Abstract
We have earlier reported that the redox-active antioxidant, vitamin C (ascorbic acid), activates the lipid signaling enzyme, phospholipase D (PLD), at pharmacological doses (mM) in the bovine lung microvascular endothelial cells (BLMVECs). However, the activation of phospholipase A2 (PLA2), another signaling phospholipase, and the modulation of PLD activation by PLA2 in the ECs treated with vitamin C at pharmacological doses have not been reported to date. Therefore, this study aimed at the regulation of PLD activation by PLA2 in the cultured BLMVECs exposed to vitamin C at pharmacological concentrations. The results revealed that vitamin C (3–10 mM) significantly activated PLA2 starting at 30 min; however, the activation of PLD resulted only at 120 min of treatment of cells under identical conditions. Further studies were conducted utilizing specific pharmacological agents to understand the mechanism(s) of activation of PLA2 and PLD in BLMVECs treated with vitamin C (5 mM) for 120 min. Antioxidants, calcium chelators, iron chelators, and PLA2 inhibitors offered attenuation of the vitamin C-induced activation of both PLA2 and PLD in the cells. Vitamin C was also observed to significantly induce the formation and release of the cyclooxygenase (COX)- and lipoxygenase (LOX)-catalyzed arachidonic acid (AA) metabolites and to activate the AA LOX in BLMVECs. The inhibitors of PLA2, COX, and LOX were observed to effectively and significantly attenuate the vitamin C-induced PLD activation in BLMVECs. For the first time, the results of the present study revealed that the vitamin C-induced activation of PLD in vascular ECs was regulated by the upstream activation of PLA2, COX, and LOX through the formation of AA metabolites involving oxidative stress, calcium, and iron.
Keywords: Phospholipase A2, Phospholipase D, Redox-active antioxidant, Lipid signaling, Endothelial cell, Arachidonate metabolite, Vitamin C
Introduction
Redox-active antioxidants of dietary origin have gained prominence not only in the human nutrition but also in pharmacology, preventive medicine, and therapeutics [1]. One such redox-active antioxidant, vitamin C (ascorbic acid), is an essential water-soluble vitamin, well known for its antiscorbutic and antioxidant functions in humans and its importance as a therapeutic agent in several pathological states, including the cardiovascular diseases [2–6]. Clinical trials, conducted so far, offer compelling evidence showing that vitamin C at pharmacological doses (mg – ~g), administered into circulation, elevates the circulating concentrations of the vitamin to mM levels, and causes the modulation of vasodilation and vascular tone in humans [6]. The role of cigarette smoke products has been implicated in the alterations of pulmonary vascular endothelium and associated pulmonary hypertension in chronic obstructive pulmonary disease [7]. Infusion of vitamin C into circulation at a dose of 10 mg/min for 120 min has been shown to improve the impairment of endothelial function in smokers [8]. Besides its antioxidant action, vitamin C also acts as a prooxidant, thus generating reactive oxygen species (ROS) and leading to oxidative stress [6]. The endothelium, a critical player in maintaining the integrity and function of the blood vessel, is an immediate target for the elevated circulating levels of prooxidant vitamin C. Hence, in our earlier study, the bovine lung microvascular endothelial cells (BLMVECs) have been used as the most appropriate model EC system, and the study has shown that vitamin C at pharmacological doses (mM) causes oxidative stress and loss of redox-dependent cell viability [6].
Phospholipases are membrane phospholipid hydrolases, which catalyze the generation of the bioactive lipid second messenger molecules, capable of playing crucial roles in cellular signaling [9]. Phospholipase D (PLD), an important member of the lipid signaling enzyme family, ubiquitously present in the mammalian cells, preferentially hydrolyzes phosphatidylcholine (PC) to generate phosphatidic acid (PA) and choline wherein the former is converted into several bioactive lipids [10–12]. Activation of PLD induced by agonists is a critical modulator of mammalian cellular signaling [10, 13–15].
Phospholipase A2 (PLA2) is another important membrane phospholipid-hydrolyzing enzyme that cleaves membrane phospholipids at the sn-2 position to release the free unsaturated fatty acid and lysophospholipid [16]. The unsaturated fatty acid thus released, typically arachidonic acid (AA), acts as a substrate for cyclooxygenases (COXs) and lipoxygenases (LOXs), which catalyze the formation of the potent bioactive AA metabolites including prostaglandins (PGs) and leukotrienes (LTs) which actively participate in the inflammatory cascades under the tight regulation of PLA2 [16–17]. PLA2, a very important housekeeping enzyme in membrane formation and repair, and its involvement in downstream AA metabolites in cardiovascular diseases have been gaining attention [17–19].
Studies have revealed that oxidants such as the ROS induce the activation of PLD in different cell systems including the vascular ECs and smooth muscle cells [14, 20–31]. Earlier, we have shown that vitamin C, at pharmacological doses, induces activation of PLD in BLMVECs through the oxidative stress and signaling cascades [31]. Reports have been made that oxidants and oxidative stress also induce the activation of PLA2 [32, 33]. However, the activation of PLA2 and the subsequent induction of formation of COX- and LOX-catalyzed bioactive AA metabolites by vitamin C, at pharmacological doses in the vascular ECs, have not been reported so far. Although the regulation of agonist-induced PLD activation is complex, the roles of PLA2 and COX- and LOX-catalyzed AA metabolites in the vitamin C-induced activation of PLD in vascular ECs have not been shown thus far [10, 34–36]. Therefore, in the current study, we investigated the regulation of vitamin C-induced PLD activation in the vascular BLMVECs by the upstream activation of PLA2 and formation of the COX- and LOX-catalyzed AA metabolites. For the first time, the results of the current study revealed that the vitamin C-induced activation of PLD in the vascular ECs was regulated by the upstream activation of PLA2 (AA release) and formation of COX- and LOX-catalyzed metabolites.
Materials and methods
Materials
BLMVECs (passage 4) (VEC Technologies, NY, USA). Phosphate-buffered saline (PBS) (Biofluids Inc., Rockville, MD). Minimal essential medium (MEM), nonessential amino acids, trypsin, fetal bovine serum (FBS), penicillin/streptomycin, DMEM phosphate-free modified medium, L-ascorbic acid, L-ascorbyl-2-phosphate, L-ascorbyl-2-sulfate, D-glucose, D-gluconic acid lactone, nonessential amino acids, trypsin-EDTA, disodium ethylenediaminetetracetic acid (Na2-EDTA), ethylene glycolbis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), BAPTA-AM (BAPTA) diethylenetriaminepentaacetic acid (DTPA), epigallocatechin gallate, N-acetylcysteine (NAC), δ-gluconolactone, bovine liver catalase, bovine erythrocyte superoxide dismutase (SOD), quinacrine, propyl gallate, and dimethyl sulfoxide (DMSO) (Sigma Chemical Co., St. Louis, MO). Phosphatidylbutanol (PBt) (Avanti Polar Lipids, Alabaster, AL). [32P]orthophosphate (carrier-free) (New England Nuclear, Wilmington, DE). Dehydroascorbic acid and desferal (Calbiochem, San Diego, CA). Endothelial cell growth factor (Upstate Biotechnology, Lake Plack, NY). [3H]arachidonic acid (AA) (American Radiolabeled Chemicals, Inc., St. Louis, MO). Arachidonyl trifluoromethyl ketone (AACOCF3), ibuprofen, indomethacin, nimesulide, baicalein, CDC, caffeic acid, eicosatriynoic acid (ETI), and enzyme immunoassay kits (EIA) for the determination of total prostaglandins, thromboxane B2 (TXB2), 8-isoprostane, and leukotriene B4 (LTB4) (Cayman Chemical Co., Ann Arbor, MI). Arachidonic acid (AA) (NuCheck Prep Inc., Elysian, MN).
Cell culture
BLMVECs were grown to confluence in MEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin and streptomycin, 5 μg/ml endothelial cell growth factor, and 1% nonessential amino acids at 37°C in a 95% air-5% CO2 atmosphere as described earlier [31]. BLMVECs, from passages 7 to 15, were used in the experiments. ECs from each primary T-75 cm flask were detached with 0.05% trypsin, resuspended in fresh medium, and sub-cultured in 35 mm or 60 mm sterile dishes or T-75 cm sterile flasks in complete medium to ~95% confluence under 95% air-5% CO2 at 37°C for treatment with vitamin C (L-ascorbic acid) and desired pharmacological agents. MEM containing vitamin C and other pharmacological agents were carefully adjusted to pH 7.4 for cellular treatments.
