Summary
Borrelia burgdorferi , the causative agent of Lyme disease (along with closely related genospecies), is in the deeply branching spirochete phylum. The bacterium is maintained in nature in an enzootic cycle that involves transmission from a tick vector to a vertebrate host and acquisition from a vertebrate host to a tick vector. During its arthropod sojourn, B. burgdorferi faces a variety of stresses, including nutrient deprivation. Here, we review some of the spirochetal factors that promote persistence, maintenance and dissemination of B. burgdorferi in the tick, and then focus on the utilization of available carbohydrates as well as the exquisite regulatory systems invoked to adapt to the austere environment between blood meals and to signal species transitions as the bacteria traverse their enzootic cycle. The spirochetes shift their source of carbon and energy from glucose in the vertebrate to glycerol in the tick. Regulation of survival under limiting nutrients requires the classic stringent response in which RelBbu controls the levels of the alarmones guanosine tetraphosphate and guanosine pentaphosphate (collectively termed (p)ppGpp), while regulation at the tick–vertebrate interface as well as regulation of protective responses to the blood meal require the two-component system Hk1/Rrp1 to activate production of the second messenger cyclic-dimeric-GMP (c-di-GMP).
Introduction
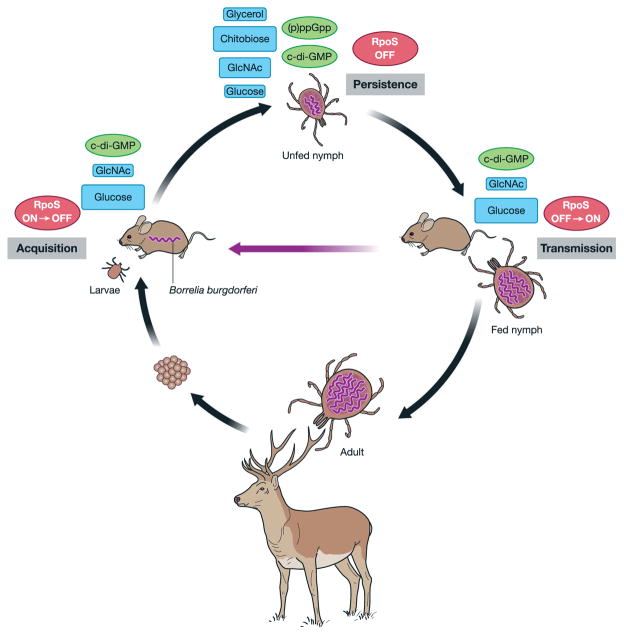
Several species of hard ticks in the genus Ixodes acquire and transmit the morphologically serpentine spirochetes Borrelia burgdorferi sensu lato as a crucial component of an enzootic cycle (Fig. 1) (Lane et al., 1991; Piesman and Schwan, 2010; Radolf et al., 2012). A few of these Borrelia genospecies, historically including B. burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii, cause Lyme disease (Burgdorfer et al., 1982; Benach et al., 1983; Steere et al., 1983), an emerging infection with a global distribution (Mead, 2015). Ixodes larvae acquire B. burgdorferi while feeding on infected vertebrates. Spirochetes reside in the midgut as larvae molt into nymphs, and then migrate to the salivary glands when the nymph feeds, at which point they transmit to the vertebrate host. Spirochetes migrate out of the midgut by, first, adhering to the midgut epithelium and, second, penetrating the intercellular junctions to access the hemocoel (Dunham-Ems et al., 2009). B. burgdorferi must persist in the nutrient-limited tick as well as evade the vertebrate and tick immune systems; these host-specific responses are associated with a sea change of gene expression (Samuels, 2011; Radolf et al., 2012; Iyer et al., 2015).
Fig. 1.
The enzootic cycle of B. burgdorferi. Acquisition: larval ticks must acquire B. burgdorferi by feeding on an infected vertebrate as the bacterium is not transovarially transmitted. Persistence: intracellular second messengers (p)ppGpp and c-di-GMP regulate persistence in the tick by various mechanisms, including modulation of carbohydrate preference, while the molecular gatekeeper RpoS is absent in unfed ticks. Transmission: following the molt into nymphs, infected ticks can transmit B. burgdorferi to uninfected hosts, completing the cycle. Adapted from Brisson et al. (2012). GlcNAc, N-acetyl glucosamine.
