Abstract
Background
Convergence insufficiency (CI) is a common binocular vision deficit after a sport-related concussion (SRC). CI may result in visual discomfort and vision-mediated functional difficulties such as slowed reading and compromised attention, leading to impaired academic, work, and sport performance.
Purpose
To test the reliability of repeated near point of convergence (NPC) measurements in a sample of athletes after an SRC; compare the symptoms and cognitive impairment of athletes with normal NPC to those with CI after an SRC; and explore the relationship among age, sex, learning disability, migraine history, and CI.
Study Design
Cross-sectional study; Level of evidence, 3.
Methods
A total of 78 athletes (mean age, 14.31 ± 2.77 years) who were seen a mean 5.79 ± 5.63 days after an SRC were administered 3 trials of an NPC assessment, along with neurocognitive (Immediate Post-Concussion Assessment and Cognitive Testing [ImPACT]) and symptom assessments. Patients were divided into normal NPC (NPC ≤5 cm; n = 45) and CI (NPC >5 cm; n = 33) groups. Intraclass correlation coefficients (ICCs) and repeated-measures analyses of variance (ANOVAs) assessed the consistency of NPC across the 3 trials. The ANOVAs were employed to examine differences on neurocognitive composites and symptoms between the normal NPC and CI groups. Stepwise regressions (controlling for age and symptom scores on the Post-Concussion Symptom Scale [PCSS]) were conducted to evaluate the predictive utility of the NPC distance for neurocognitive impairment.
Results
Groups did not differ on demographic or injury characteristics. NPC differed between trial 1 and trials 2 (P = .02) and 3 (P = .01) for the CI group but not the normal NPC group. Internal consistency was high across NPC measurements (ICC range, 0.95–0.98). Patients with CI performed worse on verbal memory (P = .02), visual motor speed (P = .02), and reaction time (P = .001, η2 = .13) and had greater total symptom scores (P = .02) after the injury. Results of hierarchical regression revealed that the NPC distance contributed significantly to the model for reaction time (P < .001).
Conclusion
CI was common (~42%) in athletes evaluated within 1 month after an SRC. Athletes with CI had worse neurocognitive impairment and higher symptom scores than did those with normal NPC. Clinicians should consider routinely screening for NPC as part of a comprehensive concussion evaluation to help inform treatment recommendations, academic accommodations, and referrals for vision therapy.
Keywords: concussion, eye injuries, convergence insufficiency, neurocognitive impairment
Sport-related concussion (SRC) continues to be a significant health concern, affecting as many as 3.8 million athletes each year in the United States.23 Among the myriad effects of concussions are symptoms (eg, headache, dizziness, nausea)13,24 as well as cognitive (eg, memory, reaction time, processing speed),2 vestibular (balance, saccades, vestibular ocular reflex),12,29 and vision or oculomotor29 impairments. Posttraumatic vision or oculomotor problems are reported in 30% to 65% of patients with a mild traumatic brain injury (TBI)6 and in nearly 30% of patients with an SRC.22 Vision and oculomotor-related symptoms and impairment after an SRC may include diplopia, blurred vision, difficulty tracking a moving target or reading, headaches, general asthenopia, and, to a lesser extent, dizziness and nausea.7 These symptoms and impairments may result in visual discomfort and vision-mediated functional difficulties, such as slowed reading impairing academic and occupational performance. Oculomotor problems can also negatively affect recovery by reducing the effectiveness of other interventions, such as cognitive rehabilitation.7 The pathophysiology of these problems is complex, involving the disruption of function in the midbrain, cerebellum, pons, and multiple regions of the cerebral cortex.17,18,26,31,32 As such, damage to one part of the system can affect multiple components of vision functioning. Given these findings in other TBI populations, research to better understand vision and oculomotor-related impairments and symptoms after an SRC is warranted.
One emerging oculomotor-related outcome after an SRC involves convergence insufficiency (CI), as measured by abnormal near point of convergence (NPC). CI is a common binocular vision disorder characterized by exophoria (tendency for eyes to deviate outward from the midline) greater at near than at distance, a receded NPC, and reduced positive fusional vergence (convergence amplitudes) at near.35 For a recent and comprehensive review of CI, please see Cooper and Jamal.11 Although variations in diagnostic criteria exist,11 rates of CI in the healthy population average 5%, with a range from 1% to 33%.35 In contrast, rates of CI in patients after a brain injury have been estimated from 42% to 43% in civilian populations and 23% to 46% in military populations.1,4,7,8 Researchers recently reported that NPC measurements were abnormal (ie, >5 cm) in 45% of athletes after an SRC.29 These researchers speculated that CI might also contribute to other SRC-related symptoms and cognitive impairment. However, the nature of the relationships between NPC and other concussion-related outcomes, including symptom reports and cognitive impairment, is unknown.