Phospholipase D activation in intact ECs
BLMVECs in 35-mm dishes (5 × 105 cells/dish) were prelabelled with [32P]orthophosphate (5 μCi/ml) in DMEM phosphate-free medium containing 2% fetal bovine serum for 12–14 h [31]. Cells were washed with MEM and incubated at 37°C in 1 ml of MEM containing 0.05% butanol in the absence and presence of desired concentrations of vitamin C (mM) for different lengths of time (0–120 min) under a humidified 95% air-5% CO2 atmosphere. In some experiments, wherever required, ECs prelabelled with [32P]orthophosphate were pretreated for 1 h with the selected pharmacological inhibitors prior to the exposure to MEM alone or MEM containing vitamin C alone or MEM containing vitamin C plus the selected pharmacological inhibitor(s) at the desired concentrations for 2 h. The incubations were terminated by the addition of methanol:conc. HCl (100:1, by vol.). Lipids were extracted essentially according to the method of Bligh and Dyer procedure as described previously [23, 31]. The [32P]-labeled phosphatidylbutanol (PBt) formed from the PLD activation and transphosphatidylation reaction, as an index of PLD activity in intact cells, was separated by the thin-layer chromatography (TLC) [23, 31]. Radioactivity associated with the [32P]-PBt was determined by the liquid scintillation counting, and data were expressed as DPM normalized to 106 counts in the total cellular lipid extract.
Assay of release of arachidonic acid and PLA2 activation
Release of AA from the cellular membrane phospholipids is widely assayed as an index of PLA2 activity [18, 37]. BLMVECs in 35-mm dishes (5 × 105 cells/dish) were labeled with carrier-free [3H]AA (5 μCi/ml) in complete EC media containing 10% FBS, nonessential amino acids, antibiotic, and growth factor for 12 h at 37°C in 5% CO2-95% air. The radioactive medium was removed by aspiration, cells were thoroughly washed twice with pre-warmed MEM (1 ml each time), and cells were incubated in MEM alone or MEM containing vitamin C (mM) at the desired concentrations for specified lengths of time (0–120 min). Wherever required, ECs prelabelled with [3H]AA were pretreated for 1 h with the selected pharmacological inhibitors prior to the exposure to MEM alone or MEM containing vitamin C alone or MEM containing vitamin C plus the selected pharmacological inhibitor(s) at the desired concentrations for 2 h. At the end of the incubation period, the amount/extent of AA released into the medium, as an index of PLA2 activity, was determined by liquid scintillation counting and expressed as DPM of [3H]/dish.
Determination of cyclooxygenase- and lipoxygenase-catalyzed formation of arachidonic acid metabolites
The COX- and LOX-catalyzed formation of AA metabolites in BLMVECs cultured in 35-mm dishes (5 × 105 cells/dish), following their exposure to vitamin C at different concentrations (mM) in MEM for 1 and 2 h, was determined by utilizing the commercially available EIA kits (Cayman Chemical Co., Ann Arbor, MI) according to Mazerik et al. [37]. Release of total prostaglandins, thromboxane A2 (measured as TXB2), 8-isoprostane, and LTB4 by cells was determined according to the manufacturer’s recommendations. The extent of release of AA metabolites from the cells was expressed as pg/ml medium/35-mm dish.
Assay of lipoxygenase activity
The in vitro activity of AA LOX in BLMVECs was assayed utilizing the standard spectrophotometric method to determine the extent of formation of conjugated dienes in the substrate (AA) according to Khanna et al. [38]. Following the treatment of BLMVECs with vitamin C (0–10 mM) in MEM in 35-mm dishes (5 × 105 cells/dish), the cells were detached with a cell scrapper and lysed in Tris-HCl (100 mM, pH 7.4) buffer. The final assay mixture (1 ml) contained 10 μM of AA and cell lysate (500 μg of protein) in 100 mM Tris-HCl buffer (pH 7.4). At the end of 5 min of incubation at 37°C, absorbance of the reaction mixture was measured at 234 nm (conjugated diene formation) against appropriate blanks. The activity of AA Lox was expressed as the conversion of AA into conjugated dienes (absorbance at 234 nm) by the enzyme in the cells.
Statistical analysis of data
Standard deviation (SD) for each data point was calculated from triplicate samples. Data were subjected to one-way analysis of variance and pair-wise multiple comparisons were done by Dunnett’s method with the significance set at P < 0.05.
Results
Vitamin C activates PLA2 in dose- and time-dependent manner
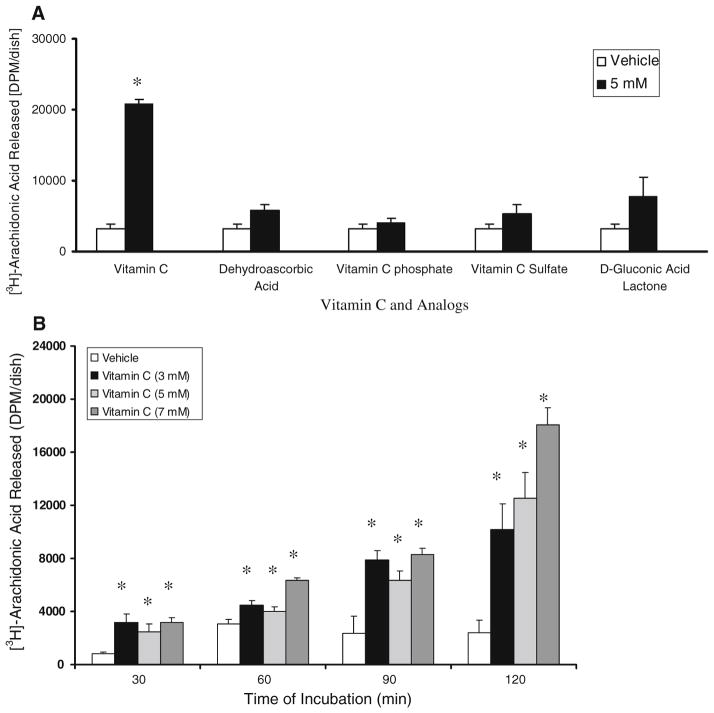
The release of AA by BLMVECs upon treatment with vitamin C was determined as the index of PLA2 activity. Our initial study revealed that only vitamin C (5 mM), but none of its analogs at the same concentration (dehydroascorbic acid, vitamin C phosphate, vitamin C sulfate, and D-gluconic acid lactone, the precursor of vitamin C), caused a significant activation of PLA2 (6.5-fold increase of AA release as compared to the vehicle-treated control cells) in BLMVECs at 120 min of incubation (Fig. 1A). The results of this experiment also suggested that (i) the ene-diol structure of vitamin C was essential for PLA2 activation and (ii) the vitamin C-induced activation of PLA2 could not be attributed to the osmotic stress caused by the vitamin at pharmacological concentrations utilized in this study as none of the vitamin C analogs with similar molecular weight was effective in causing the enzyme activation to the similar extent as caused by vitamin C at the same tested concentration. As shown in Fig. 1B, vitamin C induced the activation of PLA2 in a dose-and time-dependent fashion. Even at 30 min of treatment, vitamin C (3, 5, and 7 mM) significantly induced PLA2 activation as compared to the same in vehicle-treated control cells. At 90 min of treatment of cells, vitamin C induced a significant activation of PLA2 (3.3-, 2.7-, and 3.5-fold increase in AA release at 3, 5, and 7 mM of vitamin C, respectively) as compared to the same in the vehicle-treated control cells. At 120 min of exposure of cells to vitamin C, a significant dose-dependent increase in the activation of PLA2 was noticed (4.2-, 5.2-, and 7.5-fold increase in AA release at 3, 5, and 7 mM of vitamin C, respectively) as compared to the same in the vehicle-treated control cells (Fig. 1B).
Fig. 1.
Vitamin C activates PLA2 in a dose- and time-dependent manner. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [3H]AA (5 μCi/ml) in MEM for 12 h. After removing the [3H]-containing medium, (A) cells were incubated in MEM or MEM containing vitamin C (5 mM) and different analogs of vitamin C (5 mM) for 120 min and (B) cells were incubated with MEM or MEM containing different concentrations of vitamin C (3, 5, and 7 mM) for 0, 30, 60, and 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, release of [3H]AA into the medium from cells (as an index of PLA2 activity) was determined as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls
Antioxidants attenuate vitamin C-induced PLA2 activation
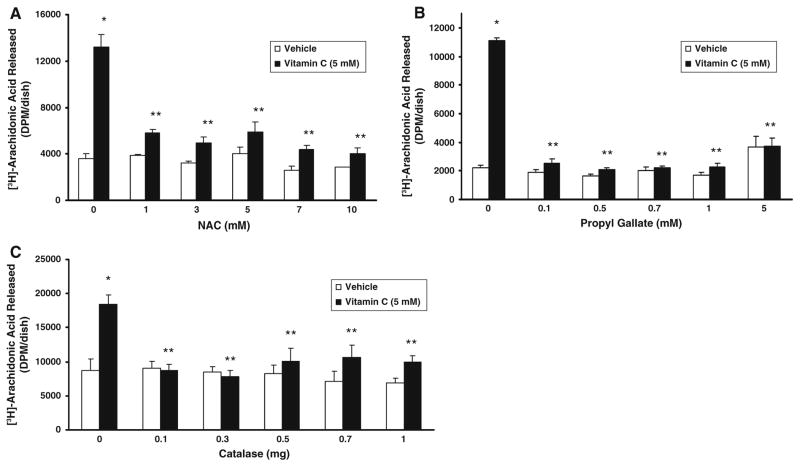
As we have shown earlier that vitamin C induces PLD activation in BLMVECs through ROS generation [6], here, we investigated whether ROS were involved in the vitamin C-induced activation of PLA2 in BLMVECs. The thiol antioxidant, N-acetylcysteine (NAC, 1–10 mM), phenolic antioxidant, propyl gallate (0.1–5 mM), and catalase (0.1–1 mg/dish), significantly and effectively attenuated the vitamin C (5 mM)-induced PLA2 activation (AA release) (Fig. 2A–C). The antioxidants were highly effective in keeping the activation of PLA2 in the vitamin C-treated cells at the same basal extent of activation observed in the vehicle-treated control cells. The results also revealed that ROS (including H2O2) were involved in the vitamin C-induced activation of PLA2 in BLMVECs.