B. burgdorferi has a sparse set of metabolic pathways, but is flush with transporters to obtain nutrients from its environment (Fig. 2) (Fraser et al., 1997; Gherardini et al., 2010; Corona and Schwartz, 2015). Spirochete burdens within the larval midgut substantially increase in response to the nutrient-rich, digested blood meal; however, these nutrients are drained in a few weeks and the spirochetes have to manage for months post-molt until the nymphs feed on a second vertebrate (Sonenshine, 1991; Kung et al., 2013). The physiological capacity of B. burgdorferi is encoded on a relatively small, but exceedingly complex genome comprising a ~950-kb linear chromosome and a suite of ~20 to 25 unique linear and circular plasmids (lp’s and cp’s, respectively) ranging in size from 5 to 56 kb (Fraser et al., 1997; Brisson et al., 2012). The chromosome and plasmids carry several gene products required for persistence in the tick (Kenedy et al., 2012; Kung et al., 2013), including the outer membrane lipoproteins OspA, OspB and BptA as well as a transporter and enzymes involved in utilizing glycerol. The expression of the genes required during the tick phase of the enzootic cycle is predominantly controlled by three interacting regulatory systems: the RpoN–RpoS alternative sigma factor cascade (which was previously reviewed in Samuels, 2011; Radolf et al., 2012), the second messenger cyclic-dimeric-GMP (c-di-GMP) and the alarmones guanosine pentaphosphate and guanosine tetraphosphate ((p)ppGpp).
Fig. 2.
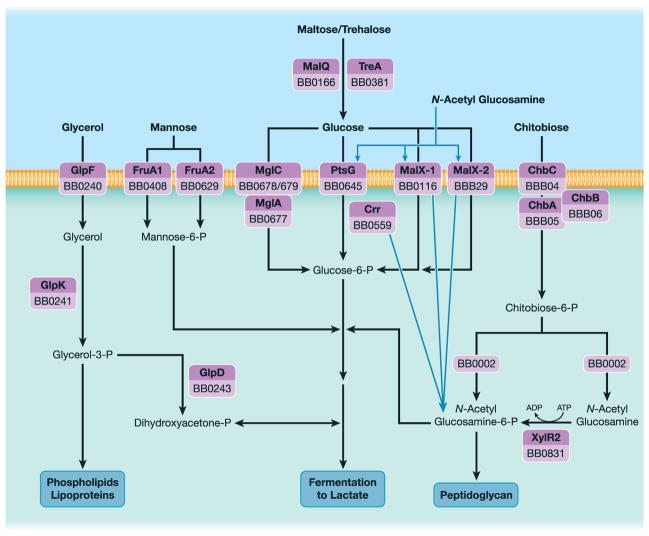
Schematic diagram of carbohydrate transport and metabolism based on functional studies and/or bioinformatics. Adapted from Corona and Schwartz (2015).
Gene products required in the tick
Several B. burgdorferi plasmids, including lp54, lp25 and lp28-4, have been implicated in infection and/or persistence in ticks. lp54, the 54-kb linear plasmid, encodes numerous differentially expressed lipoproteins, many of which are known to support spirochete infection in the vector (see below). lp25 also carries genes required for mouse and tick infection (reviewed in Kung et al., 2013): pncA (bbe22), which encodes a nicotinamidase that is essential for growth in mammals (Purser et al., 2003), and bptA (bbe16), which encodes a putative lipoprotein of unknown function that is required for persistence in ticks (Revel et al., 2005). Other genes on lp25 must be important, as lp25-deficient strains complemented with either pncA or both pncA and bptA are acquired by larvae at reduced levels and do not persist through intermolt (Gilmore et al., 2014). B. burgdorferi lacking lp28-4 also display a reduced survival in ticks and diminished tick-to-mouse transmission (reviewed in Kung et al., 2013).
Lipoproteins, referred to as outer surface proteins (Osps), are the most abundant component of the B. burgdorferi proteome (Kenedy et al., 2012). Many of these Borrelia-specific proteins are vital for persistence in, and transmission through, ticks via interaction(s) with host or vector factors (Kenedy et al., 2012; Kung et al., 2013). OspA and OspB (BBA15 and BBA16, respectively) are encoded by an operon on lp54 and synthesized primarily during the vector phase in vivo: they are dominant lipoproteins that have crucial roles in persistence within the tick. OspA interacts with TROSPA, a tick protein required for spirochete colonization of the gut epithelium (Pal et al., 2004a), and binds plasminogen (Fuchs et al., 1994). OspA is thought to protect spirochetes in the feeding tick gut from host-derived bactericidal antibodies (Battisti et al., 2008).
