Abstract
Ewing sarcoma is the second most prevalent malignant primary bone tumor but constitutes only a small proportion of cardiac metastases. We present a case of asymptomatic Ewing sarcoma metastatic to the right ventricle.
A 36-year-old man presented for evaluation and resection of a pedunculated right ventricular cardiac tumor. Three years before, he had been diagnosed with translocation-negative Ewing sarcoma, for which he had undergone chemotherapy and amputation of the left leg below the knee. We resected the right ventricular tumor. Analysis of the resected mass supported the diagnosis of metastatic Ewing sarcoma. Postoperative transthoracic echocardiograms showed normal biventricular size and function. One year later, the patient had no recurrence of the sarcoma. In addition to discussing this case, we review the relevant medical literature.
Keywords: Heart neoplasms/secondary/surgery; heart ventricles/surgery; neoplasm invasiveness; oncogene proteins, fusion; sarcoma, Ewing/genetics/pathology/surgery; treatment outcome
Cardiac tumors have been found in 0.001% to 0.3% of autopsy cases.1 Approximately 25% of primary cardiac tumors are malignant.1 Metastases to the heart are 20 to 40 times more prevalent than are primary cardiac malignancies.1 The estimated incidence of metastasis to the heart is 10% to 15.4%; lung tumors, lymphomas, and carcinomas of the breast are the typical metastatic sources.2,3
Ewing sarcoma is the second most frequent primary malignant bone tumor (after osteosarcoma) but accounts for only a small proportion of cardiac metastases.4–6 Its metastatic incidence in the right ventricle (RV) is not precisely known. We present the case of a patient who had asymptomatic Ewing sarcoma metastatic to the RV.
Case Report
In December 2013, a 36-year-old asymptomatic man presented for evaluation of a mass in his RV. Three years earlier, because of translocation-negative Ewing sarcoma, he had undergone below-the-knee amputation of the left leg and consequent chemotherapy. Two years later, he was found to have a lung metastasis. Genetic analysis of the resected lung tissue identified mechanistic target of rapamycin (mTOR) E1799K, Ewing sarcoma breakpoint region 1 (EWSR1) gene fusion with nuclear factor of activated T-cell cytoplasmic 2 gene (NFATc2), and topoisomerase 1 (TOP1) amplification lesions. These were consistent with Ewing sarcoma and prompted further chemotherapy.
Three months after the lung resection, magnetic resonance images (MRI) of the lungs revealed a 3.5 × 2.6-cm enhancing mass in the RV (Fig. 1), and the patient presented at our institution for possible resection of the mass. He was exceptionally physically fit and had no history of cardiac diseases. Results of positron emission tomography (PET) confirmed the presence of a metabolically active tumor (Fig. 2). The patient underwent cardiac evaluation and was cleared for surgery.
Fig. 1.
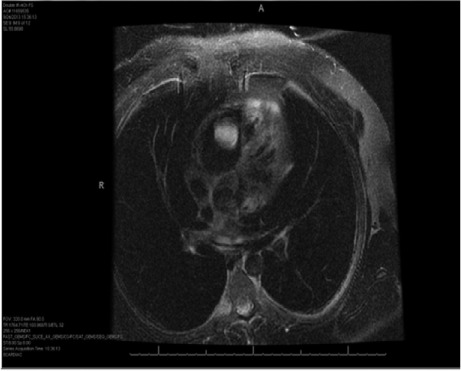
Magnetic resonance image (T2 fat-saturated axial planar view) shows an enhancing mass in the right ventricle.
Fig. 2.
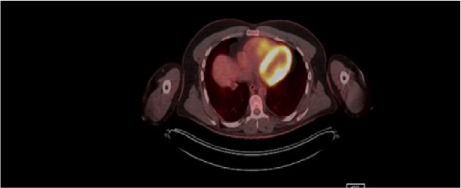
Fused positron emission tomographic–computed tomographic image with fluorodeoxyglucose (axial plane) shows a metabolically active tumor in the right ventricle.
An intraoperative transesophageal echocardiogram showed a preserved left ventricular (LV) ejection fraction. Other than a pedunculated mass attached to the RV, there were no structural abnormalities of the heart.
Under direct vision, with a normothermic beating heart, the tumor was resected with negative margins and no residual mass. Right atrial biopsies were also performed (with 2-mm negative margins on a frozen section). The total cardiopulmonary bypass time was 22 min. Postoperative transthoracic echocardiograms (TTEs) showed normal LV and RV size and function.
The morphology and immunohistochemical stains of the resected mass were consistent with a metastatic Ewing sarcoma tumor deposit. It was composed of highly malignant cells with moderate nuclear pleomorphism, a high nuclear-to-cytoplasmic ratio, frequent mitoses (16 per 10 high-power fields), and clear-to-finely vacuolated cytoplasm surrounding a central nucleus. The tumor architecture varied from solid sheets to cords and rare Homer Wright rosettes. Foci had a pale mucoid background and zones of necrosis (Fig. 3). Special staining for periodic acid-Schiff (PAS) revealed cytoplasmic glycogen in tumor cells (PAS with diastase sensitivity). The Ki-67 proliferative index was 5% to 40%. The tumor cells were positive for cluster of differentiation (CD) 99 and negative for CD45, S100 protein, pancytokeratin, and desmin (the vast majority of the tumor cells were negative for p53 staining).
