Abstract
Weight loss maintenance is a significant challenge in obesity treatment. During maintenance the “costs” of adhering to weight management behaviors may outweigh the “benefits.” This study examined the efficacy of a novel approach to weight loss maintenance based on modifying the cost-benefit ratio. Individuals who achieved a 5% weight loss (N=75) were randomized to one of three, 10-month maintenance interventions. All interventions were delivered primarily via the Internet. The Standard arm received traditional weight maintenance strategies. To increase benefits, or rewards, for maintenance behaviors, the two cost-benefit intervention conditions received weekly monetary rewards for self-monitoring and social reinforcement via e-coaching. To decrease behavioral costs (boredom) and increase novelty, participants in the cost-benefit conditions also monitored different evidence-based behaviors every two weeks (e.g., Weeks 1 & 2: steps; Week 3 & 4: red foods). The primary difference between the cost-benefit interventions was type of e-coach providing social reinforcement: Professional (CB Pro) or Peer (CB Peer). Study procedures took place in Providence, RI from 2013–2014. Retention was 99%. There were significant group differences in weight regain (p=.01). The Standard arm gained 3.5±5.7kg. In contrast, participants in CB Pro and CB Peer lost an additional 1.8±7.0kg and 0.5±6.4kg, respectively. These results suggest that an Internet delivered cost-benefit approach to weight loss maintenance may be effective for long-term weight control. In addition, using peer coaches to provide reinforcement may be a particularly economic alternative to professionals. These data are promising and provide support for a larger, longer trial.
Clinicaltrials.gov Identifier
Keywords: Weight loss maintenance, cost-benefit, incentives, Internet, e-coaching, peers, professionals, variety
Introduction
Lifestyle interventions for obesity treatment yield excellent short-term weight losses, which reduce risk for diabetes and improve cardiovascular disease risk factors.[1, 2] However, weight regain begins shortly following treatment and within 2 to 3 years, most individuals regain nearly all of the weight that they lost.[3] As such, one of the next major challenges in obesity treatment is to develop efficacious interventions for weight loss maintenance.[4]
A recent NIH meeting on the problem of weight loss maintenance highlighted the multiple barriers, including both physiological and behavioral factors, that make weight loss maintenance so challenging.[4] Since weight regain is to a large extent attributable to decreased adherence to prescribed regimens, one explanation for the decline in adherence is that over time there is a shift in the perceived costs of adherence relative to the perceived benefits.[3–6] During the initial period of active weight loss, there are many powerful benefits, or reinforcers, that promote continued adherence. Weight loss itself and seeing progress on the scale is inherently reinforcing; improvements in health, mood, and appearance occur; and social reinforcement, or compliments from family, friends, and intervention staff, motivate behavior change.[1, 5–10] However, during the period of weight loss maintenance, there is a substantial decrease in benefits for weight management behaviors and an increase in behavioral costs. Weight loss, the most reinforcing aspect of treatment slows or stops.[3, 9] Social reinforcement from interventionists, family, and friends drops off.[6, 7, 11] Moreover, health, mood, and appearance improvements plateau.[6, 8] At the same time that these salient reinforcers decrease, behavioral costs increase. Participants report becoming bored with their weight management regimen (e.g., calorie counting) and that their weight management requires more effort.[11–14] According to behavior change theory, this shift in the cost-benefit ratio (decreasing rewards and increasing response costs) results in a decline in adherence over time, and weight regain occurs.[15, 16] However, the shift in the cost-benefit ratio has never been targeted to improve weight loss maintenance outcomes.
