Abstract
Functional MRI studies have helped to elucidate underlying mechanisms in complex neurological and neuropsychiatric disorders. Disease processes often involve complex large-scale network interactions, extending beyond the presumed main disease focus. Given both the complexity of the clinical phenotype and the underlying dysfunctional brain circuits, so called pharmaco-fMRI (ph-MRI) studies probe pharmacological effects on functional neuro-anatomy, and can help to determine early treatment response, mechanisms of drug efficacy and side effects, and potentially advance CNS drug development. In this review, we discuss recent ph-MRI research in three major neuropsychiatric and neurological disorders and associated network alterations, namely selective serotonin and noradrenergic reuptake inhibitors in affective disorders and emotional processing circuits; antiepileptic drugs in epilepsy and cognitive networks; and stimulants in attention-deficit/hyperactivity disorder and networks of attention control. We conclude that ph-MRI studies show consistent and reproducible changes on disease relevant networks, and prove sensitive to early pharmacological effects on functional anatomy associated with disease. Further CNS drug research and development would benefit greatly from improved disease phenotyping, or biomarkers, using advanced imaging techniques.
Abbreviations: ACC, anterior cingulate cortex; AED, antiepileptic drugs; ADHD, attention-deficit/hyperactivity disorder; BOLD, blood oxygen level-dependent signal; CBZ, carbamazepine; CNS, central nervous system; DAT, dopamine transporter; JME, juvenile myoclonic epilepsy; LEV, levetiracetam; LTG, lamotrigine; NaRI, noradrenergic reuptake inhibitors; OXC, oxcarbazepine; Ph-MRI, pharmacological functional MRI; SSRI, selective serotonin reuptake inhibitors; TLE, temporal lobe epilepsy; TMS, transcranial magnetic stimulation; TPM, topiramate; VPA, valproate; ZNS, zonisamide
Keywords: Pharmacological, Functional MRI, Neuroimaging, CNS drug research, Biomarker
1. Introduction
In functional MRI (fMRI), reproducible patterns of activation or deactivation elicited by motor, cognitive or other tasks could be identified, such as the default mode network, a set of brain regions, which are commonly deactivated during goal-directed tasks (Bullmore, 2012, Raichle et al., 2001). This enables to explore disease effects on regional and network functional anatomy, and vice versa, may help to establish consistent (functional) imaging phenotypes of CNS diseases. These are particularly in demand given the somewhat dissatisfying trial-and-error approach when it comes to medication choices, without being able to tailor drugs a priori to the individual patient's disease phenotype. Indeed, what we have learned from fMRI studies in these disorders so far is that disease processes often involve complex large-scale network interactions, extending beyond the presumed main disease focus. Imaging phenotypes may therefore provide surrogate markers to, firstly, investigate drug effects at a network level in so called pharmaco-fMRI (ph-MRI) studies and, secondly, to early establish treatment efficacy, dose-response relationships and cognitive side-effects of CNS drugs (Nathan et al., 2014).
2. Pharmaco-fMRI: concept and challenges
Ph-MRI is a promising emerging application to assess regional network effects of and treatment response to specific medications. Several methodological difficulties have to be considered:
FMRI indirectly probes neuronal activity by measuring the Blood Oxygenation Level Dependent (BOLD) activity, which results from changes in levels of deoxyhaemoglobin in response to local metabolic demands of neuronal function (Logothetis, 2008).
The signal change in fMRI related to a drug is low; hence drug effects are generally studied as an interaction effect in task-related fMRI, i.e. task-related activation patterns for a drug are compared to those without the drug or placebo. Drugs can influence the BOLD signal both at a neuronal and vascular level complicating the interpretation of the effects observed. Approaches to quantify those effects on BOLD contrast include measurements of physiological changes in brain perfusion with blood flow measurements with arterial spin labelling (Borsook et al., 2013). As the BOLD signal is contaminated by low-frequency noise, detection of slowly evolving medication effects can be challenging (Mehta and O'Daly, 2011).
Ph-MRI has the major advantage that it can investigate effects of pharmacological agents at a network level and remotely from regions of highest target receptor densities, whereas PET and molecular studies can define target receptor occupancy and affinity without necessarily translating effects to large-scale networks (Mehta and O'Daly, 2011). Hence ph-MRI enables a “system evaluation” of networks underlying behavioural effects of a drug, independent of its biochemical mechanism of action. CNS drugs often target several receptor sub-types with varying regional distribution, and drug efficacy may differ across these targets. Functional MRI can monitor the combined effect of these interactions across multiple brain regions (Borsook et al., 2006); hence ph-MRI has the potential to provide “mechanism-related activation maps” (Nathan et al., 2014) to serve as targets for testing of drug effects. A further advantage is that fMRI does not use ionizing radiation and has no known biological side effects.
