Abstract
Academic investigators are generating a plethora of insights and technologies that have the potential to significantly improve patient care. However, to address the imperative to improve the quality, cost and access to care with ever more constrained funding, the efficiency and the consistency with which they are translated into cost effective products and/or services need to improve. Healthcare commercialization programs (HCPs) are described and proposed as an option that institutions can add to their portfolio to improve translational research. In helping teams translate specific healthcare innovations into practice, HCPs expand the skillset of investigators and enhance an institution’s innovation capacity. Lessons learned are shared from configuring and delivering HCPs, which build on the fundamentals of the National Science Foundation’s Innovation Corps program, to address the unique challenges in supporting healthcare innovations and innovators.
Keywords: Valley of death, I-Corps, commercialization, translational research, healthcare innovation
Healthcare Commercialization Programs (HCPs): Filling a gap in an institution's portfolio of ways to improve the efficiency of translating healthcare innovations from academia into practice.

I. The Growing Imperative and Challenges in Commercializing Academic Medical Research
As has been the case in most other industries, the promise of new technologies and innovations has been cited as the way to address the healthcare industry’s current challenges; in particular, to circumvent the so-called “Iron Triangle”, simultaneously improving access, cost, and quality of care [1]. At the same time, research based institutions across the globe are experiencing significant stress from more competition for less funding. From FY 2003 to 2015, for example, the NIH lost 22% of its capacity to fund research due to budget cuts, sequestration, and inflationary losses. This resulted in fewer grants and discoveries, along with talented scientists and investigators leaving research and/or the US [2].
As such, it is more important than ever to increase the efficiency and effectiveness with which investments in fundamental research and development translate into products, services, and procedures that improve the health and wellbeing of people around the world. Examples abound of the power of technology and innovation to enable disruptive step changes in performance while simultaneously slashing costs. Moore’s law in semiconductors, which projects a doubling of CPU capacity every 18 to 24 months, typifies the exponential power of technology. Innovator and futurist Ray Kurzweil extended Moore’s Law to show that when a specific technology platform approaches some kind of physical limit, a new one emerges to extend the exponential growth, bypassing perceived barriers [3].
However, the healthcare reality is of decreasing efficiencies in translating R&D into practice. Eroom’s Law typifies the experience in the pharma industry: rather than show an improvement, the trend for new drug approvals by the US Food and Drug Administration per inflation-adjusted US dollars spent on R&D is a decrease of 50% over 9 years – a negative rather than positive exponential growth [4]. While no generally accepted “law” exists for healthcare as a whole, labor productivity in healthcare, for example, is in decline, with technology not improving productivity as it has in other industries [5].
Successful innovation in healthcare requires navigating a long and challenging journey between an unmet need or discovery generated from basic research to a viable commercial product or service [6]. While there are many potential stumbling points along the way, the so-called Valley of Death that exists between academic research and commercialization of a new product is often cited as a key obstacle for health innovations. The Valley of Death has been shown to occur most frequently in the presence of non-economic investments, such as government expenditures on early stage, basic research without attention to the likelihood of commercially motivated investment at later stages if successful [7].
Technologies that emerge from academia which do not have a promising commercialization pathway and clearly articulated value proposition are simply not considered by financially-motivated investors and, as a result, languish in the lab. Financial investors are not typically impressed with the great technical results that investigators focus on developing and are rewarded for publishing – they expect them. They understand the reality is that the vast majority of new ventures fail not because of flawed science (“technical risk”), but because the market does not perceive a need or will not pay for the product or service (“market risk”) [8].
Many academic investigators and research teams are simply not prepared or motivated to think beyond their work in the lab to anticipate or address commercial issues such as market risk. Some are even discouraged from considering commercial issues while conducting research due to lingering concerns about aligning too closely with industry. A commonly held misconception by many investigators is that once they are successful publishing results in a high impact journal, the commercial value will be so self-evident that companies will flock to get commercial rights. As a result, many leave commercialization related issues to be addressed only after a technology is shown to work (i.e. proof-of-concept demonstrated), expecting them to be “transferred” to a company that will bring a product or service into practice.