The present trial tested an intervention designed to target the problematic cost-benefit ratio associated with weight loss maintenance. Individuals who achieved a clinically meaningful weight loss (≥5%) in an Internet-based program (Phase I) were randomly assigned to one of three 10-month, Internet-based weight loss maintenance interventions (Phase II): (a) a cost-benefit intervention involving a professional coach, (b) a cost-benefit intervention involving a peer coach, or (c) a standard approach. The cost-benefit interventions included treatment components that were designed to sustain benefits for engaging in weight management behaviors over time –namely, financial incentives and social reinforcement from either a professional or peer coach as well as approaches designed to reduce boredom and thus behavioral costs. The primary hypothesis was that both cost-benefit approaches, regardless of coach type,[17] would yield significantly less weight regain compared to the standard approach over the 10 month maintenance program.
Methods
Design overview
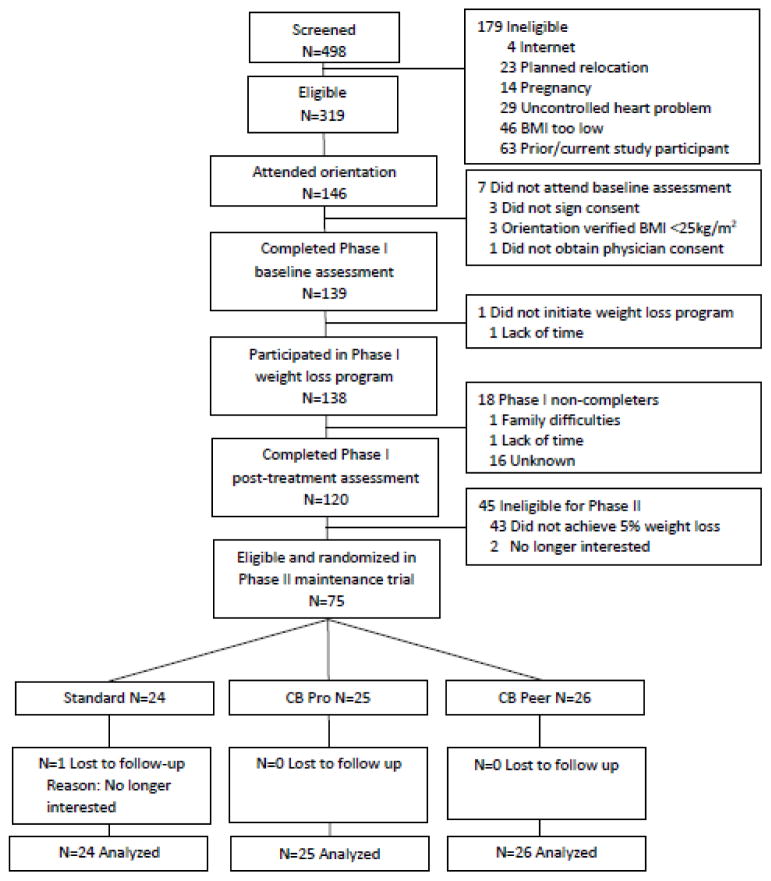
This trial involved two phases. Phase I was an 8 week weight loss phase for all participants. Those who lost >=5% of initial body weight during Phase I were eligible for Phase II, the maintenance trial. Phase II was a randomized trial comparing three different 10-month maintenance programs: (a) a standard maintenance approach (Standard), (b) a cost-benefit approach in which social reinforcement was provided by a professional coach (CB Pro), or (c) a cost-benefit approach in which social reinforcement was provided by a peer coach (CB Peer). The primary outcome was weight regain from the beginning of the maintenance program (randomization) to its end (month 10; Figure 1). This trial was executed at a research center in Providence, RI from 2013–2014. This center includes group rooms and exam rooms where weights and heights were privately assessed. All participants provided written informed consent to participate and were enrolled by study staff.
Figure 1.
Participant flow (Providence, RI, 2013–2014).
Phase 1
A total of N=138 individuals were recruited and participated in a web-based behavioral weight loss program. Eligibility criteria included age 18–70, BMI>=25kg/m2, no current or planned pregnancy or uncontrolled medical condition (e.g., heart problem), no planned relocation or previous study participation, willingness to engage in an Internet weight loss program, and English-speaking. Those who reported a medical condition affected by weight loss were required to provide physician consent to participate.