Given the rate of CI after an SRC and associated functional impairment and symptoms, screening for CI using the NPC distance is gaining favor among clinicians as part of a comprehensive clinical concussion evaluation. Researchers and clinicians have recently developed a screening tool designed to evaluate vestibular and oculomotor impairments after a concussion that includes a measurement of NPC.29 While the reliability of NPC has been established in middle school–aged children,33 the reliability of NPC measurements after an SRC is not established. The reported frequency, intensity, duration of symptoms, and cognitive impairment secondary to CI after an SRC are also largely unknown. Moreover, the influence of established risk factors for poor outcomes after an SRC (eg, demographic variables such as age, sex, attention deficit hyperactivity disorder [ADHD], learning disability [LD], and migraine history) has not been explored in the context of posttraumatic CI. Therefore, the objectives and hypotheses of the current study were as follows: (1) to test the reliability of repeated NPC measurements in a sample of athletes after an SRC (we expected that NPC measurements would be reliable across administrations); (2) to compare the symptoms and cognitive impairment of athletes with normal NPC to those with CI after an SRC (we expected that athletes with CI would report more symptoms and demonstrate greater cognitive impairment); and (3) to explore the relationship among age, sex, ADHD, LD, migraine history, and posttraumatic CI.
METHODS
Participants
We conducted a prospective cohort study of 78 athletes (45 male, 33 female) seen at a concussion clinic for initial evaluation of an SRC within 30 days of injury. Patients were consecutively enrolled in the study during the fall of 2014. Patients were excluded from the study if they met ≥1 of the following criteria: invalid neurocognitive testing, brain surgery, neurological disorder, premorbid vestibular or visual dysfunction (eg, benign paroxysmal positional vertigo, unilateral or bilateral vestibular hypofunction, strabismus, diplopia, saccadic/pursuit deficiencies), treatment for substance abuse, and/or psychiatric disorder.
Instrumentation
Concussion
A concussion was defined as a complex pathophysiological process affecting the brain, induced by biomechanical forces and involving an alteration in mental status with or without loss of consciousness.27 Practically, concussions were diagnosed by a licensed medical professional (eg, physician, neuropsychologist, certified athletic trainer) trained in the assessment and treatment of concussions using the following criteria: (1) clear mechanism of injury; (2) presence of ≥1 signs (eg, loss of consciousness, posttraumatic amnesia, disorientation/confusion, balance difficulties) and/or symptoms (eg, headache, dizziness, nausea, sleep disruption, concentration problems) at the time of injury; and (3) current, ongoing symptoms and/or impairment (eg, cognitive, vestibular difficulties).
Near Point of Convergence
Consistent with previous research for both children and adults,25,35 NPC was measured in centimeters from the tip of the nose. All measurements were conducted with a fixation stick that included a target letter in a 12-point font and a standard Gulick anthropometric tape measure. The participant was asked to “focus on the target letter at arm’s length and slowly bring it toward the tip of his/her nose while focusing on the target with both eyes” (see the Appendix of Mucha et al29 for the full administration procedure). The participant was instructed to stop moving the target when he or she saw 2 distinct images or when the examiner observed an outward deviation of 1 eye. NPC was recorded as the mean of 3 separate measurement trials that were consecutively administered without rest. In accordance with previous literature,35 mean NPC measurements ≤5 cm were considered normal, and NPC measurements >5 cm were considered abnormal and defined as CI for the purpose of this study.
