Abstract
Little is known about the frequency and clinical implications of postoperative atrial fibrillation in military veterans who undergo coronary artery bypass grafting (CABG). We examined long-term survival data, clinical outcomes, and associated risk factors in this population.
We retrospectively reviewed baseline, intraoperative, and postoperative data from 1,248 consecutive patients with similar baseline risk profiles who underwent primary isolated CABG at a Veterans Affairs hospital from October 2006 through March 2013. Multivariable logistic regression identified predictors of postoperative atrial fibrillation. Kaplan-Meier analysis was used to evaluate long-term survival (the primary outcome measure), morbidity, and length of hospital stay.
Postoperative atrial fibrillation occurred in 215 patients (17.2%). Independent predictors of this sequela were age ≥65 years (odds ratios [95% confidence intervals], 1.7 [1.3–2.4] for patients of age 65–75 yr and 2.6 [1.4–4.8] for patients >75 yr) and body mass index ≥30 kg/m2 (2.0 [1.2–3.2]). Length of stay was longer for patients with postoperative atrial fibrillation than for those without (12.7 ± 6.6 vs 10.3 ± 8.9 d; P ≤0.0001), and the respective 30-day mortality rate was higher (1.9% vs 0.4%; P=0.014). Seven-year survival rates did not differ significantly.
Older and obese patients are particularly at risk of postoperative atrial fibrillation after CABG. Patients who develop the sequela have longer hospital stays than, but similar long-term survival rates to, patients who do not.
Keywords: Atrial fibrillation/epidemiology/etiology/mortality; coronary artery bypass/adverse effects; hospitals, veterans/statistics & numerical data; length of stay; postoperative complications; predictive value of tests; proportional hazards models; retrospective studies; risk assessment; treatment outcome
Postoperative atrial fibrillation (POAF), the most prevalent arrhythmic sequela of cardiac surgery, has plagued postoperative management for decades. It occurs in 25% to 50% of patients after coronary artery bypass grafting (CABG).1,2 Although POAF is generally considered to be a temporary problem related to surgery, it can be life-threatening and is associated with increased morbidity and mortality rates.3–7
The precise pathophysiology of POAF after heart surgery is not known. Multiple baseline and intraoperative risk factors have been associated with POAF, such as age, a history of hypertension, obesity, diabetes mellitus, inflammation, and longer pump and cross-clamp times.2,8–10
Even though there are multiple, conflicting studies on POAF in civilians, only one previous study—conducted in the 1990s—examined this condition in CABG patients in the Veterans Affairs (VA) hospital population.4 It is also well documented that the VA population of cardiac surgical patients has a risk profile that differs significantly from that of civilians: they are predominately male and have higher prevalences of chronic obstructive pulmonary disease (COPD), peripheral vascular disease, smoking, and triple-vessel disease. Thus, study results in the civilian population do not generally mirror outcomes in the VA population.11 For these reasons, we examined the effects of POAF in VA patients who underwent isolated CABG.
Patients and Methods
After institutional review board approval and waiver of individual consent were granted, we retrospectively reviewed data from all patients who had undergone primary isolated CABG from October 2006 through March 2013 at the Michael E. DeBakey VA Medical Center (MEDVAMC). We excluded any patient who had a preoperative history of paroxysmal or persistent atrial fibrillation (AF), combined valve procedures, aortic aneurysm surgery, or any other concomitant cardiac surgical procedure. Of the 1,277 patients initially identified, 29 were excluded because data about AF were incomplete or missing from their records; thus, our study cohort consisted of 1,248 patients (Table I).
TABLE I.
Baseline Characteristics of the 1,248 Patients
Patients' medical records and the VA Surgical Quality Improvement Project (VASQIP) database were reviewed for demographic data, risk profiles, symptoms, surgical treatments, and operative morbidity and mortality rates. (The VASQIP prospectively collects risk and outcome data on all patients who undergo cardiac operations at the 40 VA cardiac surgery centers.) In addition, the patients' electronic charts were reviewed to supplement the information already in the VASQIP database. The day on which POAF occurred was also noted from the electronic chart and after review of the electrocardiogram. The primary study variable was the new onset of AF after CABG.
