Abstract
Optimizing antipsychotic pharmacotherapy is often challenging due to significant variability in effectiveness and tolerability. Genetic factors influencing pharmacokinetics and pharmacodynamics may contribute to some of this variability. Research studies have characterized these pharmacogenetic relationships, and some genetic markers are now available as clinical tests. These advances in pharmacogenetics research and test availability have great potential to improve clinical outcomes and quality of life in psychiatric patients. For clinicians considering using pharmacogenetics, it is important to understand the clinical implications and also the limitations of markers included in currently available tests. This review focuses on pharmacokinetic and pharmacodynamic gene variants that are currently available in commercial genetic testing panels. Associations of these variants with clinical efficacy and adverse effects, as well as other clinical implications, in antipsychotic pharmacotherapy are discussed.
Keywords: antipsychotics, pharmacodynamics, pharmacogenetics, pharmacokinetics, polymorphism, pychosis
Abstract
Con frecuencia constituye un desafío optimizar la farmacoterapia antipsicótica debido a la significativa variabilidad en la eficacia y tolerabilidad. Los factores genéticos que influyen en la farmacocinética y farmacodinámica pueden contribuir en parte a esta variabilidad. Los estudios de investigación han caracterizado estas relaciones farmacogenéticas y actualmente existen algunos marcadores genéticos como pruebas clínicas. Estos avances en la investigación farmacogenética y la disponibilidad de pruebas tienen un gran potencial para mejorar los resultados clínicos y la calidad de vida en los pacientes psiquiátricos. Para los clínicos que consideren el empleo de la farmacogenética es importante que tengan en cuenta las consecuencias clínicas y también las limitaciones de los marcadores con las pruebas disponibles en la actualidad. Esta revisión se focaliza en la farmacocinética y la farmacodinámica de las variantes génicas que están actualmente disponibles en paneles comerciales de pruebas genéticas. Se discuten las asociaciones de estas variantes con la eficacia clínica y los efectos adversos, como también otras repercusiones clínicas en la farmacoterapia antipsicótica.
Abstract
La variabilité significative de l'efficacité et de la tolérance des traitements antipsychotiques rend leur optimisation souvent difficile. Cette variabilité peut être due en partie à des facteurs génétiques influant sur la pharmacocinétique et la pharmacodynamique. Des études scientifiques ont caractérisé ces relations pharmacogénétiques, et certains marqueurs génétiques sont maintenant disponibles sous forme de tests cliniques. Ces avancées (recherche pharmacogénétique et disponibilité des tests) peuvent améliorer considérablement les résultats cliniques et la qualité de vie des patients psychiatriques. Les médecins s'intéressant à la pharmacogénétique doivent comprendre les implications cliniques et les limites des marqueurs des tests actuellement disponibles. Cet article s'intéresse principalement à la pharmacocinétique et à la pharmacodynamique des variants géniques proposés aujourd'hui dans les différents tests génétiques commercialisés. Nous analysons dans la pharmacothérapie antipsychotique la relation de ces variants avec l'efficacité clinique et les effets indésirables, ainsi que d'autres implications cliniques.
Introduction
Antipsychotics were first introduced in the 1950s to treat psychotic disorders and are now also used in the treatment of other psychiatric conditions. Although antipsychotic medications often need to be used long-term, 1-year discontinuation rates are 32% to 74%, which illustrates a need to improve these treatments.1,2 Treatment discontinuation can be partially explained by inadequate response and intolerability. For many patients, treatment selection remains a “trial-and-error” process, requiring multiple medication changes and dose adjustments to achieve a safe and effective balance between therapeutic response and tolerability. The widely observed variability among patients with identical treatment regimens results from multiple factors that can alter both drug metabolism (pharmacokinetics) and drug action (pharmacodynamics). A wide range of clinical and demographic factors (eg, age, sex, ethnicity, disease severity, diet, tobacco/alcohol use, and concurrent medications) can influence variability in both therapeutic and adverse event outcomes. Additionally, genetic variability can contribute to inherited differences in drug action and metabolism, and there is a growing need to understand how to consider this information in clinical settings. To date, pharmacogenetic research related to antipsychotic treatment has identified numerous polymorphisms across multiple genes involved in both antipsychotic pharmacokinetics and pharmacodynamics, culminating in the availability of commercially available pharmacogenetic tests within the United States and other countries.3 Some antipsychotic agents (eg, aripiprazole, brexpiprazole, iloperidone, and pimozide) have “actionable” pharmacogenetic language listed on their product labels, but guidelines of how best to interpret and apply this information are lacking. The Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG) are working on guidelines for applying or interpreing pharmacogenetic information in clinical practice. The European Pharmacogenetics Implementation Consortium (Eu-PIC) is also working to integrate pharmacogenetic information into clinical care; however, presently no CPIC and Eu-PIC guidelines and only a few DPWG guidelines (for aripiprazole, risperidone, and haloperidol) exist for antipsychotic medications.4 It is therefore important to understand the clinical implications and limitations of pharmacogenes that are now accessible for clinical use.
Pharmacogenetic testing in psychiatry is not yet a standard of practice, but its utilization is increasing and may play a role in advancing a precision-medicine approach through optimizing antipsychotic treatment and dosing strategies. However, its clinical utility remains an ongoing area of investigation. Understanding the clinical implications of these tests requires clinicians to understand of the underlying biology of pharmacogenetic variants, as well as their associations with treatment outcomes. This review focuses on genetic markers relevant to antipsychotic therapy known to be currently available on pharmacogenetic test panels. We identified clinically available pharmacogenetic test panels (CAPTPs) from the Genetic Test Registry5 as well as more broadly through Internet search queries for “pharmacogenetic test” or “pharmacogenomic test.” Testing labs or panels identifying either specific antipsychotic drugs or, more broadly, psychiatric conditions, were examined for genes and variants therein. Whereas our paper elaborates more detailed information on commercially available pharmacogenetic tests, the paper by Eap in this issue (p 313) has a stronger focus on their clinical application.
Pharmacogenetic factors influencing antipsychotic pharmacokinetics
Pharmacogenetic research has traditionally focused on genetic markers involved in the metabolism of antipsychotic medications through hepatic enzyme pathways, primarily by cytochrome P450 (CYP) enzymes. Genetic variation within drug-metabolizing enzymes may result in altered metabolic activity, which may affect pharmacokinetic parameters of antipsychotics. Among the multiple CYP enzymes in the liver, the CYP1, CYP2, and CYP3 families are the most relevant for the biotransformation of antipsychotic drugs and were identified as central to many psychiatric-focused pharmacogenetic test panels.
Genetic variants in metabolism enzymes often result in functional activity changes used to define or predict drug metabolizer phenotypes. Polymorphisms in drug metabolism genes that may affect function include single-nucleotide polymorphisms (SNPs), as well as gene duplications or deletions, small insertions/deletions, or larger copy number variants that affect gene expression or protein conformation. The National Center for Biotechnology Information (NCBI) Single-Nucleotide Polymorphism Database (dbSNP), a public repository for SNP data, assigns unique reference SNP identifiers (rs number) for each variant. For drug-metabolizing enzyme genes, combinations of these polymorphisms are used to define alleles, which historically have a star “*” designation. Diplotypes (star allele combinations, eg, CYP2D6 *l/*4) are then used for assignment of genetically defined metabolizer groups, which are generally classified into four main categories. “Extensive/ normal metabolizers (EMs/NMs)” display normal enzyme activity, characterized by usually either two normal functional alleles or one normal and one partly reduced-function allele. “Intermediate metabolizers (IMs)” display partly reduced enzyme activity, characterized by one decreased-function and one no-function allele or one normal-function allele and a decreased- or no-function allele.6 “Poor metabolizers (PMs)” display little or no enzyme activity, characterized by two nonfunctional alleles. Lastly, “rapid and ultrarapid metabolizers (UMs)” display increased enzyme activity, characterized by multiple copies of functional alleles or alleles with greater-than-normal activity. The allele combinations for the classification of IMs are gene-specific. The following section reviews some of the clinically relevant aspects of pharmacogenetics related to antipsychotic drug metabolism (Table I).