As a result, academic innovators pursuing translational research still often approach it with the mindset of doing science. They do not develop an appreciation for which of the many paths available to them is most likely to lead to patient impact and hence financial return for investors. Today’s challenging funding situation, with historically low pay-lines, creates even more pressure for investigators, particularly new ones, to focus on novelty and quickly move on to new areas once publishable results have been generated [9]. This often leaves institutional technology transfer offices (TTOs) with the very challenging task of seeking licensees to technologies that have been shown to work, but with no clear commercial value and without much, if any, technical support. It leaves funders with successfully completed projects that advance no further.
Simultaneously, financially driven investors are becoming less willing to take risks, particularly risks that they cannot control or may only pay-off in the longer term [10]. They are looking to invest in opportunities for which they are well positioned to manage the risks, such as implementation related issues. They do not want to take risks in areas for which they have no control, such as market acceptance, reimbursement and regulatory acceptance.
As a result of these increasing pressures from both sides, the Valley of Death has become harsher and wider. The only teams surviving the commercialization journey through today’s more treacherous Valley of Death are ones with the most robust preparation, demonstrably de-risking both the technology and the market to succeed in attracting investors.
II. The Role of Academic Institutions in Supporting Translational Research
Universities and academic medical centers are facing increasing competition to not only win scarcer research funding, but also to attract the best investigators and students. To compete successfully at both, they need to do more in preparing and supporting those investigators interested in translating research into practice: excellence in science is still required, but is no longer sufficient. Investigators that wish to expand beyond basic research need new skills and resources that can help them focus on creating and developing technologies that have a better chance of surviving the commercialization journey long enough to engage investors and get products to the market.
One approach being taken at some institutions is to add a business development function to an institution’s TTO, expanding its role to include “promoter” as well as “guardian” of its intellectual property (IP). While this approach can be very effective in advancing some projects, it does not address the fundamental issue of improving the commercial readiness or attractiveness of technologies emerging from labs. In addition, balancing the roles of guardian and promoter of IP in a single cost center with increasingly constrained budgets can add an obstacle to commercialization which discourages all but the few that are selected for support.
Another approach taken by institutions, including Stanford BioDesign and the Coulter Foundation as well as by funders, including the NIH and the NSF, are creating programs to assist academic investigators advance technologies toward commercialization while they are conducting their research. They are providing educational opportunities to help investigators understand, anticipate and address commercialization challenges. Efforts range from didactic educational programs to accelerators which provide hands-on skills development and bridge/product development funding as well as support in the form of mentoring and “pitch” competitions, the goal being to help teams achieve a commercial exit: the point at which financially motivated investors such as angels, VCs and strategic players invest [11].
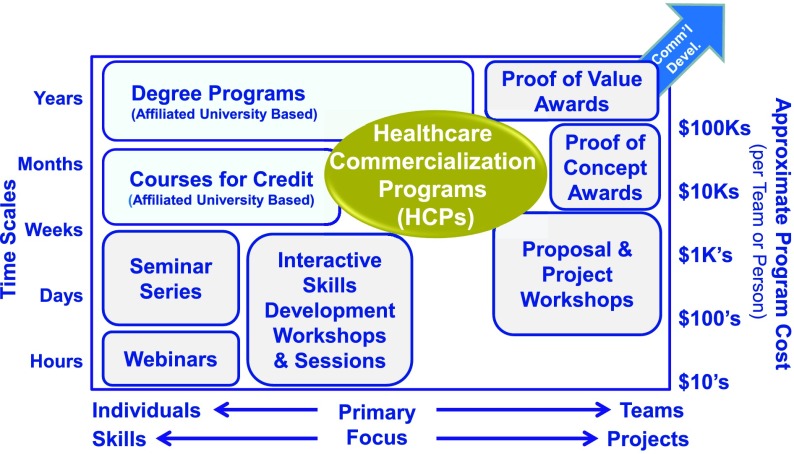
Figure 1 schematically represents a range of these approaches taken by organizations to improve the effectiveness of the translational research they support.
FIGURE 1.
The gap filled by HCPs in the portfolio approaches used by academic programs to improve the impact of translational research they support.
The horizontal axis represents the typical focus of the effort. At one end of the spectrum the focus is on building the skills of individuals, such as university based degree programs. The other end of the spectrum is focused on helping advance projects, such as accelerator programs. The vertical axis represents the typical intensity of the efforts. At one end of the spectrum are activities that take a few hours and cost very little to deliver, such as webinars. At the other end are programs that take years and cost in the hundreds of thousands of dollars, such as university based degree programs.
