Abstract
Cardiac lipomas are rare and usually present as benign, encapsulated masses outside the heart; however, they can also be found within the atria. No single theory—including molecular genetic mutation—adequately explains why this occurs. Extensive career experience and broadened knowledge in embryology and cardiac physiology have helped us to develop a hypothesis based on invagination of extracardiac tumors. This report describes a vexing case of a giant right atrial lipoma, from 1985, in which the diagnosis was made incidentally during management of a patient's acute limb ischemia. In addition, we discuss the imaging and treatment of cardiac lipoma.
Keywords: Diagnostic imaging/methods; heart atria/pathology; heart neoplasms/diagnosis; incidental findings; lipoma/diagnosis/pathology/surgery/ultrasonography; models, cardiovascular; rare diseases
The first report of cardiac lipoma appeared in 18561; since then, only a few hundred cases have been reported.2,3 Cardiac lipomas are isolated cases among larger series of cardiac neoplasms.4 Chen and colleagues5 and Lombardi and associates6 proposed that primary tumors of the heart arise from gene mutations and the proliferation of various cell types in the myocardium; however, no unifying hypothesis regarding the development of intra-atrial giant lipomas has been proved. Modern imaging techniques, including improvements in echocardiography, have simplified diagnosis7; the addition of spiral computed tomography (CT) and magnetic resonance imaging (MRI) adds clarity to the extent of these tumors. Recently reported cases of cardiac lipomas in unusual locations attest to the complex management necessary to treat these rare lesions.8,9 Cardiac surgeons continue to search for exact mechanisms of how fatty tumors can appear within the chambers of the heart. Adipogenesis after injury and cardiomyopathy is being investigated.5,6 Modern theories have heretofore focused on fibroadipocytic replacements of myocytes within the intraventricular septum or ventricles.
We postulate the invagination of a well-formed lipoma, presumably originating from connective tissue in the space behind the Sondergaard groove,10 as a component of a theory to explain the lipoma's ultimate intra-atrial location. The Sondergaard groove (or Waterston groove) is the surface manifestation of the interatrial septum, a plane important to surgeons as a landmark for mitral valve replacement and the closure of atrial septal defects.10 In 2002, Ho and colleagues11 described epicardial tissue in the Sondergaard groove. Along with atrial muscle fibers, epicardial tissue was “sandwiched between the fold, frequently containing the arterial supply to the sinus node.”11 The authors proposed that this sandwiching of adjacent epicardial tissue was the source of adipocytes that compose giant atrial lipomas. We report an illustrative case of cardiac lipoma that perplexed the senior author (WGR) for almost 3 decades and gave rise to this new invagination hypothesis.
Case Report
In 1985, a 67-year-old woman sustained sudden severe pain in her left foot and ankle, consistent with acute limb ischemia. Thromboembolectomy of a popliteal artery occlusion restored the patient's blood flow, and pathologic analysis of the specimen yielded the diagnosis of thrombus. During the patient's examination, a chest radiograph showed an irregular shadow in the heart's right border, so a cardiac angiogram was obtained (Fig. 1). This revealed a large, space-occupying lesion in the right atrium.
Fig. 1.
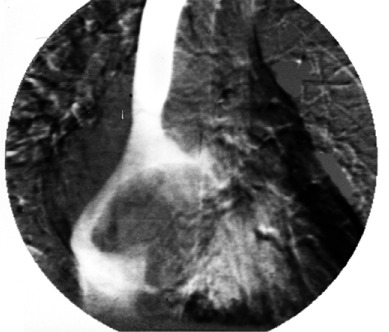
Cardiac angiogram reveals the space-occupying lesion in the right atrium.
The patient was admitted to Saint Joseph Hospital (Denver, Colo) for treatment of the right atrial mass. Her personal and family medical histories were noncontributory, her vital signs were normal, and cardiac examination yielded no significant abnormalities. An electrocardiogram showed frequent premature atrial beats, atrial conduction delay, and normal ventricular conduction. An echocardiogram confirmed the mass, and the patient was scheduled for surgery.