During Phase I, all participants were offered a web-based weight loss program. This program started with a one-hour group session during which all participants received their weight loss goal (1–2 lbs/week; 5% overall), dietary goals (<250lbs: 1200–1500kcals/day, 30–40g fat/day; >250 lbs: 1500–1800kcals/day, 40–50g fat/day), and physical activity goal (250min/week). They were also taught how to accurately self-monitor their weight, calories, and physical activity. After the one-time group session, the entire weight loss intervention was delivered via the web and included weekly multimedia videos based on the Diabetes Prevention Program[18] and a self-monitoring platform where participants entered weight, diet, and activity information and received automated, tailored feedback on their progress. We have published extensively on this program; results show that it yields clinically meaningful weight losses.[19–21]
Phase II
Participants who lost ≥5% of initial body weight at the end of Phase I (N=75) were eligible to participate in Phase II, the actual maintenance trial. A 5% weight loss threshold was chosen because individuals must have lost weight in order to maintain it and 5% is clinically meaningful.[22] Participants were randomly assigned by the study statistician to one of the three maintenance interventions. Simple computerized randomization was used. Time between end of Phase I and start of Phase II was 20.7±0.7 days (range=20–25 days). During this time all assessment and randomization procedures were conducted. Participants were informed of their randomization assignment at their Phase II group session (see below). The single group session offered to each maintenance group was conducted by a PhD or Masters level interventionist with training in behavioral weight control.
Standard
The Standard intervention included a single, one-hour group session plus periodic email contact. During the group session, participants in Standard were taught traditional evidence-based weight loss maintenance strategies, including self-regulation skills.[23] Intervention staff highlighted the importance of self-monitoring weight, diet, and activity; continuing to eat a low calorie/low fat diet; and engaging in 300 minutes of moderate to vigorous physical activity each week.[24, 25] They were instructed that if their weight was trending up, they should resume strategies used for weight loss, including prepackaged meals and meal replacements. During the group session, participants also completed a goal setting activity in which they specified their weekly weight, calorie, and activity goals depending on whether they wanted to continue to lose weight or maintain; developed a plan to achieve the goals; self-selected a reward for goal achievement; and brainstormed problem solving solutions in the event that they did not meet their goals. Intervention staff also encouraged participants in this arm to engage in specific behavioral strategies associated with long-term weight control including breakfast eating, reducing sedentary time, and developing a consistent eating and activity routine.[26–28] In addition to this one-time group session, all Standard participants received monthly informational emails from a research assistant that included problem solving strategies to promote physical activity and reduce caloric intake plus access to healthy, low calorie recipes.
Cost-benefit approach with a professional coach (CB Pro)
CB Pro participants also attended one group session during which they were oriented to their program and encouraged to engage in core weight maintenance strategies as noted above. After this one-hour session, CB Pro participants also received their entire intervention via email.
The CB intervention included several components designed to address the high cost-benefit ratio thought to undermine weight loss maintenance.[3, 5–7] Specifically, to increase “benefits,” or rewards, for weight management, two universal reinforcers were used: social reinforcement and monetary reinforcement. To decrease boredom or “costs” of long-term adherence, the maintenance program included a variety of different evidence-based strategies for weight loss maintenance, and the specific behavioral strategy changed every two weeks. Each of these approaches is detailed below.
Given that self-monitoring is consistently associated with better maintenance outcomes,[25, 29] both the social and financial reinforcers were provided contingent on self-monitoring. Each week participants self-monitored their weight and the prescribed diet or activity behavior for at least 5 days, and emailed the self-monitoring data to their professional coach (a registered dietitian with training in behavioral weight control), they received an email from their coach providing social reinforcement (support, encouragement). If they did not email the information, no social reinforcement or contact from the coach was provided.