Fig. 2.
Antioxidants attenuate vitamin C-induced PLA2 activation. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [3H]AA (5 μCi/dish) in MEM for 12 h. After removing the [3H]-containing medium, cells were pretreated with MEM or MEM containing (A) NAC (1–10 mM) and (B) propyl gallate (0.1–5 mM) for 1 h and then exposed to MEM in absence and presence of vitamin C (5 mM) for 120 min under a humidified atmosphere of 95% air-5% CO2 at 37°C. Simultaneously, (C) cells were also treated with MEM or MEM containing vitamin C (5 mM) or MEM containing catalase (0.1–1.0 mg/ml) or catalase (0.1–1.0 mg/ml) + vitamin C (5 mM) for 120 min under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, release of [3H]AA into the medium from cells (as an index of PLA2 activity) was determined as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with the vehicle-treated cells. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
Calcium chelators and PLA2 inhibitors attenuate vitamin C-induced PLA2 activation
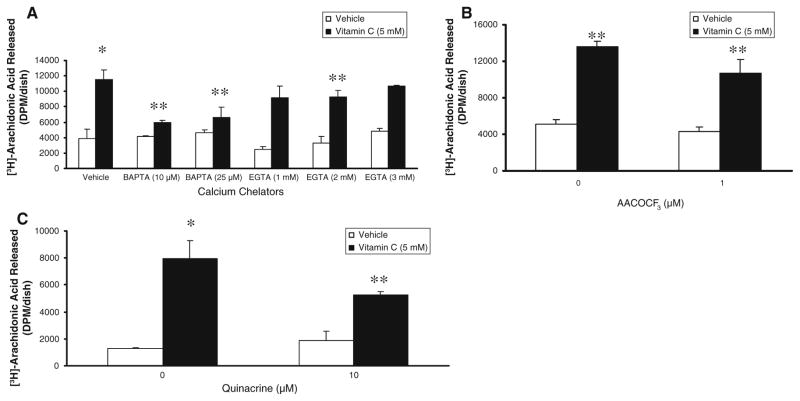
As calcium is essential for the activity of cPLA2, here, we investigated the role of calcium in the vitamin C-induced PLA2 activation. As shown in Fig. 3A, the intracellular calcium quencher, BAPTA, was more effective even at the tested dose of 10 μM in almost completely and significantly (48% of inhibition) attenuating the vitamin C (5 mM)-induced PLA2 activation at 120 min of treatment as compared to the effectiveness of the extracellular calcium chelator, EGTA (1 and 2 mM), which caused 20% of attenuation of the same in BLMVECs. These results revealed that although extracellular calcium was involved, the role of intracellular calcium in the vitamin C-induced activation of PLA2 in BLMVECs was clearly evident. We further investigated to establish the extent of involvement of cPLA2 in the vitamin C (5 mM)-induced release of AA by BLMVECs by utilizing the cPLA2-specific inhibitor, AACOCF3 and general PLA2 inhibitor, quinacrine. AA-COCF3 and quinacrine significantly caused 21.5% and 34% of inhibition, respectively, of the vitamin C-induced PLA2 activation in BLMVECs at 120 min of treatment (Fig. 3B, C) which further suggested that in addition to cPLA2, other isoforms of PLA2, such as the iPLA2 (calcium-independent isoform), would be also activated by vitamin C in ECs contributing to the release of AA.
Fig. 3.
Calcium chelators and PLA2 inhibitors attenuate vitamin C-induced PLA2 activation. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [3H]AA (5 μCi/dish) in MEM for 12 h. After removing the [3H]-containing medium, cells were pretreated with MEM or MEM containing (A) BAPTA (10 and 25 μM) or (B) AACOCF3 (1 μM) or (C) quinacrine (10 μM) for 1 h and then exposed to MEM in absence and presence of vitamin C (5 mM) for 120 min under a humidified atmosphere of 95% air-5% CO2 at 37°C. Simultaneously, (A) cells were also treated with MEM or MEM containing vitamin C (5 mM) or EGTA (1, 2, and 3 mM) or EGTA (1, 2, and 3 mM) + vitamin C (5 mM) for 120 min under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, release of [3H]AA into the medium from cells (as an index of PLA2 activity) was determined as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with the vehicle-treated cells. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
Vitamin C induces PLD activation in a dose- and time-dependent manner
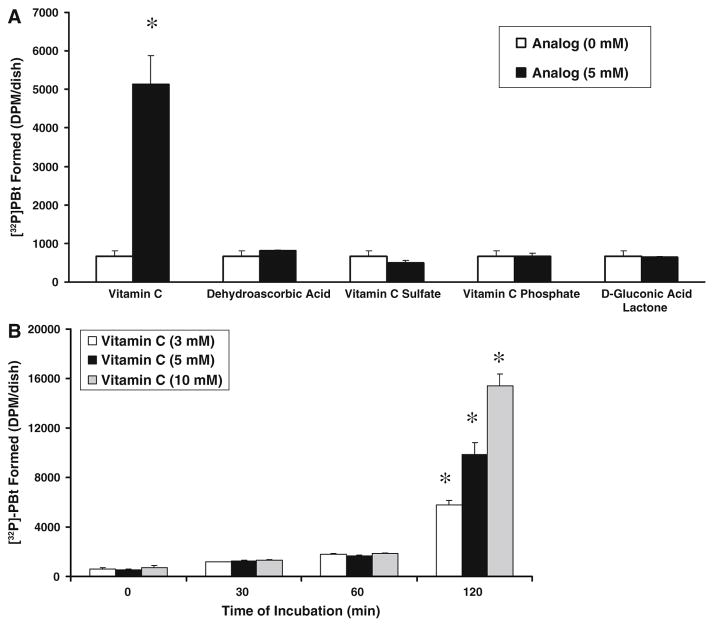
Earlier we have shown that vitamin C (mM) induces activation of PLD in BLMVECs [31]. Therefore, here, we investigated whether the analogs of vitamin C were also effective in activating PLD in ECs. As shown in Fig. 4A, none of the analogs of vitamin C (dehydroascorbic acid, vitamin C sulfate, vitamin C phosphate, and D-gluconic acid lactone), at the same concentration as that of vitamin C (5 mM) utilized, were effective in inducing the PLD activation in BLMVECs, whereas only vitamin C caused a significant activation of PLD (8-fold increase as compared with the vehicle-treated control cells). These results revealed that, as in the case of PLA2 activation, for PLD activation also (i) the ene-diol structure of vitamin C was essential and (ii) also the enzyme activation was not due to the osmotic effect caused by the mM concentrations of vitamin C utilized in the current study, because none of the vitamin C analogs with similar molecular weights at the same tested concentrations were effective in inducing PLD activation in BLMVECs. More importantly, the activation of PLA2 was evident as early as 30 min of treatment of cells with the vitamin (Fig. 1B), whereas significant, effective, and dose-dependent activation of PLD (9.6-, 18-, and 22-fold increase at 3, 5, and 10 mM doses of vitamin C) was only seen at 120 min of treatment of cells with vitamin C as compared to the same in cells exposed for 0 min to vitamin C (Fig. 4B). These results clearly indicated that the activation of PLA2 preceded the activation of PLD in BLMVECs exposed to vitamin C and further suggested a signaling role of PLA2 or its downstream AA metabolites in the regulation of PLD activation.
Fig. 4.