After a median sternotomy with use of conventional extracorporeal circulation, external examination and palpation of the heart suggested a large right atrial mass. An atriotomy exposed an orange-yellow mass that was round, smooth, and very firm (Fig. 2). Its removal necessitated dissection of the atrial wall from around the tumor's stalk, which was then transected in the posterior pericardial space. Internal examination of the heart revealed a normal atrial septum, with no patent foramen ovale, atrial septal defect, or ventricular septal defect to link the inciting event. Absent other sources of right-to-left shunting, the most likely cause appeared to be distortion of the left atrial cavity secondary to septal displacement, which impeded blood flow.
Fig. 2.
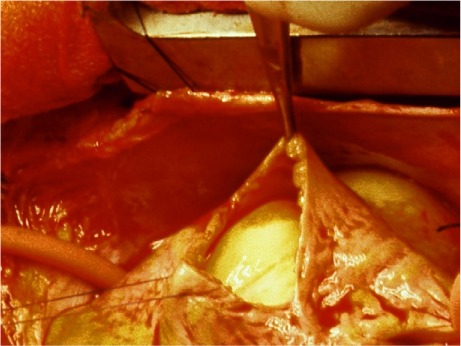
Intraoperative photograph shows the intra-atrial luminal position of the cardiac lipoma.
The anterior and posterior atriotomies were closed, and the patient was removed from extracorporeal circulation without incident. Her postoperative course was uneventful, and she was discharged from the hospital on postoperative day 10. No evidence of recurrence was seen during her early postoperative visits. The excised mass was a 7.7 × 5.5 × 4-cm lipoma, weighing 158 g and consisting of extremely dense homogenous lipoid tissue without cystic degeneration, attached to an 8 × 6 × 5.3-cm base (Fig. 3).
Fig. 3.
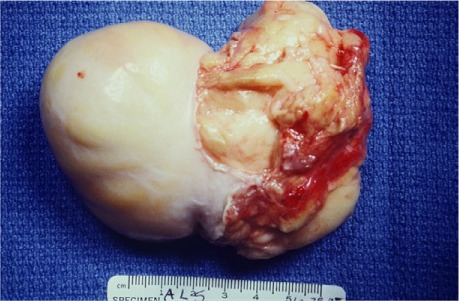
Photograph shows the 7.7 × 5.5 × 4-cm lipoma specimen, attached to an 8 × 6 × 5.3-cm base.
Discussion
Less than 10% of all benign cardiac tumors are lipomas.3 Cardiac lipomas are of mesodermal origin and histologically resemble lipomas elsewhere in the body. Approximately 50% of cardiac lipomas arise subendocardially, 25% subepicardially, and 25% from the myocardium.3,7 They have been reported in adolescents and adults.12 The tumors are typically found incidentally, because most patients with a cardiac lipoma are asymptomatic; however, valvular obstruction, cardiac compression, arrhythmias, and peripheral embolization can occur.13,14
Only a few epicardial lipomas have been reported within the atria or compressing the ventricles.15 To our knowledge, nothing unequivocally explains the presence within either atrium of adipocytes that are most typically found in extracardiac locations. Fontaine and coworkers16 analyzed fat on the right ventricular free wall and around the epicardial coronary arteries and noted adipocytes within fibrous tissue. After myocardial infarction, intramyocardial adiposity can be found in hearts that present with postinfarction ventricular tachycardia consequent to scarring.17 Cardiac adipocytes have been attributed to differentiation of cardiac precursor cells in patients who have arrhythmogenic right ventricular cardiomyopathy.6 On the other hand, large intracardiac lipomas—particularly those in the right atrium—have never been completely explained. Primary rhabdomyosarcomas of the heart have never been well documented, which suggests that differentiation of cardiac stem cells might not, by itself, account for all primary tumors of the heart, or for these cells' effective participation in myocardial regeneration after injury.
Hypothesis
We propose invagination as the mechanism for the giant right atrial tumor in the illustrative case above, involving 4 main factors: 1) the repetitive systolic contractions of the heart; 2) the thin, soft, pliable right atrial wall; 3) the firmness of the lipoma, and 4) the gradual-but-relentless growth of the tumor.