TABLE I. Common pharmacokinetic gene variants included in clinical testing panels for antipsychotic therapy. *Designation for allele; CYP, cytochrome P450; EM, extensive metabolizer; NM, normal metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; UM, ultrarapid metabolizer.
| Gene | Alleles commonly tested | Enzyme activity | Predicted phenotype | |
| *1, *2, *2A, *35 | Active | EM/NM: (1) Two active alleles (2) One active and one partially active allele | ||
| *9, *10, *17, *29 *36, *41 | Partially active | IM: (1) One active and one inactive allele (2) One inactive and one partially active allele (3) Two partially active alleles | ||
| CYP2D6 | *3, *4, *5, *6, *7, *8, *11, *12, *14, *15 | Inactive | ||
| Gene duplications | PM: Two inactive alleles UM: Three or more active alleles (active allele duplication) | |||
| *1A | Active | |||
| CYP1A2 | *1E, *1J, *1K, *4, *6, *7, *8, *11, *15, *16 | Partially active or inactive | Not uniformly/globally established | |
| *1F | Higher inducibility | |||
| CYP3A4 | *1 | Active | Not uniformly/globally established | |
| *13, *15A, *17, *22 | Inactive | |||
| CYP3A5 | *1 | Active | Not uniformly/globally established | |
| *3 | Inactive |
CYP2D6
CYP2D6 is an important hepatic enzyme that serves as the primary metabolic pathway for many antipsychotics (Tabl II).4,7,8-19 CYP2D6 activity greatly affects the biotransformation of its substrates; therefore, increases or decreases in CYP2D6 function influence pharmacokinetics and potentially impact dose-related outcomes.17
More than 1400 variants have been identified in CYP2D6 to date.20 CYP2D6*1, *2, *33, and *35 are classified as active alleles. CYP2D6 alleles *9, *10, *17, *29, *36, and *41 cause decreased enzyme activity as a result of decreased gene expression (*9, *41) or altered protein conformation (*10, *17, *36).18,19 The most common inactive allele in European populations, CYP2D6*4, has been identified with frequencies ranging from 0% in East Asian to 19% in white European populations. Other loss-of-function alleles include CYP2D6*3, *5-8, *11-16, *19-21, *38, *40, and *42. The CYP2D6 PM phenotype has been observed in approximately 7% to 10% of whites, 6% of Asians, and 3% to 8% of African-Americans.21 One unique structural characteristic of CYP2D6 is the presence of duplications or deletions of the whole gene. Deletion of the CYP2D6 gene results in the aforementioned *5 allele, whereas duplication of the gene results in an UM phenotype (*2XN), provided that functional versions of CYP2D6 are duplicated. The frequency of the UM phenotype was found to be 0% to 2% in East Asian, 1% to 10% in white, and up to 29% in some Eastern African populations.22,23
Fifteen currently approved antipsychotics are major or minor substrates of CYP2D6, with seven medications mentioning the effects of metabolizer status on pharmacokinetic parameters in the product labeling (Table II). The drug-labeling language referencing the relevance of metabolizer status for antipsychotics is broad, ranging from general comments to specific implications on dosing, particularly for newer medications. Aripiprazole, brexpiprazole, iloperidone, and pimozide labeling all mention specific dose adjustments for known CYP2D6 PMs (see Table II). DPWG guidelines also recommend dose changes for aripiprazole, risperidone, and haloperidol in CYP2D6 PMs,4 but no CPIC guidelines, at the time of writing, exist for antipsychotics.
TABLE II. Drug metabolism pathways, labeling, and guideline information, and drug exposure (AUC) implications of pharmacogenetic information in antipsychotic medications. Bold font, major metabolism pathway; *Recommendation is based on haloperidol plasma levels studies.4,17Percent Change in AUC: AUC in PM - AUC in EM/AUC in EM*100 AUC, area under the concentration-time curve; CPIC, Clinical Pharmacogenetics Implementation Consortium; DPWG, Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group; EM, extensive metabolizer; FDA, US Food and Drug Administration; N/A, not available; NS, nonsignificant; PGx, pharmacogenetic; PK, pharmacokinetic; PM, poor metabolizer.
| Drug | Metabolism pathway | PGx information included in FDA label? | CPIC guideline? | DPWG guideline? | Percent change in AUC for poor metabolizers |
| Atypical antipsychotics (second-generation [SGA]) | |||||
| Aripiprazole | CYP2D6 | YES (PM: half of usual dose) | NO | YES (PM: reduce maximum dose to 10 mg/day) | ↑ 76.9%8 |
| CYP3A4 | NO | NO | NO | N/A | |
| Asenapine | CYP1A2 | NO | NO | NO | N/A |
| UGT1A4 | NO | NO | NO | N/A | |
| CYP2D6 | NO | NO | NO | N/A | |
| CYP3A4 | NO | NO | NO | N/A | |
| Brexpiprazole | CYP2D6 | YES (PM: half of usual dose) | NO | NO | ↑ 47%8 |
| CYP3A4 | NO | NO | NO | N/A | |
| Cariprazine | CYP3A4 | NO | NO | NO | N/A |
| CYP2D6 | YES (PM: no clinically relevant effects) | NO | NO | NS8 | |
| CYP1A2 | NO | NO | NO | N/A | |
| Clozapine | CYP2D6 | YES (PM: dose reduction may be necessary) | NO | YES (No dose recommendations) | ↑ 16.8%9 |
| CYP3A4 | NO | NO | NO | N/A | |
| Iloperidone | CYP2D6 | YES (PM: half of usual dose) | NO | NO | ↑ 57.5%8 |
| CYP3A4 | NO | NO | NO | N/A | |
| Lurasidone | CYP3A4 | NO | NO | NO | N/A |
| Olanzapine | CYP1A2 | NO | NO | NO | N/A |
| CYP2D6 | NO | NO | YES (No dose recommendations) | ↑ 24.6%10 | |
| Quetiapine | CYP3A4 | NO | NO | NO | N/A |
| CYP3A5 | NO | NO | NO | ↑ 66.6%11 | |
| Risperidone | CYP2D6 | YES (PM: the PKs of active moiety are similar to EM) | NO | YES (PM: select alternative drug or be extra alert) | ↑ 641%8 |
| CYP3A4 | NO | NO | NO | N/A | |
| Ziprasidone | CYP3A4 | NO | NO | NO | N/A |
| CYP1A2 | NO | NO | NO | N/A | |
| Typical antipsychotics (first-generation [FGA]) | |||||
| Chlorpromazine | CYP2D6 | NO | NO | NO | ↑ 65%12 |
| CYP1A2 | NO | NO | NO | N/A | |
| CYP3A4 | NO | NO | NO | N/A | |
| Fluphenazine | CYP2D6 | NO | NO | NO | N/A |
| Haloperidol | CYP2D6 | NO | NO | YES* (PM: use half of usual dose or select alternative drug) | N/A |
| CYP1A2 | NO | NO | NO | N/A | |
| CYP3A4 | NO | NO | NO | N/A | |
| Loxapine | CYP1A2 | NO | NO | NO | N/A |
| CYP2D6 | NO | NO | NO | N/A | |
| CYP3A4 | NO | NO | NO | N/A | |
| Perphenazine | CYP2D6 | YES (PM: will metabolize more slowly) | NO | NO | ↑ 190% - 311%13,14 |
| Pimozide | CYP1A2 | NO | NO | NO | N/A |
| CYP2D6 | YES (PM: maximum dose of 0.05 mg/kg/ day for children and 4 mg/day for adults) | NO | NO | ↑ 153%15 | |
| CYP3A4 | NO | NO | NO | N/A | |
| Thioridazine | CYP2D6 | NO | NO | NO | ↑ 348%16 |
| CYP2C19 | NO | NO | NO | N/A | |
| Thiothixene | CYP1A2 | NO | NO | NO | N/A |
| Trifluoperazine | CYP1A2 | NO | NO | NO | N/A |
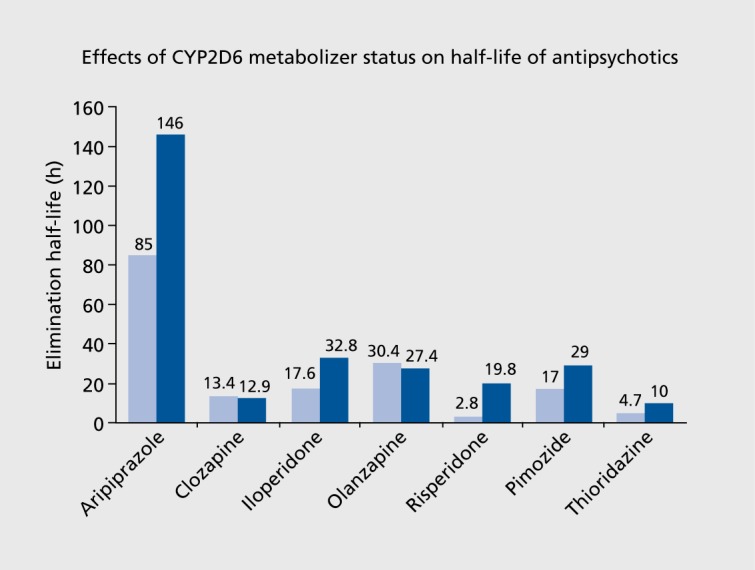
CYP2D6 metabolizer status strongly influences the metabolism of antipsychotics, leading to changes in pharmacokinetic parameters, including overall exposure as defined by the area under the concentrationtime curve (AUC), half-life, clearance, and steady-state concentration. Pharmacokinetic effects of CYP2D6 metabolizer phenotypes are described in Table II. Of note, the magnitude of influence is different in each medication that is a CYP2D6 substrate, with AUC increases ranging from 47% to 641% for the PMs. Halflife displayed an approximate twofold increase in PMs for aripiprazole, iloperidone, pimozide, and thioridazine, and a sevenfold increase for risperidone (see Figure 1).8-10,15,16
Figure 1. Pharmacokinetic effects of CYP2D6 metabolizer phenotypes on elimination half-life.8-10,15,16 light blue, extensive metabolizer; dark blue, poor metabolizer.