In addition, each week that participants self-monitored and submitted ≥5 days of weight and diet or activity information to their coach, they received a monetary reinforcement. Participants were informed that they would receive between $1–$10/week for submitting their records, but the specific schedule of reinforcers was not provided to participants in advance. To reinforce and engage participants at the outset, larger incentives were delivered at the beginning (e.g., $10, $5, $8) and incentive size varied thereafter (e.g., $1, $7, $2). If participants completed all reporting, they earned a maximum of $160 during the entire maintenance program. When participants submitted the required information to their coach, they received an email with the amount of money earned that week and their total “bank.” Weekly reminders to submit self-monitoring information were framed using regret aversion language (“Don’t miss out on your money, be sure to submit your information by Sunday at midnight”). Weight maintenance was also incentivized. Those who maintained their weight loss in full at their assessment session received $25. Thus, maximum potential earnings were $185/participant, provided at the assessment visit.
To reduce behavioral “costs” for engaging in weight management behaviors, behavioral variety was used.[12, 30, 31] Instead of counting calories for the duration of the program (as is traditionally done and was encouraged in the Standard arm), participants in the cost-benefit intervention monitored different evidence-based weight management behaviors for 2 week periods each. This approach was used to mitigate boredom and thus effort. Specifically, every two weeks, participants were emailed a new behavior to monitor (e.g., Weeks 1–2: pedometer steps; Weeks 3–4: “red foods”) and given goals for each behavior (e.g., 10,000 steps/day; limit “red foods” to 1 serving/day).
Cost-benefit approach with a peer coach (CB Peer)
The CB Peer arm involved the same one-time group session and cost-benefit components as CB Pro (social reinforcement, monetary reinforcement, and behavioral variety); however, instead of a professional coach, participants received a peer coach. Participants were randomly paired (within gender) with another individual in their arm and provided reciprocal peer coaching to one another via email. All peer coach participants received a 1.5 hour training session in how to be a reinforcing and supportive coach. During each week of the maintenance program, when peer coach dyads exchanged information about their weight over that week and adherence to the prescribed diet or activity behavior, they provided each other with social reinforcement. Moreover, while the total incentive amount was identical to CB Pro (maximum of $185/participant), the incentive structure had a social contingency. To receive the weekly incentive, BOTH participants in each peer coach dyad had to email ≥5 days of self-monitoring data to each other. Similarly, to receive the $25 weight maintenance incentive, BOTH participants had to maintain their weight loss in full.
Assessments
Assessments were conducted by research staff blinded to intervention arm.
Demographics
Participant characteristics were collected at Phase I baseline.
Adherence
To track adherence to the coaching paradigm, participants “cc’d” a research assistant on all coaching correspondence. Using these data, percentage of weeks participants submitted information to their coach was calculated. Retention was defined as completion of the final assessment visit.
Weight and height
Weight was assessed using a digital scale before and after Phase I and at the end of Phase II. Height was assessed using a stadiometer at Phase I baseline. Assessments of weight and height were completed in private exam rooms.
Statistical analyses
Statistical analyses were performed using SAS 9.4 for Windows. Analyses of variance (ANOVA) and chi-square tests were used to test for group differences in baseline characteristics and adherence. The primary outcome analysis examined weight regain over the 10 month maintenance intervention, and used an intent-to-treat framework in which missing data (n=1 participant) was imputed at .3 kg/month of weight gain over the 10 month follow-up period. This assumption is based on natural weight trajectories over time and has been used in prior studies.[32, 33] The primary outcome variable of weight regained was not normally distributed and was transformed using a rank-based normalizing transformation.[34] The transformed variable was rescaled back to the original metric before performing the ANOVA. Overall significant group differences on the primary outcome were followed by post-hoc pairwise multiple group contrasts using a stepdown bootstrap procedure that controls Type I error for multiple group contrasts at p=.05.[35, 36] Logistic regression was used to examine group differences in percent of participants achieving 5% weight loss at final assessment and percent who achieved full weight loss maintenance, defined as regaining less than 2.3kg over the 10 months of maintenance.[37] Finally, power analysis indicated that a total of N=75 participants yields .87 power assuming a 3.5kg delta between CB and Standard groups with α at .05.