Neurocognitive Impairment
The Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) is a computer-based neurocognitive test battery composed of 6 subtests designed to examine neurocognitive impairment in patients with an SRC. The ImPACT yields 4 composite scores for verbal and visual memory (measured as the percentage correct), processing speed (higher scores indicate better performance), and reaction time (measured in seconds, with lower scores indicating better performance). The ImPACT takes approximately 20 to 25 minutes to administer and has adequate reliability and validity per previous research.2,34
Concussion Symptoms
The Post-Concussion Symptom Scale (PCSS), which is embedded at the beginning of the ImPACT, is a computerized self-report inventory of 22 items representing somatic (eg, dizziness, headache), cognitive (eg, difficulty concentrating, memory problems), affective (eg, anxiety, depression), and sleep-related symptoms. Patients rate each symptom on a 7-point Likert scale from 0 (none) to 6 (severe). The PCSS has adequate reliability and validity for assessing and monitoring concussion-related symptoms.24
Procedures
The University of Pittsburgh’s institutional review board approved the study under an exempt medical records review protocol as part of our program’s Concussion Research Registry. All patients provided written informed consent before participating in the study. In cases where patients were younger than 18 years of age, both patient assent and parent/guardian consent to participate in the registry were obtained. All patients completed the measures in the following order: (1) concussion symptom inventory (ie, PCSS), (2) computerized neurocognitive testing (ie, ImPACT), and (3) NPC assessment. All measures were administered individually in a clinical examination room by licensed physical therapists trained in the assessment of concussions.
Statistical Analysis
A normal NPC group (NPC ≤5) and a CI group (NPC >5) were designated from the mean of 3 NPC measurements for each participant. With regard to demographic variables, χ2 analyses were employed to examine group differences for sex, concussion history, LD history, ADHD history, and self-reported migraine history from injury. A series of analyses of variance (ANOVAs) were employed to examine group differences in age, time from current injury, and number of previous concussions. The reliability/internal consistency of NPC across 3 measurement trials was evaluated with repeated-measures ANOVAs and intraclass correlation coefficients (ICCs). A series of ANOVAs were used to examine group differences between the CI and normal NPC groups on neurocognitive outcomes and concussion symptoms. Bonferroni correction was used to control for multiple comparisons. A series of stepwise regressions controlling for age and symptom scores on the PCSS were conducted to evaluate the predictive utility of the NPC distance for neurocognitive impairment. All statistical tests were conducted using SPSS version 22 (IBM Corp). The statistical significance level was set at P < .05 for all analyses.
RESULTS
Demographic Data
The sample included 78 concussed patients (45 male, 33 female) with a mean age of 14.31 ± 2.77 years (range, 9–24 years) seen 1 to 30 days after injury. The majority (n = 70, 90%) of the sample was enrolled within 10 days of injury (mean, 5.79 ± 5.63 days). A summary of demographic information for the overall sample is provided in Table 1. The sample was divided into normal NPC (n = 45, 57.7%) and CI (n = 33, 42.3%) groups based on the criteria discussed in the Methods section. The normal NPC and CI groups did not differ across any demographic variables, including sex (χ2(1) = 1.32, P = .25), concussion history (χ2(1) = 0.08, P = .78), LD history (χ2(1) = 0.01, P = .91), ADHD history (χ2(1) = 0.22, P = .64), migraine history (χ2(1) = 0.09, P = .76), age (F(1,77) = 0.00, P = .99), or time from injury (F(1,77) = 0.94, P = .34). Mean NPC measurements for both groups combined ranged from 0.00 cm (ie, to the tip of the nose) to 41.33 cm (mean, 6.23 ± 8.08 cm). The mean NPC measurement for the normal NPC group was 1.53 ± 1.53 cm, and the mean NPC measurement for the CI group was 12.64 ± 8.97 cm.
TABLE 1.
Demographics and Descriptive Detailsa
| Normal NPC Group (n = 45) | CI Group (n = 33) | |
|---|---|---|
| Sex, male/female, % | 64.4/35.6 | 51.5/48.5 |
| Age, y | 14.31 ± 3.13 | 14.30 ± 2.23 |
| NPC (of 3 trials), cm | 1.53 ± 1.53 | 12.64 ± 8.97 |
| No. of concussions | 0.61 ± 1.00 | 0.48 ± 0.81 |
| Prior concussions, n (%) | ||
| 0 | 31 (68.8) | 23 (69.7) |
| 1 | 7 (15.6) | 6 (18.2) |
| ≥2 | 7 (15.6) | 4 (12.1) |
| History of ADHDb | 1.91 ± 0.29 (10.6) | 1.88 ± 0.33 (11.8) |
| History of LDb | 1.94 ± 0.24 (6.4) | 1.9 ± 0.2 (5.9) |
| History of migraineb | 1.51 ± 0.51 (12.8) | 1.55 ± 0.51 (17.6) |
Values are expressed as mean ± SD unless otherwise indicated. ADHD, attention deficit hyperactivity disorder; CI, convergence insufficiency; LD, learning disability; NPC, near point of convergence.
