Structured Abstract
Purpose of the review
Numerous lines of evidence support the likelihood that inflammation drives the transition from obese/metabolically healthy to obese/type 2 diabetes (T2D). Given the temporal flexibility of inflammation in obesity-associated T2D, investigators have hypothesized that a precipitous drop in diabetogenic cytokines is critical for rapid “T2D remission” following surgery but prior to significant weight loss. We review the evidence that changes in diabetogenic cytokines play a role in outcomes of bariatric surgery, including weight loss and improved glycemic control.
Recent Findings
A 2016 indication for bariatric surgery to treat T2D integrates the large body of data showing short-term metabolic improvement. Parameters that account for improved glycemic control prior to significant weight loss, T2D recidivism over the long term, or failure of surgery to remit T2D in some patients are incompletely understood.
Summary
We review the evidence that changes in diabetogenic cytokines play a role in outcomes of bariatric surgery, including improved glycemic control. We brainstorm future research directions that may improve surgical results.
Keywords: bariatric surgery, Type 2 Diabetes, inflammation, cytokines, acute phase proteins
Introduction
Briatric surgery, most commonly Roux-en-Y gastric bypass (RYGB), is an effective intervention for morbid obesity and obesity-associated type 2 diabetes (T2D) [1–3]. Recent recommendations indicate bariatric surgery as the preferred treatment for T2D in class III obese (BMI ≥40) subjects, with consideration of surgery for class II obese people (BMI 35–39.9) with inadequately control hyperglycemia [4]. Most patients show significant metabolic improvement after bariatric surgery, with T2D remission rates of up to 70% of patients 2 yrs post-RYGB [5] and evidence for T2D remission as soon as one week post-surgery [6]. Using longitudinal data from a large registry of bariatric patients, we developed a predictive scoring system, DiaRem, which uses readily available clinical data to provide a likelihood of remission. DiaRem integrates data showing that younger patients with lower %HbA1c who are not on insulin are disproporionately likely to remit their T2D early after surgery [7, 8]. Insulin therapy, older age and high pre-surgery %HbA1c values predict low likelihood for T2D remission, clearly pointing to an essential role for a functioning β-cell population for diabetes remission post-surgery. However, a gap remains in accuracy of DiaRem, and in comprehensive identification of mechanisms that regulate T2D remission following bariatric surgery, and in our ability to develop non-surgical techniques that mimic the advantages of bariatric surgery-mediated T2D remission on an individualized basis.
Inflammation is a known complication of obesity that supports T2D development and differentiates metabolic healthy from metabolically unhealthy obesity [9]. Inflammatory status can change rapidly, due in part to the tight regulation of cytokine production [10–14], raising the possibility that a precipitous drop in diabetogenic cytokines is important for rapid post-surgical T2D remission. By extension, failure of inflammation to resolve after surgery may blunt improvements in glycemic control. A growing number of studies have measured traditional mediators of diabetogenic inflammation, including TNFα, IL-6 and CRP at various time points following bariatric surgery (see below). However, differences in experimental design of these studies, coupled with recent work that refines the generic term “inflammation” in obesity and T2D [12], suggest that longitudinal analysis of inflammation, focused on dominant inflammatory signatures of T2D and standardized surgical procedures, may improve our understanding of the role inflammation plays in surgically-induced T2D remission.
I. Mechanisms Underlying Improved Glycemic Control Following Bariatric Surgery
The ability of RYGB to “cure” T2D was first recognized by Pories and colleagues 20 years ago [2]. More recent studies showed up to 75–85% T2D remission [1, 15, 16] even before weight loss [6]. RYGB most effectively achieves a rapid improvement in glycemic control if T2D subjects are younger, have lower HbA1c levels and are not on insulin [17, 18].
Progress in identifying the mechanisms underlying improvements in glycemic control following RYGB and other types of bariatric surgery prior to significant weight loss has recently accelerated. The dominant hypothesis is that physical removal of tissues in some bariatric procedures leads to early arrival of nutrients in the distal ileum plus increased incretin secretion [19–21], which together are responsible for rapid glycemic improvement in RYGB. Alternatively, recent work in rats has shown that expansion of the small intestine in response post-surgical exposure to less fully digested food requires high amounts of glucose [22]. These data support the authors’ conclusion that the requirement for glucose for intestinal growth depletes circulating glucose, thus the clinical appearance of T2D remission. The moderate numbers of current reports have not assessed inflammation prior to significant weight loss, in part based on the expected inflammatory spike as a normal intra-surgical or immediately post-surgical event [23–25].