Vitamin C induces PLD activation in a dose- and time-dependent manner. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [32P]orthophosphate (5 μCi/dish) in DMEM-phosphate free medium for 12 h. After removing the [32P]-containing medium, (A) cells were incubated in MEM or MEM containing vitamin C (5 mM) and different analogs of vitamin C (5 mM) in presence of 0.05% butanol for 120 min and (B) cells were incubated with MEM or MEM containing different concentrations of vitamin C (3, 5, and 10 mM) in presence of 0.05% butanol for 0, 30, 60, and 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, lipids were extracted under acidic condition and PLD activity was determined as [32P]PBt formed in cells by thin-layer chromatography and liquid scintillation counting, as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls
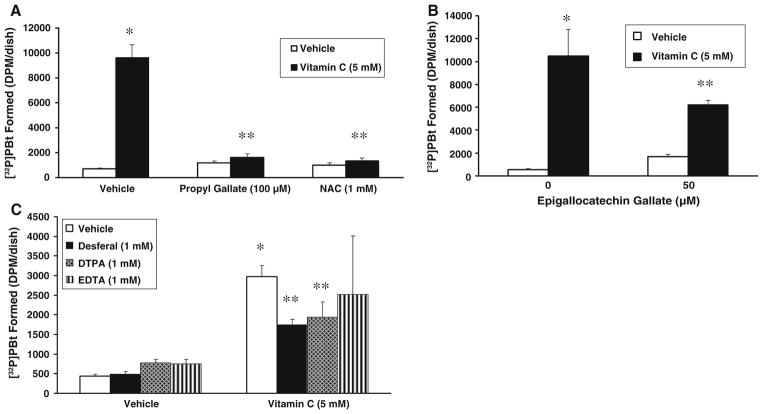
Antioxidants and iron chelators attenuate vitamin C-induced PLD activation
As we have reported earlier that vitamin C induces activation of PLD through ROS [6, 31], and the current study also revealed that the vitamin C-induced activation of PLA2 was regulated by ROS, here, we investigated the effect of antioxidants and iron chelators on the vitamin C-induced PLD activation in BLMVECs. Epigallocatechin gallate (50 μM), a well-known tea antioxidant, caused a significant attenuation (41% inhibition) of the vitamin C (5 mM)-induced PLD activation at 120 min of treatment, whereas both propyl gallate (100 μM), a phenolic antioxidant, and NAC (1 mM), the widely used thiol antioxidant, offered almost the complete attenuation of the vitamin C-induced PLD activation under identical conditions in BLMVECs (Fig. 5A, B). The iron chelators, both desferal and DTPA (1 mM), caused a significant attenuation of the vitamin C (5 mM)-induced PLD activation (41% and 15% inhibition, respectively, as compared to the vitamin C treatment alone) in cells at 120 min of incubation (Fig. 5C). These results revealed that in addition to iron, ROS were also involved in the vitamin C-induced activation of PLD in BLMVECs.
Fig. 5.
Antioxidants and iron chelators attenuate vitamin C-induced PLD activation. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [32P]orthophosphate (5 μCi/dish) in DMEM-phosphate free medium for 12 h. After removing the [32P]-containing medium, cells were pretreated for 1 h with MEM or MEM containing (A) propyl gallate (100 μM) or NAC (1 mM), (B) epigallocatechin gallate (50 μM), and (C) desferal (1 mM) and then were incubated in MEM or MEM containing vitamin C (5 mM) in presence of 0.05% butanol for 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37 °C. Cells were also treated with MEM or MEM containing vitamin C (5 mM) or DTPA or EDTA (1 mM) or DTPA or EDTA (1 mM) + vitamin C (5 mM) (C) for 120 min under identical conditions. At the end of the incubation period, lipids were extracted under acidic condition and PLD activity was determined as [32P]PBt formed in cells by thin-layer chromatography and liquid scintillation counting, as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
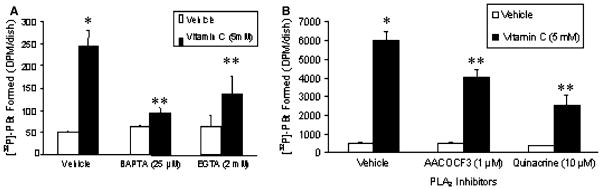
Calcium chelators and PLA2 inhibitors attenuate vitamin C-induced PLD activation
As the earlier experiments of this study showed that the intracellular calcium quencher (BAPTA), extracellular calcium chelator (EGTA), cPLA2-specific inhibitor (AA-COCF3), and the general PLA2 inhibitor (quinacrine) significantly attenuated the vitamin C-induced PLA2 activation in BLMVECs, here, we investigated the role of calcium and PLA2 in the vitamin C (5 mM)-induced PLD activation in BLMVECs. Although EGTA significantly caused a significant (44%) attenuation, BAPTA was more effective in significantly attenuating the vitamin C-induced PLD activation (62% inhibition) in BLMVECs treated for 120 min (Fig. 6A). However, AACOCF3 (1 μM) significantly caused only a 32% attenuation of the vitamin C-induced PLD activation at 120 min of exposure of BLMVECs to the vitamin, while quinacrine (10 μM) significantly caused a 58% inhibition of the same (Fig. 6B). These results clearly revealed that (i) as in the case of vitamin C-induced activation of PLA2 observed earlier in this study, the PLD activation induced by vitamin C was also regulated by both the intracellular and extracellular calcium and (ii) in addition to cPLA2, other isoforms of PLA2 such as iPLA2 might be involved in the regulation of the vitamin C-induced PLD activation. These results further strengthened the upstream regulatory role of PLA2 in the vitamin C-induced activation of PLD in BLMVECs.
Fig. 6.
Calcium chelators and PLA2 inhibitors attenuate vitamin C-induced PLD activation. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [32P]orthophosphate (5 μCi/dish) in DMEM-phosphate free medium for 12 h. After removing the [32P]-containing medium, cells were pretreated for 1 h with MEM or MEM containing (A) BAPTA (25 μM) and (B) AACOCF3 (1 μM) or quinacrine (10 μM), and then were incubated in MEM or MEM containing vitamin C (5 mM) in presence of 0.05% butanol for 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. (A) Cells were also treated with MEM or MEM containing vitamin C (5 mM) or EGTA (2 mM) or EGTA (2 mM) + vitamin C (5 mM) in presence of 0.05% butanol (A) for 120 min under identical conditions. At the end of the incubation period, lipids were extracted under acidic condition and PLD activity was determined as [32P]PBt formed in cells by thin-layer chromatography and liquid scintillation counting, as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
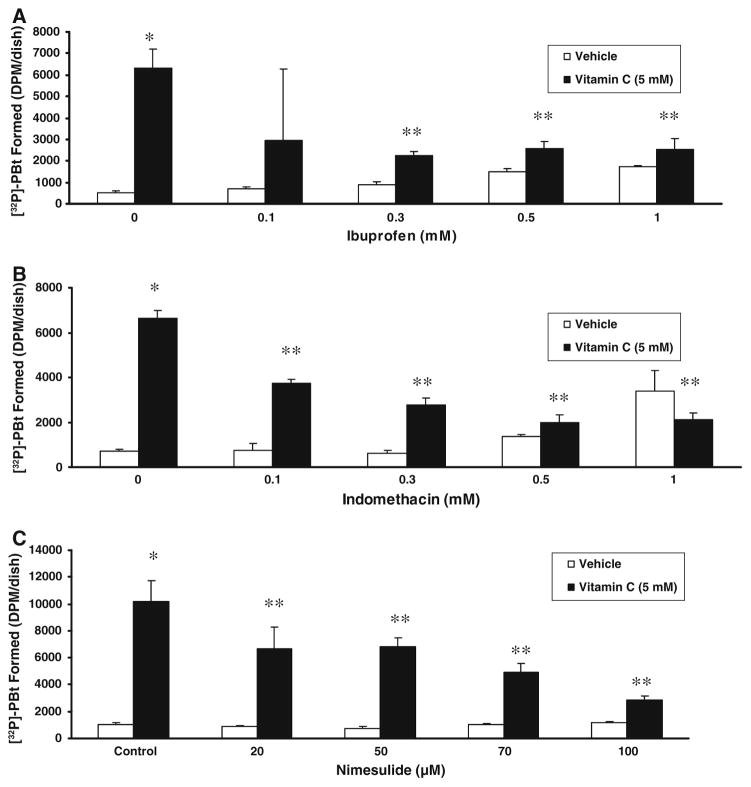
Cyclooxygenase inhibitors attenuate vitamin C-induced PLD activation
As the results of the earlier experiments of the current study showed that PLA2 (release of AA) was activated upstream of PLD activation and the latter was attenuated by the PLA2-specific inhibitors, and the PLA2-mediated release of AA provides substrate for the COX-catalyzed formation of AA metabolites such as prostaglandins, here, we investigated whether the role of COX could be involved in the PLD activation in BLMVECs exposed to vitamin C. Ibuprofen (0.1–1 mM) caused a significant and effective attenuation of the vitamin C (5 mM)-induced PLD activation in BLMVECs treated for 120 min (Fig. 7A). Another general COX inhibitor, indomethacin, in a dose-dependent fashion (0.1–1 mM), offered a significant and effective attenuation of the vitamin C (5 mM)-induced PLD activation in cells under identical conditions (Fig. 7B). Nimesulide, another COX-2-selective inhibitor (which is also known to inhibit COX-1), in a dose-dependent fashion (20–100 μM), significantly and effectively attenuated the vitamin C (5 mM)-induced PLD activation in BLMVECs treated for 120 min (Fig. 7C). These results clearly revealed that the COX-mediated AA metabolites were also involved in the regulation of the vitamin C-induced activation of PLD in BLMVECs.