Values in parentheses represent percentages.
Reliability of NPC Across Measurements
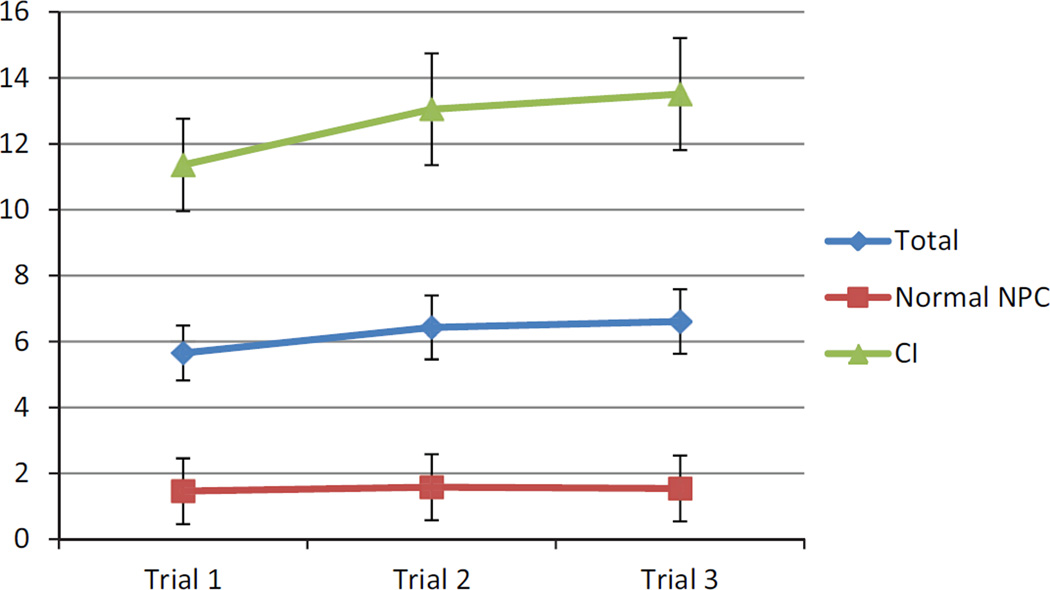
The ICCs for the normal NPC and CI groups combined for NPC measurement trials 1, 2, and 3 suggested a high degree of internal consistency, with ICCs across the 3 trials ranging from 0.95 to 0.98. The ICCs for the CI group ranged from 0.78 to 0.89, and those for the normal NPC group ranged from 0.92 to 0.97. The NPC measurements differed significantly from trial 1 to 3 (F(2,75) = 7.52, P = .001, η2 = .17), and the normal NPC group differed significantly from the CI group (F(1,76) = 66.79, P < .001, η2 = .47). Post hoc analyses revealed that trial 1 (mean, 5.65 ± 7.30 cm) differed significantly from trial 2 (mean, 6.43 ± 8.57 cm) (P = .002) and trial 3 (mean, 6.61 ± 8.63 cm) (P = .001). Trials 2 and 3 did not differ between group and trial (P = .71) (Figure 1). Post hoc analyses revealed a significant difference between trial 1 and trials 2 (P = .015) and 3 (P = .008) for the CI group but no difference between trials 2 and 3 (P = .74) (Figure 1). There were no significant differences across trials for the normal NPC group (all P > .10).
Figure 1.
Near point of convergence (NPC) for trials 1, 2, and 3. CI, convergence insufficiency.
NPC, Symptoms, and Neurocognitive Impairment
Findings from a series of ANOVAs revealed that the normal NPC and CI groups differed on neurocognitive impairment and symptoms. Effect sizes were small to medium (Table 2). Specifically, the CI group performed worse on verbal memory (F(1,76) = 6.17, P = .02, η2 = .08), processing speed (F(1,76) = 5.84, P = .02, η2 = .07), and reaction time (F(1,76) = 11.20, P = .001, η2 = .13). Participants in the CI group also reported greater PCSS total symptom scores (F(1,76) = 5.58, P = .02, η2 = .07) than did participants in the normal NPC group. Visual memory scores did not differ between groups (F(1,76) = 2.43, P = .12, η2 = .03).
TABLE 2.