Results
Participants
All participants (N=75) lost a minimum of 5% of initial weight for eligibility in the maintenance trial, with a mean percent weight loss of 8.0±2.1. There were no group differences in baseline characteristics (Table 1). Retention was 99%.
Table 1.
Participant baseline characteristics (Providence, RI, 2013–2014).
| Total (N=75) | CB Pro (N=25) | CB Peer (N=26) | Standard (N=24) | p-value | |
|---|---|---|---|---|---|
| Female, n (%) | 64 (85.3) | 22 (88.0) | 22 (84.6) | 20 (83.3) | 0.89 |
|
| |||||
| Age, mean (SD) | 48.5 (10.7) | 47.6 (11.9) | 46.9 (11.6) | 51.1 (8.2) | 0.34 |
|
| |||||
| Race, n (%) | 0.36 | ||||
| White | 66 (88.0) | 21 (84.0) | 22 (84.6) | 23 (95.8) | |
| Non-White | 9 (12.0) | 4 (16.0) | 4 (15.4) | 1 (4.2) | |
|
| |||||
| Ethnicity, n (%) | 0.42 | ||||
| Not Hispanic/Latino | 73 (97.3) | 24 (96.0) | 25 (96.2) | 24 (100.0) | |
| Hispanic/Latino | 1 (1.3) | 0 (0.0) | 1 (1.3) | 0 (0.0) | |
| Declined to answer | 1 (1.3) | 1 (1.3) | 0 (0.0) | 0 (0.0) | |
|
| |||||
| Education | 0.20 | ||||
| High School (10–12yr) | 3 (4.0) | 1 (4.0) | 2 (7.7) | 0 (0.0) | |
| Vocational (> HS) | 4 (5.3) | 0 (0.0) | 1 (3.8) | 3 (12.5) | |
| Some college (<4yr) | 13 (17.3) | 8 (32.0) | 2 (7.7) | 3 (12.5) | |
| College/Univ Grad | 29 (38.7) | 8 (32.0) | 11 (42.3) | 10 (41.7) | |
| Grad/Prof School | 26 (34.7) | 8 (32.0) | 10 (38.5) | 8 (33.3) | |
|
| |||||
| Income | 0.10 | ||||
| 0–$25,000 | 2 (2.7) | 0 (0.0) | 0 (0.0) | 2 (8.3) | |
| $25,001–$50,000 | 10 (13.3) | 6 (24.0) | 2 (7.7) | 2 (8.3) | |
| $50,001–$75,000 | 15 (20.0) | 5 (20.0) | 3 (11.5) | 7 (29.2) | |
| $75,001–$100,000 | 14 (18.7) | 5 (20.0) | 8 (30.8) | 1 (4.2) | |
| $100,001–$125,000 | 16 (21.3) | 3 (12.0) | 6 (23.1) | 7 (29.2) | |
| $125,000+ | 18 (24.0) | 6 (24.0) | 7 (26.9) | 5 (20.8) | |
|
| |||||
| Phase I % wt Loss (SD) | 8.0 (2.1) | 7.9 (1.9) | 8.3 (2.3) | 7.8 (2.1) | 0.59 |
|
| |||||
| Phase I kg wt loss (SD) | −7.2 (2.1) | −7.3 (1.9) | −7.6 (2.4) | −6.7 (1.6) | 0.23 |
|
| |||||
| Phase II baseline BMI, mean (SD) | 31.5 (5.8) | 31.6 (5.9) | 30.6 (5.0) | 29.4 (4.3) | 0.33 |
|
| |||||
| Phase II baseline weight kg, mean (SD) | 85.7 (19.5) | 88.8 (20.9) | 85.8 (19.2) | 77.5 (15.3) | 0.10 |
Adherence
Participants were highly adherent to the coaching paradigm (Table 2). Participants in CB Pro and CB Peer emailed their coach at least 5 days of self-monitoring data on 85.0% and 92.8% of intervention weeks, respectively. As a result, incentive earnings did not significantly differ by group (CB Pro: $3.43±0.78/week, CB Peer: $3.52±0.77/week, p=0.70). Similarly, the percentage of participants who emailed at least 5 days of self-monitoring information on all 40 intervention weeks, thus earning the full $160 self-monitoring incentive, did not differ by arm (CB Pro: 24%, CB Peer: 27%, p=.81). In addition, both types of coaches were highly adherent to providing feedback (CB Pro: 100% of weeks, CB Peer: 83.1% of weeks).
