-methyl-D-aspartate)-induced death in a mouse model of glaucoma [92].
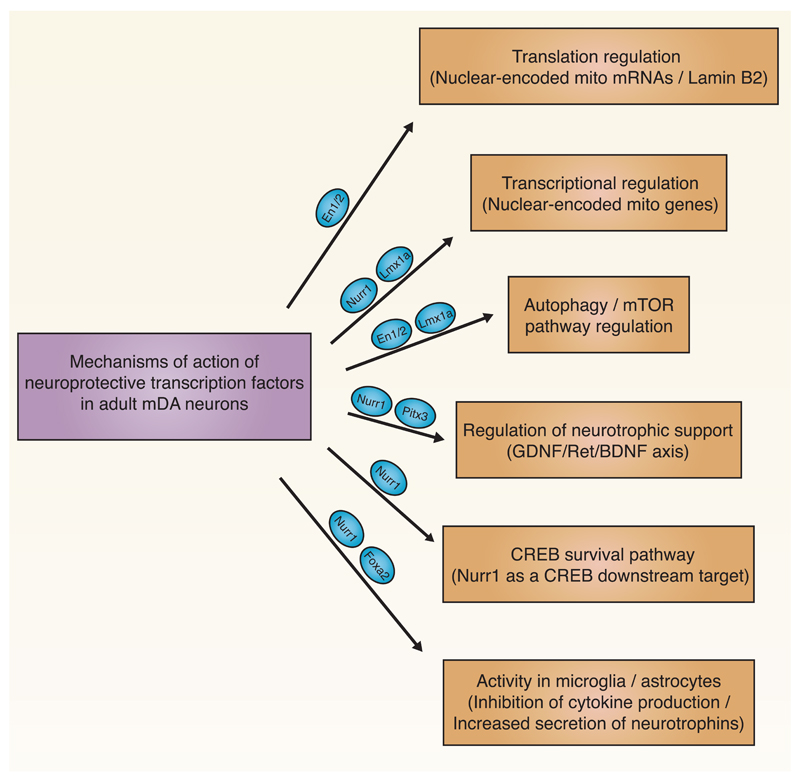
Finally, the transcription factors Nurr1, Foxa1/2 and Pitx3 might also engage other mechanisms for neuroprotection of adult mDA neurons. For instance, Nurr1/Pitx3 are involved in transcriptional regulation of the GDNF-RET-BDNF axis for neurotrophic support [93–95]. Nurr1 was also reported to be a downstream target of CREB for survival signaling [96]. Finally, Nurr1/Foxa2 might also act in microglia and astrocytes to decrease pro-inflammatory cytokine production and to increase the secretion of neurotrophic factors [97, 98]. Figure 2 provides a summary of mechanisms and signaling pathways engaged by transcription factors to confer dopaminergic neuroprotection in the adult brain.
Fig. 2.
Mechanisms and signaling pathways engaged by transcription factors to confer dopaminergic neuroprotection in the adult brain.
5. Concluding remarks
The study of genetically engineered mice with altered expression of transcription factors involved in mDA neuron development has provided valuable insights in our understanding of adult mDA neuron maintenance and PD pathogenesis, mainly related to mitochondria, autophagy and retrograde degeneration. In view of the phenotype of En1+/- mice, it will be interesting to furher explore possible connections between EN1/2 and PD. Recent studies have revealed that these transcription factors might be linked to several neuroprotective mechanisms and survival pathways in adult mDA neurons. It is interesting to point out that many interconnections between En1/2, Nurr1, Lmx1a/b, Pitx3, Foxa1/2, GDNF/Ret, BDNF, PGC-1α, nuclear-encoded mitochondrial gene expression, pro-inflammatory cytokines and PD-linked genes such as α-synuclein, Pink1 or Parkin have been recognized. It will be interesting to examine whether En1/2 could regulate any PD-linked genes. The dissection of the molecular mechanisms underlying neuroprotection conferred by transcription factors could help identifying novel therapeutic targets for PD. Thanks to their ability to transduce cells, En1/2 and Otx2 have also been used directly to protect mDA neurons in various experimental PD models [19, 20, 92]. It will be interesting to further evaluate the therapeutic potential of these transcription factors in non-human primate models of PD. More generally, protein transduction technology using cell penetrating peptides can offer an attractive alternative to viral vector-based gene delivery for therapeutic purposes [99–101].