II. The Impact of Bariatric Surgery on Diabetogenic Inflammation
Although reports showing improved glycemic control within a week of bariatric surgery have not investigated potential changes in inflammation, a moderate number of studies have measured classical markers of diabetogenic inflammation at various time points following different surgical procedures. The inflammatory markers most often measured in bariatric surgery outcome studies include: TNFα, the first inflammatory cytokine implicated in obesity-associated metabolic derangement [26]; IL-6, a pleiotropic cytokine that has complex effects on obesity-associated metabolic decline [27–29]; and CRP and serum amyloid A (SAA), two acute phase proteins that are imperfect yet commonly used indicators of inflammation [30]. Overall, the effect of surgery and/or surgically-induced weight loss on inflammation has been inconsistent [31–37].
IIA. The Effect of Bariatric Surgery on TNFα
TNFα is arguably the most frequently quantitated cytokine in serum/plasma of post-surgical patients, due to strong associations of TNFα with insulin resistance [38]. TNFα concentrations at 2 weeks, 6 months, and/or 13 months after laparoscopic RYGB (LRYGB), were indistinguishable from pre-surgical/baseline levels in two independent studies [35, 39]. A third study of samples from subjects undergoing LRYGB showed TNFα significantly increased 3 months post-surgery compared to baseline, while recapitulating the other studies’ findings of no change in TNFα in comparison at 6 and 12 month post-surgery [36]. In contrast, a fourth analysis of TNFα 3 weeks-6 months post RYGB showed a significant decrease at each time point compared to baseline [31]. This latter outcome was consistent with rat RYGB work showing that adipose tissue mRNA levels of TNFα decreased 9 weeks post-operatively compared to the sham procedure, although RYGB did not alter TNFα amounts in the rats’ livers [25]. Thus the impact of RYGB on TNFα remain controversial.
In contrast to the inconsistency of RYGB for inducing changes in TNFα across studies, the impact of laparoscopic adjustable gastric banding (LAGB) on TNFα is relatively consistent, with multiple groups showing no change in serum levels anywhere from 2 weeks up to 14 months post-operatively [40, 41] despite an improvement in markers of glucose homeostasis [42], and a pre-op correlation between insulin resistance and serum TNFα [40]. Although findings for circulating TNFα following LAGB all show no change, Moschen et al. [43] found that TNFα in subcutaneous adipose tissue dramatically dropped, and that adipose tissue TNFα mRNA correlated with improved insulin sensitivity six months after LAGB. Interestingly gastric banding-associated weight loss also reduced T cell numbers [42]. Taken together, the existing studies suggest that the exact surgical procedure (RYGB vs. LAGB) may impact the TNFα response. Perhaps most importantly, this work shows inconsistency among studies, and calls for comparisons amongst outcomes from a standardized set of surgical procedures to understand the impact of bariatric surgery on TNFα-associated inflammation.