3. Pharmaco fMRI: functional networks and specific medication effects
In the following, the effect of CNS drugs on major neuronal networks will be discussed exemplary in neuropsychiatric conditions. It is important to note that regional medication effects have usually been probed in the context of cognitive or emotional paradigms, similar to those paradigms employed to explore disease-related functional alterations at a regional and network level. Hence, activation changes attributed to medication effects have to be considered in the context of possible additional effects of disease load or activity, and the sensitivity of the task to unveil disease and medication related changes on functional anatomy.
3.1. SSRI, NaRI and emotional processing circuits
Most ph-MRI studies in affective disorders explore the effects of selective serotonin (SSRI) or noradrenergic (NaRI) reuptake inhibitors and benzodiazepines. Overall, fMRI studies in major depression, and studies employing anxiety provocation in patients with anxiety disorders report attenuation of the limbic and para-limbic regions, and enhanced activation within prefrontal networks following SSRI/NaRI treatment relative to the drug naïve state (Delaveau et al., 2011, Furmark et al., 2002, Phan et al., 2013). In healthy controls, SSRI treatment lead to attenuation of amygdala activation during a negative emotional stimuli task (Anderson et al., 2007, Harmer et al., 2006, Windischberger et al., 2010). This has been similarly reported after single dose application of anxiolytic benzodiazepines (Paulus et al., 2005). On the contrary, increased amygdala activation was observed with a short SSRI trial in healthy subjects during a positive emotional paradigm, suggesting that antidepressants may modulate abnormal emotional processing in the diseased (Norbury et al., 2009). In a behavioural study of depressed patients, changes in emotional processing within the first two weeks of SSRI/NaRI treatment were predictive of later treatment response, before therapeutic effects were observed (Tranter et al., 2009). Pre-treatment activation within limbic and fronto-cortical regions was found to be predictive of treatment success with SSRI/NaRI in anxious patients (McClure et al., 2007, Nitschke et al., 2009).
Normalization of limbic and fronto-cortical imbalance has also been observed following non-pharmacological treatment, such as cognitive behavioural therapy (Fu et al., 2008, Furmark et al., 2002) and neuro-modulation (Mayberg et al., 2005), suggesting that observed effects could represent a common treatment pathway; the specificity of antidepressant medication on the fronto-limbic network therefore remains unclear.
In resting-state functional connectivity studies in major depressive disorder, treatment response to SSRI/NaRI was associated with increased connectivity between cortico-frontal and limbic regions, likely resulting in greater control over emotion regulation (Dichter et al., 2015). A meta-analysis of fMRI studies in patients with major depression employing emotional stimuli provocation also reports enhanced deactivation of areas of the default mode, including anterior and posterior cingulate cortices, precuneus and the inferior parietal lobule (Delaveau et al., 2011). There is some evidence that antidepressant treatment may normalize hyper-connectivity within the default mode network, though without evidence of a clear correlation with clinical improvement (Gudayol-Ferré et al., 2015).
3.2. Antiepileptic drugs and higher cognitive networks
Given the variety of available anticonvulsive drugs (AED) and the heterogeneity of epilepsy syndromes in terms of networks involved, fMRI surrogate markers for early determination of treatment efficacy and likelihood of side effects are urgently needed. So far, ph-MRI studies are rare and usually of small sample sizes (Koepp, 2011). Ph-fMRI studies in epilepsy are hampered by the fact that patients usually are already on AED, therefore the study design has to control for effects of co-medication in addition to other confounders, such as disease activity or disease severity and comorbidities (Beltramini et al., 2015). Most ph-MRI studies in epilepsy patients employ cognitive tasks, likely because cognitive network alterations in epilepsy are well known, and cognitive side effects are a major factor for medication adherence (Bootsma et al., 2009, Fisher et al., 2000). AED appear to lead to attenuation of either task-related activation and/or deactivation in co-localised hubs critical to both the specific epilepsy syndrome and the network relevant to the cognitive function studied.
3.2.1. Carbamazepine and oxcarbazepine
The first ph-fMRI study in epilepsy employed a visual-spatial memory retrieval task in patients with refractory temporal lobe epilepsy (TLE) (Jokeit et al., 2001) evaluating the relationship of mesio-temporal fMRI activation and carbamazepine serum (CBZ) concentrations. The extent of task and syndrome specific fMRI activation within the medial temporal lobe was negatively correlated with the CBZ serum levels. The effect was most marked with close to toxic drug levels. However, there was no psychometric data available to relate effects to memory function in these patients.
Employing a graph theoretical approach and resting state fMRI in a TLE cohort treated with CBZ or oxcarbazepine (OXC) and comparing to those who were on other AED (Haneef et al., 2015), altered “hubness” was reported in those on CBZ/OXC, i.e. less highly connected nodes connecting distant parts of the brain. Whereas betweenness centrality, or hubness, was reduced within the limbic circuit and thalamus with CBZ/OXC use, it was increased in default mode regions, i.e. cingulate and posterior cingulate/precuneus.