Less abundant B. burgdorferi surface-associated proteins also have been implicated in supporting survival in the vector or transmission through feeding ticks; however, in most cases, their biological function(s) have yet to be determined. Many of the genes encoding these proteins are carried on lp54 along with the ospAB operon. Expression of bba03 is increased in fed ticks, and BBA03-deficient spirochetes cannot compete with wild type to efficiently transmit to mice (Bestor et al., 2012). BBA07 and BBA52 are exposed on the spirochete surface; a bba07 mutant (Xu et al., 2010b) and a bba52 mutant (Kumar et al., 2010) can infect mice and persist in ticks, but both mutants are defective in tick-mediated transmission. bba57 encodes a putative outer membrane lipoprotein and is required for early murine infection and, potentially, transmission (Yang et al., 2013b). Another lp54 gene product, Lp6.6 (BBA62), is required as pathogens enter and survive in the tick vector; bba62 is one of the most highly expressed genes in engorged nymphs (with significantly higher expression in ticks than in the mammalian host-adapted state) (Iyer et al., 2015) and bba62 mutants are defective in transmitting to naïve hosts (Promnares et al., 2009). BBA64 (P35) has also been shown to be important in persistence and transmission (reviewed in Kenedy et al., 2012). Mutants in the gene encoding the surface-localized antigen BBA66 were infectious in mice by needle inoculation, but exhibited reduced spirochete burdens and pathology in joints; bba66 mutants are acquired by larvae and persist through intermolt, but are impaired in their ability to be transmitted by nymphs (Patton et al., 2013).
Other genomic elements carry genes involved in the tick phase of the enzootic cycle. OspC (BBB19) is a dominant lipoprotein encoded by the first borrelial gene mapped to a circular plasmid, cp26 (reviewed in Brisson et al., 2012); expression of ospC is induced during nymphal transmission (Schwan et al., 1995) and is required for early murine infection (Grimm et al., 2004; Pal et al., 2004b). Like OspA, it also binds plasminogen (Lagal et al., 2006; Önder et al., 2012), which could assist migration through the vector, although there is some controversy regarding the specific role of OspC in dissemination through tick tissues (Grimm et al., 2004; Pal et al., 2004b; Fingerle et al., 2007; Dunham-Ems et al., 2012). The outer surface gene product of bbe31, carried on lp25, binds to the tick protein TRE31 and promotes migration into the hemocoel during feeding (reviewed in Kung et al., 2013). Arthritis-related protein (Arp), encoded by bbf01 on lp28-1, is a target for antibody-mediated disease resolution in the mouse model and is antigenic in humans; mutants were compromised in transmission, suggesting that Arp supports survival or dissemination through ticks (Imai et al., 2013).
Several chromosomal genes aid spirochete survival and their gene products function, albeit not always exclusively, in the tick phase of the enzootic cycle. BmtA, encoded by bb0219, is a putative manganese transporter that is essential in both ticks and mammals (reviewed in Kung et al., 2013). Surface protein P66, encoded by bb0603, has integrin-binding and channel-forming activities (reviewed in Kenedy et al., 2012); p66 mutants survive through intermolt but are noninfectious in mice by tick transmission (Ristow et al., 2012). bb0405 encodes a surface-exposed transmembrane protein and mutants were unable to transmit from ticks to mice (Kung et al., 2016). The subsurface membrane protein LA7 (p22), encoded by bb0365, supports survival in the tick (Pal et al., 2008); LA7-deficient B. burgdorferi were severely impaired in their ability to persist in feeding and quiescent ticks during transmission and intermolt (Yang et al., 2013a). Dps/NapA/BicA (BB0690) is an ortholog of bacterioferritin that is uniquely fused to a copper-binding metallothionein-like domain (Wang et al., 2012). In other bacterial species, Dps (DNA-binding protein from starved cells) is found at high levels during stationary phase, when it protects DNA as part of the cellular response to starvation. However, in vitro assays fail to demonstrate either DNA binding or protection against oxidative damage by Dps/NapA/BicA from B. burgdorferi (Li et al., 2007). On the other hand, although dps/napA/bicA mutants are infectious in mammals and can colonize the tick midgut, they do not survive prolonged periods in unfed ticks (Li et al., 2007). Wang et al. (2012) propose that Dps/NapA/BicA sequesters excess metals throughout the enzootic cycle, but dps/napA/bicA is highly expressed in fed nymphs, with significantly higher expression in ticks than in the mammalian host (Li et al., 2007; Iyer et al., 2015), and the gene is repressed during recovery from starvation in vitro (Drecktrah et al., 2015).