IIB. The Effect of Bariatric Surgery on Circulating IL-6
Interleukin-6 (IL-6) is often considered a strictly pro-inflammatory cytokine, but IL-6 has broader biologic properties, based in part on differences in the signaling cascades activated by the subunit composition of the two recognized IL-6 receptors [44, 45]. As a result of the well-known duplicity of IL-6, it is perhaps unsurprising that both gain-of-IL-6-function and loss-of-IL-6-function in mice and/or humans (knock-out, neutralizing antibody, or IL-6 infusion) improve glycemic control [27–29]. Regardless of the precise role of IL-6 in obesity and obesity-associated metabolic disease, many investigators have measured IL-6 following post-bariatric surgery weight loss. IL-6 concentrations were unchanged in T2D subjects two weeks post-RYGB and a 7% weight loss [37]. An independent measurement of IL-6 at baseline and 3,6,12 months post-RYGB indicated significant increases only at the 3 month time point [36]. In contrast, several reports agree that IL-6 decreases 12–14 months after RYGB [24, 46]; however, the studies disagreed on whether IL-6 correlated with BMI, insulin concentrations or HOMA-IR. Omentectomy during RYGB did not significantly affect IL-6 twelve months after RYGB [47]. Similar to the lack of consistency in studies measuring in IL-6 changes post-RYGB, the changes in serum IL-6 levels after LAGB varied across studies: Samaras et al. noted a transient decrease in IL-6 two weeks after LAGB, which then returned to baseline at 12 weeks, despite a significant improvement in the glycemic control of T2D patients [42]. Independent analysis similarly showed no change in IL-6 twelve months post-surgery and following significant weight loss [41, 43]. In contrast, a third study found a reduction in serum, subcutaneous adipose tissue, and liver IL-6 that correlated with improved insulin sensitivity six months after LAGB [43], consistent with other work [40]. Taken together, these studies indicate that comparisons of nearly identical surgical procedures at matched time points post-surgery will be essential to understand the impact of bariatric surgery on IL-6-mediated inflammation.
IIC. The Effect of Bariatric Surgery on Acute Phase Proteins
C-reactive protein (CRP), an acute phase liver protein that rapidly rises in response to injury or inflammation, is a traditional (albeit imperfect) measure of inflammation [48]. Generally speaking, serum CRP levels drop following bariatric surgery with a decline that correlates with weight loss as indicated by studies that measured CRP 6 months after sleeve gastrectomy [49]. Detectable decreases have also been noted as early as one month post-op [50]. CRP also significantly decreased 6 months after LAGB [43], and was similarly low for 12 months in an independent (though procedurally similar) analysis following LAGB [41]. CRP concentrations have also been measured in samples from patients who received RYGB, with investigators taking measurements at various time points ranging from 6–52 weeks post-op. At 12 months post-op, subjects averaged an 82% reduction in CRP, which was more pronounced in those who were insulin sensitive (as indicated by a HOMA-IR of <4) at baseline. The change in CRP associated with HOMA-IR but was independent of the change in body weight in these subjects [46]. In contrast, CRP reduction did not correlate with HOMA-IR fourteen months after gastroplasty, but instead independently correlated with BMI reduction [40]. Similar to the surgically-induced drop in CRP in the studies cited above, a more thorough longitudinal analysis high sensitivity CRP (hsCRP), at 3,6, and12 months post-RYGB showed progressive drops from baseline at all three time points, and correlated with BMI, insulin and HOMA-IR [36]. This work recapitulates the drop in CRP measured after RYGB-induced weight loss of 7% [37]. Finally, one study of Korean T2D subjects showed CRP decreased about the same in people with and without T2D remission following RYGB [51]. Overall, unlike the variable changes in TNFα, IL-6 and other obesity-associated inflammatory molecules (such as MCP-1, IL-8 etc.) following various weight loss surgeries, CRP generally falls, raising the possibility that bariatric procedures lower the risk of cardiovascular disease in obese subjects.
Like CRP, serum amyloid A (SAA) is an acute phase reactant synthesized by the liver in response to inflammation [52]. SAA is increased in obesity [53, 54], and obesity-associated complications including atherosclerosis [55, 56]. Twelve months after RYGB, SAA decreased by 57% in a group of 66 obese patients. The SAA reduction paralleled lower CRP, but CRP remained more significantly correlated with BMI change than did SAA [46]. A similar study looking at the relationship between T2D and SAA before and 13 months after RYGB in a small cohort of women with or without T2D found that RYGB significantly reduced circulating SAA, which correlated strongly with the reduction in body fat, but was independent of T2D status [35]. Work by Poitou et al. further reinforced these findings by demonstrating that a mixed cohort of obese RYGB and LAGB subjects had reduced serum SAA 3 months post-surgery. SAA concentrations failed to correlate with metabolic markers such as glucose, insulin and plasma lipids, but instead correlated with BMI and adipocyte volume. Importantly, serum SAA protein correlated with mRNA expression of inducible SAA (isoforms 1 and 2) from subcutaneous adipose tissue [57]. Overall, the data indicate that obesity-associated increases in SAA concentrations are related adiposity rather than insulin resistance, and that like CRP, SAA generally falls as a result of multiple weight lost surgery procedures. Taken together, these data indicate that acute phase proteins change more predictably than TNFα, IL-6, or other cytokines measured (MCP-1, IL-8 etc. [39, 58–60] following bariatric surgery (Figure 1). One exception to the variability in cytokine responses to bariatric surgery is adiponectin, a generally anti-inflammatory cytokine made by adipocytes, which uniformly increases post- surgery [36, 61].