Fig. 7.
Cyclooxygenase inhibitors attenuate vitamin C-induced PLD activity. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [32P]orthophosphate (5 μCi/dish) in DMEM-phosphate free medium for 12 h. After removing the [32P]-containing medium, cells were pretreated for 1 h with MEM or MEM containing (A) ibuprofen (0–1 mM), (B) indomethacin (0–1 mM), and (C) nimesulide (0–100 μM), and then were incubated in MEM or MEM containing vitamin C (5 mM) in presence of 0.05% butanol for 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, lipids were extracted under acidic condition and PLD activity was determined as [32P]PBt formed in cells by thin-layer chromatography and liquid scintillation counting, as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
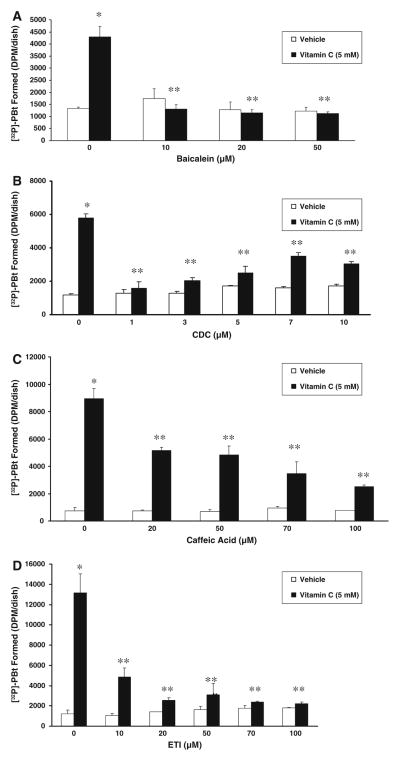
Lipoxygenase inhibitors attenuate vitamin C-induced PLD activation
The results of the earlier experiments revealed that the vitamin C-induced activation of PLD in BLMVECs was effectively attenuated by COX-specific inhibitors, further suggesting that the COX-mediated formation of AA metabolites apparently played a regulatory role in the enzyme activation. Also, the results that PLA2 inhibitors attenuated the vitamin C-induced PLD activation in ECs clearly indicated the involvement of AA in such PLD activation. Therefore, it was hypothesized that the LOX-catalyzed metabolites of AA from the membrane PLs could also activate PLD in the vitamin C-treated ECs. Hence, we examined the effect of LOX-specific inhibitors on the vitamin C (5 mM)-induced PLD activation in BLMVECs at 120 min of treatment of cells with vitamin C. As shown in Fig. 8A, B pretreatment of cells for 1 h with the 12-LOX-specific inhibitors, baicalein and CDC, even at 10 μM and 1 μM, respectively, almost completely and significantly attenuated the vitamin C-induced activation of PLD in BLMVECs, and further increasing the concentration of both baicalein and CDC up to 50 μM and 10 μM, respectively, did not appear to enhance the inhibition of PLD activation. Caffeic acid, the 5-LOX-and 12-LOX-specific inhibitor, upon pre-incubation for 1 h, from 20–100 μM dose, caused a dose-dependent and significant attenuation of the vitamin C-induced PLD activation in BLMVECs (Fig. 8C). ETI, a dual 12-LOX and COX-specific inhibitor, following pre-incubation for 1 h, caused a significant and dose-dependent attenuation of the vitamin C-induced PLD activation in BLMVECs, but at 100 μM dose, ETI caused almost the total attenuation of the PLD activation, suggesting the involvement of both COX and LOX in the vitamin C-induced PLD activation in cells (Fig. 8D). These results unequivocally demonstrated that in addition to COX, LOX also played an important role in modulating the vitamin C-induced activation of PLD in ECs through the LOX-mediated generation of AA metabolites.
Fig. 8.
Lipoxygenase inhibitors attenuate vitamin C-induced PLD activation. BLMVECs (5 × 105 cells/35-mm dish) were prelabelled with [32P]orthophosphate (5 μCi/dish) in DMEM-phosphate free medium for 12 h. After removing the [32P]-containing medium, cells were pretreated for 1 h with MEM or MEM containing (A) baicalein (0–50 μM), (B) CDC (0–10 μM), (C) caffeic acid (0–100 μM), and (D) ETI (0–100 μM), and then were incubated in MEM or MEM containing vitamin C (5 mM) in presence of 0.05% butanol for 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, lipids were extracted under acidic condition and PLD activity was determined as [32P]PBt formed in cells by thin-layer chromatography and liquid scintillation counting, as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls. ** Significantly different at P < 0.05 as compared with the vitamin C-treated cells
Vitamin C induces release of cyclooxygenase- and lipoxygenase-catalyzed arachidonic acid metabolites and activation of lipoxygenase
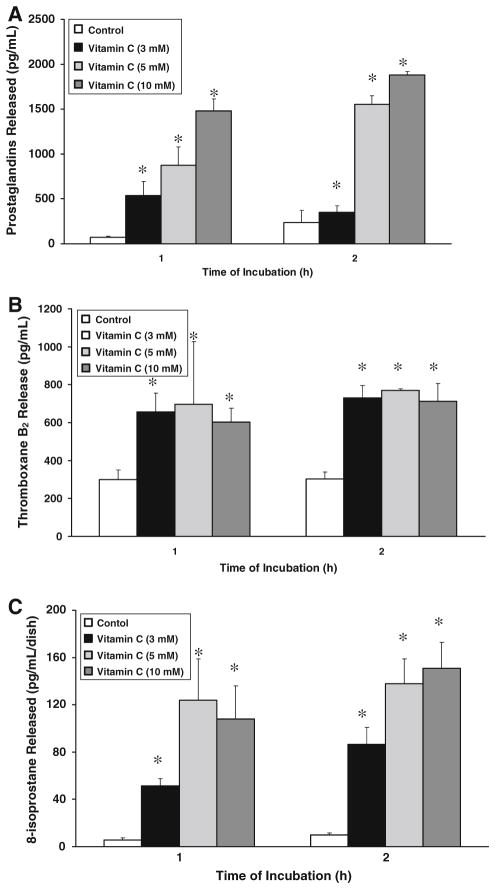
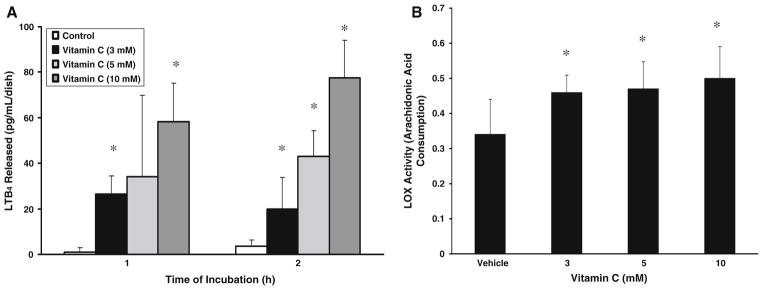
As the earlier experiments of the current study showed that vitamin C induced PLA2 activation, release of AA, and COX- and LOX-specific inhibitors attenuated the vitamin C-induced PLD activation, here, we investigated whether the vitamin induced the release of COX- and LOX-catalyzed AA metabolites and activation of AA LOX in BLMVECs. As shown in Fig. 9A, C vitamin C, in a dose-dependent (3–10 mM) and time-dependent (1 and 2 h) fashion induced a significant release of total PGs and 8-isoprostane. Vitamin C, under identical conditions, even at 3 mM concentration, significantly induced the release of TXA2 (measured as TXB2) which did not change either by increasing the dose of vitamin C (5 and 10 mM) or by prolonging the time of treatment of cells to 2 h (Fig. 9B). Vitamin C (5 mM) induced significant release of LTB4 (a LOX-formed AA metabolite) in a dose- and time-dependent fashion following treatment of cells with the vitamin (3–10 mM) for 1 and 2 h (Fig. 10A). As shown in Fig. 10B, vitamin C, even at a concentration of 3 mM, significantly induced the activation of AA LOX following incubation of BLMVECs for 2 h (Fig. 10B). Increasing the dose of vitamin C from 3 to 10 mM did not significantly enhance the AA LOX activity in BLMVECs under identical conditions. These results revealed that vitamin C induced the release of COX- and LOX-catalyzed AA metabolites and the activation of AA LOX in ECs.
Fig. 9.
Vitamin C induces the release of cyclooxygenase-catalyzed arachidonic acid metabolites. BLMVECs (5 × 105 cells/35-mm dish) were treated with MEM or MEM containing vitamin C (3–5 mM) for 60 and 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, the medium was collected and the extent of release of (A) total prostaglandins, (B) thromboxane B2, and (C) 8-isoprostane was measured as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls
Fig. 10.