Surgical Techniques and Management
During the study period, all CABG procedures at the MEDVAMC were performed through a median sternotomy and by means of standard technique. Most procedures were on-pump, with ascending aortic cannulation and dual-stage venous cannulation of the right atrium. Cardiopulmonary bypass (CPB) was achieved with a membrane oxygenator, hemodilution, and mild systemic hypothermia. Myocardial protection was afforded by intermittent cold-blood cardioplegic solution delivered retrograde and antegrade. Left ventricular (LV) sumps via the pulmonary veins were not used.
Off-pump procedures involved use of the CTS stabilization system (Guidant Corp.; Santa Clara, Calif) at near normothermia. Intracoronary shunts were used whenever possible.
To reduce blood loss, blood was re-collected with a suction cardiotomy reservoir and cell-saver in the CPB group; only a cell-saver was used in the off-pump group.
Almost all procedures involved grafting the left internal mammary artery to the left anterior descending coronary artery. The saphenous vein was used when appropriate. Proximal anastomoses were performed with use of a side-biting clamp.
After surgery, patients were transferred to a dedicated surgical intensive care unit. Heart rates, electrocardiographic variables, central venous pressures, Swan-Ganz hemodynamic variables, arterial pressures, and acid-base and blood-gas levels were continuously monitored during each patient's stay. Inotropic support enabled stable hemodynamic conditions. Any perioperative need for blood products was determined on a patient-by-patient basis; in general, blood was transfused when the patient had a hemoglobin level <8 g/dL, was actively bleeding, or was hemodynamically unstable. Fluid intake and output were monitored hourly. Continuous telemetry was used to detect arrhythmias.
Preoperatively, patients were routinely given β-blockers. This therapy was reinstated on postoperative day 1 or 2 after CABG in patients who were hemodynamically stable and were not receiving pressors. Patients in whom POAF developed were given amiodarone intravenously in a 150-mg bolus, followed by an intravenous drip for 24 hours, per protocol. Patients then took 200 mg of amiodarone orally, twice daily, for 1 month, unless this was contraindicated. Those whose POAF persisted until discharge from the hospital were prescribed warfarin (target international normalized ratio, 2–3). Cardioversion was not performed unless it was hemodynamically necessary.
Outcome Measures
The primary outcome measure was that of long-term survival. Other outcome measures included morbidity (such as renal failure, cardiac arrest, coma, stroke, ventilation >48 hr, mechanical circulatory support, perioperative myocardial infarction, tracheostomy, repeat ventilator support within 30 d, reoperation for bleeding, or repeat CPB), death, and length of stay (LOS), this last calculated as the number of days from hospital admission to discharge, as well as days from CABG to hospital discharge (our focus). Risk factors included age, smoking status, and body mass index (BMI); comorbidities, such as diabetes mellitus and COPD; laboratory values; and operative characteristics, such as on- versus off-pump CABG, number of distal anastomoses, and CPB and ischemic times. We examined long-term survival data for the entire study period. The VASQIP database had complete vital-status data, and time to death from the date of the operation was computed for patients who died before 8 April 2013. Patients who did not die before this date were censored from the results.
Statistical Analysis
We compared the baseline characteristics of patients who had POAF with those who did not, using the χ2 test for categorical variables and the t test for continuous variables. P values ≤0.05 were considered to be statistically significant. Adjustment to the P values was not made for multiple comparisons. Multivariable logistic regression modeling was used to identify predictors of POAF. A backward elimination procedure was used in which the risk factors were all entered into the model, and those that were significant at P ≤0.05 were retained in the final model. Odds ratios with 95% confidence intervals (CIs) were obtained. The C statistic representing the area under the receiver operating characteristic curve was used to measure the ability of the model to discriminate patients who had POAF from those who did not. The Hosmer-Lemeshow statistic was used to evaluate the goodness-of-fit of the model.
Multivariable linear regression modeling was used to determine whether POAF was an independent predictor of the LOS from surgery to hospital discharge. Backward elimination was used: the patients' demographic, preoperative, and intraoperative risk factors and POAF status were entered into the model, and those that were significant at P ≤0.05 were retained in the final model. Because the distribution of LOS was skewed, we used 2 approaches. First, for the 67 patients (5% of the study cohort) with a postoperative LOS longer than 20 days, we truncated their stays at 20 days. Second, we eliminated those patients with stays longer than 20 days from the analyses to see whether the same variables were retained in the model and whether the parameter estimates were similar.