Many researchers have also investigated the relationship between CYP2D6 genotype and plasma concentration of antipsychotic agents and found concentration levels to be 1.1-4.5 times higher for PMs than for EMs for aripiprazole, risperidone, haloperidol, perphenazine, and thioridazine.17 Interestingly, the product labeling for clozapine indicates that dose reduction may be necessary in CYP2D6 PMs. However, studies have shown no differences in clozapine AUC, half-life, and dose-corrected plasma concentration between CYP2D6 PMs and EMs,17 suggesting that patients treated with clozapine would not benefit from CYP2D6 pharmacogenetic testing.
There are important considerations related to pharmacokinetic implications of drug metabolism pharmacogenetics. Firstly, some antipsychotics have active metabolites (eg, aripiprazole, risperidone) and, therefore, the disposition of both parent drug and active metabolite(s) may need to be taken into account when considering clinical implications. For example, risperidone is metabolized to an equally active metabolite (9-hydroxy-risperidone) by CYP2D6. Risperidone appears to be metabolized to the active metabolite much slower in PMs than in EMs; thus, whereas the AUC of the parent drug was 169 ng-h/mL in PMs and 22.8 ng-h/ mL in EMs, the AUC of its active metabolite was, respectively, 57.5 ng-h/mL and 139 ngh/mL,8 Therefore, when considering its total active moiety (risperidone + 9-hydroxy-risperidone), the increase in AUC was only 30% , compared with a 641 % increase when considering the risperidone parent drug only (Table II). Given the complexity, DPWG guidelines recommend the use of alternative drugs or that extra caution be exercised for use of risperidone in patients who are PMs.4 A second consideration is that the impact of altered metabolizer status needs to be assessed on a drug-by-drug basis, as secondary drug metabolism pathways may impact the magnitude of effect of PM status.
Associations between CYP2D6 polymorphisms and clinical efficacy or safety have yielded conflicting results. Most clinical studies failed to show a significant relationship between CYP2D6 genotype and symptom response.24-26 Numerous study limitations present challenges in interpreting these outcomes (limitations include nonprospective study design, small numbers of patients, heterogeneous methods of phenotype assignment, heterogeneous population groups across the studies, and confounding factors, such as interaction with concomitant drugs). On the other hand, some studies have demonstrated associations with adverse effects, especially extrapyramidal symptoms.26,27 When confined to prospective studies, a meta-analysis found a significant association between tardive dyskinesia (TD) and CYP2D6 polymorphisms.26
It is premature to unequivocally conclude that CYP2D6 pharmacogenetic testing is highly predictive of clinical response in patients taking antipsychotics. However, well-established links between CYP2D6 PM status and pharmacokinetic parameters in some antipsychotic agents indicate that this information may be helpful for dosing and titration decisions.
CYP1A2
CYP1A2 represents another important enzyme responsible for the biotransformation of some antipsychotics (Table II). Many pharmacogenetic testing laboratories now offer tests for CYP1A2 polymorphisms, although pharmacogenetic information regarding CYP1A2 remains absent from product labels and guideline statements for antipsychotic medications.
Compared with genes encoding other CYP enzymes (eg, CYP2D6), the effects of CYP1A2 genetic polymorphisms on enzyme activity are not as well understood. Additionally, there is currently no universally accepted method for defining CYP1A2 metabolizer groups by genotype. Commercial pharmacogenetic testing companies estimate CYP1A2 metabolizer status based on general effects that single-nucleotide variants have on expression or activity. CYP1A2 activity can be strongly influenced by environmental factors (tobacco and diet), which makes understanding the effect of CYP1A2 genotype more difficult.28
The most commonly tested variants in CAPTPs are CYP1A2*1F, *1K, *4, *5, *6, *7, *8, *11, *15, and *16.
Some of these variants increase gene function by induction of expression (CYP1A2*1F), and others decrease (CYP1A2*1K, *4, *7, *8, *11, *15, and *16) or abolish (CYP1A2*6) enzyme activity.29
The clinical utility of CYPIA2 genotypes is unclear. One rather unique consideration for the interpretation of CYPIA2 pharmacogenetics is the significant impact of smoking on enzyme activity. Smoking rates in patients with psychosis are twice that of the general population.30 The hydrocarbons in cigarette smoke are responsible for the induction of CYP1A2 in the body, increasing the clearance of substrates such as olanzapine, clozapine, chlorpromazine, and haloperidol up to 1.6-fold.31 In fact, smoking status has a more profound effect on clozapine metabolism than any known and currently tested CYP1A2 genetic variant (CYP1A2*1C and CYP1A2*1F).32,33 Thus, parsing the environmental and genetic effects on CYP1A2 metabolism is difficult, and conflicting studies highlight the complexity and uncertainties of interpreting antipsychotic-CYP1A2 relationships.34-38
CYP3A subfamily
The CYP3A subfamily includes CYP3A4, CYP3A5, CYPSA7, and CYP3A43. These enzymes are involved in the metabolism of approximately 37% of 200 top-selling prescription drugs,39 and many antipsychotics are substrates for the CYP3A4 enzyme (Table II).7 CYP3A4 and CYP3A5 have some overlap in substrate metabolism and are the most prevalent CYP3A enzymes in adults, and thus genetic variation influencing their activity may play a role in antipsychotic pharmacokinetics.
CYP3A5 is located at chromosome 7q22.1 and contains 13 coding exons, with over 2000 variants reported to date.20 Although CYP3A4 and CYP3A5 enzyme activity is highly variable across the general population, most CYP3A4 alleles identified are either very rare or have minimal effects on CYP3A4 activity.40 CYP3A4 alleles tested for on currently available commercial pharmacogenetic panels include *1, *13, *15A, and *22. Although some structural or general enzyme activity changes have been ascribed to these variants,41 uniformly accepted metabolizer groupings for CYP3A4 are lacking.
CYP3A5 arguably has more defined data on the effects of genetic variants on enzyme expression or function. The most common loss-of-function allele in white populations, CYP3A5*3, has been identified with frequencies ranging from 94% in Europeans to 18% in African populations. Other loss-of-function alleles include CYP3A5*6 and *7. CYP3A5*2 is considered to be a reduced-function allele.42 Currently available pharmacogenetic tests assay *1 and *3 for CYP3A5.
Presently, there are no established guidelines or product labeling recommendations that address CYP3A pharmacogenetics related to antipsychotics. Although some evidence exists that decreased-function alleles increase exposure to some antipsychotics,11,43 other studies have not found such effects,44,45 making recommendations related to clinical utility premature at the present time.
ABCB1
The P-glycoprotein (P-gp) drug transporter, encoded by ABCB1, is a transmembrane transporter located throughout the body, including intestine, liver, and the blood-brain barrier.46 P-gp may affect drug absorption and distribution of substrates.47,48 Many antipsychotics, such as aripiprazole, olanzapine, risperidone, and paliperidone, are substrates of P-gp48; therefore, it may play a significant role in their disposition.