These, in combination, cause the atrial wall to be invaginated and gradually thinned over time (Fig. 4). Continued pressure by the enlarging extracardiac tumor results in incremental stretching and subsequent penetration of the atrial wall. The rarity of this tumor precludes definitive proof of our invagination theory at this time.
Fig. 4.
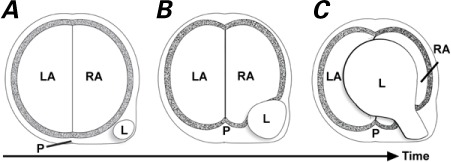
Artist's drawing shows the process of gradual invagination of the lipoma A) from the connective tissue behind the Sondergaard groove B) through the atrial wall and C) into the chamber of the right atrium.
L = lipoma; LA = left atrium; P = pericardial space; RA = right atrium
Imaging and Treatment of Cardiac Lipoma
Imaging. Transthoracic echocardiography is still the initial imaging method of choice in diagnosing cardiac tumors.12 However, it cannot distinguish lipomas from other cardiac masses of similar morphologic characteristics.7,18,19 For this reason, reports of cardiac lipoma have tended to emphasize the role of transesophageal echocardiography, contrast CT, and MRI.4 These imaging methods have improved the accurate diagnosis of cardiac lipoma because they can identify adipose tissue with minimal equivocation. The contrast between adipose tissue in lipoma and that from the cardiac parenchyma clearly distinguishes the depth of invasion and highlights the margins of the lipoma, thus enabling comprehensive preoperative planning.4
Treatment. Open resection is typically performed,20–22 through the appropriate atriotomy or ventriculotomy; however, minimally invasive techniques, including video-assisted resection, might be appropriate in selected patients.23 In treating tumors of massive size, creative reconstructions might be necessary, with the use of pericardium to patch defects in the main arterial and venous trunks.24
Giant atrial lipomas are exceedingly rare and incompletely explained. We have presented a novel hypothesis regarding the invagination and subsequent atrial erosion by a large extracardiac lipoma that penetrated a patient's intra-atrial lumen. Cardiac surgeons should consider that extracardiac masses adjacent to the atria might erode into the heart, whereas masses adjacent to the ventricles are more likely to produce extrinsic compression.
Acknowledgment
We thank Sonya E. Fogg, MLS (Manager, Library and Learning Resource Center, Texas Heart Institute, Houston), for researching our historical reference.1
Footnotes
From: Department of Surgery (Dr. Rainer), University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045; and Department of Graduate Medical Education (Drs. Bailey and Hollis), Saint Joseph Hospital, Denver, Colorado 80218
References
- 1.Albers JTH. Ein Lipom mit stellenweise vorherrschender Faserbildung in der Muskelsubstanz des Herzens und eine zweite Geschwulst mit atheromatösem Inhalt am Bulbus aortae [in German] Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1856;10(1–2):215–21. Available from: http://hdl.handle.net/2027/uc1.b3745189?urlappend=%3Bseq=225 [cited 2016 Aug 29] [Google Scholar]
- 2.Shumacker HB, Jr, Leshnower AC. Extracavitary lipoma of the heart. Operative resection. Ann Thorac Surg. 1974;18(4):411–4. doi: 10.1016/s0003-4975(10)64378-0. [DOI] [PubMed] [Google Scholar]
- 3.Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191(2):127–38. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grande AM, Ragni T, Vigano M. Primary cardiac tumors. A clinical experience of 12 years. Tex Heart Inst J. 1993;20(3):223–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114(3):454–68. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT, Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2011;109(12):1342–53. doi: 10.1161/CIRCRESAHA.111.255075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragland MM, Tak T. The role of echocardiography in diagnosing space-occupying lesions of the heart. Clin Med Res. 2006;4(1):22–32. doi: 10.3121/cmr.4.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B, Bhairappa S, Shankar SK, Prasad NM, Manjunath CN. Cardiac lipoma at unusual location -- mimicking atrial myxoma. Echocardiography. 2013;30(3):E72–4. doi: 10.1111/echo.12093. [DOI] [PubMed] [Google Scholar]