Neurocognitive Test Performance After an Injurya
| Normal NPC Group (n = 45) | CI Group (n = 33) | Total (n = 78) | |
|---|---|---|---|
| Verbal memoryb | 82.91 ± 12.66 | 74.64 ± 16.78 | 79.41 ± 15.01 |
| Visual memory | 69.18 ± 15.00 | 63.76 ± 15.41 | 66.88 ± 15.31 |
| Visual motor speedb | 33.78 ± 8.88 | 28.87 ± 8.82 | 31.70 ± 9.13 |
| Reaction timeb | 0.67 ± 0.10 | 0.79 ± 0.21 | 0.72 ± 0.16 |
| Symptom total scoreb | 24.04 ± 19.48 | 35.94 ± 24.98 | 29.08 ± 22.61 |
Values are expressed as mean ± SD. CI, convergence insufficiency; NPC, near point of convergence.
Statistically significant difference between groups (P < .05).
To predict neurocognitive composites on the ImPACT (ie, verbal and visual memory, processing speed, and reaction time), 4 hierarchical regressions were conducted that included 2 steps: (1) age, ADHD, LD, and PCSS total symptom score; and (2) NPC distance. Age, ADHD, LD, and PCSS total score accounted for 24.7% of the variance in reaction time composite scores (F(4,73) = 5.98, P < .001). Both age (P = .001) and PCSS total score (P < .001) contributed significantly to the first step; however, neither ADHD (P = .78) nor LD (P = .38) was a significant predictor in the first step. The addition of NPC distance in step 2 accounted for an additional 13.6% of the variance in reaction time (F(1,72) = 15.85, P < .001), with age (P = .002) and NPC (P < .001) contributing significantly to the model. Neither ADHD (P = .72) nor LD (P = .19) was a significant predictor in the second step for reaction time composite scores. Neither ADHD nor LD was a significant predictor of verbal memory, visual memory, or visual motor speed in either step 1 or 2 (all P > .10). Further, the addition of NPC distance in step 2 did not contribute significantly to models for verbal memory, visual memory, and visual motor speed.
DISCUSSION
This study is the first to examine the reliability of NPC measurements and their relationship to neurocognitive impairment after an SRC. Primary findings from this study suggest that CI is common after an SRC and that multiple NPC measurements are consistent. The prevalence of CI was over 40% in this sample of patients evaluated within 1 month of an SRC. This finding is consistent with prior research, which suggests that up to 46% of patients with a mild TBI are positive for CI.1,4,7,8 Our results also suggest that repeated measurements of NPC in patients after an SRC are generally reliable, with ICCs collectively ranging from 0.95 to 0.98. However, there is evidence of an increase in the NPC distance from the first measurement to both the second and third measurements for the CI group. This finding provides some support for the notion of a fatiguing process associated with repeated NPC measurements in patients with CI.14 In contrast, NPC measurements for our normal NPC group did not differ across the 3 trials, which is consistent with the NPC literature in healthy populations.21
Another key finding in the current study was that NPC measurements contributed to neurocognitive performance as a significant predictor for reaction time. Similarly, the CI group performed worse on verbal memory, visual motor speed, and reaction time and reported more symptoms than the normal NPC group. The present study complements the body of literature describing vision difficulties after concussions by quantifying the relationship between NPC and performance on a neurocognitive test battery designed to detect difficulties after a concussion. The concussed group with CI performed worse on 3 of 4 composites of neurocognitive testing compared with the concussed group with normal NPC. The absence of a significant difference on the visual memory composite is curious, although it may be related to the psychometric properties of the scale rather than true sparing of deficits in the domain of visual memory. Provided that computerized neurocognitive test batteries are visually based and include several timed subtests, our results supporting more global neurocognitive deficits are expected. Further, the relationship between reaction time and NPC previously reported by Mucha et al29 was replicated in this study. Of importance to the current study, the neural basis for reaction time/processing speed and oculomotor control involves multiple shared regions in the brain (ie, cerebellum, brain stem, diencephalon/midbrain, and aspects of the cerebral cortex).15,30
Patients with CI endorse more concussion symptoms compared with those with normal NPC. This finding may suggest that patients with posttraumatic CI may simply have more severe injuries with greater overall symptoms. Alternatively, patients with CI may be more symptomatic because of the array of visually based symptoms related to CI that overlap with items on postconcussive symptom scales (eg, headache, dizziness, concentration difficulties). Regardless of the cause of the relationship between CI and total concussion symptoms, the fact that they are related suggests that more objective NPC measurements may help augment and inform subjective symptom reports.