An emerging option for academic investigators is the development of Healthcare Commercialization Programs (HCPs). These programs attempt to strike a balance by cost-effectively advancing a project into commercialization, while simultaneously building the skills of team members so that they can learn while contributing to an entrepreneurial activity.
III. Healthcare Commercialization Programs
There have been several efforts to establish Healthcare Commercialization Programs. Two recent examples are a modified version of the I-Corps program [12], adapted in collaboration with the NIH [13] and another being the Coulter Foundation C3I program [14].
In 2013, the National Heart, Lung and Blood Institute (NHLBI) took a fresh approach to improving the effectiveness of the translation of academic based research into implementation. It established three centers to accelerate innovations from the bench to the clinic: the NIH Centers for Accelerated Innovations (NCAIs). The NCAIs are to help advance projects into patient care by providing product development funds and project facilitation, while also providing skills development to empower academic investigators with a greater understanding of the commercial path of their technologies [15].
The NCAIs were provided additional funds by the NSF in the spring and summer of 2015 to explore how the I-Corps program could help advance NCAI supported projects into practice. The University of California NCAI [16] applied its pre-existing I-Corps program and approach for teams from across the University of California network, with a focus on teams from the San Francisco area.
The Boston NCAI, B-BIC [17] (through its collaborator the Consortia to Improve Medicine with Innovation and Technology: CIMIT [18]) and Cleveland’s NCAI (The NIH Center for Accelerated Innovation at Cleveland Clinic; NCAI-CC [19]) took a different approach. They choose to blend several methodologies into a program intended to address the unique challenges facing healthcare innovations. They each built on the fundamentals of the I-Corps program while integrating commercialization experiences gained from introducing therapeutic and HealthTech products. CIMIT built on its 18+ year history and experiences facilitating more than 600 projects with its unique approach to facilitating teams as well as its work with the Coulter Foundation and academic based programs at its member institutions [20]. Cleveland Clinic incorporated specific workshops focused on therapeutics, including regulatory and intellectual property with support of the NHLBI staff. A brief overview of each and some results are outlined below:
A. CIMIT’s Commercialization Readiness Assessment and Accelerator for Solutions in Healthcare (CRAASH) Course
CIMIT’s focus is on HealthTech innovations, the overlap of medicine and engineering, including devices, diagnostics, e/digital health and big data [21]. Its initial pilot program had eight weekly sessions and was comprised of a cohort of 9 teams. Each team had a Clinical Lead, Technical Lead and Entrepreneurial Lead, which were supported by an executive from the CIMIT Accelerator Team [22]. The overall results were very promising, with four teams successfully attracting commercial funding within 6 months of completing the course, receiving more than $11M in commercial investment. Three are now pursuing licensing opportunities instead of trying to create a NewCo, and two projects are being rethought after learning of the lack of a market need. Programmatically, these “quick kills” are viewed as being a success along with the teams that are able to advance to commercialization as it saves time and resources so that teams can work on more impactful problems.
The teams all reported significant progress in a compressed period of time, and similar results have been generated in subsequent cohorts (which now run ten weekly sessions). An example of a self-reported team assessment is:
“Our experience at the CIMIT program and all the support from the faculty has been instrumental to our progress. Less than a year ago, I was an MD-PhD student with no business experience and no intentions of commercializing technology. In fact, before the course, we didn’t even know who our customer was or what our product was, let alone our business model. CIMIT taught us to make hypotheses about our business and test them rigorously by speaking to all the stakeholders in the ecosystem. Not only did we convince ourselves of who our customer was and what we should be building, all the data we gathered also convinced investors.”
B. The NCAI-CC Healthcare Commercialization Program
The NCAI-CC HCP program was seven weekly sessions, specifically designed for therapeutics. It provided specialization workshops on regulatory and intellectual property consideration with NHLBI staff and focused on the development of a Target Product Profile, an accepted tool for pharmaceutical development projects. The cohort included five teams, each with a Principal Investigator, Entrepreneurial Lead and a faculty member, one of the NCAI product development directors.
The teams all began at different baselines but advanced their understanding of their commercialization process. They defined the types and quality of data that would be required to attract a pharmaceutical company’s interest in commercial development. One team identified a path that includes formation of a new company and application for SBIR support. Two teams were a no go due to the lack of clear path.