Previous data in TLE suggests a “re-distribution” of hub regions with high betweenness centrality to mainly paralimbic and temporal association cortices (Bernhardt et al., 2011), hence suggesting a region-specific effect of CBZ/OXC on disease-related network changes.
3.2.2. Valproate
In a placebo-controlled, combined transcranial magnetic stimulation (TMS) and fMRI study, valproate (VPA) and lamotrigine (LTG) demonstrated network specific effects. When TMS was applied over the motor region, both agents reduced TMS-specific effective connectivity between the primary motor and pre-motor cortex and the primary motor and supplementary motor area (SMA). Only LTG-treatment was associated with increased effective connectivity between the left dorsolateral prefrontal cortex and anterior cingulate when TMS was applied over the prefrontal cortex (Li et al., 2011).
For VPA, similar effects are seen in juvenile myoclonic epilepsy (JME). This syndrome has been associated with increased functional and structural connectivity between central motor and prefrontal cognitive networks, likely accounting for cognitively triggered jerks, a reflex trait highly associated with the syndrome (Vollmar et al., 2011, Vollmar et al., 2012, Yacubian and Wolf, 2014). Abnormal motor cortex co-activation with cognitive networks during an fMRI working memory task in JME was shown to be modulated by disease factors, i.e. the trait was enhanced with more frequent seizures and during the morning, when seizures occur more frequently due to the typical chrono-dependency of the syndrome. Vice versa, abnormal co-activation was attenuated with increasing VPA dose, consistent with the clinical impression that VPA is effective in JME, particularly in treatment of myoclonic jerks, and not necessarily associated with cognitive side effects (Wandschneider et al., 2012).
3.2.3. Levetiracetam
Several studies attribute a favourable cognitive profile to Levetiracetam (LEV) (Helmstaedter and Witt, 2008), considered to be superior to CBZ (Helmstaedter and Witt, 2010).
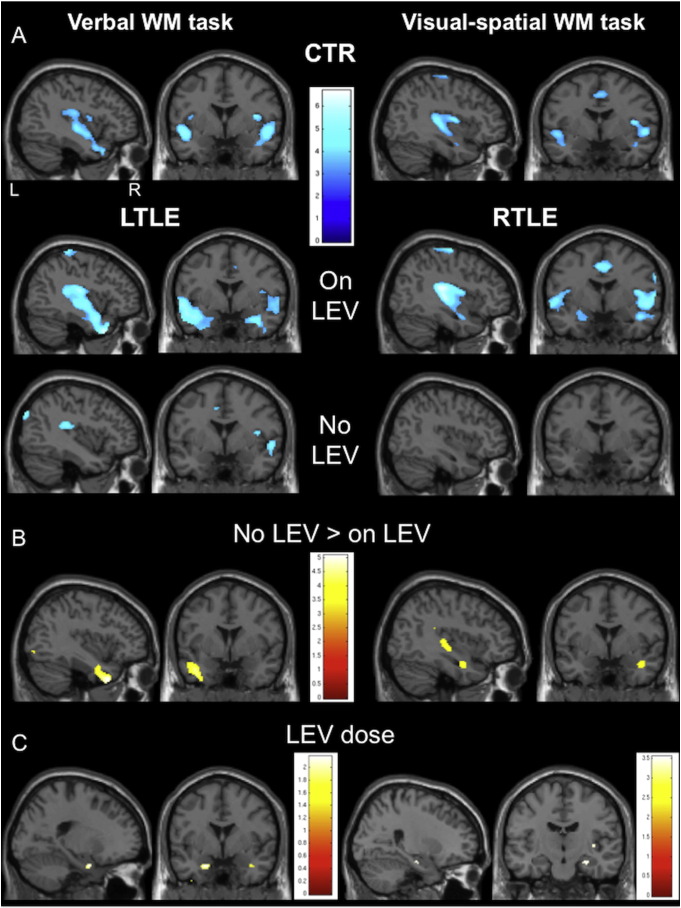
Consistent with neurobehavioural data, fMRI studies show a beneficial effect of LEV to cognitive networks. Task- and syndrome specific regional fMRI effects, as well as dose-dependency were demonstrated in a verbal and visual-spatial working memory task in left and right TLE patients, comparing those treated with to those without LEV: Patients on LEV showed an augmentation of task-related deactivation in the diseased temporal lobe compared to patients without LEV; more specifically, this effect was seen in the left mid-temporal gyrus in left TLE during the verbal, and the right hippocampus in right TLE during the visual-spatial task and became more apparent with increasing LEV dose. As patients on LEV showed similar task-related deactivation patterns to healthy controls, LEV appears to be associated with restoration of normal fMRI activation (Fig. 1). (Wandschneider et al., 2014).