Using χ2 analyses, we examined whether having POAF was associated with operative death and 30-day death. We were unable to risk-adjust these mortality data for the various patient and clinical characteristics because of the small number of deaths within these time periods. We also examined long-term survival data for patients throughout the study period. The log-rank test from the unadjusted Kaplan-Meier analysis was performed to test for a significant difference in long-term survival outcomes between patients who did and did not have POAF. The Cox proportional hazards regression model was used to determine whether differences existed between these 2 groups while controlling for characteristics that might affect long-term survival outcomes and that might have differed between the groups. Hazard ratios representing the cumulative risk of death over the entire study period were computed, along with their 95% CIs. Analyses were performed with use of SAS software version 9.4 (SAS Institute Inc.; Cary, NC).
Results
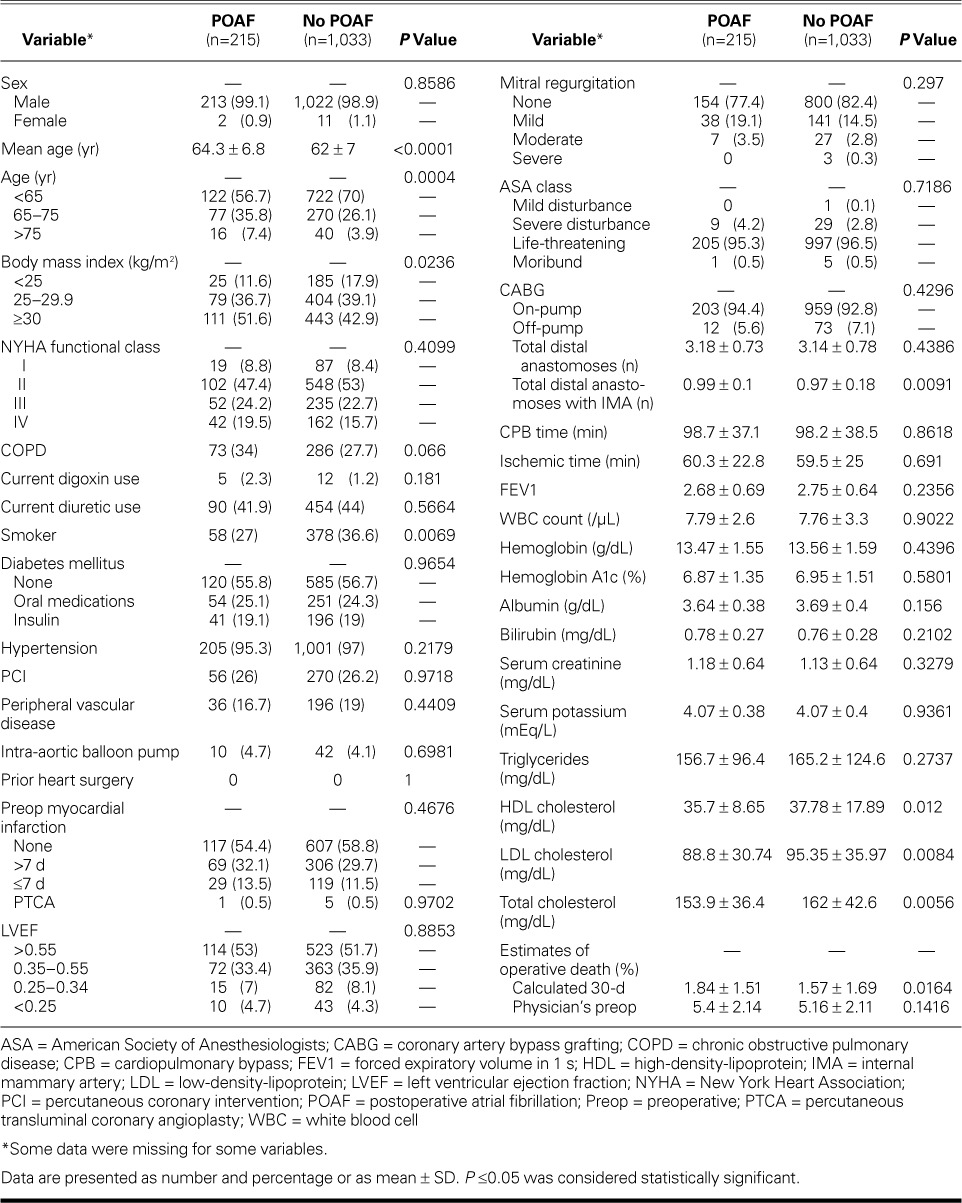
Of the 1,248 patients who underwent isolated CABG, 215 (17.2%) developed POAF and 1,033 (82.8%) did not (Table I). The mean age of the POAF group was higher than that of the non-POAF group (64 ± 7 vs 62 ± 7 yr; P <0.0001). Although all 1,248 patients had data on whether they had POAF, some data were missing for some variables.