Vitamin C induces release of lipoxygenase-catalyzed arachidonic acid metabolites and activation of arachidonic acid lipoxygenase. BLMVECs (5 × 105 cells/35-mm dish) were treated with (A) MEM or MEM containing vitamin C (3–10 mM) for 60 and 120 min and (B) MEM or MEM containing vitamin C (3–10 mM) for 120 min, under a humidified atmosphere of 95% air-5% CO2 at 37°C. At the end of the incubation period, the medium was collected and (A) the extent of release of LTB4 and (B) AA lipoxygenase activity were determined as described under Materials and Methods. Data represent mean ± SD of three independent experiments in triplicate. * Significantly different at P < 0.05 as compared with vehicle-treated controls
Discussion
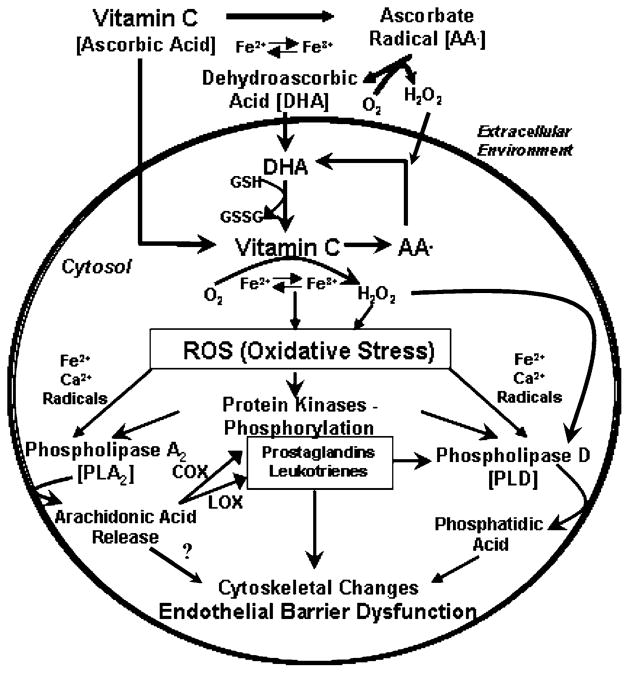
Overall, the results of the present study revealed that vitamin C at pharmacological doses (mM) induced the activation of both PLA2 and PLD through oxidant (ROS) and calcium signaling. Activation of both the enzymes was attenuated by the PLA2-specific inhibitors (general and cPLA2-specific), suggesting that PLA2 activation played an upstream regulatory role in the activation of PLD in BLMVECs. Furthermore, the results of the current study also showed that mM doses of vitamin C induced the release of COX- and LOX-catalyzed AA metabolites by cells, suggesting that activation of COX and LOX in the generation of AA metabolites from the PLA2-released AA from membrane phospholipids in BLMVECs. More noticeably, the current study also demonstrated that both the COX and LOX played a crucial role in upstream regulation of the vitamin C-induced PLD activation in BLMVECs through PLA2 activation and release of AA and through formation of COX- and LOX-catalyzed AA metabolites (Scheme 1). For the first time, this study demonstrated the cross talk among the lipid signaling enzymes including PLA2, COX, and LOX in the regulation of agonist (vitamin C)-induced activation of PLD in ECs.
Scheme 1.
Schematic representation of the putative phospholipase signaling events mediated by vitamin C in vascular ECs
Vascular endothelium plays a pivotal role in the regulation of structure and function of the blood vessel and maintains the homeostasis of the circulatory system and the entire body in general. Phospholipids of cellular membranes play an important role in the living cell as both the structural and functional entities. Phospholipases are enzymes that specifically hydrolyze the membrane phospholipids and generate bioactive lipid second messengers, which play a vital role in cellular signaling [19, 16]. Therefore, it is conceivable to hypothesize that vitamin C at pharmacological doses in circulation exerts its effects on the vascular endothelium, which in turn may contribute to the vitamin C-induced modulation/alteration of vascular functions.
Phospholipase A2 (PLA2) is an important membrane phospholipid-hydrolyzing enzyme which catalyzes the hydrolysis of the membrane phospholipids at the sn-2 position generating the free unsaturated fatty acid and lysophospholipid [16]. Thus, the unsaturated fatty acid released from the membrane phospholipids upon the action of PLA2, usually AA, is a substrate for COXs and LOXs, which mediate the formation of potentially bioactive AA metabolites such as PGs and LTs [17]. These AA metabolites of COXs and LOXs have been identified to play crucial roles in the inflammatory cascades and are tightly regulated by the activity of PLA2 [16]. PLA2 is also a very important housekeeping enzyme involved in the membrane formation and repair [18]. PLA2 has been shown to be activated by several agonists in different systems both in vitro and in vivo [17]. Roles of PLA2 and AA metabolites in cardiovascular diseases have been emerging [19]. Therefore, unregulated PLA2 activation mediated by agonists, such as vitamin C at mM concentrations, can jeopardize the endothelial function and eventually the vessel function. PLA2 in mammalian systems are broadly divided into three major classes: (i) cytosolic calcium-dependent PLA2 (cPLA2), (ii) intracellular calcium-independent PLA2 (iPLA2), and (iii) secretory calcium-dependent PLA2 (sPLA2) [19]. PLA2 acts on the sn-2 fatty acid esterified in the membrane phospholipids to release the unsaturated fatty acid and to generate the lysophospholipid [18]. The free AA thus generated acts as a substrate for COXs and LOXs [18]. The lysophospholipid with the alkyl group at the sn-1 position which is also generated from the membrane phospholipid upon the action of PLA2 is converted into the platelet activating factor (PAF). As both the eicosanoids (COX- and LOX-mediated AA metabolites) are potent bioactive lipids and key players in inflammation, PLA2 is regarded as an important lipid signaling enzyme [16, 18]. Regulation of PLA2 appears to be complex. The activity of cPLA2 has been widely studied and shown to be regulated through phosphorylation of serine which is mediated by the mitogen-activated protein kinases (MAPKs), protein kinase A (PKA), and protein kinase C (PKC) [17]. However, the regulation of activities of iPLA2 and sPLA2 is not thoroughly understood. Lipid peroxidation has been shown to simulate the activity of sPLA2 [37]. ROS have been shown to activate iPLA2 and cause release of AA in macrophages [39]. Oxidant (hydrogen peroxide)-mediated release of AA by astrocytes has been demonstrated due to activation of cPLA2 and iPLA2 [40]. PLA2 activity is regulated by cellular signaling cascades, ROS, and oxidative stress. Our earlier findings showing that vitamin C at pharmacological doses induces oxidative stress and the present study revealing that antioxidants attenuated PLA2 activation induced by mM doses of vitamin C in BLMVECs further established that vitamin C at pharmacological doses induced PLA2 activation in ECs through oxidative stress. The results of the present study had also shown that vitamin C at mM doses induced release of AA which was attenuated by calcium chelators in BLMVECs and cPLA2-specific inhibitor (AACOCF3), suggesting that vitamin C induced cPLA2 activation in EcsP. Partial inhibition of vitamin C-induced AA release by quinacrine in BLMVECs suggested activation of calcium-independent iPLA2.
AA metabolites including the LOX- and COX-generated prostanoids (PGs, thromboxane, and prostacyclin), hydroperoxy and hydroxy metabolites, and LTs have been identified as important inflammatory mediators in vascular endothelial dysfunction and atherosclerosis [41, 42]. Both the isoforms of COX, COX-1 and COX-2, convert AA into prostaglandin H2 (PGH2), which acts as a substrate for further metabolic conversion into TXA2, prostacyclin (PGI2), and PGE2 in vascular cells [44, 45]. LOXs exist in three different isoforms including 5-LOX, 12-LOX, and 15-LOX, which convert AA in mammalian cells released from membrane phospholipids upon the action of PLA2 into AA metabolites such as hydroperoxyeicosatetraenoic acids (HPETEs) and LTs [46, 47]. The LOX-derived AA metabolites act as potent bioactive lipid signaling molecules in cells including ECs [48, 49]. Isoprostanes (PG-like molecules), generated by the free radical-mediated oxidation of AA in vivo and in cellular systems, serve as biomarkers of oxidative stress [50]. One such isoprostane, 8-isoprostane, is also utilized as a marker of oxidative stress in the mammalian cells [51]. The current study also demonstrated the formation of 8-isoprostane in BLMVECs following the treatment with vitamin C at pharmacological doses, further suggesting that vitamin C-induced oxidative stress in ECs, which could also be responsible for the activation of downstream PLA2 and PLD. Influx of calcium, activation of cPLA2, and release of AA have been shown to play a crucial role in COX-mediated generation of AA metabolites in the vascular ECs [43]. The results of the current study clearly revealed the vitamin C-induced formation of LOX- and COX-generated AA metabolites in BLMVEC and further suggested the activation of LOXs and COXs and formation of AA-derived inflammatory mediators in ECs by vitamin C.