Surprisingly, in the current study, the CI and normal NPC groups did not differ on any of the demographic variables (ie, age, sex) or preexisting conditions (eg, LD, ADHD, migraine). Previous research suggests that normative NPC measures are equivalent across sex and age groups among healthy children and adults, with an established clinical cutoff of 5 cm from the nose.21,25,35 As such, our findings regarding age and sex are consistent with the extant literature. However, these same demographic groups (eg, female patients, younger children) are reported to be at risk for poor outcomes or prolonged recovery after an SRC.5,39 Based on our preliminary findings, it appears that CI may not be a consequence of a concussion that is affected by age or sex, despite evidence of comorbidities in healthy samples. Some studies have suggested that LD/ADHD and developmental or congenital CI are comorbid, although the nature of the relationship is unclear.3 For example, eliminating CI may or may not have an effect on reading.11 A recent case series suggested that CI may result from migraines.37 However, our findings suggest that CI after an SRC may be independent of any preexisting conditions known to influence concussion risk and outcomes.
Clinical Implications
Measuring NPC is a relatively quick and easy procedure to screen for CI after a concussion. The current findings suggest that NPC should be integrated into a comprehensive, multifaceted concussion evaluation. However, NPC or any other single clinical tool should not be used as a “standalone measure” for diagnostic purposes. Moreover, our findings suggest that there may be subtle relationships among different assessment modalities. For example, low scores on certain cognitive tests and high symptom scores may be related to CI after an SRC. In addition, because congenital or developmental CI is observed in approximately 5% of the healthy population and is related to other ocular conditions, it is necessary to consider NPC in the context of clinical history.
Our findings support the conceptualization of a concussion as a multifaceted injury,9,16 which calls for individualized, targeted clinical care. Many symptoms specific to ocular-motor difficulties after concussions, such as headache, fatigue, distractibility/attentional concerns, difficulty with more visually based academic work (eg, mathematics, reading), asthenopia/pressure behind the eyes, and blurred vision/focus, are consistent with CI. Because symptoms secondary to CI appear to be triggered by the extended time engaged in visually based activities, such as computer work or reading, a better understanding of CI and its effects on patients may help inform academic accommodations.20 Depending on the severity of visually based difficulties, the authors suggest extra time for homework and the addition of breaks interspersed throughout the academic day/homework periods to reduce visual strain that contributes to symptoms. Referral to a specialist (eg, behavioral neuro-optometrist) may be warranted in cases where CI does not resolve with accommodations, although the timeline for referral is unclear. While the efficacy of vision therapy has not been empirically examined for patients with a concussion or TBI, it is effective in treating congenital CI, leading to improved NPC and functional outcomes (eg, reading comprehension).10,19,38 Control groups in these studies failed to improve across the study duration, suggesting that CI may persist when not actively treated.
Future Directions and Research
Future research should extend the present study with samples of both younger aged and older aged patients. Little is known about the spontaneous recovery and functional adaptation of CI after a concussion. Therefore, researchers should examine NPC across different postinjury time intervals including acute, subacute, and chronic. The examination of symptom clusters (eg, cognitive, physical) in patients with CI may help with the identification of problems and treatment recommendations. Similarly, the influence of the most commonly practiced concussion management strategy, cognitive and physical rest,36 on CI is unknown. Based on our findings that NPC fatigues with consecutive trials, visually based work may also fatigue NPC or worsen CI. Related to this notion, vision therapy for CI has anecdotally been effective among patients after a concussion, although no empirical support exists to date. Further, evaluating visually based academic accommodations in this subset of patients may aid in the understanding of academic difficulties and lead to evidence-based practice for making accommodations. Because of alternative causes of CI (eg, developmental, pseudotumor, posttraumatic),28 further research is needed to discern preexisting from posttraumatic CI. Our study did not measure divergence or recovery, which is a component of some diagnostic criteria for CI.21,25 Divergence or recovery of binocularity is performed by slowly moving the target away from the patient until the patient reports 1 target or when the examiner sees the eyes regain triangulation on the target.25 Patients may have normal NPC but also have abnormal divergence that may be clinically meaningful. Future research should consider these related measures of CI.