Predictors of Survival. The 30-day mortality rate was higher in the POAF group than in the non-POAF group (1.9% vs 0.3%; P=0.015) (Table II). However, Kaplan-Meier analysis did not reveal a significant difference in 7-year survival rates between the 2 groups (Fig. 1). Results of Cox proportional hazard regression showed that COPD, peripheral vascular disease, LV ejection fraction <0.35, tracheostomy, and stroke were significant independent predictors of poor survival outcomes (Table III).
TABLE II.
Outcomes in the 1,248 Patients
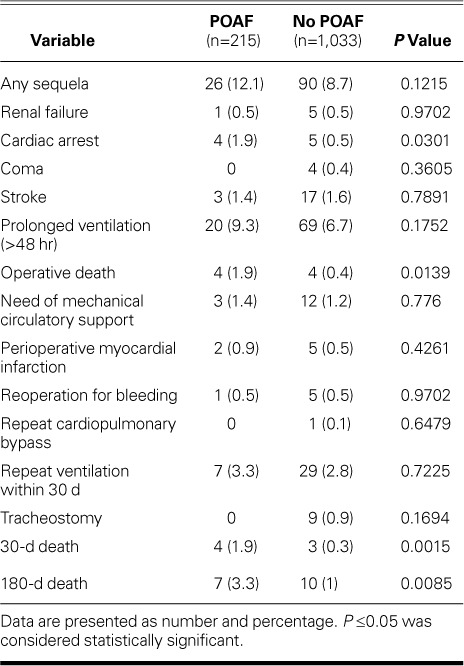
Fig. 1.
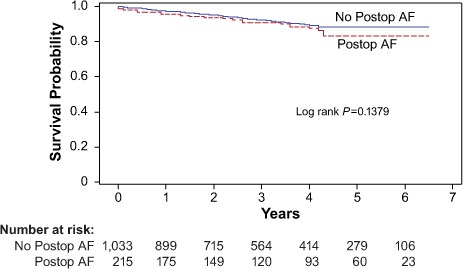
Graph shows Kaplan-Meier analysis of survival outcomes and number at risk in the 1,248 patients who had and did not have postoperative atrial fibrillation (Postop AF).
TABLE III.
Predictors of Poor Survival Outcomes after Coronary Artery Bypass Grafting
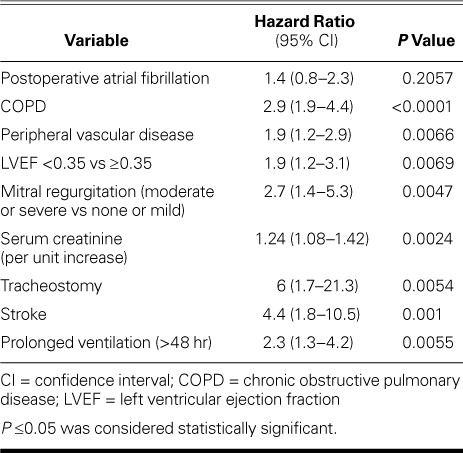
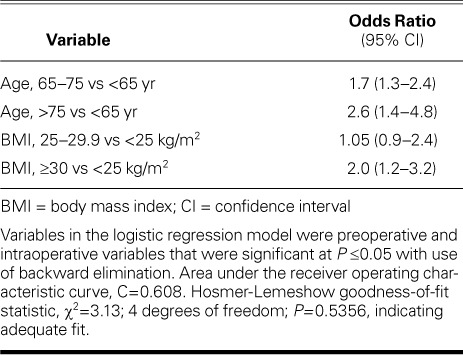
Predictors of Postoperative Atrial Fibrillation. Independent predictors of POAF were older age and higher BMI. Patients 65 to 75 years of age had 1.7 times higher odds (95% CI, 1.3–2.4) of having POAF than did patients younger than 65 years, and patients older than 75 years had 2.6 times higher odds (95% CI, 1.4–4.8) than did those younger than 65 years. The odds of POAF were twice as high (95% CI, 1.2–3.2) for obese patients (BMI, ≥30 kg/m2) as for patients of normal weight (Table IV).