Carbon utilization
B. burgdorferi, an extreme auxotroph, lacks genes encoding enzymes for the citric acid cycle and oxidative phosphorylation, deriving energy instead from the fermentation of sugars through glycolysis (Fraser et al., 1997; Gherardini et al., 2010). In vitro, the spirochetes are able to utilize a limited range of simple and complex carbohydrates for energy (von Lackum and Stevenson, 2005; Hoon-Hanks et al., 2012). These substrates enter the cell primarily via the phosphotransferase system (PTS) (Fraser et al., 1997; von Lackum and Stevenson, 2005; Corona and Schwartz, 2015), a multicomponent carbohydrate uptake system that couples sugar-specific transport across the cytoplasmic membrane with sugar phosphorylation. The borrelial genome encodes one Enzyme I (EI) component (bb0558) and two putative Histidine phosphocarrier protein (Hpr) paralogs (bb0448, bb0557) that form a phoshorelay system coupled to multiple membrane-associated, sugar-specific Enzyme II (EII) transporters (Fraser et al., 1997; Corona and Schwartz, 2015). Glucose, the preferred carbon source, is predicted to be imported by two glucose-/maltose-specific (BB0116/MalX-1 and BBB29/MalX-2) and/or the glucose-specific (BB0645/PtsG) PTS transporters and a non-PTS ABC-type transporter with homology to the Mgl system in Escherichia coli (Death and Ferenci, 1993; von Lackum and Stevenson, 2005; Gherardini et al., 2010) (Fig. 2). Glucose-6-phosphate is then funneled into glycolysis for energy generation or used as a substrate for synthesis of membrane phospholipids, lipoproteins and nucleic acids (Gherardini et al., 2010). Recently, Khajanchi et al. (2016) demonstrated that ptsG mutants are avirulent in mice by syringe inoculation, suggesting that spirochetes rely heavily on this transporter in vivo. In ticks, spirochetes must compete for blood meal-derived glucose with rapidly differentiating midgut epithelial cells that are avidly engulfing the blood meal. Consequently, spirochetes must modify their metabolism to take advantage of alternate carbon sources available within the midgut. Consistent with this notion, B. burgdorferi possesses systems for uptake and utilization of a number of alternate carbon sources (von Lackum and Stevenson, 2005; Gherardini et al., 2010; Corona and Schwartz, 2015), including glycerol (Glp) (Pappas et al., 2011), glucose disaccharides, like trehalose (MalQ and TreA) (Hoon-Hanks et al., 2012), and chitobiose (Chb), an N-acetyl glucosamine (GlcNAc) dimer derived from chitin, the major component of tick cuticle (Tilly et al., 2001; 2004; Rhodes et al., 2009; Sze et al., 2013). Glp-deficient organisms show significantly reduced survival within feeding ticks and, following the molt, strongly diminished capacity to transmit to mice via tick bite (He et al., 2011; Pappas et al., 2011; Caimano et al., 2015). Surprisingly, neither the Chb system nor MalQ is required for survival during the blood meal or tick-to-mammal transmission (Tilly et al., 2004; Hoon-Hanks et al., 2012). Given that glucose and β-glucoside transporters share considerable sequence and structural similarity (Saier et al., 1988; McCoy et al., 2015), one or more of the glucose transporters likely promote uptake of GlcNAc (Sze et al., 2013), an essential building block for peptidoglycan, or disaccharides. The genes encoding FruA2 (predicted to transport mannose), ChbC and MalX-2 are more highly expressed in ticks than in mammals (Iyer et al., 2015).
B. burgdorferi does not contain a homolog of cAMP receptor protein (also called catabolite activator protein), which is used by gram-negative bacteria to couple PTS-mediated sugar transport to gene regulation (catabolite repression) (Fraser et al., 1997). Instead, the spirochete has evolved novel mechanisms for regulating central carbohydrate metabolism through the enzootic cycle (Pappas et al., 2011).