In mammalian cells, two predominant isoforms of PLD, PLD1 and PLD2, have been identified, cloned, and characterized [34]. Several distinct cofactors including Arf, Rho, Cdc42, phosphatidylinositol 4,5-bisphosphate, and detergents have been shown to activate PLD in vitro with an isoform specificity [31]. Oxidants including the ROS have been shown to stimulate the activity of PLD in several mammalian cell systems in culture, including the vascular smooth muscle cells and ECs [31]. Cell signaling kinases such as the p38 MAPK, extracellular signal-regulated kinases (ERKs), and Src kinase have been identified to play a role in the regulation of oxidant-mediated activation of PLD in the bovine pulmonary artery ECs (BPAECs) [31]. Nevertheless, the activation of PLD by different agonists has been shown to be regulated by cellular calcium, PKC, heterotrimeric G proteins, small molecular weight G proteins, protein tyrosine kinases, and protein tyrosine phosphatases [31]. Apparently, the involvement of these signal mediators in the regulation of activation of PLD is isoform-, agonist-, and cell-specific. Earlier, we have also reported that the oxidant-mediated activation of PLD in vascular ECs involves alterations in the thiol-redox status [31]. Recently, we have also shown that vitamin C at mM pharmacological concentrations causes loss of redox-dependent cell viability through oxidative stress and induces PLD activation through oxidative stress and MAP kinases in BLMVECs [6, 31]. The results of the current study revealed that the vitamin C-induced PLD activation in BLMVECs also involved calcium, ROS, and iron, which are typical players in oxidative stress.
In MC3T3-E1 cells, the PGF2α-stimulated PLD activation has been shown to be associated with DAG formation [52]. The involvement of G proteins has been reported in the PGF2α-induced activation of PLD in osteoblast-like cells [53]. PGD2, another COX-catalyzed AA metabolite, has been shown to activate PLD in osteoblast-like cells through calcium/calmodulin signaling [54]. In MC3T3-E1 cells, PGE2 has been observed to activate PLD through the GTP-binding protein and calcium [55]. Contrastingly, in UMR-106 cells, the 12-otetradecanoylphorbol 13-acetate (TPA)-induced PLD activation has been shown to be regulated by phosphatidate phosphohydrolase/DAG lipase pathway but not by COX-generated PGE2 [56]. A cross talk between PLD2 and PLA2/COX-2-mediated PG formation in HEK293 cells has been observed [57]. Linoleate hydroperoxide generated by the soybean LOX has been reported to activate PLD in the BPAECs [23]. In rat luteal cells, the PGF2α-stimulated PLD activation has been shown to be attenuated by the LOX-specific inhibitors (NDGA and ETYA) suggesting that LOX is involved in the PGF2α-induced PLD activation through the generation of LOX-catalyzed AA metabolites [58]. The results of the current study were in agreement with these earlier reports that both the COX- and LOX-generated AA metabolites derived from the PLA2-released AA from the membrane phospholipids are key regulators in the vitamin C-induced PLD activation in BLMVECs (Scheme 1). Nonetheless, other signaling players such as protein kinases, calcium, and G proteins have not been ignored in the vitamin C-induced EC PLD activation.
The roles of phospholipases and COXs in vascular diseases and ischemic tissue injury are becoming increasingly evident [59, 60]. The three important isoforms of LOX (5-LOX, 12-LOX, and 15-LOX) have been shown to be associated with several human diseases including the myocardial diseases [46–49]. The role of PLD in vascular disorders has been emerging [31]. Vitamin C, although it appears to protect the vascular endothelium, as a prooxidant induces oxidative stress in circulation at pharmacological (supra-physiological) concentrations achieved by the parenteral administration of vitamin C in humans who are critically ill [61]. As established in the current study, the activation and cross talk among PLA2, COXs, LOXs, and PLD to generate the bioactive lipid messengers in the cultured ECs under the treatment with vitamin C at pharmacological concentrations insist a thorough investigation with an emphasis on lipid signaling in endothelium in vivo in animal models, including humans receiving supra-physiological doses of the redox-active antioxidant parenterally. As vitamin C, at pharmacological doses, has been emerging as a pro-drug in selectively killing cancer cells [63–63], the results of the current study appear to offer insights into the effects of supra-physiological doses of vitamin C on angiogenesis in the neoplastic tissues.
Acknowledgments
This work was supported by the grants from the National Institutes of Health (NIH RO1 NS42617, HL 067176-05, NIDDKD RO1 DK056363, and EB 004031) and the funds from the Dorothy M. Davis Heart and Lung Research Institute and the Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine of the Ohio State University College of Medicine.
Contributor Information
Emily Steinhour, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Shariq I. Sherwani, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Jessica N. Mazerik, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Valorie Ciapala, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Elizabeth O’Connor Butler, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Jason P. Cruff, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Ulysses Magalang, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Sampath Parthasarathy, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Chandan K. Sen, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Clay B. Marsh, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Periannan Kuppusamy, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA.
Narasimham L. Parinandi, Email: Narasimham.parinandi@osumc.edu, Lipid Signaling and Lipidomics Laboratory, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA. Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, The Ohio State University, 473 W. 12th Avenue, Columbus, OH 43210, USA
References
- 1.Halvorsen BL, Carlsen MH, Phillips KM, Bohn SK, Holte K, Jacobs DR, Jr, et al. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 3.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. doi: 10.1016/S0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 4.May JM, Qu ZC, Li X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem Pharmacol. 2001;62:873–881. doi: 10.1016/S0006-2952(01)00736-5. [DOI] [PubMed] [Google Scholar]
- 5.Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. Can Med Assoc J. 2001;164:353–355. [PMC free article] [PubMed] [Google Scholar]
- 6.Varadharaj S, Watkins T, Cardounel AJ, Garcia JG, Zweier JL, Kuppusamy P, et al. Vitamin C-induced loss of redox-dependent viability in lung microvascular endothelial cells. Antioxid Redox Signal. 2005;7:287–300. doi: 10.1089/ars.2005.7.287. [DOI] [PubMed] [Google Scholar]
- 7.Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 8.Hirai N, Kawano H, Hirashima O, Motoyama Y, Moriyama Y, Sakamoto T, et al. Insulin resistance and endothelial dysfunction in smokers: effects of vitamin C. Am J Physiol Heart Circ Physiol. 2000;279:H1172–H1178. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- 9.Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- 10.Exton JH. Regulation of phospholipase. Biochim Biophys Acta. 1999;1439:175–186. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 11.Billah MM, Lapetina EG, Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid: A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981;256:5399–5403. [PubMed] [Google Scholar]
- 12.Brindley DN, Waggoner DW. Phosphatidate phosphohydrolase and signal transduction. Chem Phys Lipids. 1996;80:45–57. doi: 10.1016/0009-3084(96)02545-5. [DOI] [PubMed] [Google Scholar]
- 13.Exton JH. New developments in phospholipase D. J Biol Chem. 1997;272:15579–15582. doi: 10.1074/jbc.272.25.15579. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan V. Oxidants and signal transduction in vascular endothelium. J Lab Clin Med. 1995;125:126–137. [PubMed] [Google Scholar]
- 15.Singer WD, Brown HA, Jiang X, Sternweis PC. Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem. 1996;271:4504–4510. doi: 10.1074/jbc.271.13.7412. [DOI] [PubMed] [Google Scholar]
- 16.Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipases in generating lipid second messengers in signal transduction. FASEB J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborti S. Phospholipase A2 isoforms: a perspective. Cell Signal. 2003;15:637–665. doi: 10.1016/S0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 18.Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2000;531:2–6. doi: 10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 19.Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischemia/hypoxia. Acta Physiol (Oxf) 2006;187:75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiss Z, Anderson WH. Hydrogen peroxide regulates phospholipase D-mediated hydrolysis of phosphatidylethanolamine and phosphatidylcholine by different mechanisms in NIH 3T3 fibroblasts. Arch Biochem Biophys. 1994;311:430–436. doi: 10.1006/abbi.1994.1258. [DOI] [PubMed] [Google Scholar]