Table 2.
Adherence and weight outcomes by intervention arm (Providence, RI 2013–2014).
| CB Pro (N=25) | CB Peer (N=26) | Standard (N=24) | |
|---|---|---|---|
| Adherence | |||
| Weeks (out of 40) 5 days of self-monitoring data submitted, n (%) | 34 (85.0)a | 37 (92.8)a | - |
| Weekly earnings, mean (SD) | $3.43 (0.78)a | $3.52 (0.77)a | - |
| Earned maximum self-monitoring incentive, n (%) | 6 (24)a | 7 (27)a | - |
| Phase II weight regain/maintenance | |||
| Kg weight change, mean (SD) | −1.8 ± 7.0a | −0.5 ± 6.4a | +3.5 ± 5.7b |
| % weight change, mean (SD) | −1.6 ± 7.4a | −0.3 ± 6.0a | +4.0 ± 6.3b |
| Maintained weight loss in full, n (%) | 18 (72)a | 19 (73)a | 8 (33)b |
| Maintained a clinically meaningful (≥5%) weight loss, n (%) | 19 (76)a | 17 (65)a,b | 9 (38)b |
| Overall weight loss (Phase I start to Phase II end) | |||
| % wt loss, mean (SD) | 9.7 (7.1)a | 8.6 (6.3)a | 4.0 (6.3)b |
| Kg weight loss, mean (SD) | −9.1 (9.9)a | −8.1 (5.7)a | −3.2 (4.1)b |
Note: Within variable values with different superscripts (e.g., a vs. b) differ significantly from one another at p<.05. Values with the same superscripts (e.g., a vs. a) do not significantly differ.
Weight Regain
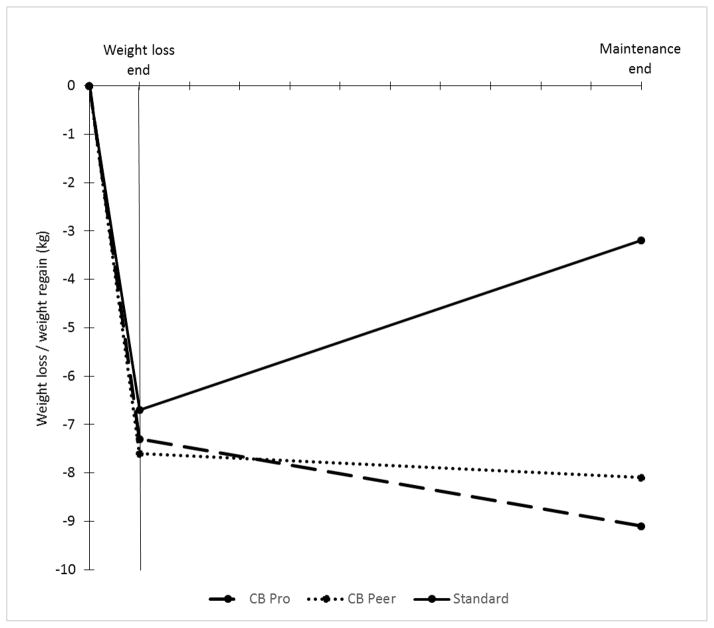
There was an overall significant group effect (p=.01) for weight regain over the maintenance period (Table 2). CB Pro (M=−1.8±7.0) and CB Peer (M=−0.5±6.4) regained significantly (p=0.012 and p=0.049, respectively) less weight than Standard (M=3.5±5.7), but were not different from each other (p=0.47).