Figure 1.
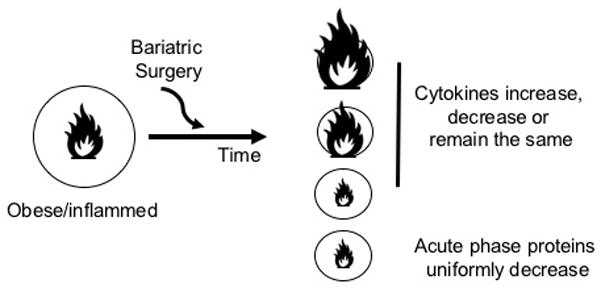
Changes in inflammatory biomarkers following bariatric surgery. Left: Surgical patients are selected based on obesity as indicated by the large circle. Surgical patients with T2D are also inflamed as indicated by the large flame. Following bariatric surgery, the majority of patients lose weight, as indicated by the decreased size of the circle. Right: multiple outcomes for the post-surgical change in inflammation have been reported. Cytokine changes are controversial and study-dependent, as indicated by the size of the flame (top three outcomes). Acute phase proteins decrease and are often correlated with weight loss or metabolic health as assess by HOMA-IR or glycemic control.
III. Conclusions
The relationships among measures of post-surgical cytokines, acute phase proteins, and metabolic health improvements, including T2D remission, remain poorly understood. The widely predicted drop in T2D-promoting inflammation following bariatric surgery-induced weight loss has been inconsistently supported over numerous studies that differ in surgical procedure, post-surgical time point and tissue studied. It is possible that fundamental differences in mechanisms that regulate inflammatory cytokines (TNFα, IL-6, IL-8 etc.) and acute phase proteins (CRP and SAA) together with the different surgical procedures explain the unpredictability of the inflammation outcomes, and may also explain the inconsistency of the relationships between both types of inflammatory markers and measures of metabolic improvement. It is equally possible that technical differences in sample collection/handling account for the varying outcomes, including the small sample number (and unreported power) used for many studies. Our preliminary work indicates that, in contrast to the recognizable cytokine signatures we found by stimulating PBMCs from obese/T2D subjects [12], cytokine signatures in plasma or serum yields weak suggestions of relationships between obesity and measures of metabolic health, perhaps due in part to low signal-to-noise ratios. We predict a more comprehensive analytical screen on stimulated PBMCs as we published [12], with cells collected before and at multiple time points after bariatric surgery, both separate and integrated analysis of the different surgical techniques, and addition of metabolic variables to the multivariate analyses may shed light on the role inflammation plays in outcomes following various types of bariatric surgery (Table 1). Given the ability of cytokine profiles from stimulated PBMCs to predict clinical disease status [12], pre-surgical inflammatory profiling may also help identify people who are likely to maximally benefit from a given surgical approach, and perhaps most importantly, to predict those for whom bariatric surgery will not trigger significant weight loss or T2D remission. Finally, in light of new data showing that surgically-induced T2D remission may not be permanent, especially in non-whites [62], coupled with the general paradigm that inflammation is critical for metabolic decline in obese individuals [9], analysis of inflammatory profiles may be important for understanding permanency of metabolic improvement post-surgery. Determining relationships amongst inflammatory mediators, pancreatic beta cell function and T2D remission will be absolutely essential towards shifting the standard of care to maximally benefit patients.
Table 1.