Examples of the self-reported assessments include:
“Taught to think differently about our technology – with a business/practical perspective never learned in graduate school or as a postdoc”
“Helped prepare me for industry – I became better suited to write business reports such as a strategic plan focused for upper management”
“It was not until we talked to clinicians, patients and regulatory experts that we were able to capture a holistic view of how our technology fit in the therapeutic ecosystem.”
IV. Lessons Learned
The CIMIT and NCAI-CC faculty coordinated activities in developing, delivering and critiquing their programs. Despite the differences between the programs due to the different clinical foci, the faculty developed consensus “lessons learned”. While there is strong agreement that there is no single “right way” to run an HCP, the following points are intended to help guide others wishing to develop an HCP. The lessons reinforce the applicability of some of the fundamentals of the I-Corps program as well as ways that an HCP should differ from I-Corps to address the unique needs and challenges of healthcare innovators and innovations.
A. Building on the I-Corps Fundamentals
The fundamental premise of the I-Corps program was found to apply very well to healthcare: get team members out of the lab to talk with stakeholders, customers in particular, and apply the scientific approach of developing, testing and validating hypotheses about the business. There are many facets of the I-Corps program that were found to apply; some key examples include:
-
•
A weekly goal of around 10 exploratory interviews with a range of stakeholders needs to be stressed, and may be the most challenging as well as valuable thing for participants to learn. Most successful investigators are good advocates and teachers, re-learning to listen to feedback is often a challenge, particularly when based on partial information.
-
•
The flipped-classroom structure with off-line assignments and weekly two-hour interactive session focused on practical applications of the materials and on teams presenting their work for robust feedback in a mix of physical and web-based meetings is time-efficient and allows participants to continue with their “day jobs”.
-
•
Programs should run at least eight to ten weeks to allow time for a sufficient number of interviews along with the potential of at least one “pivot” - a change in direction based on validated feedback from the market.
B. Unique Issues for HCPs
It is not surprising that an effective HCP would differ from the I-Corps program since it was developed with a different focus. I-Corps was designed to support Principal Investigator led academic teams with SBIR awards in a broad range of industries. While many commercialization challenges transcend industries, there are some unique challenges facing healthcare innovators that require simultaneous attention in an HCP.
Some key examples include:
-
1.
Buying Dynamics: The user of the products/services (User) and those responsible for making a buying decision (Economic Buyer) in healthcare are usually not the same people, can be very hard to identify and may change from one institution to the next. In addition, Users and Economic Buyers frequently have competing or misaligned objectives. This is in contrast, for example, to selling to consumers who make the purchasing decision and use the products.
-
2.
Reimbursement Complexity: Reimbursement for products/services in healthcare is a complex and highly regulated process. This is in contrast to most industries in which the price a company can charge is established by a free market.
-
3.
Regulatory Pathway: While regulations exist in every industry, the impact that early and what may appear to be subtle decisions can have a major impact on the level of testing and validation that a medical product or service must undergo before being approved for sale or use. The decision can mean the difference of years before introduction and 10’s of millions of dollars of investment and therefore commercial viability. The option to start with a minimally viable product (MVP) and iterate with customer experiences to improve it is not the same as it is in industries with products that have less potential for harm, and are therefore less regulated.
-
4.
Culture: Healthcare professionals, be they doctors, nurses, therapists, technicians, etc. must invest many years and a great deal of money into education in order to practice. The option for them to leave a position to focus on a start-up comes at a very high professional and personal risk and therefore is often not practical. However, their constant engagement in a development effort and focus on addressing important unmet clinical needs is critical to success. Their engagement must be managed within their other time constraints, which are generally only increasing, and done in a way consistent with their expectations and culture.
-
5.
Funding Requirements: While there is a wide range of funding required to develop a new product across industries, healthcare innovations are typically at the higher cost end. While the low-range may be $100K for an App and a few $Million for a consumer device, it now costs about $2 Billion to bring a new drug to the market [4]. In 2010, medical devices typically cost between $31 Million for those that require a 510(k) and $94 Million for higher-risk devices requiring the PMA regulatory pathway (excluding reimbursement and sales/marketing activities) [23]. As such, more resources, both quantity and magnitude are typically needed for a healthcare innovation to survive the Valley of Death. Investors with smaller dollars are therefore less inclined to invest due to the potentially negative impact that later stage investments can have on the value of their investment.
C. Distinguishing Features of an Effective HCP
Below are some key distinguishing features and attributes the authors suggest to build on the I-Corps fundamentals to address these healthcare specific issues in constructing and conducting an effective HCP:
-
1.