The phenotypes of several genetically engineered mouse models of PD strongly revive the idea that mDA neurons may degenerate in a retrograde fashion [78], and this could be a common feature of many neurodegenerative disorders [102]. It should be pointed out that most of the strategies tested so far to achieve neuroprotection in PD were targeted to save the neuronal cell bodies, and much less efforts were devoted to prevent the retrograde degeneration process, which might actually precede the loss of neuronal somas. The mechanisms underlying death of neuronal cell bodies and those involved in axon degeneration might be quite different [78]. In this regard, it is noteworthy that overexpression of a constitutively active form of Akt was shown to strongly suppress retrograde degeneration of dopaminergic axons in a neurotoxin (6-OHDA) rodent model of injury, and this protection was linked to mTOR signalling and inhibition of macroautophagy [103]. The phenotypic correction of retrograde degeneration in Lmx1a conditional knockout mice by rapamycin is also quite compelling [17]. It will be interesting to examine in future studies if En1/2 or Otx2 protein transduction can prevent retrograde degeneration of mDA neurons in experimental PD models.
Acknowledgements
We thank Michel Volovitch, Ariel Di Nardo and Alain Joliot for useful discussions. The work was supported by Région Ile de France, Fondation Bettencourt Schueller, GRL program N°2009-00424 and ERC Advanced Grant HOMEOSIGN n°339379.
Abbreviations
- En1/2
Engrailed-1/Engrailed-2
- DA
dopamine
- DAT
dopamine transporter
- mDA
mesencephalic dopaminergic
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 6-OHDA
6-hydroxydopamine
- PD
Parkinson disease
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- 1.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AH. Aetiopathogenesis of Parkinson's disease. J Neurol. 2011;258:S307–10. doi: 10.1007/s00415-011-6016-y. [DOI] [PubMed] [Google Scholar]
- 3.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 4.Prakash N, Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J Physiol. 2006;575:403–10. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 6.Blaess S, Ang SL. Genetic control of midbrain dopaminergic neuron development. Wiley Interdiscip Rev Dev Biol. 2015;4:113–34. doi: 10.1002/wdev.169. [DOI] [PubMed] [Google Scholar]
- 7.Simon HH, Saueressig H, Wurst W, Goulding MD, O'Leary DD. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J Neurosci. 2001;21:3126–34. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon HH, Thuret S, Alberi L. Midbrain dopaminergic neurons: control of their cell fate by the engrailed transcription factors. Cell Tissue Res. 2004;318:53–61. doi: 10.1007/s00441-004-0973-8. [DOI] [PubMed] [Google Scholar]
- 9.Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131:3229–36. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- 10.Alavian KN, Sgado P, Alberi L, Subramaniam S, Simon HH. Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. Neural Dev. 2009;4:11. doi: 10.1186/1749-8104-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs J, Stettler O, Alvarez-Fischer D, Prochiantz A, Moya KL, Joshi RL. Engrailed signaling in axon guidance and neuron survival. Eur J Neurosci. 2012;35:1837–45. doi: 10.1111/j.1460-9568.2012.08139.x. [DOI] [PubMed] [Google Scholar]
- 12.Alavian KN, Jeddi S, Naghipour SI, Nabili P, Licznerski P, Tierney TS. The lifelong maintenance of mesencephalic dopaminergic neurons by Nurr1 and engrailed. J Biomed Sci. 2014;21:27. doi: 10.1186/1423-0127-21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33:85–9. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs J, Mueller JC, Lichtner P, Schulte C, Munz M, Berg D, Wullner U, Illig T, Sharma M, Gasser T. The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging. 2009;30:731–8. doi: 10.1016/j.neurobiolaging.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Rissling I, Strauch K, Hoft C, Oertel WH, Moller JC. Haplotype analysis of the engrailed-2 gene in young-onset Parkinson's disease. Neurodegener Dis. 2009;6:102–5. doi: 10.1159/000207796. [DOI] [PubMed] [Google Scholar]
- 16.Haubenberger D, Reinthaler E, Mueller JC, Pirker W, Katzenschlager R, Froehlich R, Bruecke T, Daniel G, Auff E, Zimprich A. Association of transcription factor polymorphisms PITX3 and EN1 with Parkinson's disease. Neurobiol Aging. 2011;32:302–7. doi: 10.1016/j.neurobiolaging.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Laguna A, Schintu N, Nobre A, Alvarsson A, Volakakis N, Jacobsen JK, Gomez-Galan M, Sopova E, Joodmardi E, Yoshitake T, Deng Q, et al. Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson's disease. Nat Neurosci. 2015;18:826–35. doi: 10.1038/nn.4004. [DOI] [PubMed] [Google Scholar]