Regulation by (p)ppGpp and c-di-GMP
Although repression of the alternative sigma factor RpoS allows for induction of important tick phase genes, like ospAB (reviewed in Samuels, 2011; Radolf et al., 2012), the purine signaling molecule c-di-GMP and stringent response alarmones (p)ppGpp are indispensible master regulators of B. burgdorferi biology in the tick (Fig. 1). Modulating the levels of these second messengers in response to external signals alters the transcriptional landscape, as well as many other cellular processes, adapting the spirochete to the challenges of a dynamic and hostile environment in the tick midgut (Caimano et al., 2011; 2015; He et al., 2011; Kostick et al., 2011; Sultan et al., 2011; Bugrysheva et al., 2015; Drecktrah et al., 2015). Classically, (p)ppGpp synthesis is triggered by an accumulation of uncharged tRNAs during nutrient stress, but a shortage of carbon, iron, phosphate or fatty acids can also induce the stringent response (reviewed in Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Hauryliuk et al., 2015). (p)ppGpp alters RNA polymerase activity both directly, by binding to the initiating holoenzyme, and indirectly, through sigma factor competition, to alter the transcriptome, including rRNA levels (reviewed in Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Hauryliuk et al., 2015). In B. burgdorferi, the bifunctional enzyme RelBbu adjusts (p)ppGpp levels in response to nutrient stress, although the specific extracellular signals remain to be identified (Bugrysheva et al., 2003; Concepcion and Nelson, 2003; Drecktrah et al., 2015).
relBbu mutants are unable to synthesize (p)ppGpp (Bugrysheva et al., 2005; Drecktrah et al., 2015) and are compromised for persistence in the tick between blood meals and/or during nymph feeding, but retain the ability to infect mice (Drecktrah et al., 2015). The (p)ppGpp-dependent transcriptome, determined using a relBbu mutant, included a number of persistence-related genes induced by the stringent response, such as the glycerol uptake and metabolism (glp) operon (Bugrysheva et al., 2015; Drecktrah et al., 2015). The glp operon is induced in ticks and by glycerol in vitro; survival of glp mutants is compromised in the tick, highlighting the importance of glycerol as a signal and carbon source in this part of the enzootic cycle (He et al., 2011; Pappas et al., 2011). Glycerol presumably enters the cytoplasm through the glycerol uptake facilitator (GlpF) and is phosphorylated by a putative glycerol kinase (GlpK) to produce glycerol-3-phosphate. Glycerol-3-phosphate may be either used in the biosynthesis of phospholipids and lipoproteins or shuttled to glycolysis via conversion to dihydroxyacetone phosphate by a putative glycerol-3-phosphate dehydrogenase (GlpD) (Fraser et al., 1997; Corona and Schwartz, 2015). A number of studies indicate that the glp genes (bb0240–bb0243) form an operon (He et al., 2011; Pappas et al., 2011; Bugrysheva et al., 2015; Caimano et al., 2015), although (p)ppGpp uniquely affects expression of the constituent genes: glpF and glpK are induced while glpD is repressed (Drecktrah et al., 2015). Thus, the stringent response may direct glycerol toward production of phospholipids and lipoproteins instead of glycolysis, which would require GlpD. In addition, (p)ppGpp upregulates other genes encoding proteins required for persistence in the tick: OspA, Dps/NapA/BicA and PncA (Drecktrah et al., 2015).
The Hk1/Rrp1 two-component system (TCS) consists of a membrane-bound hybrid histidine kinase and a cytoplasmic response regulator with diguanlyate cyclase activity (Caimano et al., 2011; 2015; He et al., 2011; Kostick et al., 2011). The Hk1 periplasmic sensor consists of three ligand-binding domains, each with homology to ABC transporter periplasmic solute-binding proteins (Bauer et al., 2015). Analyses of the Hk1 sensor point to amino acids and/or their derivatives as potential activating ligands (Bauer et al., 2015). Upon ligand binding, Hk1 is thought to mediate a signal transduction cascade that culminates in phosphorylation of Rrp1. In turn, phosphorylated Rrp1 catalyzes the synthesis of c-di-GMP (Ryjenkov et al., 2005), a ubiquitous bacterial second messenger associated with a wide range of lifestyle control networks, including the transition from planktonic to sessile states, biofilm formation, cell cycle progression, and virulence (reviewed in Römling et al., 2013). In contrast to the RpoN/RpoS pathway, which activates genes required during the vertebrate host phase (Samuels, 2011; Radolf et al., 2012), the Hk1/Rrp1 TCS functions exclusively during the tick phase of the enzootic cycle: spirochetes lacking either Hk1 or Rrp1 are virulent in mice but rapidly destroyed within feeding larvae and nymphs (Caimano et al., 2011; He et al., 2011; Kostick et al., 2011).