Variables that may explain the inconsistencies in outcomes of inflammatory changes following bariatric surgery and possible solutions
Variables that may explain the inconsistencies in post-surgical measures of inflammation, and possible solution to these identified challenges.
| Variable | Solution [Reference] |
|---|---|
| Surgical Technique (RYGB, GB, SG etc.) | Group studies using very similar techniques in analysis |
| Time Points | Limit analysis to quarterly time points after initial inflammatory surge (2 wks+ post-surgery) |
| Tissue Source | Use PBMCs or adipose tissue; signal:noise of serum/plasma limits value [12,25,42,43,57] |
| Cytokines Measured | Focus on cytokines validated to dominate diabetogenic inflammation [12] |
| Pre-Surgical Metabolic State | Characterize subjects according to clinical research standards (IV glucose tolerance or clamps) rather than %HbA1c etc. [37] |
| Pre-Surgical Inflammatory State | Focus on cytokines validated to dominate diabetogenic inflammation [12] |
| Analytical Tool | Use constrained and unconstrained multivariate approaches in addition to traditional regression analyses to assess inflammation [12] |
IV. Key Points
Inflammation causes the transition from obese and metabolically healthy to obese and metabolically unhealthy.
Measures of inflammatory changes following bariatric surgery, including TNFα, IL-6, and acute phase proteins (CRP and SAA) are inconsistent despite the demonstrated impact of surgery on weight loss and T2D remission.
Inflammatory proteins shown to predict T2D through multivariate analytical approaches have not been tested for impact on T2D remission following bariatric surgery.
Longitudinal studies, coupled with an appreciation of potential differences in inflammation due to differences in surgical techniques and sample timing will be absolutely essential to assess the importance of inflammation in bariatric surgery outcomes, including T2D remission and T2D recurrence following transient remission.
Acknowledgments
Financial support and sponsorship: Supported by R01 DK108056 and The Boston University CTSI UL1TR001430.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
VI. References and Recommended reading
Papers of particular interest, published within the annual period of review, (18 months/2015–2016) have been highlighted as:
* of special interest
** of outstanding interest
- 1.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Annals of surgery. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Annals of surgery. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 350–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yska JP, van Roon EN, de Boer A, et al. Remission of Type 2 Diabetes Mellitus in Patients After Different Types of Bariatric Surgery: A Population-Based Cohort Study in the United Kingdom. JAMA Surg. 2015:1–8. doi: 10.1001/jamasurg.2015.2398. [DOI] [PubMed] [Google Scholar]
- **4.Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. This statement provides very recent clinical guidelines summarizing when to consider surgical options in the diabetes treatment algorithm. [DOI] [PubMed] [Google Scholar]
- 5.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 7.Still CD, Wood GC, Argyropoulos G. DiaRem score: external validation--authors’ reply. Lancet Diabetes Endocrinol. 2014;2:13. doi: 10.1016/S2213-8587(13)70201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **8.Wood GC, Mirshahi T, Still CD, Hirsch AG. Association of DiaRem Score With Cure of Type 2 Diabetes Following Bariatric Surgery. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.0251. The authors show their vailidated score generated from data generally available in medical records allow personalization of the decision to perform RYGB surgery towards the major outcome of T2D remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis GV, Obin MS. ‘Metabolically healthy obesity’: origins and implications. Mol Aspects Med. 2013;34:59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HM, Kim JJ, Kim HJ, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Ip B, Cilfone NA, Belkina AC, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity (Silver Spring) 2016;24:102–112. doi: 10.1002/oby.21243. This primary paper uses an unbiased approach to define T cell inflammatory profiles that distiguish and predict T2D. This work indicates that a more comprehensive analysis (i.e. adding myeloid cells as a second imoprtant source of inflamamtion in T2D) will define “diabetogenic inflammation” in T2D for the first time to identify physiologically important targets for therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolajczyk BS. B cells as under-appreciated mediators of non-auto-immune inflammatory disease. Cytokine. 2010;50:234–242. doi: 10.1016/j.cyto.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2:38–45. doi: 10.1016/S2213-8587(13)70070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2014;22:888–894. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patriti A, Facchiano E, Sanna A, et al. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14:840–848. doi: 10.1381/0960892041590818. [DOI] [PubMed] [Google Scholar]