- 21.Min DS, Kim EG, Exton JH. Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J Biol Chem. 1998;273:29986–29994. doi: 10.1074/jbc.273.45.29986. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan V, Garcia JG. Agonist-induced activation of phospholipase D in bovine pulmonary artery endothelial cells: regulation by protein kinase C and calcium. J Lab Clin Med. 1993;121:337–347. [PubMed] [Google Scholar]
- 23.Natarajan V, Taher MM, Roehm B, Parinandi NL, Schmid HH, Kiss Z, et al. Activation of endothelial cell phospholipase D by hydrogen peroxide and fatty acid hydroperoxide. J Biol Chem. 1993;268:930–937. [PubMed] [Google Scholar]
- 24.Natarajan V, Scribner WM, Hart CM, Parthasarathy S. Oxidized low density lipoprotein-mediated activation of phospholipase D in smooth muscle cells: a possible role in cell proliferation and atherogenesis. J Lipid Res. 1995;36:2005–2016. [PubMed] [Google Scholar]
- 25.Natarajan V, Scribner WM, Vepa S. Regulation of phospholipase D by tyrosine kinases. Chem Phys Lipids. 1996;80:103–116. doi: 10.1016/0009-3084(96)02548-0. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan V, Vepa S, Verma RS, Scribner WM. Role of protein tyrosine phosphorylation in H2O2-induced activation of endothelial cell phospholipase D. Am J Physiol. 1996;271:L400–L408. doi: 10.1152/ajplung.1996.271.3.L400. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan V, Scribner WM, Vepa S. Phosphatase inhibitors potentiate 4-hydroxynonenal-induced phospholipase D activation in vascular endothelial cells. Am J Respir Cell Mol Biol. 1997;17:251–259. doi: 10.1165/ajrcmb.17.2.2623. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan V, Vepa S, Shamlal R, Al-Hassani M, Ramasarma T, Ravishankar HN, et al. Tyrosine kinases and calcium dependent activation of endothelial cell phospholipase D by diperoxovanadate. Mol Cell Biochem. 1998;183:113–124. doi: 10.1023/A:1006872230910. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan V, Scribner WM, Vepa S. Reactive oxygen species signaling through regulation of protein tyrosine phosphorylation in endothelial cells. Am J Respir Cell Mol Biol. 1997;17:251–259. [Google Scholar]
- 30.Parinandi NL, Scribner WM, Vepa S, Shi S, Natarajan V. Phospholipase D activation in endothelial cells is redox sensitive. Antioxid Redox Signal. 1999;1:193–210. doi: 10.1089/ars.1999.1.2-193. [DOI] [PubMed] [Google Scholar]
- 31.Varadharaj S, Steinhour E, Hunter MG, Watkins T, Baran CP, Magalang U, et al. Vitamin C-induced activation of phospholipase D in lung microvascular endothelial cells: Regulation by MAP kinases. Cell Signal. 2006;18:1396–1407. doi: 10.1016/j.cellsig.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Gissel H. The role of Ca2+ in muscle cell damage. Ann N Y Acad Sci. 2005;1066:166–180. doi: 10.1196/annals.1363.013. [DOI] [PubMed] [Google Scholar]
- 33.Muralikrishna AR, Hatcher JF. Phospholipase A2 reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(3):376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Frohman MA, Sung TC, Morris AJ. Mammalian phospholipase D structure and regulation. Biochim Biophys Acta. 1999;1439:175–186. doi: 10.1016/s1388-1981(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan V, Scribner WM, Morris AJ, Roy S, Vepa S, Yang J, et al. Role of p38 MAP kinase in diperoxovanadate-induced phospholipase D activation in endothelial cells. Am J Physiol. 2001;281:L435–L449. doi: 10.1152/ajplung.2001.281.2.L435. [DOI] [PubMed] [Google Scholar]
- 36.Cummings R, Parinandi NL, Wang L, Usatyuk P, Natarajan V. Phospholipase D/phosphatidic acid signal transduction: role and physiological significance in lung. Mol Cell Biochem. 2002;234–235:99–109. doi: 10.1023/A:1015944828973. [DOI] [PubMed] [Google Scholar]
- 37.Mazerik JN, Mikkilineni H, Kuppusamy VA, Steinhour E, Peltz A, Marsh CB, et al. Mercury activates phospholipase A2 and induces arachidonic acid metabolites in vascular endothelial cells. Toxicol Mech Methods. 2007;17(9):541–557. doi: 10.1080/15376510701380505. [DOI] [PubMed] [Google Scholar]
- 38.Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J Neurochem. 2006;98:1474–1386. doi: 10.1111/j.1471-4159.2006.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez J, Moreno J. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Arch Biochem Biophys. 2001;392(2):257–252. doi: 10.1006/abbi.2001.2439. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Yu S, Sun AY, Sun GY. Oxidant-mediated AA release from astrocytes involves cPLA2 and iPLA2. Free Radic Biol Med. 2003;34(12):1531–1543. doi: 10.1016/S0891-5849(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 41.Reiss AB, Edelman SD. Recent insights into the role of prostanoids in antherosclerotic vascular disease. Curr Vasc Pharmacol. 2006;4:395–408. doi: 10.2174/157016106778521652. [DOI] [PubMed] [Google Scholar]
- 42.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvasc Res. 2005;69:107–127. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Antoniotti S, Fiorio PA, Pregnolato S, Mottola A, Lovisolo D, Munaron L. Control of endothelial cell proliferation by calcium influx and arachidonic acid metabolism: a pharmacological approach. J Cell Physiol. 2003;197(3):370–378. doi: 10.1002/jcp.10359. [DOI] [PubMed] [Google Scholar]
- 44.Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of procyclin synthesis by COX-2. J Immunol. 2001;167(5):2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 45.Flavahan NA. Balancing prostanoid activity in the human vascular system. Trends Pharmacol Sci. 2006;28(3):106–110. doi: 10.1016/j.tips.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, et al. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. J Clin Invest. 1996;97:806–813. doi: 10.1172/JCI118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Lipoxygenase inhibitors as potential cancer preventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483. [PubMed] [Google Scholar]
- 48.Tang DG, Chen YQ, Honn KV. Arachidonic acid lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitman S, Gezginci M, Timmermann BN, Holman TR. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem. 2002;45:2659–2661. doi: 10.1021/jm0201262. [DOI] [PubMed] [Google Scholar]
- 50.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 51.Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(3):245–251. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama T, Sakai T, Nozawa Y, Oka N. Prostoglandin F2 alpha-stimulated phospholipase D activation in osteoblast-like MC3T3-E1 cells: involvement in sustained 1,2-diacylglycerol production. Biochem J. 1994;298:479–484. doi: 10.1042/bj2980479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozawa O, Suzuki A, Kotoyori J, Tokuda H, Watanabe Y, Ito Y, et al. Prostaglandin F2-alpha activates phospholipase D independently from activation of protein kinase C in osteoblast-like cells. J Cell Biochem. 1994;55:373–379. doi: 10.1002/jcb.240550315. [DOI] [PubMed] [Google Scholar]
- 54.Imamura Y, Kozawa O, Suzuki A, Watanabe Y, Saito H, Oiso Y. Mechanism of phospholipase D activation induced by prostaglandin D2 in osteoblast-like cells: function of Ca2+/cal-modulin. Cell Signal. 1995;7:45–51. doi: 10.1016/0898-6568(94)00059-K. [DOI] [PubMed] [Google Scholar]
- 55.Oiso Y, Suzuki A, Kozawa O. Effect of prostaglandin E2 on phospholipase D activity in osteoblast-like MC3T3-E1 cells. J Bone Miner Res. 1995;10:1185–1190. doi: 10.1002/jbmr.5650100807. [DOI] [PubMed] [Google Scholar]
- 56.Kaneki H, Yokozawa J, Fujieda M, Mizuochi S, Ishikawa C, Ide H. Phorbol ester-induced production of prostaglandin E2 from phosphatidylcholine through the activation of phospholipase D in UMR-106 cells. Bone. 1998;23(3):213–222. doi: 10.1016/S8756-3282(98)00100-8. [DOI] [PubMed] [Google Scholar]
- 57.Uenu N, Murakami M, Kudo I. Functional crosstalk between phospholipase D(2) and signaling phospholipase A(2)/cyclooxygenase-2-mediated prostaglandin biosynthetic pathways. FEBS Lett. 2000;475:242–246. doi: 10.1016/S0014-5793(00)01691-4. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto H, Endo T, Kiya T, Goto T, Sagae S, Ito E, et al. Activation of phospholipase D by prostaglandin F2-alpha in rat luteal cells and effects of inhibitors of arachidonic acid metabolism. Prostaglandins. 1995;50:201–211. doi: 10.1016/0090-6980(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 59.Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P. Phospholipase A2 in vascular disease. Circ Res. 2001;89:298–304. doi: 10.1161/hh1601.095598. [DOI] [PubMed] [Google Scholar]
- 60.Phillis JW, O’Regan MH. The role of phospholipases, cyclooxyegnases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/CritRevNeurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 61.McGregor GP, Biesalski HK. Rationale and impact of vitamin C in clinical nutrition. Curr Opin Nutr Metab Care. 2006;9:697–703. doi: 10.1097/01.mco.0000247478.79779.8f. [DOI] [PubMed] [Google Scholar]
- 62.Duconge J, Miranda-Massari JR, Gonzalez MJ, Taylor PR, Riordan HD, Riordan NH, et al. Vitamin C pharmacokinetics after continuous infusion in a patient with prostate cancer. Ann Pharmacother. 2007;41:1082–1083. doi: 10.1345/aph.1H654. [DOI] [PubMed] [Google Scholar]
- 63.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug tp deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]