Fig. 3.
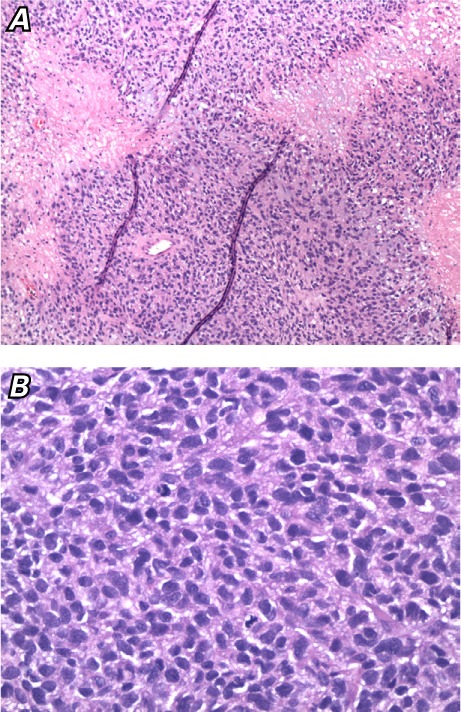
Photomicrographs show tumor cells with A) necrosis (H & E, orig. ×20) and B) a high mitotic rate (H & E, orig. ×40).
The patient recovered uneventfully, was discharged from the hospital on postoperative day 10, and continued medical management by his oncologist. One year later, he had experienced no recurrence of his disease.
Discussion
In 1921, James Ewing described classic Ewing sarcoma as a tumor of small round cells with high nuclear-to-cytoplasmic ratios arrayed in sheets, with scant pale eosinophilic to amphophilic cytoplasm, round nuclei with equally distributed granular chromatin, and inconspicuous nucleoli.7 Mitotic activity is often low, and cytoplasmic glycogen is usually present (PAS-positive granules persist after diastase digestion).4,7 A CD99 (cell-surface glycoprotein p30/32MIC2) positivity is representative of Ewing sarcoma. Translocation between chromosomes 11 and 22 is present in 85% of patients.4,8 Mapping of breakpoint regions by means of fluorescence in situ hybridization or reverse transcription-polymerase chain reaction often identifies chimeric genes formed between EWSR1 and various fusion partners, such as the friend leukemia integration site 1 gene (FLI 1) (93%), ETS-related gene (ERG) (5%), ETS-variant gene (ETV) 1, ETS-variant gene 4 (E1AF), fifth Ewing sarcoma variant (FEV), and zinc-finger sarcoma gene (ZSG). Chimera formation between ESWR1 and NFATc2 genes also occurs but is rare.8
Primary Ewing sarcoma usually occurs in the pelvis, long bones of the lower extremities, and chest wall.5 The tumor metastasizes predominantly by the hematogenous route to the lung, bone, and bone marrow, as is seen in 25% of patients.4,5,9 Patients with localized disease at presentation have an approximate 67% chance of cure. The event-free survival rate is around 30% for patients who have only pulmonary metastases, and it is less than 20% for patients whose disease is more diffuse.4
Cardiac metastases from this tumor can follow a venal route or result from surrounding local tumor invasion.6 Two case reports from the same center described cardiac invasion by local mediastinal Ewing sarcomas and the use of CorMatrix® porcine submucosal tissue grafts (CorMatrix Cardiovascular; Roswell, Ga) for pericardial reconstruction.10 Metastases to the RV have occluded the RV outflow tract.6 Cardiac metastasis has been the initial presentation of Ewing sarcoma.11 Primary cardiac Ewing sarcoma with LV and RV involvement or with only RV origin has also been described.12,13 Isolated metastatic Ewing sarcoma has been reported as a pedunculated intracardiac mass in the left atrium one year after lung surgery for Ewing sarcoma.14 Total artificial heart support has been used as a rescue strategy in a few cases of cardiac sarcoma other than Ewing sarcoma.15
Cardiac symptoms result from invasion of the myocardium, valves, and pericardium by this tumor. Patients can present with no symptoms, with mild fatigue or weight loss, or with symptoms of myocardial ischemia or congestive heart failure; in addition, thromboembolism and arrhythmias can occur.13
Computed tomography (CT) and cardiac-gated MRI are superior to TTE in detecting the presence, location, and extent of masses.5,16 Fluorodeoxyglucose PET fused with CT is better for determining the anatomic localization of cancer spread.5 The diagnosis of cardiac tumors by means of endomyocardial biopsy has rarely been described. Therapy for Ewing sarcoma embraces local control of the tumor (surgical intervention, radiation, or both) and systemic chemotherapy. Anti-angiogenic therapy is promising.4
Primary malignant tumors in the heart are treated with resection and heart reconstruction. Cardiac surgery is, in general, contraindicated in the presence of metastases. However, it is a valid option for preventing life-threatening complications or embolization, and for palliation.10 Heart transplantation is a possible option if the cardiac sarcoma metastases are not resectable, if the patient is free of other metastatic disease, and if life expectancy can thus be substantially extended.10,12,16 Regardless of successful surgical resection with or without adjuvant therapy, the prognosis of these patients is poor.