Limitations
Although the present study provides both theoretical and clinically practical insights into one aspect of posttraumatic oculomotor impairment after a concussion, it is not without methodological limitations. Our sample size was relatively small, and the discussion on potential risk factors, including LD, ADHD, and migraine, is limited and should be examined in a larger sample in the future. The normal NPC and CI groups were created by artificially dichotomizing a continuous variable, which could potentially contribute to differences observed in ANOVA versus regression results. Patients were recruited from a specialty concussion clinic with a relatively large time interval (1–30 days after injury), which introduces the possibility of selection bias (eg, more severe injury, protracted recovery). As such, the rate of CI in the current study may be artificially inflated. Further, approximately a third of our sample had a history of concussions, and it is unknown if these participants had prior CI or if they had received any treatment previously (eg, vision therapy). Only 1 type of NPC measurement was used in the current study, which may limit the generalizability or utility of findings to other studies/clinicians measuring NPC in different ways (eg, from the nose vs from the forehead, with an accommodative target vs penlight).11,35 Further, the prevalence of CI in the general population is poorly understood because of the lack of population-based studies and variation in diagnostic criteria,11 which create a challenge for clinicians to discern normal from abnormal NPC after a concussion. Based on the literature, a cutoff of NPC >5 cm was used to indicate CI for our sample. This lower threshold for NPC may have resulted in higher false positive rates for CI in the current study. Finally, baseline neurocognitive testing was not available to ensure group equivalency before injury.
CONCLUSION
The current findings suggest that NPC can be reliably measured after an SRC. CI was common (~42%) in the current sample of athletes evaluated within 1 month after an SRC. Patients with CI had worse neurocognitive impairment and higher symptom scores than those with normal NPC. After controlling for age, ADHD, LD, and total symptom score, the NPC distance contributed 13.6% of the variance in reaction time performance. However, there is no evidence for a relationship between concussion-related risk factors such as sex, concussion history, LD/ADHD, migraine history, and posttraumatic CI. Clinicians should consider routinely screening for NPC as part of a comprehensive concussion evaluation to help inform treatment recommendations, academic accommodations, and referrals for vision therapy.
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: This research was supported in part by a grant to the University of Pittsburgh from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (1K01DC012332-01A1). M.W.C. is a cofounder and 10% shareholder of ImPACT Applications Inc.
Footnotes
Investigation performed at the University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1.Alvarez TL, Kim EH, Vicci VR, Dhar SK, Biswal BB, Barrett A. Concurrent vision dysfunctions in convergence insufficiency with traumatic brain injury. Optom Vis Sci. 2012;89(12):1740–1751. doi: 10.1097/OPX.0b013e3182772dce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr WB, McCrea M. Sensitivity and specificity of standardized neurocognitive testing immediately following sports concussion. J Int Neuropsychol Soc. 2001;7(6):693–702. doi: 10.1017/s1355617701766052. [DOI] [PubMed] [Google Scholar]
- 3.Borsting E, Rouse M, Chu R. Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry. 2005;76(10):588–592. doi: 10.1016/j.optm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Brahm KD, Wilgenburg HM, Kirby J, Ingalla S, Chang C-Y, Goodrich GL. Visual impairment and dysfunction in combat-injured service-members with traumatic brain injury. Optom Vis Sci. 2009;86(7):817–825. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- 5.Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102(5):856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- 6.Capa-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177(7):804–813. doi: 10.7205/milmed-d-12-00061. [DOI] [PubMed] [Google Scholar]
- 7.Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han M, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2007;78(4):155–161. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Cohen M, Groswasser Z, Barchadski R, Appel A. Convergence insufficiency in brain-injured patients. Brain Inj. 1989;3(2):187–191. doi: 10.3109/02699058909004551. [DOI] [PubMed] [Google Scholar]
- 9.Collins M, Kontos A, Reynolds E, Murawski C, Fu F. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235–246. doi: 10.1007/s00167-013-2791-6. [DOI] [PubMed] [Google Scholar]