Among the over 12 000 variants identified in the ABCB1 gene region,20 three SNPs (rsll28503 [1236C>T], rs2032582 [2677G>T],and rsl045642 [3435C>T]) are the most commonly studied variants, and results are inconclusive regarding SNP-phenotype associations. Some studies suggested that the phenotype of these silent polymorphisms may result from protein folding and stability rather than from changes in gene expression, and the effect of the structural alterations is expected to be more substrate-specific.49,50 In this regard, true relationships between ABCB1 genotype and pharmacokinetics of antipsychotic agents, as well as clinical outcomes, are unclear. Moreover, the high overlap in specificity of substrates for P-gp with CYP3A4 makes understanding the effect of the P-gp genotype more difficult.51 Preliminary evidence suggests that patients who have the rare allele of those three polymorphisms may have better symptom response outcomes, higher risks of adverse events (olanzapine, risperidone), and higher plasma levels or AUCs of antipsychotic agents (olanzapine, risperidone, and clozapine). However, as results across studies are mixed, the clinical utility of examining these variants is still uncertain.48 A few pharmacogenetic testing laboratories now offer ABCB1-polymorphism testing of those three common SNPs for some other medical conditions, including epilepsy, cardiovascular diseases, and inflammatory bowel disease, and also with regard to antidepressant treatment. But to date there are no established guidelines or product labels that provide recommendations for ABCBI pharmacogenetics related to antipsychotic use.
Pharmacodynamic genes and antipsychotic response
In addition to drug metabolizing enzymes and drug transporters, pharmacogenetic research has also focused on genes encoding therapeutic targets of antipsychotic pharmacotherapies, with an emphasis on dopamine and serotonin neurotransmitter systems thought to be dysregulated in psychotic disorders.52,53
Some of these markers are now included in CAPTPs (Table III),54-60 although they have yet to be included in drug labels or guidelines. Variants in pharmacodynamic genes have been important for understanding underlying mechanisms related to both disease presentation and treatment response. Some pharmacodynamic gene variants may be related to clinical response or tolerability to antipsychotic drugs and have also been investigated for relationships with disease risk, disease phenotypes, and risk for other nonpsychiatric conditions. The following sections review genes and variants associated with antipsychotic treatment outcomes and for which clinical tests are currently available. Putative mechanisms, summaries of association data, and other considerations will be discussed.
TABLE III. Common pharmacodynamic gene variants included in clinical testing panels for antipsychotic treatment response. 1000 genomes superpopulations are as follows: African (AFR; n=661), Mixed American (AMR; n=347), East Asian (EAS; n=504), European (EUR; n=503), and South Asian (SAS; n=489). Association data for each variant is summarized from the most recently published meta-analysis. US, upstream.
| Gene | Variant description | Variant classification | Frequency 1000 genomes populations | Association data | |||||
| AFR | AMR | EAS | EUR | SAS | All | ||||
| COMT | c.472 G>A, V158M rs4680 | missense | 0.28 A | 0.38 A | 0.28 A | 0.50 A | 0.44 A | 0.37 A | Homozygous Met allele increased clinical response [OR=1.37, 95% CI: 1.02-1.85]81 |
| DRD2/ ANKK1 | c.2137G>A, E713K Taq1A; rs1800497 | missense | 0.33 A | 0.39 A | 0.41 A | 0.19 A | 0.31 A | 0.33 A | Taq1A_A2 allele increased risk of tardive dyskinesia [OR=1.30, 95% CI: 1.09-1.55]57,58 |
| DRD2 | c.-486_-485 insC -141C ins/del rs1799732 | 5'-US promoter region | 0.57 C Del | 0.16 C Del | 0.14 C Del | 0.08 C Del | 0.13 C Del | 0.24 C Del | -141 Del_C decreased treatment response [OR=0.65, 95% CI: 0.43-0.97]56 |
| DRD3 | c.25A>G, S9G rs6280 | missense | 0.82 G | 0.43 G | 0.31 G | 0.34 G | 0.42 G | 0.49 G | Gly allele increased risk of tardive dyskinesia [OR=1.17, 95%CI: 1.01-1.37]72 |
| HTR2A | c.102C>T, S34S rs6313 | synonymous | 0.39 T | 0.35 T | 0.59 T | 0.44 T | 0.42 T | 0.44 T | C allele increased risk of tardive dyskinesia [OR=1.64, 95%CI: 1.17-2.23]86 |
| HTR2C | -759 C>T rs3813929 | 5'-US promoter region | 0.01 T | 0.12 T | 0.15 T | 0.16 T | 0.28 T | 0.13 T | C allele increased risk of weight gain [OR: 2.70; 95%CI: 1.46-5.01]100 |
Dopamine system: DRD2
All currently approved antipsychotics have varying degrees of antagonistic action at dopamine D2 receptors in the brain. Dopamine receptor genes have therefore received much attention as strong candidates in antipsychotic pharmacogenetic association studies. The D2 receptor is expressed in the brain,61 and D2 antagonism by antipsychotics in the mesolimbic dopamine pathway is thought to be responsible for the reduction in positive symptoms of schizophrenia, and antagonism in the nigrostriatal pathway is related to movement disorder side effects.
Over over 4000 variants in DRD2 have been identified20; however, at present only two: the TaqlA polymorphism (rs1800497) and -141 Ins/Del (c.-486_-485insC;rsl799732) are available on CAPTPs. It is important to be aware that DRD2 has also been associated with several nonpharmacogenetic phenotypes, including risk for attention-deficit/hyperactivity disorder (ADHD), alcohol dependence, schizophrenia, Tourette syndrome, and others.62-65
TaqlA nomenclature is derived from the restriction endonuclease enzyme originally used to identify this SNP. The “Al” allele of TaqlA— now known to be located 10 kb downstream of DRD2 within the coding region of the ankyrin repeat and kinase domain-containing 1 (ANKK1) gene (c.2137G>A, Glu713Lys)— is associated with D2 expression and reduced striatal receptor density.66-68 The A1 allele is in linkage disequilibrium with two other DRD2 intronic variants (rs2283265 and rsl076560), which influence DRD2 splicing.69 Associations between the TaqlA_Al allele and increased symptom response have been reported, but metaanalysis of this relationship showed no significant association.57 With respect to side effects, meta-analyses indicate an approximately 30% increase in the odds of developing TD in TaqlA_A2 allele carriers (predominantly first-generation antipsychotics).55,56 Additionally, studies have also shown correlations between other side effects, including hyperprolactinemia and weight gain, and TaqlA genotype.70-72
The DRD2 -141 Ins/Del polymorphism is located in the promoter region approximately 485 nucleotides upstream of the coding start site and consists of a deletion or insertion of a cytosine base. The -141 Del_C allele is correlated with altered D2 expression58,73 and decreased antipsychotic treatment response.74-76 Though conflicting results exist, meta-analysis suggests that -141 Del_C carriers are approximately 35% less likely to respond than insertion homozygotes.57 There are other DRD2 variants, (A-241G [c.-585A>G; rsl799978]; Ser311Cys [c.932 C>G; rsl801028]; and TaqlB [-882 G>A; rsl079597]) that have been examined, with mixed results, in association studies, but they are not currently available as clinical tests.52,53
Dopamine system: DRD3
In addition to the dopamine D2 receptors, antipsychotics show similar affinity for the dopamine D3 receptor. D3-receptor expression increases in the striatum after antipsychotic treatment,77-79 suggesting that the gene encoding the D3 receptor (DRD3) may be important for pharmacogenetic investigation. Over 4000 DRD3 variants have been identified.20 Pharmacogenetic research related to antipsychotic treatment has focused on a missense variant that results in a serine-to-glycine amino acid substitution (Ser9Gly, c.25A>G, rs6280). Multiple studies have investigated the relationship between the Ser9Gly polymorphism and antipsychotic response; however, they have produced conflicting results, with a meta-analysis observing a nonsignificant trend between the Ser9 allele and decreased antipsychotic response (odds ratio=0.82; 95% confidence interval, 0.65-1.04).80 Additionally, multiple studies have reported a significant association between Ser9Gly and TD52; however, three separate meta-analyses provide inconsistent findings with only a modest to nonexistent effect for the Gly9 allele with increased TD risk.58,81,82
Dopamine system: COMT
Catechol-0-methyltransferase (COMT) is a key enzyme for dopamine clearance and metabolic termination of dopamine activity and has been investigated in antipsychotic association studies.