Fig. 1.
(Wandschneider et al., 2014). Group comparisons between patients with and without Levetiracetam (LEV) during two working memory (WM) fMRI paradigms.
Group maps of areas of task-related deactivation networks in controls and all patients during the left- and right-lateralising task, are demonstrated. Whereas healthy controls and patients on LEV show similar patterns of deactivation, patients without LEV show less deactivation in the medial temporal lobe areas than both controls and patients on LEV in either lateralising task (Fig. A). During the verbal WM task, left TLE patients without LEV significantly fail to deactivate the left mid-temporal gyrus (Fig. B; left TLE without LEV > left TLE with LEV, p < 0.001, 20 voxels threshold extent). During the right-lateralising visual-spatial task, patients with right TLE who are not treated with LEV fail to deactivate the right hippocampus (Fig. B; right TLE without LEV > right TLE with LEV, p < 0.001, 20 voxels threshold extent).
A post-hoc analysis in patients treated with LEV demonstrated a dose-dependent effect of mesial temporal lobe deactivation through LEV. The lower the LEV dose, the lesser the right hippocampus is deactivated during the visual-spatial WM task (Fig. C; p < 0.001, 20 voxels threshold extent). A similar dose effect is observed in left TLE patients during the verbal WM task at a lower level of significance (Fig. C; p < 0.05, uncorrected): The left > right hippocampus becomes less strongly deactivated with lower LEV dose. CTR = healthy controls; LEV = levetiracetam; LTLE = left temporal lobe epilepsy; RTLE = right temporal lobe epilepsy; WM = working memory.
Though out-of-scanner neurobehavioural data, including frontal lobe cognitive measures, did not differ between those treated with LEV and those without, LEV modulated fMRI deactivation patterns can be interpreted as a beneficial drug effect. This is corroborated by data in healthy subjects and epilepsy patients where progressive deactivation of mesial temporal structures during cognitive tasks is observed with improved performance (Cousijn et al., 2012, Stretton et al., 2013).
Functional MRI data from individuals with amnestic mild cognitive impairment, which is associated with a risk of Alzheimer's disease, demonstrated that dysfunctional, increased hippocampal activation in the dentate gyrus/CA3 was normalized by low-dose LEV treatment with improvement of memory performance (Bakker et al., 2012, Bakker et al., 2015). Hence across different disease entities, LEV appears to have a localisation-specific effect, which is both relevant to seizure generation and propagation, and cognitive performance via a major hub of the default mode network.
3.2.4. Topiramate and zonisamide
For TPM, cognitive dysfunction has been described in patients with epilepsy, migraine and healthy controls, characterised by reduced attention, psychomotor speed, short-term memory and more specifically, impairment of expressive language and working memory. These deficits are noted even after single-dose administration and on steady-dose in mono- or combination therapy, irrespective of seizure control. Psychometric measures consistently improve after significant dose reduction or discontinuation. (Bootsma et al., 2008, Martin et al., 1999, Meador et al., 2005, Mula and Trimble, 2009, Thompson et al., 2000) Zonisamide treatment leads to similar, probably less pronounced neurocognitive impairment (Mula and Trimble, 2009, Ojemann et al., 2001).
Topiramate is the AED most studied in ph-MRI trials, though in relatively small cohorts. Five fMRI studies employed expressive language tasks in two healthy subjects, five to 16 epilepsy and ten migraine patients after a single dose or on steady-state TPM treatment. Combined results convey a pattern of reduced activation in language relevant regions, i.e. dominant inferior and middle frontal gyri, superior temporal gyrus (De Ciantis et al., 2008, Jansen et al., 2006, Szaflarski and Allendorfer, 2012), and a failure to deactivate task-negative regions, including the default mode network (Szaflarski and Allendorfer, 2012, Tang et al., 2016, Yasuda et al., 2013). Successful task execution in general has been associated with effective deactivation of task-negative areas (Raichle et al., 2001, Seghier and Price, 2012).
Both AED contain a sulfa moiety and specific detrimental effects on verbal intellectual abilities have also been described in a related drug, sulthiame (Dodrill, 1975). Sulfa-compound containing drugs share the carbonic anhydrase inhibition mechanism and the mechanism underlying the similar fMRI changes in TPM and ZNS may be comparable to acetazolamide, another carbonic anhydrase inhibitor, which has been shown to increase blood flow with constant oxygen consumption, leading to an enhancement of the resting BOLD and decrease of the activation BOLD (Bruhn et al., 1994).