Team Composition: Teams need to include the perspectives and proactive engagement from the clinical as well as the technical and business environments. While all team members need to share work and responsibilities, the person with the business/entrepreneurial focus needs to take the lead in coordinating the team’s efforts as well as organize the outreach and implementation of stakeholder interviews. Clinicians are critical team members, and being time efficient is crucial to enable their participation. This approach differs from I-Corp that has fixed team member roles and only includes a technical and entrepreneurial lead along with an industry mentor supplied by the team. Successful implementation requires that each team member understands and is prepared to put in the time required, a total of about 40 hours/team/week. Senior faculty members should be discouraged from taking a team member slot if they intend to just oversee the work.
-
2.
Executive Faculty: Given the inter-related complexities associated with healthcare innovations and need to balance multiple trade-off’s simultaneously to effectively de-risk projects, the authors urge that the HCP faculty presenting materials be healthcare industry experts – and use healthcare examples. In particular, rather than having team-supplied mentors, the HCP should be responsible for supplying qualified, experienced industry veterans to provide teams with consistent feedback and guidance. To be cost effective, the same individuals can deliver most of the program’s customized content as well as support teams. They need to allocate significant time, typically ~4 hours per week/team. It is important that in addition to the work they each do with a specific team that they work as group in providing regular synthesized feedback to teams. While increasing the cost, having experienced executives with a diversity of backgrounds (technical, regulatory, operational, marketing, manufacturing, etc.) working together helps teams understand trade-offs and make better decisions to efficiently and cost effectively de-risk projects. A key to successful implementation is finding and recruiting these individuals, which is a challenge. The authors suggest tapping into existing entrepreneurial networks – locally or nationally – to get access to people with the diversity of skills needed.
-
3.
Content: The content used in HCPs should be designed to help teams address the specific challenges facing healthcare innovations. It should cover the complex buying dynamics, reimbursement complexities, practice workflow implications, regulatory consequences and constraints in addition to traditional entrepreneurial topics. The author’s experience is that there is enough difference between Pharma/Biotech and HealthTech, both in terms of content and the expertise of faculty and industry experts, to justify separate programs. Fortunately, there is a great deal of content already freely available. For example, the Coulter Foundation and Stanford BioDesign have well developed, publically available HealthTech content for reference, so there is little need to create new content. We suggest curating a repository using the participants’ feedback that can be accessed by team members as a reference library, not only during but also after the program.
-
4.
Style: Like I-Corps, teams need frank, objective feedback. But unlike the rough treatment that is part of the I-Corps pedagogy, faculty must also develop strong rapport with the team members. They should be seen as being part of the team and not as outside assessors. Keeping objective metrics (like the number of interviews) visible keeps pressure on teams and engenders some inter-team competition. Team-team interactions are encouraged and generate some of the key learning opportunities by seeing the challenges and mistakes of other teams, as well as successful strategies they employ to overcome them.
-
5.
Ecosystem Leverage: In addition to the above ways an HCP should be conducted, the context in which an HCP is used can help address the funding challenges. The primary goal of the program is for each team to establish if they can deliver against a validated value proposition with a convincing “pitch” that articulates the narrative of a viable commercialization pathway – or conclude that a “Quick Kill” is appropriate. It is critical that teams start their journey into the Valley of Death with a convincing pitch, but even better if they have some resources as they start their journey. So, rather than conduct an HCP as a stand-alone activity at the end of a funding cycle (such as the I-Corps program after SBIR funding), the authors strongly encourage HCPs to be conducted as an integral part of an ecosystem or program, like the NCAI program, while its other resources are available to assist teams advance. This approach can significantly improve funding efficiency by applying the benefits of an HCP early, for example as a “Phase 0” award. This helps focus available resources on the teams with the highest likelihood of translational success and focus teams on tasks that are most needed to advance projects to commercially relevant, as well as technically and clinically important milestones.
V. Conclusions
HCPs offer institutions and funders an additional option to improve the efficiency with which the healthcare projects they support reach practice and improve people’s health. It offers a cost effective way for them to increase the likelihood of selecting and advancing projects to commercialization and patient impact while also helping build innovation capacity. They teach academic based investigators important skills in a learn-by-doing mode to enable them to contribute to entrepreneurial activities without attempting to turn them into entrepreneurs. While no single approach for HCPs is being advocated, the lessons learned synthesized from a diversity of programs can act as a guide for those interested in establishing a HCP to maximize the impact of their available resources.