TABLE IV.
Predictors of Postoperative Atrial Fibrillation
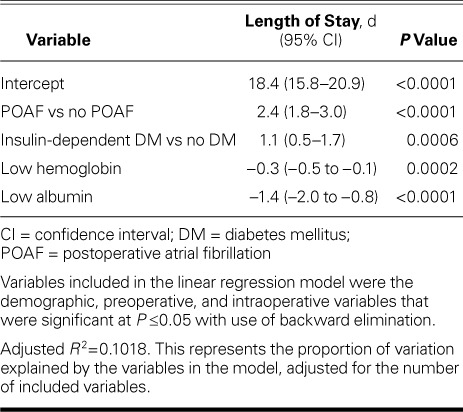
Length of Stay. Postoperative LOS was significantly longer in the POAF group (12.7 ± 6.6 vs 10.3 ± 8.9 d; P ≤0.0001). Furthermore, POAF was a significant predictor of postoperative LOS in the multivariable model that included preoperative and intraoperative risk factors (P <0.0001). Length of stay for patients with POAF was 2.4 days longer (95% CI, 1.8–3.0) than for those who did not have POAF. Other variables significantly associated with longer LOS were diabetes mellitus treated with insulin (these patients had a mean LOS 1.1 days longer than did nondiabetic patients) (95% CI, 0.5–1.7 d), and lower hemoglobin and albumin levels (Table V).
TABLE V.
Predictors of Longer Length of Stay after Coronary Artery Bypass Grafting
Discussion
Preoperative AF is a well-known risk factor for morbidity and death in patients who undergo CABG.12 In contrast, POAF, which is a prevalent sequela of cardiac surgery, has been described as a benign, self-remitting entity.12–15 More recently, however, POAF has emerged as a more sinister problem than was originally concluded.
Almassi and colleagues4 associated POAF with a greater risk of early death (5.95% vs 2.95%) and 6-month death (9.4% vs 4.2%). Likewise, Villareal and associates7 correlated POAF with both operative and 5-year death; the 5-year survival rate was 74% in patients with POAF versus 87% in those without (P <0.0001). In that study, the association with late death remained significant even after early death was excluded, and Kaplan-Meier survival curves involving ≤6 years of follow-up diverged, suggesting that clinical recognition of POAF identifies an ongoing or persistent effect. Similarly, Ahlsson and colleagues3 and Mariscalco and Engstrom5 found elevated early and 8-year mortality rates in patients who had POAF. However, in other studies,13–15 a similar elevation in the short-term mortality rate was not observed. Furthermore, Landymore and Howell16 performed Holter monitoring on their hospital-discharged patients and observed a very low incidence of AF, describing it as a transient phenomenon. In the current study of veterans, we noticed higher early mortality rates in the POAF patients; however, long-term (7-yr) mortality rates were not significantly different between the 2 groups.
The mechanisms that underlie the association between POAF and death are at best hypothetical. In the immediate postoperative period, the hemodynamic insult caused by loss of atrial kick certainly might contribute. The mechanisms leading to long-term death are even more difficult to ascertain after the patient is discharged from the hospital. The development of congestive heart failure, the occurrence of disabling stroke or other embolic catastrophes, and certain adverse drug effects (such as proarrhythmia from antiarrhythmic drugs and hemorrhage from anticoagulants) might all contribute. To further complicate issues, most studies on POAF—including ours—do not mention whether the patients still had AF upon follow-up evaluation. Very few investigators have looked at the persistence of AF, and the study involving Holter monitoring on discharged patients yielded a very low prevalence of post-discharge AF.16 Furthermore, the effect of POAF on LV dysfunction is not entirely clear.