- 18.Prochiantz A, Di Nardo AA. Homeoprotein signaling in the developing and adult nervous system. Neuron. 2015;85:911–25. doi: 10.1016/j.neuron.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnier L, Le Pen G, Hartmann A, Bizot JC, Trovero F, Krebs MO, Prochiantz A. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J Neurosci. 2007;27:1063–71. doi: 10.1523/JNEUROSCI.4583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Fischer D, Fuchs J, Castagner F, Stettler O, Massiani-Beaudoin O, Moya KL, Bouillot C, Oertel WH, Lombes A, Faigle W, Joshi RL, et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat Neurosci. 2011;14:1260–6. doi: 10.1038/nn.2916. [DOI] [PubMed] [Google Scholar]
- 21.Nordstrom U, Beauvais G, Ghosh A, Pulikkaparambil Sasidharan BC, Lundblad M, Fuchs J, Joshi RL, Lipton JW, Roholt A, Medicetty S, Feinstein TN, et al. Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson's disease. Neurobiol Dis. 2015;73:70–82. doi: 10.1016/j.nbd.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sgado P, Alberi L, Gherbassi D, Galasso SL, Ramakers GM, Alavian KN, Smidt MP, Dyck RH, Simon HH. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proc Natl Acad Sci U S A. 2006;103:15242–7. doi: 10.1073/pnas.0602116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson's disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12:359–66. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–31. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden JP, Demaro JA, 3rd, Knoten A, Hoshi M, Pehek E, Johnson EM, Jr, Gereau RWt, Jain S. Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. J Neurosci. 2013;33:17095–107. doi: 10.1523/JNEUROSCI.0890-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson's disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543–54. doi: 10.1016/j.tins.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Lesage S, Lohmann E, Tison F, Durif F, Durr A, Brice A. Gene symbol: PARK2. Disease: Parkinsonism, juvenile, autosomal recessive. Hum Genet. 2008;123:114. [PubMed] [Google Scholar]
- 28.Verstraeten A, Theuns J, Van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet. 2015;31:140–9. doi: 10.1016/j.tig.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 30.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–61. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bezard E, Yue Z, Kirik D, Spillantini MG. Animal models of Parkinson's disease: limits and relevance to neuroprotection studies. Mov Disord. 2013;28:61–70. doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid- hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–75. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- 33.Di Giovannantonio LG, Di Salvio M, Acampora D, Prakash N, Wurst W, Simeone A. Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability. Dev Biol. 2013;373:176–83. doi: 10.1016/j.ydbio.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Gotz S, Vogt Weisenhorn DM, Simeone A, Wurst W, Prakash N. A WNT1-regulated developmental gene cascade prevents dopaminergic neurodegeneration in adult En1 mice. Neurobiol Dis. 2015;82:32–45. doi: 10.1016/j.nbd.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Veenvliet JV, Dos Santos MT, Kouwenhoven WM, von Oerthel L, Lim JL, van der Linden AJ, Koerkamp MJ, Holstege FC, Smidt MP. Specification of dopaminergic subsets involves interplay of En1 and Pitx3. Development. 2013;140:3373–84. doi: 10.1242/dev.094565. [DOI] [PubMed] [Google Scholar]
- 36.Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson's disease: an update. Front Neuroanat. 2014;8:152. doi: 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulin JF, Zou J, Drouin-Ouellet J, Kim KY, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014;9:930–43. doi: 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 39.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–62. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–10. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Eilam R, Peter Y, Elson A, Rotman G, Shiloh Y, Groner Y, Segal M. Selective loss of dopaminergic nigro-striatal neurons in brains of Atm-deficient mice. Proc Natl Acad Sci U S A. 1998;95:12653–6. doi: 10.1073/pnas.95.21.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, Neumann M, Yuan X, Grummt I, Schutz G, Parlato R. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–60. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-beta. Nat Neurosci. 2013;16:613–21. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17:357–66. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–82. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–53. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pissadaki EK, Bolam JP. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease. Front Comput Neurosci. 2013;7:13. doi: 10.3389/fncom.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–30. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, Backman CM, Larsson NG, Olson L, Gellhaar S, Galter D, Lupica CR. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease. FASEB J. 2011;25:1333–44. doi: 10.1096/fj.10-173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:1–12. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 53.Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol. 2000;12:400–6. doi: 10.1016/s0955-0674(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 54.Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol. 2003;4:814–9. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- 55.Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wizenmann A, Brunet I, Lam JSY, Sonnier L, Beurdeley M, Zarbalis K, Weisenhorn-Vogt D, Weiln C, Dwivedy A, Joliot A, Wurst W, et al. Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron. 2009;64:355–366. doi: 10.1016/j.neuron.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–20. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 58.Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, Prochiantz A. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 2013;3:1815–23. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lesaffre B, Joliot A, Prochiantz A, Volovitch M. Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish. Neural Develop. 2007;2 doi: 10.1186/1749-8104-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Lullo E, Haton C, Le Poupon C, Volovitch M, Joliot A, Thomas JL, Prochiantz A. Paracrine Pax6 activity regulates oligodendrocyte precursor cell migration in the chick embryonic neural tube. Development. 138:4991–5001. doi: 10.1242/dev.066282. [DOI] [PubMed] [Google Scholar]
- 61.Nedelec S, Foucher I, Brunet I, Bouillot C, Prochiantz A, Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2004;101:10815–20. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topisirovic I, Borden KL. Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): an unexpected relationship. Histol Histopathol. 2005;20:1275–84. doi: 10.14670/HH-20.1275. [DOI] [PubMed] [Google Scholar]
- 63.Stettler O, Joshi RL, Wizenmann A, Reingruber J, Holcman D, Bouillot C, Castagner F, Prochiantz A, Moya KL. Engrailed homeoprotein recruits the adenosine A1 receptor to potentiate ephrin A5 function in retinal growth cones. Development. 2012;139:215–24. doi: 10.1242/dev.063875. [DOI] [PubMed] [Google Scholar]
- 64.Yoon BC, Jung H, Dwivedy A, O'Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–64. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Court FA, Coleman MP. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012;35:364–72. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Gehrke S, Wu Z, Klinkenberg M, Sun Y, Auburger G, Guo S, Lu B. PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab. 2015;21:95–108. doi: 10.1016/j.cmet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taymans JM, Nkiliza A, Chartier-Harlin MC. Deregulation of protein translation control, a potential game-changing hypothesis for Parkinson's disease pathogenesis. Trends Mol Med. 2015;21:466–72. doi: 10.1016/j.molmed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Chartier-Harlin MC, Dachsel JC, Vilarino-Guell C, Lincoln SJ, Lepretre F, Hulihan MM, Kachergus J, Milnerwood AJ, Tapia L, Song MS, Le Rhun E, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89:398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhungel N, Eleuteri S, Li LB, Kramer NJ, Chartron JW, Spencer B, Kosberg K, Fields JA, Stafa K, Adame A, Lashuel H, et al. Parkinson's disease genes VPS35 and EIF4G1 interact genetically and converge on alpha-synuclein. Neuron. 2015;85:76–87. doi: 10.1016/j.neuron.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badura M, Braunstein S, Zavadil J, Schneider RJ. DNA damage and eIF4G1 in breast cancer cells reprogram translation for survival and DNA repair mRNAs. Proc Natl Acad Sci U S A. 2012;109:18767–72. doi: 10.1073/pnas.1203853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadkhodaei B, Alvarsson A, Schintu N, Ramskold D, Volakakis N, Joodmardi E, Yoshitake T, Kehr J, Decressac M, Bjorklund A, Sandberg R, et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci U S A. 2013;110:2360–5. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoekstra EJ, von Oerthel L, van der Linden AJ, Schellevis RD, Scheppink G, Holstege FC, Groot-Koerkamp MJ, van der Heide LP, Smidt MP. Lmx1a is an activator of Rgs4 and Grb10 and is responsible for the correct specification of rostral and medial mdDA neurons. Eur J Neurosci. 2013;37:23–32. doi: 10.1111/ejn.12022. [DOI] [PubMed] [Google Scholar]