Acknowledgment
The authors would like to acknowledge the support and guidance offered by the faculty at the NSF I-Corps Program, NHLBI and NIBIB as well as the many team members who participated in the programs and provided feedback to make them better. In particular, the experience and insights of the executive faculty of the CIMIT CRAASH Course, the Cleveland Clinical program and the Coulter Foundation’s C3i program made the programs possible. In addition, each reviewer added significant insights that we hope are reflected.
Biographies

John M. Collins received the B.S. degree in mechanical engineering from the Rensselaer Polytechnic Institute in 1980, and the M.S. and Ph.D. degrees in mechanical engineering from the Massachusetts Institute of Technology in 1982. He held a leadership position with TIAX LLC and Arthur D Little, Inc. He is currently the Chief Operating Officer with the Consortia for Improving Medicine with Innovation and Technology (CIMIT), and the consortium of the greater Boston area’s premier academic medical centers and universities along with a growing network of international affiliates. He is also a frequent Speaker. He holds over 20 U.S. patents. His focus is to advance CIMIT’s mission of improving patient care by facilitating collaboration among clinicians, researchers, and entrepreneurs to accelerate the healthcare innovation cycle for novel products, services, and procedures that improve healthcare.

Ofer Reizes received the Ph.D. degree in molecular pharmacology from the UT-Southwestern Medical Center, Dallas. He holds a post-doctoral fellowship with the Boston Children’s Hospital and the Harvard Medical School. In 2001, he was with Procter & Gamble Pharmaceuticals, Inc., to lead drug discovery efforts in obesity and metabolic diseases, progressing several obesity targets from early discovery to preclinical evaluation. In 2006, he joined the Cleveland Clinic Foundation, where he studies the interface between obesity and cancer. His research has been authored in Cell, the Journal of Clinical Investigation, and the Proceedings of the National Academy of Sciences. He has presented at multiple national and international meetings, including the Endocrine Society and the American Diabetes Association. He has consulted for several biotechnology companies. He is currently a Co-Inventor of a patent titled Methods and Reagents for Regulating Obesity. He is also the Director of Skills Development with the NIH Center for Accelerated Innovations, Cleveland Clinic Foundation.

Michael K. Dempsey received the B.S.E.E. degree from the University of Michigan. He is currently the Entrepreneur in residence with the Consortia for Improving Medicine with Innovation and Technology (CIMIT), the Director with the CIMIT Accelerator Program, the Co-Executive Director with the Center for Biomedical and Interventional Technology, Yale University, and a Faculty Member with MIT, where he lead academic innovators through the commercialization journey and teach students the fundamentals of building medical companies. He is an Interim CEO with CIMIT and Yale, where he leads a team of highly experienced med-tech executives who join the academic team with up to a full-time commitment and for as long as two years. He is also the PI on several NIH SBIR grants. He has dozens of patents on medical devices, has founded three companies, and has received a citation from the Commissioner of the FDA for exceptional initiative and leadership to protect the public health.
Funding Statement
This work was supported in part by the National Science Foundation under Award IIP-1450161 and Award 1450218 and the National Heart, Lung and Blood and Sleep Foundation under Grant 5 U54 EB015408 and Grant 1U54HL119810-01.