Transcriptomic analyses of an rrp1 mutant grown in vitro suggest that, during tick feeding, c-di-GMP promotes utilization of alternate carbon sources for glycolysis and biosynthesis of phospholipids and peptidoglycan (Rogers et al., 2009; He et al., 2011; Caimano et al., 2015). Most notably, c-di-GMP is required for expression of the glp operon via PlzA (Caimano et al., 2015), the only c-di-GMP effector protein identified to date in B. burgdorferi (Freedman et al., 2010; Pitzer et al., 2011; He et al., 2014). In addition, c-di-GMP upregulates the expression of genes encoding known or putative PTS transporters for GlcNAc (MalX-2) and chitobiose (ChbC), presumably to take advantage of copious amounts of GlcNAc and chitobiose secreted by tick midgut epithelial cells for the formation of the peritrophic membrane and cuticle (Sonenshine, 1991). Loss of c-di-GMP also results in reduced expression of AckA, the acetate kinase required for generation of acetyl phosphate, which gives rise to acetyl-CoA, the starting point for synthesis of peptidoglycan by way of the mevalonate pathway (Xu et al., 2010a; Van Laar et al., 2012). Presumably, the dramatic phenotypes displayed by hk1 and rrp1 mutants within feeding ticks stem, at least in part, from simultaneous dysregulation of both carbon metabolism and cell envelope biogenesis (Sze et al., 2013; Caimano et al., 2015).
In addition to its role in carbon metabolism, c-di-GMP also upregulates the expression of multiple genes encoding outer surface lipoproteins, including several OspE/BbCRASP paralogs, which have been shown to inhibit complement-mediated lysis by binding factor H and related proteins (reviewed in Kraiczy and Stevenson, 2013). Other surface-associated lipoproteins within the Hk1/Rrp1 regulon (such as the Mlps) may help to protect spirochetes from potentially borreliacidal molecules (including antimicrobial peptides and reactive oxygen species) encountered within the midgut during tick feeding (Caimano et al., 2015).
These two nucleotide messengers converge on common pathways important for persistence, such as glycerol utilization, indicating there is likely some degree of coordination. While there is no known direct link between (p)ppGpp and c-di-GMP, a closer examination of their metabolic pathways indicates shared substrates and products. Both enzymes are in competition for the common substrate GTP; thus, an increase in the synthetic activity of RelBbu or Rrp1 may decrease the ability of the other enzyme to produce its respective messenger. Alternatively, pyrophosphate (PPi) released during c-di-GMP synthesis could potentially increase (p)ppGpp levels by product inhibition of RelBbu activity, as PPi is formed when (p)ppGpp is hydrolyzed. Thus, c-di-GMP and (p)ppGpp levels could vary inversely (or cooperatively) depending on the intracellular conditions.
Conclusions and perspectives
Borreliologists and medical entomologists have deciphered many of the molecular mechanisms wielded by the spirochete to adapt to its vector and host, as well as to be maintained in its natural enzootic cycle, which requires being transmitted from a feeding tick to a naïve host and being acquired from an infected vertebrate by a naïve vector. We have described some gene products, including those involved in carbon utilization, and how they are regulated so that B. burgdorferi can survive in the tick between blood meals. Perhaps surprisingly, as Borrelia is not known for doing business as usual in model organisms (Samuels and Radolf, 2009), the spirochete takes advantage of the stringent response, the classic bacterial response to nutrient deprivation.
Many, if not most, of the B. burgdorferi gene products involved in tick infectivity display little to no homology to proteins of known function. Therefore, the biological significance of the vast majority of these factors, especially their mechanistic role in spirochete survival in the vector or transmission between species, is enigmatic. There remains much to learn regarding the unexpected regulation of individual genes in the glp operon as well as the coordination of metabolic pathways as the spirochete cycles back and forth between vector and host. We sincerely hope that this Microreview provides the background, and a conceptual framework, for further research on the interaction of B. burgdorferi with its tick vector.
Acknowledgments
We apologize to authors whose studies were not cited in this review because of space limitations. We thank Ira Schwartz and Bob Gilmore for thoughtful review of the manuscript, and Utpal Pal, Justin Radolf and Ira Schwartz for useful discussions. Studies in our laboratories have been supported by National Institutes of Health grants AI051486 (to D.S.S.), AI88131 (to D.D. and D.S.S.), AI29735 (to Justin D. Radolf and M.J.C.), and AI080615 (to Utpal Pal) as well as an American Heart Association grant 16GRNT27740039 (to M. J.C.). The authors declare no conflict of interest.