3.3. Stimulants and networks involved in attention control
Attention-deficit/hyperactivity disorder is characterised by inattention, impulsiveness and hyperactivity, and in behavioural studies, associated with deficits in task inhibition, attention, working memory and timing. Probing the underlying regional functional anatomy, main tasks employed in fMRI studies of ADHD are Stop or Go/No-Go tasks, measuring the ability to suppress an already triggered motor response; time discrimination tasks, during which the subject has to decide which of displayed items stays on the screen for the longer time; and n-back working memory tasks. (Rubia et al., 2014) Abnormal regional activation patterns during these tasks have been reported in the fronto-parietal cognitive and attentional networks, the default mode, striatal and limbic regions (Cortese et al., 2012, Rubia, 2011).
Using a Go/No-Go task with high or low levels of reward for correct answers, Liddle et al. (2011) demonstrated that motivation can modulate effects of methylphenidate, a dopamine reuptake inhibitor, via the default mode network in ADHD. Task induced deactivations were observed in the healthy control cohort independently from the level of incentives. In patients, methylphenidate and high incentive had similar effects on activation patterns and performance: Patients on methylphenidate did not differ from controls, irrespective of incentive levels, i.e. medication normalized their raised motivational threshold. When off medication and at low incentive, there was attenuated deactivation within the default mode network in ADHD, which normalized with increased incentive. This data suggests that the default mode network is modulated by dopamine, even though it does not include regions with high density dopaminergic projections, such as the striatum (Mehta, 2011). As corroborated by healthy control data from a combined [11C]cocaine PET and fMRI study employing an attentional paradigm, increased DAT availability in the striatum, resulting in lower dopamine levels at the synapse, was associated with attenuated deactivation of the precuneus, and increased DAT in the caudate was associated with less deactivation of the precuneus with increasing attentional load; vice versa lower DAT availability as in ADHD patients on methylphenidate likely modulates deactivation in the default mode network facilitating attention (Tomasi et al., 2009). Indeed, a rewarded working memory fMRI study in healthy controls contrasting the effects of methylphenidate and the noradrenaline reuptake inhibitor atomoxetine demonstrated that both drug effects were context-dependent and showed an interaction with the degree of incentive: In the reward context, both drugs lead to attenuation of working memory networks and enhanced task-dependent deactivation in the default mode network in comparison to placebo. By contrast, during non-rewarded trials, only methylphenidate lead to increased activity in working memory regions and attenuated default mode deactivation compared to placebo, which were similar activation patterns when contrasting reward to non-reward (Marquand et al., 2011). Thus, dynamic interactions between motivational state and drug effects have to be considered in ph-MRI studies.
4. The use of ph-MRI in CNS drug development
This topic has recently received expert reviews (Borsook et al., 2006, Borsook et al., 2013, Nathan et al., 2014).
Challenges for CNS drug research include the complexity of neuronal networks, and misclassification, or incorrect phenotyping of patients, particularly in psychiatric conditions. In addition, outcome measures often rely on subjective ratings, and even if there are objective clinical measures, such as seizure frequency, their quantification is dependent on the patient's or relatives' accounts and often unreliable (Cook et al., 2013). Ph-MRI hence poses a unique opportunity for CNS drug research to measure drug effects on a functional network level in the living brain, both in healthy and diseased.
During Phase 1 drug trials to evaluate drug safety, dose range and side effects in a small group of people, ph-MRI could help to determine potential side effects by capturing e.g. effects on cognitive networks, and dose ranges by e.g. exploring minimal doses that lead to significant changes in neuronal circuits (Borsook et al., 2013). In combination with a ligand-based approach, such as PET, dosing ranges can be refined by determining how much receptor occupancy (PET) will produce the desired functional network effects in ph-MRI (Borsook et al., 2013). Whole-brain information on functional drug effects early on in CNS trials can also help to tailor subsequent and larger-scale trials accordingly. When evaluating new agents in patient populations, from Phase 2 trials onwards, using effects on functional networks in ph-MRI as endpoint measures has the potential to produce more consistent and objective data with less variance than e.g. subjective ratings on efficacy and side effects, and therefore could reduce required sample sizes considerably. This would be even more efficient, if imaging is used to better define the diseased population a priori by characterizing certain imaging biomarkers of disease; drug effects on these with direct gain of insight into their underlying mechanisms, as well as effective drug response could then be studied by ph-MRI (Engel, 2011, Wiedemann, 2011). Such biomarkers could be used for proof-of-concept studies to early identify promising novel compounds (Nathan et al., 2014), even in the absence of initial symptom relief as one commonly observes e.g. in antidepressant treatment. Clinical trials can be hampered by natural fluctuations in disease severity, leading to a falsely perceived benefit or failure of a substance. Ph-MRI can therefore help to disentangle disease states and drug effects.
Beyond trials for potentially new CNS drugs, longitudinal imaging studies can capture long-term drug effects on brain circuits, in order to understand whether chronic drug exposure can eventually lead to normalization of brain function, and mechanisms in non-responders, as well as the evolution of treatment resistance to a substance that previously lead to long-term symptom benefit, as e.g. observed in epilepsy.