- 73.Vives-Bauza C, Przedborski S. Mitophagy: the latest problem for Parkinson's disease. Trends Mol Med. 2011;17:158–65. doi: 10.1016/j.molmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–56. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pickrell AM, Pinto M, Hida A, Moraes CT. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. J Neurosci. 2011;31:17649–58. doi: 10.1523/JNEUROSCI.4871-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51:S2–9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 77.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–25. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12:826–8. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pham AH, Meng S, Chu QN, Chan DC. Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum Mol Genet. 2012;21:4817–26. doi: 10.1093/hmg/dds311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, Bezard E. Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson's disease. Mol Neurobiol. 2003;28:209–18. doi: 10.1385/MN:28:3:209. [DOI] [PubMed] [Google Scholar]
- 83.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease. Brain. 2012;135:2058–73. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Perez AM, Claramonte-Clausell B, Sanchez-Andres JV, Herrero MT. Parkinson's disease and autophagy. Parkinsons Dis. 2012;2012:429524. doi: 10.1155/2012/429524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci. 2013;36:418–28. doi: 10.1016/j.tins.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan PY, Yue Z. Genetic causes of Parkinson's disease and their links to autophagy regulation. Parkinsonism Relat Disord. 2014;20(Suppl 1):S154–7. doi: 10.1016/S1353-8020(13)70037-3. [DOI] [PubMed] [Google Scholar]
- 87.Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM. Development and characterization of a new Parkinson's disease model resulting from impaired autophagy. J Neurosci. 2012;32:16503–9. doi: 10.1523/JNEUROSCI.0209-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panman L, Papathanou M, Laguna A, Oosterveen T, Volakakis N, Acampora D, Kurtsdotter I, Yoshitake T, Kehr J, Joodmardi E, Muhr J, et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 2014;8:1018–25. doi: 10.1016/j.celrep.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 89.Di Salvio M, Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N, Wurst W, Simeone A. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci. 2010;13:1481–8. doi: 10.1038/nn.2661. [DOI] [PubMed] [Google Scholar]
- 90.Borgkvist A, Puelles E, Carta M, Acampora D, Ang SL, Wurst W, Goiny M, Fisone G, Simeone A, Usiello A. Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Mol Cell Neurosci. 2006;31:293–302. doi: 10.1016/j.mcn.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 91.Chung CY, Licznerski P, Alavian KN, Simeone A, Lin Z, Martin E, Vance J, Isacson O. The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons. Brain. 2010;133:2022–31. doi: 10.1093/brain/awq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torero Ibad R, Rheey J, Mrejen S, Forster V, Picaud S, Prochiantz A, Moya KL. Otx2 promotes the survival of damaged adult retinal ganglion cells and protects against excitotoxic loss of visual acuity in vivo. J Neurosci. 2011;31:5495–503. doi: 10.1523/JNEUROSCI.0187-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallen AA, Castro DS, Zetterstrom RH, Karlen M, Olson L, Ericson J, Perlmann T. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci. 2001;18:649–63. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- 94.Galleguillos D, Fuentealba JA, Gomez LM, Saver M, Gomez A, Nash K, Burger C, Gysling K, Andres ME. Nurr1 regulates RET expression in dopamine neurons of adult rat midbrain. J Neurochem. 2010;114:1158–67. doi: 10.1111/j.1471-4159.2010.06841.x. [DOI] [PubMed] [Google Scholar]
- 95.Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N, Le W. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J Neurosci. 2011;31:12802–15. doi: 10.1523/JNEUROSCI.0898-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J, Spiegelman BM, Perlmann T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci U S A. 2010;107:12317–22. doi: 10.1073/pnas.1007088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oh SM, Chang MY, Song JJ, Rhee YH, Joe EH, Lee HS, Yi SH, Lee SH. Combined Nurr1 and Foxa2 roles in the therapy of Parkinson's disease. EMBO Mol Med. 2015 doi: 10.15252/emmm.201404610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–96. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- 100.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, Shim JW, Jo AY, Kim BW, Lee H, Lee SH, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–35. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–7. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 103.Cheng HC, Kim SR, Oo TF, Kareva T, Yarygina O, Rzhetskaya M, Wang C, During M, Talloczy Z, Tanaka K, Komatsu M, et al. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–35. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]