The incidence of Ewing sarcoma metastases in the RV is not precisely known, but with improvement in noninvasive techniques, more cases might be diagnosed in asymptomatic patients, which could prompt more efficient treatment by means of early surgical resection.
Acknowledgments
The authors thank Dr. Christina Paruthi and Dr. Aleksandar Gavric for assistance with article preparation.
Footnotes
From: Center for Advanced Heart Failure (Drs. Gregoric, Kar, Loyalka, Petrovic, and Thangam) and Department of Pathology and Laboratory Medicine (Drs. Buja and Zhao), University of Texas Health Science Center at Houston/Memorial Hermann Hospital–Texas Medical Center, Houston, Texas 77030
References
- 1.Ostrowski S, Marcinkiewicz A, Kosmider A, Jaszewski R. Sarcomas of the heart as a difficult interdisciplinary problem. Arch Med Sci. 2014;10(1):135–48. doi: 10.5114/aoms.2014.40741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinder M, Charafeddine A, Parnell AS, DiBardino DJ, Knudson JD. Osteosarcoma with cardiac metastasis in a 22-year-old man: a case report and review of cardiac tumors. Congenit Heart Dis. 2014;9(5):E147–52. doi: 10.1111/chd.12113. [DOI] [PubMed] [Google Scholar]
- 3.Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65(6):1456–9. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing's sarcoma family of tumors: current management. Oncologist. 2006;11(5):503–19. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 5.Coccia P, Ruggiero A, Rufini V, Maurizi P, Attina G, Marano R et al. Cardiac metastases of Ewing sarcoma detected by 18F-FDG PET/CT. J Pediatr Hematol Oncol. 2012;34(3):236–8. doi: 10.1097/MPH.0b013e318242754d. [DOI] [PubMed] [Google Scholar]
- 6.Flinn RM, Foyle A, Montague TJ. Extraskeletal Ewing's sarcoma with fatal cardiac metastasis. CMAJ. 1985;133(10):1017–8. [PMC free article] [PubMed] [Google Scholar]
- 7.The classic. Diffuse endothelioma of bone. By James Ewing. 1921. Clin Orthop Relat Res. 1984;(185):2–5. [PubMed] [Google Scholar]
- 8.Szuhai K, Ijszenga M, de Jong D, Karseladze A, Tanke HJ, Hogendoorn PC. The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009;15(7):2259–68. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- 9.Grier HE. The Ewing family of tumors. Ewing's sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44(4):991–1004. doi: 10.1016/s0031-3955(05)70541-1. [DOI] [PubMed] [Google Scholar]
- 10.Paul S, Ramanathan T, Cohen DM, Lebenthal A, Zellos L, Araki SF, Sugarbaker DJ. Primary Ewing sarcoma invading the heart: resection and reconstruction. J Thorac Cardiovasc Surg. 2007;133(6):1667–9. doi: 10.1016/j.jtcvs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chandramohan NK, Hussain MB, Nayak N, Kattoor J, Pandey M, Krishnankutty R. Multiple cardiac metastases from Ewing's sarcoma. Can J Cardiol. 2005;21(6):525–7. [PubMed] [Google Scholar]
- 12.Higgins JC, Katzman PJ, Yeager SB, Dickerman JD, Leavitt BJ, Tischler MD, Battle RW. Extraskeletal Ewing's sarcoma of primary cardiac origin. Pediatr Cardiol. 1994;15(4):207–8. doi: 10.1007/BF00800678. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury UK, Saxena A, Ray R, Sheil A, Reddy SM, Agarwal S, Mittal C. Salvage one and one-half ventricular repair and resection of Ewing's sarcoma of cardiac origin. Hellenic J Cardiol. 2010;51(1):71–3. [PubMed] [Google Scholar]
- 14.da Cunha CR, Santos PC, de Conti DO, Atik FA. Ewing's sarcoma presenting as an isolated intra-cardiac mass. Eur J Cardiothorac Surg. 2012;41(6):1398. doi: 10.1093/ejcts/ezr238. [DOI] [PubMed] [Google Scholar]
- 15.Bruckner BA, Rodriguez LE, Bunge R, Motomura T, Estep JD, Loebe M, Reardon MJ. Large cardiac tumor managed with resection and two ventricular assist devices. Ann Thorac Surg. 2014;97(1):321–4. doi: 10.1016/j.athoracsur.2013.04.136. [DOI] [PubMed] [Google Scholar]
- 16.Agaimy A, Rosch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928–38. [PMC free article] [PubMed] [Google Scholar]