5. Conclusion
Pharmaco-fMRI studies, show consistent and reproducible changes on disease relevant networks and prove sufficiently sensitive to detect dose-dependent network changes early, prior to clinical change, and can predict long-term efficacy, providing exciting new tools for novel CNS drug research.
It remains uncertain, how specific these effects are for single compounds: observed effects on abnormal functional anatomy are likely to represent common pathways for several drug classes or even non-pharmacological treatment, such as SSRI, NaRI, benzodiazepines and cognitive-behaviour therapy in affective disorders.
Overall inferences on specific drugs and diseases are difficult, as studies employed a variety of tasks. Observed effects on task-related deactivations or the default mode network appear to be more robust and reproducible than task-active functional anatomy. Furthermore, drug efficacy is probably best studied via functional connectivity analysis, whereas task-based regional activation may be more appropriate for evaluation of specific cognitive side effects.
Pharmaco-fMRI studies have been used primarily in treatment research of psychiatric diseases and pain studies. For these entities, treatment research is reliant on subjective measures and in need of objective endpoints. Ph-MRI studies have the potential to improve treatment decisions for complex neurological conditions, such as epilepsy, where choice of treatment is done mainly in a trial-and-error fashion.
Funding
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR UCLH/UCL Biomedical Research Centre funding scheme.
References
- Anderson I.M., Del-Ben C.M., Mckie S., Richardson P., Williams S.R., Elliott R., Deakin J.F.W. Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport. 2007;18:1351–1355. doi: 10.1097/WNR.0b013e3282742115. [DOI] [PubMed] [Google Scholar]
- Bakker A., Krauss G.L., Albert M.S., Speck C.L., Jones L.R., Stark C.E., Yassa M.A., Bassett S.S., Shelton A.L., Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Albert M.S., Krauss G., Speck C.L., Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramini G.C., Cendes F., Yasuda C.L. The effects of antiepileptic drugs on cognitive functional magnetic resonance imaging. Quant. Imaging Med. Surg. 2015;5:238–246. doi: 10.3978/j.issn.2223-4292.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Chen Z., He Y., Evans A.C., Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex N.Y.N. 2011;1991(21):2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bootsma H.P.R., Ricker L., Diepman L., Gehring J., Hulsman J., Lambrechts D., Leenen L., Majoie M., Schellekens A., de Krom M., Aldenkamp A.P. Long-term effects of levetiracetam and topiramate in clinical practice: a head-to-head comparison. Seizure. 2008;17:19–26. doi: 10.1016/j.seizure.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Bootsma H.P., Ricker L., Hekster Y.A., Hulsman J., Lambrechts D., Majoie M., Schellekens A., de Krom M., Aldenkamp A.P. The impact of side effects on long-term retention in three new antiepileptic drugs. Seizure. 2009;18:327–331. doi: 10.1016/j.seizure.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Borsook D., Becerra L., Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat. Rev. Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Borsook D., Becerra L., Fava M. Use of functional imaging across clinical phases in CNS drug development. Transl. Psychiatry. 2013;3 doi: 10.1038/tp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn H., Kleinschmidt A., Boecker H., Merboldt K.D., Hänicke W., Frahm J. The effect of acetazolamide on regional cerebral blood oxygenation at rest and under stimulation as assessed by MRI. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1994;14:742–748. doi: 10.1038/jcbfm.1994.95. [DOI] [PubMed] [Google Scholar]
- Bullmore E. The future of functional MRI in clinical medicine. NeuroImage. 2012;62:1267–1271. doi: 10.1016/j.neuroimage.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Cook M.J., O'Brien T.J., Berkovic S.F., Murphy M., Morokoff A., Fabinyi G., D'Souza W., Yerra R., Archer J., Litewka L., Hosking S., Lightfoot P., Ruedebusch V., Sheffield W.D., Snyder D., Leyde K., Himes D. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12:563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H., Rijpkema M., Qin S., van Wingen G.A., Fernández G. Phasic deactivation of the medial temporal lobe enables working memory processing under stress. NeuroImage. 2012;59:1161–1167. doi: 10.1016/j.neuroimage.2011.09.027. [DOI] [PubMed] [Google Scholar]
- De Ciantis A., Muti M., Piccolini C., Principi M., Di Renzo A., De Ciantis R., Frondizi D., Iannone G., Ottaviano P., Piccirilli M. A functional MRI study of language disturbances in subjects with migraine headache during treatment with topiramate. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2008;29(Suppl. 1):S141–S143. doi: 10.1007/s10072-008-0906-5. [DOI] [PubMed] [Google Scholar]