- 20.Manning S, Pucci A, Batterham RL. GLP-1: a mediator of the beneficial metabolic effects of bariatric surgery? Physiology (Bethesda) 2015;30:50–62. doi: 10.1152/physiol.00027.2014. [DOI] [PubMed] [Google Scholar]
- **21.Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care. 2016;39:893–901. doi: 10.2337/dc16-0145. This manuscript reviews the constellation of factors that likely mediate post-bariatric surgery glycemic imporvement towards identifying non-surgical therapies that might mimic surgically-associated T2D remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Ortega FJ, Vilallonga R, Xifra G, et al. Bariatric surgery acutely changes the expression of inflammatory and lipogenic genes in obese adipose tissue. Surg Obes Relat Dis. 2016;12:357–362. doi: 10.1016/j.soard.2015.08.498. The authors collect subcutaneous and visceral adipose tissue at the beginning and end of RYGB to show surgery acutely changes expression of multiple lipogenic and inflamamtory genes. [DOI] [PubMed] [Google Scholar]
- *24.Lindegaard KK, Jorgensen NB, Just R, et al. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetology & metabolic syndrome. 2015;7:12. doi: 10.1186/s13098-015-0012-9. This longitudinal analysis examined serveral inflmmatory markers following RYGB, with analysis of samples 1 wk post-surgery potentially shedding insights into the almost immediate post-surgical T2D remission noted for some patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rideout DA, Peng Y, Rakita SS, et al. Roux-en-Y gastric bypass alters tumor necrosis factor-alpha but not adiponectin signaling in immediate postoperative period in obese rats. Surg Obes Relat Dis. 2010;6:676–680. doi: 10.1016/j.soard.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 27.Kraakman MJ, Allen TL, Whitham M, et al. Targeting gp130 to prevent inflammation and promote insulin action. Diabetes Obes Metab. 2013;15(Suppl 3):170–175. doi: 10.1111/dom.12170. [DOI] [PubMed] [Google Scholar]
- 28.Matthews VB, Allen TL, Risis S, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- 29.Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 30.Castoldi G, Galimberti S, Riva C, et al. Association between serum values of C-reactive protein and cytokine production in whole blood of patients with Type 2 diabetes. Clin Sci (Lond) 2007;113:103–108. doi: 10.1042/CS20060338. [DOI] [PubMed] [Google Scholar]
- 31.Miller GD, Nicklas BJ, Fernandez A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2011;7:618–624. doi: 10.1016/j.soard.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netto BD, Bettini SC, Clemente AP, et al. Roux-en-Y Gastric Bypass Decreases Pro-inflammatory and Thrombotic Biomarkers in Individuals with Extreme Obesity. Obes Surg. 2014 doi: 10.1007/s11695-014-1484-7. [DOI] [PubMed] [Google Scholar]
- 33.Montecucco F, Lenglet S, Quercioli A, et al. Gastric bypass in morbid obese patients is associated with reduction in adipose tissue inflammation via N-oleoylethanolamide (OEA)-mediated pathways. Thromb Haemost. 2015;113:838–850. doi: 10.1160/TH14-06-0506. [DOI] [PubMed] [Google Scholar]
- 34.Viana EC, Araujo-Dasilio KL, Miguel GP, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-alpha. Prospective clinical trial. Obes Surg. 2013;23:1252–1261. doi: 10.1007/s11695-013-0894-2. [DOI] [PubMed] [Google Scholar]
- 35.Catalan V, Gomez-Ambrosi J, Ramirez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–1474. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- 36.Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–955. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]
- *37.Kratz M, Hagman DK, Kuzma JN, et al. Acute improvements in glycemic control after gastric bypass occur despite persistent adipose tissue inflammation. Obesity. 2016 doi: 10.1002/oby.21524. In Press. This work is a first attempt to more comprehensively assess longitudinal post-RYGB changes in inflammation in a well-characterized clinical population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- *39.Sams VG, Blackledge C, Wijayatunga N, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surgical endoscopy. 2015 doi: 10.1007/s00464-015-4638-3. These authors report a longitudinal analysis of inflammatory cytokines in serum and, most uniquely, subcutaneous adipose tissue following RYGB and gastric banding. [DOI] [PubMed] [Google Scholar]