- 10.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49(6):673. [PubMed] [Google Scholar]
- 11.Cooper J, Jamal N. Convergence insufficiency: a major review. Optometry. 2012;83(4):137–158. [PubMed] [Google Scholar]
- 12.Corwin DJ, Wiebe DJ, Zonfrillo MR, et al. Vestibular deficits following youth concussion. J Pediatr. 2015;166(5):1221–1225. doi: 10.1016/j.jpeds.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covassin T, Elbin R, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 14.Davies C. Orthoptic treatment in convergence insufficiency. Can Med Assoc J. 1946;55(1):47. [PMC free article] [PubMed] [Google Scholar]
- 15.De Beaumont L, Brisson B, Lassonde M, Jolicoeur P. Long-term electrophysiological changes in athletes with a history of multiple concussions. Brain Inj. 2007;21(6):631–644. doi: 10.1080/02699050701426931. [DOI] [PubMed] [Google Scholar]
- 16.Ellis MJ, Leddy JJ, Willer B. Physiological, vestibulo-ocular and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain Inj. 2015;29(2):238–248. doi: 10.3109/02699052.2014.965207. [DOI] [PubMed] [Google Scholar]
- 17.Gamlin PD, Yoon K. An area for vergence eye movement in primate frontal cortex. Nature. 2000;407(6807):1003–1007. doi: 10.1038/35039506. [DOI] [PubMed] [Google Scholar]
- 18.Gnadt JW, Mays LE. Neurons in monkey parietal area LIP are tuned for eye-movement parameters in three-dimensional space. J Neurophysiol. 1995;73(1):280–297. doi: 10.1152/jn.1995.73.1.280. [DOI] [PubMed] [Google Scholar]
- 19.Grisham J. Visual therapy results for convergence insufficiency: a literature review. Am J Optom Physiol Optics. 1988;65(6):448–454. doi: 10.1097/00006324-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Halstead ME, McAvoy K, Devore CD, et al. Returning to learning following a concussion. Pediatrics. 2013;132(5):948–957. doi: 10.1542/peds.2013-2867. [DOI] [PubMed] [Google Scholar]
- 21.Hayes GJ, Cohen BE, Rouse MW, De Land PN. Normative values for the nearpoint of convergence of elementary schoolchildren. Optom Vis Sci. 1998;75(7):506–512. doi: 10.1097/00006324-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kontos AP, Elbin R, Schatz P, et al. A revised factor structure for the post-concussion symptom scale baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375–2384. doi: 10.1177/0363546512455400. [DOI] [PubMed] [Google Scholar]
- 23.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–174. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 25.Maples WC, Hoenes R. Near point of convergence norms measured in elementary school children. Optom Vis Sci. 2007;84(3):224–228. doi: 10.1097/OPX.0b013e3180339f44. [DOI] [PubMed] [Google Scholar]
- 26.Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984;51(5):1091–1108. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- 27.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 28.McGregor ML. Convergence insufficiency and vision therapy. Pediatr Clin North Am. 2014;61(3):621–630. doi: 10.1016/j.pcl.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Mucha A, Collins MW, Elbin R, et al. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions preliminary findings. Am J Sports Med. 2014;42(10):2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierrot-Deseilligny C, Milea D, Müri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Prince SJ, Pointon AD, Cumming BG, Parker AJ. The precision of single neuron responses in cortical area V1 during stereoscopic depth judgments. J Neurosci. 2000;20(9):3387–3400. doi: 10.1523/JNEUROSCI.20-09-03387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rambold H, Neumann G, Helmchen C. Vergence deficits in pontine lesions. Neurology. 2004;62(10):1850–1853. doi: 10.1212/01.wnl.0000125331.95849.62. [DOI] [PubMed] [Google Scholar]
- 33.Rouse MW, Borsting E, Deland PN. Reliability of binocular vision measurements used in the classification of convergence insufficiency. Optom Vis Sci. 2002;79(4):254–264. doi: 10.1097/00006324-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91–99. doi: 10.1016/j.acn.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Scheiman M, Gallaway M, Frantz KA, et al. Nearpoint of convergence: test procedure, target selection, and normative data. Optom Vis Sci. 2003;80(3):214–225. doi: 10.1097/00006324-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304–307. doi: 10.1136/bjsports-2013-092190. [DOI] [PubMed] [Google Scholar]
- 37.Singman EL, Matta NS, Silbert DI. Convergence insufficiency associated with migraine: a case series. Am Orthoptic J. 2014;64(1):112–116. doi: 10.3368/aoj.64.1.112. [DOI] [PubMed] [Google Scholar]
- 38.Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. J Rehabil Res Develop. 2013;50:1223–1240. doi: 10.1682/JRRD.2012.12.0235. [DOI] [PubMed] [Google Scholar]
- 39.Zuckerman SL, Odom M, Lee YM, Forbes J, Sills AK, Solomon G. 145 sport-related concussion and age: number of days to neurocognitive baseline. Neurosurgery. 2012;71(2):E558. [Google Scholar]