In various studies, POAF has been associated with a 2- to 4-fold increased risk of stroke at 30 days, a 4- to 5-day increase in hospital LOS, and an increase in the cost of care of approximately $10,000 per patient.17–22 We too observed longer LOS in patients who had POAF, but we did not observe a significantly higher stroke rate, and we think that the additional LOS might be related to other factors, such as the time required to achieve rhythm and rate control in these patients. This further reinforces the observation that LOS and complications of POAF might have other causes in addition to embolic phenomena and their sequelae.
Wang and colleagues23 from the Framingham Heart Study and multiple other investigators have shown that obesity is an important predictor of AF development in adults. These investigators further proposed that left atrial enlargement causes the association between obesity and AF in obese patients.23–25 However, other putative mechanisms of POAF might also contribute to its higher incidence in obese patients, including ventricular remodeling,26 elevated plasma volume,27 disturbances in autonomic tone,28 ventricular diastolic dysfunction,29 and enhanced neurohormonal activation.30 In addition, increased oxidative stress,31 lipoapoptosis,32 or both, which are seen as adiposity increases, might lead to myocardial structural changes (including atrial changes), increasing the likelihood of AF. Likewise, our study shows a clear relationship between AF and higher BMI.
Older age is one of the strongest predictors of POAF. Mathew and co-authors6 have documented that, for every decade of life, there is a 75% increase in the odds of developing POAF; and they concluded that, on the basis of age alone, anyone older than age 70 is automatically at high risk of developing AF. Advanced age is associated with degenerative and inflammatory modifications in atrial anatomy (such as dilation and fibrosis). These alter atrial electrophysiologic properties by shortening the effective refractory period, by dispersing refractoriness and altering conduction (thus causing abnormal automaticity), and by causing anisotropic conduction.33 These processes act as potential substrates for POAF.34
Patients with POAF have been shown in some studies to have a higher incidence of cardiac arrest.17,18 In our series, the incidence of cardiac arrest during the index hospitalization was significantly higher in patients in whom POAF developed, further supporting the possibility that patients with POAF are sicker and more prone to life-threatening complications than are patients without POAF.
This study is limited by its retrospective and single-center nature. Although our query was designed to include all cases of POAF, the incidence of POAF was 17.2%, which is significantly lower than that in other reports. Furthermore, autopsy data were unavailable, and we had no data on immediate causes of death. Thus, we used all-cause death. We also did not evaluate the use of β-blockers, because almost all the patients began β-blocker therapy on postoperative day 1 or 2. However, strengths of our study are its long follow-up period and our robust database with attainment of nearly 100% follow-up.
In conclusion, POAF—the most prevalent arrhythmic sequela of CABG—increases short-term mortality rates, morbidity, and LOS in military veterans. Although its effect on long-term survival outcomes is not entirely clear, much work remains in the prevention and management of this condition in order to improve CABG outcomes.
Acknowledgment
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript.
Footnotes
From: Division of Cardiothoracic Surgery (Drs. Bakaeen, Cornwell, Omer, Petersen, and Rosengart) and VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (Dr. Petersen), Michael E. DeBakey Veterans Affairs Medical Center; Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery (Drs. Bakaeen, Bakshi, Cornwell, Coselli, LeMaire, Omer, Pattakos, Preventza, Rachlin, and Rosengart) and Department of Medicine (Dr. Petersen), Baylor College of Medicine; and Department of Cardiovascular Surgery (Drs. Bakaeen, Coselli, LeMaire, Pattakos, Preventza, and Rosengart), Texas Heart Institute; Houston, Texas 77030
This study was supported in part by the use of facilities and resources of the U.S. Department of Veterans Affairs; Veterans Health Administration; Health Services Research and Development Service; and VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs, the U.S. government, or Baylor College of Medicine.