- Delaveau P., Jabourian M., Lemogne C., Guionnet S., Bergouignan L., Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J. Affect. Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodrill C.B. Effects of sulthiame upon intellectual, neuropsychological, and social functioning abilities among adult epileptics: comparison with diphenylhydantoin. Epilepsia. 1975;16:617–625. doi: 10.1111/j.1528-1157.1975.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Engel J. Biomarkers in epilepsy: introduction. Biomark. Med. 2011;5:537–544. doi: 10.2217/bmm.11.62. [DOI] [PubMed] [Google Scholar]
- Fisher R.S., Vickrey B.G., Gibson P., Hermann B., Penovich P., Scherer A., Walker S. The impact of epilepsy from the patient's perspective I. Descriptions and subjective perceptions. Epilepsy Res. 2000;41:39–51. doi: 10.1016/s0920-1211(00)00126-1. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Williams S.C.R., Cleare A.J., Scott J., Mitterschiffthaler M.T., Walsh N.D., Donaldson C., Suckling J., Andrew C., Steiner H., Murray R.M. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Furmark T., Tillfors M., Marteinsdottir I., Fischer H., Pissiota A., Långström B., Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch. Gen. Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Gudayol-Ferré E., Peró-Cebollero M., González-Garrido A.A., Guàrdia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front. Hum. Neurosci. 2015;9:582. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneef Z., Levin H.S., Chiang S. Brain graph topology changes associated with anti-epileptic drug use. Brain Connect. 2015;5:284–291. doi: 10.1089/brain.2014.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer C.J., Mackay C.E., Reid C.B., Cowen P.J., Goodwin G.M. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol. Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Witt J.-A. The effects of levetiracetam on cognition: a non-interventional surveillance study. Epilepsy Behav. EB. 2008;13:642–649. doi: 10.1016/j.yebeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Witt J.-A. Cognitive outcome of antiepileptic treatment with levetiracetam versus carbamazepine monotherapy: a non-interventional surveillance trial. Epilepsy Behav. EB. 2010;18:74–80. doi: 10.1016/j.yebeh.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Jansen J.F.A., Aldenkamp A.P., Marian Majoie H.J., Reijs R.P., de Krom M.C.T.F.M., Hofman P.A.M., Eline Kooi M., Nicolay K., Backes W.H. Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav. EB. 2006;9:181–185. doi: 10.1016/j.yebeh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Jokeit H., Okujava M., Woermann F.G. Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol. 2001;1:6. doi: 10.1186/1471-2377-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp M.J. Gender and drug effects on neuroimaging in epilepsy. Epilepsia. 2011;52(Suppl. 4):35–37. doi: 10.1111/j.1528-1167.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- Li X., Large C.H., Ricci R., Taylor J.J., Nahas Z., Bohning D.E., Morgan P., George M.S. Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor or prefrontal effective connectivity. Psychiatry Res. 2011;194:141–148. doi: 10.1016/j.pscychresns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Liddle E.B., Hollis C., Batty M.J., Groom M.J., Totman J.J., Liotti M., Scerif G., Liddle P.F. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J. Child Psychol. Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Marquand A.F., De Simoni S., O'Daly O.G., Williams S.C.R., Mourão-Miranda J., Mehta M.A. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011;36:1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Kuzniecky R., Ho S., Hetherington H., Pan J., Sinclair K., Gilliam F., Faught E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Lozano A.M., Voon V., McNeely H.E., Seminowicz D., Hamani C., Schwalb J.M., Kennedy S.H. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McClure E.B., Adler A., Monk C.S., Cameron J., Smith S., Nelson E.E., Leibenluft E., Ernst M., Pine D.S. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- Meador K.J., Loring D.W., Vahle V.J., Ray P.G., Werz M.A., Fessler A.J., Ogrocki P., Schoenberg M.R., Miller J.M., Kustra R.P. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology. 2005;64:2108–2114. doi: 10.1212/01.WNL.0000165994.46777.BE. [DOI] [PubMed] [Google Scholar]
- Mehta M.A. Commentary: the only way is down. Augmented deactivation of the default mode network by increased catecholamine transmission–a general mechanism? Reflections on Liddle et al. (2011) J. Child Psychol. Psychiatry. 2011;52:772–773. doi: 10.1111/j.1469-7610.2011.02401.x. [DOI] [PubMed] [Google Scholar]
- Mehta M.A., O'Daly O.G. Pharmacological application of fMRI. Methods Mol. Biol. Clifton NJ. 2011;711:551–565. doi: 10.1007/978-1-61737-992-5_28. [DOI] [PubMed] [Google Scholar]
- Mula M., Trimble M.R. Antiepileptic drug-induced cognitive adverse effects: potential mechanisms and contributing factors. CNS Drugs. 2009;23:121–137. doi: 10.2165/00023210-200923020-00003. [DOI] [PubMed] [Google Scholar]