- 40.Kopp HP, Kopp CW, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042–1047. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 41.Laimer M, Ebenbichler CF, Kaser S, et al. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes Relat Metab Disord. 2002;26:659–662. doi: 10.1038/sj.ijo.0801970. [DOI] [PubMed] [Google Scholar]
- 42.Samaras K, Viardot A, Botelho NK, et al. Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia. 2013;56:2564–2572. doi: 10.1007/s00125-013-3033-7. [DOI] [PubMed] [Google Scholar]
- 43.Moschen AR, Molnar C, Geiger S, et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 44.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Cron L, Allen T, Febbraio MA. The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. J Exp Biol. 2016;219:259–265. doi: 10.1242/jeb.129213. [DOI] [PubMed] [Google Scholar]
- 46.Holdstock C, Lind L, Engstrom BE, et al. CRP reduction following gastric bypass surgery is most pronounced in insulin-sensitive subjects. Int J Obes (Lond) 2005;29:1275–1280. doi: 10.1038/sj.ijo.0803000. [DOI] [PubMed] [Google Scholar]
- 47.Herrera MF, Pantoja JP, Velazquez-Fernandez D, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010;33:1413–1418. doi: 10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hakeam HA, O’Regan PJ, Salem AM, et al. Inhibition of C-reactive protein in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:456–460. doi: 10.1007/s11695-008-9729-y. [DOI] [PubMed] [Google Scholar]
- 50.Mallipedhi A, Prior SL, Barry JD, et al. Changes in inflammatory markers after sleeve gastrectomy in patients with impaired glucose homeostasis and type 2 diabetes. Surg Obes Relat Dis. 2014;10:1123–1128. doi: 10.1016/j.soard.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 51.Kim MK, Jang EH, Hong OK, et al. Changes in serum levels of bone morphogenic protein 4 and inflammatory cytokines after bariatric surgery in severely obese korean patients with type 2 diabetes. International journal of endocrinology. 2013;2013:681205. doi: 10.1155/2013/681205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Buck M, Gouwy M, Wang JM, et al. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2015 doi: 10.1016/j.cytogfr.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, He X, Shi X, et al. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res. 2010;59:323–334. doi: 10.1007/s00011-010-0163-y. [DOI] [PubMed] [Google Scholar]
- 54.Yang RZ, Lee MJ, Hu H, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King VL, Thompson J, Tannock LR. Serum amyloid A in atherosclerosis. Curr Opin Lipidol. 2011;22:302–307. doi: 10.1097/MOL.0b013e3283488c39. [DOI] [PubMed] [Google Scholar]
- 56.Kotani K, Satoh N, Kato Y, et al. A novel oxidized low-density lipoprotein marker, serum amyloid A-LDL, is associated with obesity and the metabolic syndrome. Atherosclerosis. 2009;204:526–531. doi: 10.1016/j.atherosclerosis.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Poitou C, Coussieu C, Rouault C, et al. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 2006;14:309–318. doi: 10.1038/oby.2006.40. [DOI] [PubMed] [Google Scholar]
- 58.Schernthaner GH, Kopp HP, Kriwanek S, et al. Effect of massive weight loss induced by bariatric surgery on serum levels of interleukin-18 and monocyte-chemoattractant-protein-1 in morbid obesity. Obes Surg. 2006;16:709–715. doi: 10.1381/096089206777346763. [DOI] [PubMed] [Google Scholar]
- 59.Schernthaner GH, Kopp HP, Krzyzanowska K, et al. Soluble CD40L in patients with morbid obesity: significant reduction after bariatric surgery. Eur J Clin Invest. 2006;36:395–401. doi: 10.1111/j.1365-2362.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 60.Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94:450–458. doi: 10.3945/ajcn.111.013771. [DOI] [PubMed] [Google Scholar]
- 61.Hindle AK, Edwards C, McCaffrey T, et al. Reactivation of adiponectin expression in obese patients after bariatric surgery. Surgical endoscopy. 2010;24:1367–1373. doi: 10.1007/s00464-009-0780-0. [DOI] [PubMed] [Google Scholar]
- **62.Istfan N, Anderson WA, Apovian C, et al. Racial differences in weight loss, hemoglobin A, and blood lipid profiles after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016 doi: 10.1016/j.soard.2015.12.028. This work analyzes racial differences in glycemic improvement following RYGB, and shows that subjects of African descent are more likely to lose the initial post-surgical imrpovement in glycemic control 2 years post-RYGB. [DOI] [PubMed] [Google Scholar]