References
- 1.Cox JL. A perspective of postoperative atrial fibrillation in cardiac operations. Ann Thorac Surg. 1993;56(3):405–9. doi: 10.1016/0003-4975(93)90871-e. [DOI] [PubMed] [Google Scholar]
- 2.Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2011;141(5):1305–12. doi: 10.1016/j.jtcvs.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Ahlsson A, Bodin L, Fengsrud E, Englund A. Patients with postoperative atrial fibrillation have a doubled cardiovascular mortality. Scand Cardiovasc J. 2009;43(5):330–6. doi: 10.1080/14017430802702291. [DOI] [PubMed] [Google Scholar]
- 4.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226(4):501–13. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariscalco G, Engstrom KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88(6):1871–6. doi: 10.1016/j.athoracsur.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 6.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 7.Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742–8. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Ascione R, Lloyd CT, Underwood MJ, Lotto AA, Pitsis AA, Angelini GD. Inflammatory response after coronary revascularization with or without cardiopulmonary bypass. Ann Thorac Surg. 2000;69(4):1198–204. doi: 10.1016/s0003-4975(00)01152-8. [DOI] [PubMed] [Google Scholar]
- 9.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96(10):3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 10.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 11.Grover FL, Shroyer AL, Hammermeister K, Edwards FH, Ferguson TB, Jr, Dziuban SW, Jr et al. A decade's experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg. 2001;234(4):464–74. doi: 10.1097/00000658-200110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahian DM, Edwards FH. Statistical risk modeling and outcomes analysis. Ann Thorac Surg. 2008;86(5):1717–20. doi: 10.1016/j.athoracsur.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Kalavrouziotis D, Buth KJ, Ali IS. The impact of new-onset atrial fibrillation on in-hospital mortality following cardiac surgery. Chest. 2007;131(3):833–9. doi: 10.1378/chest.06-0735. [DOI] [PubMed] [Google Scholar]
- 14.Lee JK, Klein GJ, Krahn AD, Yee R, Zarnke K, Simpson C et al. Rate-control versus conversion strategy in postoperative atrial fibrillation: a prospective, randomized pilot study. Am Heart J. 2000;140(6):871–7. doi: 10.1067/mhj.2000.111104. [DOI] [PubMed] [Google Scholar]
- 15.Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94(3):331–5. [PubMed] [Google Scholar]
- 16.Landymore RW, Howell F. Recurrent atrial arrhythmias following treatment for postoperative atrial fibrillation after coronary bypass operations. Eur J Cardiothorac Surg. 1991;5(8):436–9. doi: 10.1016/1010-7940(91)90191-l. [DOI] [PubMed] [Google Scholar]
- 17.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94(3):390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 18.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 19.Hogue CW, Jr, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100(6):642–7. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 20.Reed GL, 3rd, Singer DE, Picard EH, DeSanctis RW. Stroke following coronary-artery bypass surgery. A case-control estimate of the risk from carotid bruits. N Engl J Med. 1988;319(19):1246–50. doi: 10.1056/NEJM198811103191903. [DOI] [PubMed] [Google Scholar]
- 21.Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000;23(3):155–9. doi: 10.1002/clc.4960230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor GJ, Malik SA, Colliver JA, Dove JT, Moses HW, Mikell FL et al. Usefulness of atrial fibrillation as a predictor of stroke after isolated coronary artery bypass grafting. Am J Cardiol. 1987;60(10):905–7. doi: 10.1016/0002-9149(87)91045-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 24.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 25.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112(21):3247–55. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 26.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266(2):231–6. [PubMed] [Google Scholar]
- 27.Messerli FH, Ventura HO, Reisin E, Dreslinski GR, Dunn FG, MacPhee AA, Frohlich ED. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982;66(1):55–60. doi: 10.1161/01.cir.66.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Pelat M, Verwaerde P, Merial C, Galitzky J, Berlan M, Montastruc JL, Senard JM. Impaired atrial M(2)-cholinoceptor function in obesity-related hypertension. Hypertension. 1999;34(5):1066–72. doi: 10.1161/01.hyp.34.5.1066. [DOI] [PubMed] [Google Scholar]
- 29.Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, Leonetti F. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002;10(8):767–73. doi: 10.1038/oby.2002.104. [DOI] [PubMed] [Google Scholar]
- 30.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001;79(1):21–9. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 31.Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23(1):67–74. doi: 10.1038/sj.ijo.0800761. [DOI] [PubMed] [Google Scholar]
- 32.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97(4):1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103(5):769–77. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 34.Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988;62(4):811–32. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]