Over 1900 variants have been identified in COMT.20 Most studies have focused on a common missense variant, Vall58Met (c.472 G>A, rs4680), which is now included on some CAPTPs. The Met allele confers thermoinstability of the COMT enzyme, decreasing dopamine catabolism, and thereby increasing dopamine in areas of the brain, such as the prefrontal cortex.83 Correlations have been observed between the Met allele and increased clinical response to antipsychotics, greater improvement in negative symptoms, and cognitive function.84-89 Conflicting results exist, but two recently published meta-analyses confirmed significant associations between homozygous Met allele carriers and a 37% increase in the odds of clinical response, which may be driven by effects on positive symptoms and atypical antipsychotic treatment.54,90 Early studies suggested that the Met allele may be protective against TD; however, meta-analyses have presented conflicting or nonsignificant results.55,91
Serotonin system genes
Second-generation or “atypical” antipsychotics (SGAs) have higher affinities for serotonin 2A receptors (5HT2A) than for D2 receptors, a pharmacodynamic characteristic thought to have implications for both the efficacy and tolerability profiles of these medications.92,93 As a result, many pharmacogenetic studies have investigated genes governing or influencing serotonin transmission. Of the serotonin receptors, the 5-HT2A and 5-HT2C subtypes have been the primary targets of investigations into antipsychotic response and tolerability and are now included on some clinically available test panels.
Serotonin system: HTR2A
The serotonin 2A receptor, encoded by HTR2A, is the most extensively studied serotonin-receptor target of SGAs. Currently, two polymorphisms of HTR2A are represented on commercially available pharmacogenetic test platforms. The first, T102C (c.l02C>T,S34S;rs6313), has shown weak associations with SGA response, with the T allele resulting in a modest (36%) increase in the odds of response.94 Additionally, studies have also suggested a relationship between the 102C allele and a modestly increased TD risk.59 The functional consequence of this synonymous polymorphism remains unknown, but the C allele has been associated with lower HTR2A expression resulting either from suspected epigenetic mechanisms95 or due to a second polymorphism in tight linkage disequilibrium. The G allele of this linked SNP,A-1438G (rs6311), is associated with decreased promoter activity,96,97 as well as with antipsychotic response; however, results are inconsistent.98 Other SNPs in HTR2A including His452Tyr (c.1354C>T, H452Y; rs6314) and c.6142211 G>A (rs7997012) have also been correlated with antipsychotic treatment response and side effects,94,99-103 but with inconsistent results and uncertain clinical implications.59,104
Serotonin system: HTR2C
The serotonin 2C receptor, encoded by HTR2C, has also been extensively studied because of its critical involvement in mediating the effect of antipsychotics on weight gain, negative symptoms, and possibly cognitive function.105 Although there are other nonsynonymous SNPs potentially relevant to treatment outcomes that reside in HTR2C,106 the variant that appears on some CAPTPs is -759 T/C (rs3813929), which has been extensively investigated for associations with antipsychotic-induced weight gain. It is well known that the 5-HT2C receptor is involved in regulation of food intake, and 5-HT2C antagonists cause increases in food intake and weight gain.105 In regard to the C-759T polymorphism, the C allele has been consistently associated with a 240% to 270% increased odds in the risk of weight gain when meta-analyses examine findings across multiple studies.60,107 Studies indicate that the C-759T polymorphism may affect transcription factor binding, with the T allele associated with greater expression and less weight gain.108,109 Knockout rodent studies indicate that an absence of 5-HT2C receptors is related to greater feeding activity and adipose disposition.110 Antipsychotics with the greatest affinities for 5-HT2C receptors (ie, clozapine and olanzapine) are also associated with the greatest weight gain, a risk that seems to be modestly lower in persons carrying the higher-expressing form of the gene. Owing to the strength and replicability of this association, the C-759T polymorphism has been informative for understanding one mechanistic piece underlying antipsychotic weight gain. Although it appears on some CAPTPs, the clinical utility of this SNP requires further study. Specifically, whether selecting antipsychotic therapy based on this SNP results in a more favorable weight gain profile than usual care is not known.
Other development and regulatory system genes
Despite the evidence for the relationships between specific variants within dopamine and serotonin genes, antipsychotic treatment response, and tolerability, there may arguably be other pharmacodynamics genes that contribute to these outcomes. This evident need has led to numerous investigations in other genes that influence neuronal function, neurotransmitter signaling, and neurotransmitter disposition. Currently, these genes are not included in clinically available pharmacogenetic tests and thus not included for detailed discussion in this review. Nonetheless, genetic variations in BDNF, GRM3, HSPG2, HTR1A, LEP, LEPR, MC4R, MTHFR, SULT4A1, and ZNF804A (and others) have shown some promising associations with aspects of antipsychotic symptom improvement and side effects.52,53,98
Clinical implementation of pharmacogenetics in psychiatry
Progress in the field of antipsychotic pharmacogenetics has been substantial over the past 20 years, resulting in the identification of numerous associations between antipsychotic drug efficacy and tolerability with various genetic markers in both pharmacokinetic and pharmacodynamic pathways. This has led to excitement about using this knowledge in the clinic where there is a dire need to improve treatment outcomes. Commercial pharmacogenetic testing companies have now made this technology accessible by selectively including genetic variants into antipsychotic-related genetic testing panels. However, decisions about which variants to include in commercial panels are made by the testing companies. Although the selection of variants is often based on an evaluation of scientific literature, there are currently no standards to guide this process.
Governmental agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) now emphasize that drug manufacturers should include pharmacogenetic information in product labeling when appropriate. Additionally, groups including CPIC, DPWG, and Eu-PIC have worked to establish guidelines about how to use available genetic information to guide treatment. Currently, DPWG guidelines provide actionable recommendations for aripiprazole, risperidone, and haloperidol (see Table II).4 CPIC and Eu-PIC have not yet published pharmacogenetic recommendations for antipsychotics. Moreover, clinical validity and utility of these pharmacogenetic testing panels are not well evaluated for antipsychotics. Clinical use thus remains limited by the knowledge gap between laboratory and translational research and clinical practice. In order to decrease this gap, additional work is in progress to determine how and when testing might be useful, as well as whether pharmacogenetics-guided dosing or drug selection strategies are better than usual care.
Currently, testing laboratories report the results with varying degrees of interpretation, decision support, and even therapeutic recommendations. However, it is challenging to integrate pharmacogenetic testing data into clinical records because of the complexity and heterogeneity of the information. In order to address this issue, a number of groups, including the Institute of Medicine Roundtable on Translating Genomic-Based Research for Health and CPIC have worked to standardize the terminology of pharmacogenetic testing. In spite of this, standardized terms and languages are still lacking; thus, each clinician still needs to navigate the testing results and enter them into medical records without standardized guides for this process.
Another important consideration affecting the clinical utility of antipsychotic-related pharmacogenetics has to do with the differences in genomic coverage across pharmacogenetic testing methodologies. Initially, pharmacogenetic testing was conducted by the single-gene approach. Multigene panels, analyzing multiple genes or genetic variants in a single assay, have now become available and are offered by numerous clinical laboratories.3 Testing multiple pharmacogenetic variants relevant to a specific treatment or therapeutic area in a single assay is more cost-effective and can provide valuable preemptive pharmacogenetic information (applicable to medications that patients potentially receive during their lifetime).111 Furthermore, technologies for genetic detection and discovery continue to rapidly advance and decrease in cost, leading to the availability of whole exome and genome sequencing methodology in clinical genetics laboratories. These technological advancements have allowed for a more comprehensive genomic coverage, but create challenges for the integration of this information into electronic medical records and in how to translate large amounts of genomic data into clinically relevant and actionable information required for drug and dosing selection in antipsychotic treatment.