- Nathan P.J., Phan K.L., Harmer C.J., Mehta M.A., Bullmore E.T. Increasing pharmacological knowledge about human neurological and psychiatric disorders through functional neuroimaging and its application in drug discovery. Curr. Opin. Pharmacol. 2014;14:54–61. doi: 10.1016/j.coph.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Oathes D.J., Johnstone T., Whalen P.J., Davidson R.J., Kalin N.H. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am. J. Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R., Taylor M.J., Selvaraj S., Murphy S.E., Harmer C.J., Cowen P.J. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology. 2009;206:197–204. doi: 10.1007/s00213-009-1597-1. [DOI] [PubMed] [Google Scholar]
- Ojemann L.M., Ojemann G.A., Dodrill C.B., Crawford C.A., Holmes M.D., Dudley D.L. Language disturbances as side effects of topiramate and zonisamide therapy. Epilepsy Behav. EB. 2001;2:579–584. doi: 10.1006/ebeh.2001.0285. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Feinstein J.S., Castillo G., Simmons A.N., Stein M.B. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch. Gen. Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Coccaro E.F., Angstadt M., Kreger K.J., Mayberg H.S., Liberzon I., Stein M.B. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol. Psychiatry. 2013;73:329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rubia K., Alegria A.A., Cubillo A.I., Smith A.B., Brammer M.J., Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol. Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Price C.J. Functional heterogeneity within the default network during semantic processing and speech production. Front. Psychol. 2012;3:281. doi: 10.3389/fpsyg.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton J., Winston G.P., Sidhu M., Bonelli S., Centeno M., Vollmar C., Cleary R.A., Williams E., Symms M.R., Koepp M.J., Thompson P.J., Duncan J.S. Disrupted segregation of working memory networks in temporal lobe epilepsy. NeuroImage Clin. 2013;2:273–281. doi: 10.1016/j.nicl.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Allendorfer J.B. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. EB. 2012;24:74–80. doi: 10.1016/j.yebeh.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Xia W., Yu X., Zhou B., Wu X., Lui S., Luo C., Huang X., Ouyang L., Chen Q., Gong Q., Zhou D. Altered cerebral activity associated with topiramate and its withdrawal in patients with epilepsy with language impairment: an fMRI study using the verb generation task. Epilepsy Behav. EB. 2016;59:98–104. doi: 10.1016/j.yebeh.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Thompson P.J., Baxendale S.A., Duncan J.S., Sander J.W. Effects of topiramate on cognitive function. J. Neurol. Neurosurg. Psychiatry. 2000;69:636–641. doi: 10.1136/jnnp.69.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D., Wang R., Telang F., Wang G.-J., Chang L., Ernst T., Fowler J.S. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter R., Bell D., Gutting P., Harmer C., Healy D., Anderson I.M. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J. Affect. Disord. 2009;118:87–93. doi: 10.1016/j.jad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Vollmar C., O'Muircheartaigh J., Barker G.J., Symms M.R., Thompson P., Kumari V., Duncan J.S., Janz D., Richardson M.P., Koepp M.J. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain J. Neurol. 2011;134:1710–1719. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar C., O'Muircheartaigh J., Symms M.R., Barker G.J., Thompson P., Kumari V., Stretton J., Duncan J.S., Richardson M.P., Koepp M.J. Altered microstructural connectivity in juvenile myoclonic epilepsy: the missing link. Neurology. 2012;78:1555–1559. doi: 10.1212/WNL.0b013e3182563b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandschneider B., Thompson P.J., Vollmar C., Koepp M.J. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia. 2012;53:2091–2098. doi: 10.1111/epi.12003. [DOI] [PubMed] [Google Scholar]
- Wandschneider B., Stretton J., Sidhu M., Centeno M., Kozák L.R., Symms M., Thompson P.J., Duncan J.S., Koepp M.J. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology. 2014;83:1508–1512. doi: 10.1212/WNL.0000000000000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann K. Biomarkers in development of psychotropic drugs. Dialogues Clin. Neurosci. 2011;13:225–234. doi: 10.31887/DCNS.2011.13.2/kwiedemann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windischberger C., Lanzenberger R., Holik A., Spindelegger C., Stein P., Moser U., Gerstl F., Fink M., Moser E., Kasper S. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. NeuroImage. 2010;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Yacubian E.M., Wolf P. Praxis induction. Definition, relation to epilepsy syndromes, nosological and prognostic significance. A focused review. Seizure. 2014;23:247–251. doi: 10.1016/j.seizure.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Yasuda C.L., Centeno M., Vollmar C., Stretton J., Symms M., Cendes F., Mehta M.A., Thompson P., Duncan J.S., Koepp M.J. The effect of topiramate on cognitive fMRI. Epilepsy Res. 2013;105:250–255. doi: 10.1016/j.eplepsyres.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]