- 9.Grech R, Mizzi A, Grech S. Compression of the superior vena cava by an interatrial septal lipoma: a case report. Case Rep Pulmonol. 2013;2013:945726. doi: 10.1155/2013/945726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sondergaard T, Gotzsche H, Ottosen P, Schultz J. Surgical closure of interatrial septal defects by circumclusion. Acta Chir Scand. 1955;109(3–4):188–96. [PubMed] [Google Scholar]
- 11.Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res. 2002;54(2):325–36. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg MK, Chang IL, Silverman NH, Ramamoorthy C, Chan FP. Images in cardiovascular medicine. Near sudden death from cardiac lipoma in an adolescent. Circulation. 2006;113(21):e778–9. doi: 10.1161/CIRCULATIONAHA.105.589630. [DOI] [PubMed] [Google Scholar]
- 13.Censi S, Squeri A, Baldelli M, Parizi ST. Ischemic stroke and incidental finding of a right atrial lipoma. J Cardiovasc Med (Hagerstown) 2013;14(12):905–6. doi: 10.2459/JCM.0b013e328364bf8b. [DOI] [PubMed] [Google Scholar]
- 14.Khalili A, Ghaffari S, Jodati A, Shokoohi B, Pourafkari L. Giant right atrial lipoma mimicking tamponade. Asian Cardiovasc Thorac Ann. 2015;23(3):317–9. doi: 10.1177/0218492313504938. [DOI] [PubMed] [Google Scholar]
- 15.Xie LX, Chen YS, Liu SY. A giant cardiac lipoma associated with ventricular inversion and ventricular aneurysm: ultrasonography and CT imaging findings. Chest. 2012;141(1):241–4. doi: 10.1378/chest.10-2987. [DOI] [PubMed] [Google Scholar]
- 16.Fontaine G, Fontaliran F, Zenati O, Guzman CE, Rigoulet J, Berthier JL, Frank R. Fat in the heart. A feature unique to the human species? Observational reflections on an unsolved problem. Acta Cardiol. 1999;54(4):189–94. [PubMed] [Google Scholar]
- 17.Pouliopoulos J, Chik WW, Kanthan A, Sivagangabalan G, Barry MA, Fahmy PN et al. Intramyocardial adiposity after myocardial infarction: new implications of a substrate for ventricular tachycardia. Circulation. 2013;128(21):2296–308. doi: 10.1161/CIRCULATIONAHA.113.002238. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Jung HO. Anatomic variants mimicking pathology on echocardiography: differential diagnosis. J Cardiovasc Ultrasound. 2013;21(3):103–12. doi: 10.4250/jcu.2013.21.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King SJ, Smallhorn JF, Burrows PE. Epicardial lipoma: imaging findings. AJR Am J Roentgenol. 1993;160(2):261–2. doi: 10.2214/ajr.160.2.8424329. [DOI] [PubMed] [Google Scholar]
- 20.Mu J, Li X, Zhang J, Bo P. An unusual case of a giant cardiac lipoma. Acta Cardiol. 2014;69(4):469–70. doi: 10.1080/ac.69.4.306670. [DOI] [PubMed] [Google Scholar]
- 21.Kanemitsu S, Hirano K, Shimono T, Shimpo H. A case of a large cardiac lipoma with coronary artery disease. Asian Cardiovasc Thorac Ann. 2014;22(5):604–6. doi: 10.1177/0218492313479953. [DOI] [PubMed] [Google Scholar]
- 22.Jedlinski IM, Bugajski P, Greberski K, Kalawski R, Slomczynski M. Large asymptomatic cardiac lipoma localised in superior vena cava inflow: three-year follow-up [in Polish] Kardiol Pol. 2014;72(3):285. doi: 10.5603/KP.2014.0056. [DOI] [PubMed] [Google Scholar]
- 23.Patris V, Argiriou M, Lama N, Sakellaridis T, Charitos C. Trans-aortic excision of intraventricular lipoma with the assistance of arthroscopic camera. J Thorac Dis. 2013;5(4):E140–3. doi: 10.3978/j.issn.2072-1439.2013.08.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu SB, Zhu J, Liu Y, Wang RP. Surgical treatment of a giant symptomatic cardiac lipoma. J Thorac Oncol. 2013;8(10):1341–2. doi: 10.1097/JTO.0b013e3182a12a6a. [DOI] [PubMed] [Google Scholar]