Conclusion
There is a great need to improve treatment and dosing selection for patients with psychotic disorders, and improving the precision of our treatments, in part through pharmacogenetic testing, has great potential to offer a more personalized approach to treatment decisions in clinical psychiatry. Genetic variations in specific drug metabolism markers may be helpful for dosing and titration approaches because of their impact on pharmacokinetics; however, product-labeling language relating to specific gene variants varies across different antipsychotic medications. Genetic variants related to antipsychotic pharmacodynamics are now included in CAPTPs, although they remain absent from drug labels or guidelines. The clinical application of antipsychotic pharmacogenetics is in its early stages but seems to be one promising approach to help us improve antipsychotic pharmacotherapy. More research is needed on using test information prospectively, although some information obtained retrospectively may help in characterizing patterns of tolerability or response for a given patient. We can begin to provide valuable information that is needed for the development and implementation of pharmacogenetic guidelines necessary for common clinical utility in antipsychotic use through: (i) increased genomics education relevant for clinicians; (ii) development of efficient infrastructure between research and clinical practice; and (iii) advancing current prediction algorithms to include both comprehensive genomic information and pertinent clinical variables.
Selected abbreviations and acronyms
- ABCB1
ATP binding cassette subfamily D member 1
- AUC
area under the concentration-time curve
- CPATP
clinically available pharmacogenetic test panel
- CPIC
Clinical Pharmacogenetics Implementation Consortium
- CYP
cytochrome P450
- DPWG
Dutch Pharmacogenetics Working Group
- DRD2
dopamine D2 receptor gene
- EM
extensive metabolizer
- P-gp
P-glycoprotein
- PM
poor metabolizer
- SNP
single-nucleotide polymorphism
- TD
tardive dyskinesia
Disclosures: The authors have declared no potential conflicts of interest.
Contributor Information
Seenae Eum, College of Pharmacy, Department of Experimental and Clinical Pharmacology; University of Minnesota, Minneapolis, Minnesota, USA.
Adam M. Lee, College of Pharmacy, Department of Experimental and Clinical Pharmacology; University of Minnesota, Minneapolis, Minnesota, USA.
Jeffrey R. Bishop, College of Pharmacy, Department of Experimental and Clinical Pharmacology; College of Medicine, Department of Psychiatry; University of Minnesota, Minneapolis, Minnesota, USA.
REFERENCES
- 1.Lieberman JA., Tollefson G., Tohen M., et al Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA., Stroup TS., McEvoy JP., et al Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Bousman CA., Hopwood M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry. 2016;3(6):585–590. doi: 10.1016/S2215-0366(16)00017-1. [DOI] [PubMed] [Google Scholar]
- 4.Swen JJ., Nijenhuis M., de Boer A., et al Pharmacogenetics: from bench to byte— an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 5.Genetic Test Registry Bethesda, MD: National Center for Biotechnology Information, US National Library of Medicine. Available at: http:// www.ncbi.nlm.nih.gov/gtr/. Accessed February 19, 2016. [Google Scholar]
- 6.Scott SA., Sangkuhl K., Stein CM., et al Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacabelos R., Hashimoto R., Takeda M. Pharmacogenomics of antipsychotics efficacy for schizophrenia. Psychiatry Clin Neurosci. 2011;65(1):3–19. doi: 10.1111/j.1440-1819.2010.02168.x. [DOI] [PubMed] [Google Scholar]
- 8. FDA Approved Drug Products. Rockville, MD: US Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cder/ drugsatfda/. Accessed February 4, 2016. [Google Scholar]
- 9.Dahl ML., Llerena A., Bondesson U., Lindström L., Bertilsson L. Disposition of clozapine in man: lack of association with debrisoquine and S-mephenytoin hydroxylation polymorphisms. Br J Clin Pharmacol. 1994;37(1):71–74. doi: 10.1111/j.1365-2125.1994.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hägg S., Spigset O., Lakso HA., Dahlqvist R. Olanzapine disposition in humans is unrelated to CYP1A2 and CYP2D6 phenotypes. Eur J Clin Pharmacol. 2001;57(6-7):493–497. doi: 10.1007/s002280100343. [DOI] [PubMed] [Google Scholar]
- 11.Kim KA., Joo HJ., Lee HM., Park JY. Influence of ABCB1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of quetiapine in healthy volunteers. Pharmacogenet Genomics. 2014;24(1):35–42. doi: 10.1097/FPC.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 12.Sunwoo YE., Ryu J., Jung H., et al Disposition of chlorpromazine in Korean healthy subjects with CYP2D6 wild type and *10B mutation. Clin Pharmacol Ther. 2004;75(2):P90–P90. [Google Scholar]
- 13.Dahl-Puustinen ML., Liden A., Aim C., Nordin C., Bertilsson L. Disposition of perphenazine is related to polymorphic debrisoquin hydroxylation in human beings. Clin Pharmacol Ther. 1989;46(1):78–81. doi: 10.1038/clpt.1989.109. [DOI] [PubMed] [Google Scholar]
- 14.Ozdemir V., Bertilsson L., Miura J., et al CYP2D6 genotype in relation to perphenazine concentration and pituitary pharmacodynamic tissue sensitivity in Asians: CYP2D6-serotonin-dopamine crosstalk revisited. Pharmacogenet Genomics. 2007;17(5):339–347. doi: 10.1097/FPC.0b013e32801a3c10. [DOI] [PubMed] [Google Scholar]
- 15.Desta Z., Kerbusch T., Flockhart DA. Effect of clarithromycin on the pharmacokinetics and pharmacodynamics of pimozide in healthy poor and extensive metabolizers of cytochrome P450 2D6 (CYP2D6). Clin Pharmacol Ther. 1999;65(1):10–20. doi: 10.1016/S0009-9236(99)70117-7. [DOI] [PubMed] [Google Scholar]
- 16.von Bahr C., Movin G., Nordin C., et al Plasma levels of thioridazine and metabolites are influenced by the debrisoquin hydroxylation phenotype. Clin Pharmacol Ther. 1991;49(3):234–240. doi: 10.1038/clpt.1991.22. [DOI] [PubMed] [Google Scholar]
- 17.Ravyn D., Ravyn V., Lowney R., Nasrallah HA. CYP450 pharmacogenetic treatment strategies for antipsychotics: a review of the evidence. Schizophr. fles. 2013;149(1-3):1–14. doi: 10.1016/j.schres.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Owen RP., Sangkuhl K., Klein TE., Altman RB. Cytochrome P450 2D6. Pharmacogenet Genomics. 2009;19(7):559–562. doi: 10.1097/FPC.0b013e32832e0e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48(11):689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20. Database of Single Nucleotide Polymorphisms (dbSNP) Bethesda, MD: National Center for Biotechnology Information, National Library of Medicine. Available at: http://www.ncbi.nlm.nih.gov/projects/SNP/snp... ref.cgi showRare=on&chooseRs=coding&go=Go&locusld=18Q6. Accessed February 19, 2016. [Google Scholar]
- 21.Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22. doi: 10.1046/j.1365-2362.33.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 22.Sistonen J., Sajantila A., Lao O., Corander J., Barbujani G., Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17(2):93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]
- 23.Aklillu E., Persson I., Bertilsson L., Johansson I., Rodrigues F., IngelmanSundberg M. Frequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther. 1996;278(1):441–446. [PubMed] [Google Scholar]
- 24.Muller DJ., Brandl EJ., Hwang R., et al The AmpliChip® CYP450 test and response to treatment in schizophrenia and obsessive compulsive disorder: a pilot study and focus on cases with abnormal CYP2D6 drug metabolism. Genet Test Mol Biomarkers. 2012;16(8):897–903. doi: 10.1089/gtmb.2011.0327. [DOI] [PubMed] [Google Scholar]
- 25.Grossman I., Sullivan PF., Walley N., et al Genetic determinants of variable metabolism have little impact on the clinical use of leading antipsychotics in the CATIE study. Genet Med. 2008;10(10):720–729. doi: 10.1097/GIM.0b013e3181863239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleeman N., Dundar Y., Dickson R., et al Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: systematic review and meta-analyses. Pharmacogenomics J. 2011;11(1):1–14. doi: 10.1038/tpj.2010.73. [DOI] [PubMed] [Google Scholar]
- 27.Brockmöller J., Kirchheiner J., Schmider J., et al The impact of the CYP2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin Pharmacol Ther. 2002;72(4):438–452. doi: 10.1067/mcp.2002.127494. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen BB., Brix TH., Kyvik KO., Brosen K. The interindividual differences in the 3-demethylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12(6):473–478. doi: 10.1097/00008571-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Thorn CF., Aklillu E., Klein TE., Altman RB. PharmGKB summary: very important pharmacogene information for CYP1A2. Pharmacogenet Genomics. 2012;22(1):73–77. doi: 10.1097/FPC.0b013e32834c6efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartz SM., Pato CN., Medeiros H., et al Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry. 2014;71(3):248254–glycoprotein: implications for drug delivery and activity in cancer chemotherapy. doi: 10.1001/jamapsychiatry.2013.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai HD., Seabolt J., Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469–494. doi: 10.2165/00023210-200115060-00005. [DOI] [PubMed] [Google Scholar]