References
- Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer WJ, Luthra A, Zhu G, Radolf JD, Malkowski MG, Caimano MJ. Structural characterization and modeling of the Borrelia burgdorferi hybrid histidine kinase Hk1 periplasmic sensor: a system for sensing small molecules associated with tick feeding. J Struct Biol. 2015;192:48–58. doi: 10.1016/j.jsb.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Bast TF, Habicht GS, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bestor A, Rego ROM, Tilly K, Rosa PA. Competitive advantage of Borrelia burgdorferi with outer surface protein BBA03 during tick-mediated infection of the mammalian host. Infect Immun. 2012;80:3501–3511. doi: 10.1128/IAI.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J, Dobrikova EY, Sartakova ML, Caimano MJ, Daniels TJ, Radolf JD, et al. Characterization of the stringent response and relBbu expression in Borrelia burgdorferi. J Bacteriol. 2003;185:957–965. doi: 10.1128/JB.185.3.957-965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Bryksin AV, Godfrey HP, Cabello FC. Borrelia burgdorferi rel is responsible for generation of guanosine-3′-diphosphate-5′-triphosphate and growth control. Infect Immun. 2005;73:4972–4981. doi: 10.1128/IAI.73.8.4972-4981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Pappas CJ, Terekhova DA, Iyer R, Godfrey HP, Schwartz I, Cabello FC. Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One. 2015;10:e0118063. doi: 10.1371/journal.pone.0118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, et al. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun. 2015;83:3043–3060. doi: 10.1128/IAI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion MB, Nelson DR. Expression of spoT in Borrelia burgdorferi during serum starvation. J Bacteriol. 2003;185:444–452. doi: 10.1128/JB.185.2.444-452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona A, Schwartz I. Borrelia burgdorferi: carbon metabolism and the tick-mammal enzootic cycle. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.MBP-0011-2014. MBP-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux ZD, Swanson MS. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- Death A, Ferenci T. The importance of the binding-protein-dependent Mgl system to the transport of glucose in Escherichia coli growing on low sugar concentrations. Res Microbiol. 1993;144:529–537. doi: 10.1016/0923-2508(93)90002-j. [DOI] [PubMed] [Google Scholar]
- Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog. 2015;11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, Radolf JD. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012;8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle V, Goettner G, Gern L, Wilske B, Schulte-Spechtel U. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int J Med Microbiol. 2007;297:97–107. doi: 10.1016/j.ijmm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, Marconi RT. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol Med Microbiol. 2010;58:285–294. doi: 10.1111/j.1574-695X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F, Boylan J, Lawrence K, Skare J. Metabolism and physiology of Borrelia. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 103–138. [Google Scholar]
- Gilmore RD, Brandt KS, Hyde JA. pncA and bptA are not sufficient to complement Ixodes scapularis colonization and persistence by Borrelia burgdorferi in a linear plasmid lp25-deficient background. Infect Immun. 2014;82:5110–5116. doi: 10.1128/IAI.02613-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, et al. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang JJ, Ye M, Lou Y, Yang XF. Cyclic di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi. Infect Immun. 2014;82:445–452. doi: 10.1128/IAI.01238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon-Hanks LL, Morton EA, Lybecker MC, Battisti JM, Samuels DS, Drecktrah D. Borrelia burgdorferi malQ mutants utilize disaccharides and traverse the enzootic cycle. FEMS Immunol Med Microbiol. 2012;66:157–165. doi: 10.1111/j.1574-695X.2012.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai D, Holden K, Velazquez EM, Feng S, Hodzic E, Barthold SW. Influence of arthritis-related protein (BBF01) on infectivity of Borrelia burgdorferi B31. BMC Microbiol. 2013;13:100. doi: 10.1186/1471-2180-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D, Jr, Corona A, Iacobas DA, et al. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol. 2015;95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Lenhart TR, Akins DR. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol. 2012;66:1–19. doi: 10.1111/j.1574-695X.2012.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajanchi BK, Odeh E, Gao L, Jacobs MB, Philipp MT, Lin T, Norris SJ. Phosphoenolpyruvate phosphotransferase system components modulate gene transcription and virulence of Borrelia burgdorferi. Infect Immun. 2016;84:754–764. doi: 10.1128/IAI.00917-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P, Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: structure, function and regulation of gene expression. Ticks Tick Borne Dis. 2013;4:26–34. doi: 10.1016/j.ttbdis.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis. 2010;201:1084–1095. doi: 10.1086/651172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung F, Anguita J, Pal U. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol. 2013;8:41–56. doi: 10.2217/fmb.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung F, Kaur S, Smith AA, Yang X, Wilder CN, Sharma K, et al. A Borrelia burgdorferi surface-exposed transmembrane protein lacking detectable immune responses supports pathogen persistence and constitutes a vaccine target. J Infect Dis. 2016;213:1786–1795. doi: 10.1093/infdis/jiw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lackum K, Stevenson B. Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol Lett. 2005;243:173–179. doi: 10.1016/j.femsle.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lagal V, Portnoï D, Faure G, Postic D, Baranton G. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 2006;8:645–652. doi: 10.1016/j.micinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, Eggers CH, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007;63:694–710. doi: 10.1111/j.1365-2958.2006.05550.x. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Levin EJ, Zhou M. Structural insight into the PTS sugar transporter EIIC. Biochim Biophys Acta. 2015;1850:577–585. doi: 10.1016/j.bbagen.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Önder Ö, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, Brisson D. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem. 2012;287:16860–16868. doi: 10.1074/jbc.M111.290775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, de Silva AM, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004a;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004b;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Dai J, Li X, Neelakanta G, Luo P, Kumar M, et al. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J Infect Dis. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7:e1002102. doi: 10.1371/journal.ppat.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton TG, Brandt KS, Nolder C, Clifton DR, Carroll JA, Gilmore RD. Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission. Infect Immun. 2013;81:2488–2498. doi: 10.1128/IAI.00140-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Schwan TG. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 251–278. [Google Scholar]
- Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. Analysis of the Borrelia burgdorferi cyclic-di-GMP-binding protein PlzA reveals a role in motility and virulence. Infect Immun. 2011;79:1815–1825. doi: 10.1128/IAI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Promnares K, Kumar M, Shroder DY, Zhang X, Anderson JF, Pal U. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol Microbiol. 2009;74:112–125. doi: 10.1111/j.1365-2958.2009.06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel AT, Blevins JS, Almazán C, Neil L, Kocan KM, de la Fuente J, et al. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci U S A. 2005;102:6972–6977. doi: 10.1073/pnas.0502565102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RG, Coy W, Nelson DR. Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol. 2009;9:108. doi: 10.1186/1471-2180-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, et al. The β3-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol Microbiol. 2012;85:1105–1118. doi: 10.1111/j.1365-2958.2012.08160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Yamada M, Erni B, Suda K, Lengeler J, Ebner R, et al. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988;2:199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Samuels DS, Radolf JD. Who is the BosR around here anyway? Mol Microbiol. 2009;74:1295–1299. doi: 10.1111/j.1365-2958.2009.06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. New York: Oxford University Press; 1991. p. 447. [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, Motaleb MA. Analysis of the HD-GYP domain cyclic-di-GMP phosphodiesterase reveals a role in motility and enzootic life cycle of Borrelia burgdorferi. Infect Immun. 2011;79:3273–3283. doi: 10.1128/IAI.05153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, Li C. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect Immun. 2013;81:1775–1787. doi: 10.1128/IAI.00050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Elias AF, Errett J, Fischer E, Iyer R, Schwartz I, et al. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J Bacteriol. 2001;183:5544–5553. doi: 10.1128/JB.183.19.5544-5553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Grimm D, Bueschel DM, Krum JG, Rosa P. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 2004;4:159–168. doi: 10.1089/1530366041210738. [DOI] [PubMed] [Google Scholar]
- Van Laar TA, Lin YH, Miller CL, Karna SLR, Chambers JP, Seshu J. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PLoS One. 2012;7:e38171. doi: 10.1371/journal.pone.0038171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Lutton A, Olesik J, Vali H, Li X. A novel iron- and copper-binding protein in the Lyme disease spirochaete. Mol Microbiol. 2012;86:1441–1451. doi: 10.1111/mmi.12068. [DOI] [PubMed] [Google Scholar]
- Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, et al. Role of acetylphosphate in activation of the Rrp2–RpoN–RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010a;6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, He M, He JJ, Yang XF. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect Immun. 2010b;78:2910–2918. doi: 10.1128/IAI.00372-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hegde S, Shroder DY, Smith AA, Promnares K, Neelakanta G, et al. The lipoprotein La7 contributes to Borrelia burgdorferi persistence in ticks and their transmission to naïve hosts. Microbes Infect. 2013a;15:729–737. doi: 10.1016/j.micinf.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Qin J, Promnares K, Kariu T, Anderson JF, Pal U. Novel microbial virulence factor triggers murine Lyme arthritis. J Infect Dis. 2013b;207:907–918. doi: 10.1093/infdis/jis930. [DOI] [PMC free article] [PubMed] [Google Scholar]