References
- [1].Christensen C. M. (Mar. 2011). A Disruptive Solution for Health Care. Harvard Business Review. [Online]. Available: https://hbr.org/2011/03/a-disruptive-solution-for-heal.html [Google Scholar]
- [2].Federation of American Societies for Experimental Biology. NIH Research Funding Trends, accessed on May 2, 2016. [Online]. Available: http://www.faseb.org/Science-Policy-and-Advocacy/Federal-Funding-Data/NIH-Research-Funding-Trends.aspx
- [3].Kurzweil R. The Law of Accelerating Returns, accessed on Mar. 7, 2001. [Online]. Available: http://www.kurzweilai.net/the-law-of-accelerating-returns [Google Scholar]
- [4].Scannell J. W., Blanckley A., Boldon H., and Warrington B., “Diagnosing the decline in pharmaceutical R&D efficiency,” Nature Rev. Drug Discovery, vol. 11, no. , pp. 191–200, Mar. 2012. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/22378269 [DOI] [PubMed] [Google Scholar]
- [5].Carew D. G. (Feb. 2013). Tracking Healthcare Cost. Progressive Policy Institute. [Online]. Available: http://www.progressivepolicy.org/wp-content/uploads/2013/02/02.2013_Carew_Tracking-Healthcare-Cost-Growth-Through-a-New-Measure-of-Productivity.pdf [Google Scholar]
- [6].Parrish J. A., Schachter S., Ford-Carleton P., Dempsey M., Spiliotis D., and Collins J. (Jan-Feb 2014). Accelerating the Innovation Cycle. IEEE Pulse. [Online]. Available: http://pulse.embs.org/january-2014/accelerating-the-innovation-cycle [DOI] [PubMed] [Google Scholar]
- [7].Beard T. R., Ford G. S., Koutsky T. M., and Spiwak L. J., “A valley of death in the innovation sequence: An economic investigation,” Oxford J., vol. 18, no. 5, pp. 343–356, 2009. [Google Scholar]
- [8].CB Insights. (Feb. 2016). 156 Startup Failure Post-Mortems. [Online]. Available: https://www.cbinsights.com/blog/startup-failure-post-mortem/
- [9].Urban Institute. The NIH Funding Crisis is Really a Biomedical Research Workforce Crisis, accessed on May 6, 2015. [Online]. Available: http://www.urban.org/urban-wire/nih-funding-crisis-really-biomedical-research-workforce-crisis
- [10].Ernst & Young. Pulse of the Industry: Medical Technology Report, Financing Trends: Mind the Gap, accessed on May 2, 2016. [Online]. Available: http://www.ey.com/US/en/Industries/Life-Sciences/Pulse-of-the-industry-medical-technology-report-2013-Financing-trends-reveal-growing-funding-gap
- [11].Ostrovsky A. and Barnett M., “Accelerating change: Fostering innovation in healthcare delivery,” Healthcare, vol. 2, no. 1, pp. 9–13, Mar. 2014. [DOI] [PubMed] [Google Scholar]
- [12].National Science Foundation. I-Corps NSF Innovation Corps. Retrieved From, accessed on Nov. 28, 2015. [Online]. Available: http://www.nsf.gov/news/special_reports/i-corps/about.jsp
- [13].Ledford H., “Biotech boot camp,” Nature, vol. 519, no. 7544, pp. 402–405, 2015. [DOI] [PubMed] [Google Scholar]
- [14].Coulter W. H. Coulter College, accessed on May 2, 2016. [Online]. Available: http://whcf.org/coulterfoundation-programs/translational-research/coulter-college/ [Google Scholar]
- [15].National Heart, Lung and Blood Institute. About NCAI and Reach, accessed on May 2, 2016. [Online]. Available: http://ncai.nhlbi.nih.gov/ncai/aboutncai/mission
- [16].University of California Center for Accelerated Innovation, accessed on May 2, 2016. [Online]. Available: http://uccai.ctsi.ucla.edu
- [17].Boston Biomedical Innovation Center. Boston Biomedical Innovation Consortium, accessed on May 2, 2016. [Online]. Available: http://www.b-bic.org
- [18].Consortia for Improving Medicine with Innovation and Technology. CIMIT: Center for Integration of Medicine & Innovative Technology, accessed on May 2, 2016. [Online]. Available: http://www.cimit.org
- [19].Cleveland Clinic. NHS Center for Accelerated Innovation at Cleveland Clinic, accessed on May 2, 2016. [Online]. Available: http://www.ncai-cc.ccf.org
- [20].Schachter S., Collins J., Dempsey M., Spiliotis D., and Parrish J., “Deep innovation in the medical domain a La boston’s CIMIT,” Venture Philanthropy, vol. 3, pp. 20–30, 2016. [Google Scholar]
- [21].Consortia for Improving Medicine with Innovation and Technology. CRAASH Course, accessed on May 2, 2016. [Online]. Available: http://cimitcolab.org/web/craash
- [22].Consortia for Improving Medicine with Innovation and Technology. CIMIT Accelerator Program, accessed on May 2, 2016. [Online]. Available: http://www.cimit.org/services-accelerator.html
- [23].Makower J. (2010). FDA Impact on U.S. Medical Technology Innovation: A Survey of Over 200 Medical Technology Companies. [Online]. Available: http://advamed.org/res.download/30 [Google Scholar]