- 32.van der Weide J., Steijns LS., van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13(3):169–172. doi: 10.1097/00008571-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Jaquenoud Sirot E., Knezevic B., Morena GP., et al ABCB1 and cytochrome P450 polymorphisms: clinical pharmacogenetics of clozapine. J Clin Psychopharmacol. 2009;29(4):319–326. doi: 10.1097/JCP.0b013e3181acc372. [DOI] [PubMed] [Google Scholar]
- 34.Ozdemir V., Kalow W., Okey AB., et al Treatment-resistance to clozapine in association with ultrarapid CYP1 A2 activity and the C— >A polymorphism in intron 1 of the CYP1A2 gene: effect of grapefruit juice and lowdose fluvoxamine. J Clin Psychopharmacol. 2001;21(6):603–607. doi: 10.1097/00004714-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Eap CB., Bender S., Jaquenoud Sirot E., et al Nonresponse to clozapine and ultrarapid CYP1 A2 activity: clinical data and analysis of CYP1 A2 gene. J Clin Psychopharmacol. 2004;24(2):214–219. doi: 10.1097/01.jcp.0000116646.91923.2f. [DOI] [PubMed] [Google Scholar]
- 36.Perera V., Gross AS., Polasek TM., et al Considering CYP1 A2 phenotype and genotype for optimizing the dose of olanzapine in the management of schizophrenia. Expert Opin Drug Metab Toxicol. 2013;9(9):1115–1137. doi: 10.1517/17425255.2013.795540. [DOI] [PubMed] [Google Scholar]
- 37.Nozawa M., Ohnuma T., Matsubara Y., et al The relationship between the response of clinical symptoms and plasma olanzapine concentration, based on pharmacogenetics: Juntendo University Schizophrenia Projects (JUSP). Ther Drug Monit. 2008;30(1):35–40. doi: 10.1097/FTD.0b013e31816336fd. [DOI] [PubMed] [Google Scholar]
- 38.Czerwensky F., Leucht S., Steimer W. CYP1A2*1D and *1F polymorphisms have a significant impact on olanzapine serum concentrations. Ther Drug Monit. 2015;37(2):152–160. doi: 10.1097/FTD.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 39.Zanger UM., Turpeinen M., Klein K., Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal BioanalChem. 2008;392(6):1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 40.Whirl-Carrillo M., McDonagh EM., Hebert JM., et al Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. CYP3A4 allele nomenclature. Stockholm, Sweden: The Human Cytochrome P450 (CYP) Allele Nomenclature Database. Available at: http:// www.cypalleles.ki.se/cyp3a4.htm. Accessed February 19, 2016. [Google Scholar]
- 42.Lamba J., Hebert JM., Schuetz EG., Klein TE., Altman RB. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. 2012;22(7):555–558. doi: 10.1097/FPC.0b013e328351d47f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigos KL., Bies RR., Pollock BG., Lowy JJ., Zhang F., Weinberger DR. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620–625. doi: 10.1038/mp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakken GV., Molden E., Hermann M. Impact of genetic variability in CYP2D6, CYP3A5, and ABCB1 on serum concentrations of quetiapine and N-desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2015;37(2):256–261. doi: 10.1097/FTD.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 45.Soderberg MM., Molden E., Dahl ML. No influence of CYP3A43 rs472660G> A on steady-state serum olanzapine concentrations in white psychiatric patients. Pharmacogenet Genomics. 2014;24(5):272–275. doi: 10.1097/FPC.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 46.Thiebaut F., Tsuruo T., Hamada H., Gottesman MM., Pastan I., Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eichelbaum M., Fromm MF., Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit. 2004;26(2):180–185. doi: 10.1097/00007691-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Moons T., de Roo M., Claes S., Dom G. Relationship between P-glycoprotein and second-generation antipsychotics. Pharmacogenomics. 2011;12(8):1193–1211. doi: 10.2217/pgs.11.55. [DOI] [PubMed] [Google Scholar]
- 49.Kimchi-Sarfaty C., Oh JM., Kim IW., et al A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 50.Fung KL., Pan J., Ohnuma S., et al MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74(2):598–608. doi: 10.1158/0008-5472.CAN-13-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wacher VJ., Wu CY., Benet al LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Moi Carcinog. 1995;13(3):129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 52.Zhang JP., Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol. 2011;7(1):9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pouget al JG., Muller DJ. Pharmacogenetics of antipsychotic treatment in schizophrenia. Methods Mol Biol. 2014;1175:557–587. doi: 10.1007/978-1-4939-0956-8_14. [DOI] [PubMed] [Google Scholar]
- 54.Huang E., Zai CC., Lisoway A., et al Catechol-O-methyltransferase Val1 58Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizo-affective disorder patients: a meta-analysis. 2016 Apr 29. Epub ahead of print. doi:10.1093/ijnp/ pyv132. Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakker PR., van Harten PN., van Os J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry. 2008;13(5):544–556. doi: 10.1038/sj.mp.4002142. [DOI] [PubMed] [Google Scholar]
- 56.Zai CC., De Luca V., Hwang RW., et al Meta-analysis of two dopamine D2 receptor gene polymorphisms with tardive dyskinesia in schizophrenia patients. Mol Psychiatry. 2007;12(9):794–795. doi: 10.1038/sj.mp.4002023. [DOI] [PubMed] [Google Scholar]
- 57.Zhang JP., Lencz T., Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry. 2010;167(7):763–772. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai H-T., North KE., West SL., Poole C. The DRD3 rs6280 polymorphism and prevalence of tardive dyskinesia: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):57–66. doi: 10.1002/ajmg.b.30946. [DOI] [PubMed] [Google Scholar]
- 59.Lerer B., Segman RH., Tan EC., et al Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol. 2005;8(3):411–425. doi: 10.1017/S1461145705005389. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JP., Malhotra AK. Pharmacogenetics of antipsychotics: recent progress and methodological issues. Expert Opin Drug Metab Toxicol. 2013;9(2):183–191. doi: 10.1517/17425255.2013.736964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Missale C., Nash SR., Robinson SW., Jaber M., Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 62.Pan YQ., Qiao L., Xue XD., Fu JH. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: a meta-analysis. Neurosci Lett. 2015;590:101–105. doi: 10.1016/j.neulet.2015.01.076. [DOI] [PubMed] [Google Scholar]
- 63.Yuan A., Su L., Yu S., Li C., Yu T., Sun J. Association between DRD2/ANKKI TaqlA polymorphism and susceptibility with Tourette syndrome: a meta-analysis. PLoS One. 2015;10(6):e0131–060. doi: 10.1371/journal.pone.0131060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao J., Pan YQ., Ding M., Pang H., Wang BJ. Association between >DRD2 (rs1799732 and rs1801028) and ANKK1 (rs1800497) polymorphisms and schizophrenia: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(1):1–13. doi: 10.1002/ajmg.b.32281. [DOI] [PubMed] [Google Scholar]
- 65.Wang F., Simen A., Arias A., Lu QW., Zhang H. A large-scale meta-analysis of the association between the ANKKI/DRD2 TaqlA polymorphism and alcohol dependence. Hum Genet. 2013;132(3):347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson J., Thomas N., Singleton A., et al D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7(6):479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Pohjalainen T., Rinne JO., Nägren K., et al The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3(3):256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 68.Jönsson EG., Nöthen MM., Grunhage F., et al Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Bertolino A., Fazio L., et al Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104(51):20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller DJ., Zai CC., Sicard M., et al Systematic analysis of dopamine receptor genes (DRD1-DRD5) in antipsychotic-induced weight gain. Pharmacogenomics J. 2012;12(2):156–164. doi: 10.1038/tpj.2010.65. [DOI] [PubMed] [Google Scholar]
- 71.Calarge CA., Ellingrod VL., Acion L., et al Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics. 2009;19(5):373–382. doi: 10.1097/FPC.0b013e328329a60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young RM., Lawford BR., Barnes M., et al Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2 *A1 allele. Br J Psychiatry. 2004;185:147–151. doi: 10.1192/bjp.185.2.147. [DOI] [PubMed] [Google Scholar]
- 73.Arinami T., Gao M., Hamaguchi H., Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6(4):577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki A., Kondo T., Mihara K., et al The -141C Ins/Del polymorphism in the dopamine D2 receptor gene promoter region is associated with anxiolytic and antidepressive effects during treatment with dopamine antagonists in schizophrenic patients. Pharmacogenetics. 2001;11(6):545–550. doi: 10.1097/00008571-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Yamanouchi Y., Iwata N., Suzuki T., Kitajima T., Ikeda M., Ozaki N. Effect of DRD2, 5-HT2A, and COMT genes on antipsychotic response to risperidone. Pharmacogenomics J. 2003;3(6):356–361. doi: 10.1038/sj.tpj.6500211. [DOI] [PubMed] [Google Scholar]
- 76.Lencz T., Robinson DG., Xu K., et al DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in firstepisode schizophrenia. patients. Am J Psychiatry. 2006;163(3):529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 77.Joyce JN., Gurevich EV. D3 receptors and the actions of neuroleptics in the ventral striatopallidal system of schizophrenics. Ann N Y Acad Sci. 1999;877:595–613. doi: 10.1111/j.1749-6632.1999.tb09291.x. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz JC., Diaz J., Pilon C., Sokoloff P. Possible implications of the dopamine D3 receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31(2-3):277–287. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 79.Sokoloff P., Diaz J., Le Foil B., et al The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5(1):25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 80.Hwang R., Zai C., Tiwari A., et al Effect of dopamine D3 receptor gene polymorphisms and clozapine treatment response: exploratory analysis of nine polymorphisms and meta-analysis of the Ser9Gly variant. Pharmacogenomics J. 2010;10(3):200–218. doi: 10.1038/tpj.2009.65. [DOI] [PubMed] [Google Scholar]
- 81.Bakker PR., van Harten PN., van Os J. Antipsychotic-induced tardive dyskinesia and the Ser9Gly polymorphism in the DRD3 gene: a meta analysis. Schizophr Res. 2006;83(2-3):185–192. doi: 10.1016/j.schres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Lerer B., Segman RH., Fangerau H., et al Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology. 2002;27(1):105–119. doi: 10.1016/S0893-133X(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 83.Chen J., Lipska BK., Halim N., et al Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bertolino A., Caforio G., Blasi G., et al Interaction of COMT Val108/158Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161(10):1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 85.Bertolino A., Caforio G., Blasi G., et al COMT Val1 58Met polymorphism predicts negative symptoms response to treatment with olanzapine in schizophrenia. Schizophr Res. 2007;95(13):253–255. doi: 10.1016/j.schres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Molero P., Ortuño F., Zalacain M., Patiño-García A. Clinical involvement of catechol-O-methyltransferase polymorphisms in schizophrenia spectrum disorders: influence on the severity of psychotic symptoms and on the response to neuroleptic treatment. Pharmacogenomics J. 2007;7(6):418–426. doi: 10.1038/sj.tpj.6500441. [DOI] [PubMed] [Google Scholar]
- 87.Woodward ND., Jayathilake K., Meltzer HY. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr Res. 2007;90(13):86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Pelayo-Terán JM., Pérez-Iglesias R., Vazquez-Bourgon J., et al Catechol-O-methyltransferase Val158Met polymorphism and negative symptoms after acute antipsychotic treatment in first-episode non-affective psychosis. Psychiatry Res. 2011;185(1-2):286–289. doi: 10.1016/j.psychres.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Weickert TW., Goldberg TE., Mishara A., et al Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol Psychiatry. 2004;56(9):677–682. doi: 10.1016/j.biopsych.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Chen H., Tu J., Ni P., Zhang W., Xu L. COMT genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis [in Chinese], Zhong Nan Da Xue Xue Bao YiXue Ban. 2015;40(6):623–631. doi: 10.11817/j.issn.1672-7347.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 91.LvZ, Rong B., Tong X., etal The association between COMT Val158Met gene polymorphism and antipsychotic-induced tardive dyskinesia risk. Int J Neurosci. 2015:1–7. doi: 10.3109/00207454.2015.1089504. [DOI] [PubMed] [Google Scholar]
- 92.Meltzer HY., Massey BW., Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol. 2012;13(8):1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- 93.Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- 94.Arranz MJ., Munro J., Sham P., et al Meta-analysis of studies on genetic variation in 5-HT2A receptors and clozapine response. Schizophr Res. 1998;32(2):93–99. doi: 10.1016/s0920-9964(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 95.Polesskaya 00., Aston C., Sokolov BP. Allele C-specific methylation of the 5-HT2A receptor gene: evidence for correlation with its expression and expression of DNA methylase. DNMT1. J Neurosci Res. 2006;83(3):362–373. doi: 10.1002/jnr.20732. [DOI] [PubMed] [Google Scholar]
- 96.Parsons MJ., D'Souza UM., Arranz MJ., Kerwin RW., Makoff AJ. The -1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry. 2004;56(6):406–410. doi: 10.1016/j.biopsych.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 97.Myers RL., Airey DC., Manier DH., Shelton RC., Sanders-Bush E. Polymorphisms in the regulatory region of the human serotonin 5-HT2A receptor gene (HTR2A) influence gene expression. Biol Psychiatry. 2007;61(2):167–173. doi: 10.1016/j.biopsych.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 98.Blanc 0., Brousse G., Meary A., Leboyer M., Llorca PM. Pharmacogenetic of response efficacy to antipsychotics in schizophrenia: pharmacodynamic aspects. Review and implications for clinical research. Fundam Clin Pharmacol. 2010;24(2):139–160. doi: 10.1111/j.1472-8206.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- 99.Arranz MJ., Collier DA., Munro J., et al Analysis of a structural polymorphism in the 5-HT2A receptor and clinical response to clozapine. NeurosciLett. 1996;217(2-3):177–178. [PubMed] [Google Scholar]
- 100.Hazelwood LA., Sanders-Bush E. His452Tyr polymorphism in the human 5-HT2A receptor destabilizes the signaling conformation. Mol Pharmacol. 2004;66(5):1293–1300. [PubMed] [Google Scholar]
- 101.Masellis M., Basile V., Meltzer HY., et al Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology. 1998;19(2):123–132. doi: 10.1016/S0893-133X(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 102.Arranz MJ., Munro J., Owen MJ., et al Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol Psychiatry. 1998;3(1):61–66. doi: 10.1038/sj.mp.4000348. [DOI] [PubMed] [Google Scholar]
- 103.aika B., Leucht S., Heres S., Schneider H., Steimer W. Pharmacogenetics and olanzapine treatment: CYP1A2*1F and serotonergic polymorphisms influence therapeutic outcome. Pharmacogenomics J. 2010;10(1):20–29. doi: 10.1038/tpj.2009.32. [DOI] [PubMed] [Google Scholar]
- 104.Tang H., McGowan 00., Reynolds GP. Polymorphisms of serotonin neurotransmission and their effects on antipsychotic drug action. Pharmacogenomics. 2014;15(12):1599–1609. doi: 10.2217/pgs.14.111. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds GP., Kirk SL. Metabolic side effects of antipsychotic drug treatment-pharmacological mechanisms. Pharmacol Ther. 2010;125(1):169–179. doi: 10.1016/j.pharmthera.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Veenstra-VanderWeele J., Anderson GM., Cook EH Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410(2-3):165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 107.Sicard MN., Zai CC., Tiwari AK., et al Polymorphisms of the HTR2Cgene and antipsychotic-induced weight gain: an update and meta-analysis. Pharmacogenomics. 2010;11(11):1561–1571. doi: 10.2217/pgs.10.123. [DOI] [PubMed] [Google Scholar]
- 108.Wallace TJ., Zai CC., Brandl EJ., Muller DJ. Role of 5-HT2C receptor gene variants in antipsychotic-induced weight gain. Pharmgenomics Pers Med. 2011;4:83–93. doi: 10.2147/PGPM.S11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hill MJ., Reynolds GP. Functional consequences of two HTR2C polymorphisms associated with antipsychotic-induced weight gain. Pharmacogenomics. 2011;12(5):727–734. doi: 10.2217/pgs.11.16. [DOI] [PubMed] [Google Scholar]
- 110.Tecott LH., Sun LM., Akana SF., et al Eating disorder and epilepsy in mice lacking 5-HT2Cserotonin receptors. Nature. 1995;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 111.Relling MV., Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]