Weight Loss Maintenance
A logistic regression analysis compared percent of participants in each group who achieved full weight loss maintenance at month 10, defined as regaining 2.3kg or less.[37] A significantly greater percentage of participants in CB Pro (72%) and CB Peer (73%) maintained their weight loss in full compared to Standard (33%) (p’s≤0.008). The CB Pro and CB Peer groups did not significantly differ (p=0.931).
We also compared the percent of participants in each group who met the 5% weight goal at the end of both Phase I and Phase II. A significantly greater percentage of participants (76%) in CB Pro maintained a 5% weight loss or greater (p=0.008, OR=5.3, 95% CI: 1.5, 18.1) compared to Standard (37.5%). The percentage of participants in CB Peer (65.4%) was at a marginally significant level versus Standard (p=0.052, OR=3.1, 95% CI: 1.0, 10.0). CB Pro and CB Peer groups did not significantly differ from one another (p=0.408, OR=1.7, 95% CI: 0.5, 5.7).
Discussion
According to behavior change theory, weight regain may be explained by a significant shift in the cost-benefit ratio for engaging in weight management behaviors over time. This is the first study to test whether an intervention package that targets the problematic cost-benefit shift improves weight loss maintenance outcomes. Results showed that this intervention, which included provision of ongoing benefits, or rewards, for weight management behaviors via social and monetary reinforcement and reduction in boredom and thus behavioral “costs” by having participants monitor a variety of evidence-based behaviors was effective in improving long-term weight outcomes. Both groups given the cost-benefit intervention continued on average to actually lose weight over the 10 month maintenance period, whereas the standard group had a 3kg weight gain. Thus, this novel intervention, targeting both sides of the cost-benefit ratio, was successful in improving maintenance.
Another important finding from this study was that peer-provided social reinforcement was just as effective as professional for weight loss maintenance. Whereas previous studies have added professional e-coaching to improve outcomes in web-based weight loss programs,[38, 39] this is the first study to test peer e-coaching. Our results showing that peers are as effective as professionals suggest that peers may be a particularly efficient and economical method of maintaining the minimal costs of web programs while also optimizing engagement and efficacy for long-term weight control.
One of the primary reinforcers used in this study – financial incentives – has been used to improve weight loss outcomes but has not been extensively examined for weight loss maintenance.[40] From the 1970s through the 1990s, Jeffery and colleagues conducted a series of financial incentive trials showing that the addition of financial rewards to behavioral weight loss treatment improves weight loss outcomes in the short-term.[40–42] Recently, Volpp and colleagues have continued this line of work with similar effects.[43] In a recent review of the incentive weight loss literature, Jeffery indicated that there is sufficient evidence to conclude that financial incentives improve the efficacy of weight loss programs in the short term; however, studies are needed that evaluate the impact of financial incentives for weight loss maintenance.[40] Results from our trial provide evidence that financial rewards may be beneficial for weight loss maintenance. In addition, findings from the peer arm suggest that making incentive payouts contingent upon dyadic partner performance yield excellent adherence to critical weight management behaviors (i.e., self-monitoring) and maintenance of lost weight. This is consistent with previous findings showing that social contingencies are particularly effective and perhaps more effective than individual contingencies.[44]
Social reinforcement was also used to increase “benefits” for long-term weight management with promising results. These findings are consistent with those of previous studies showing that extending contact with intervention staff and group members significantly improves weight loss maintenance outcomes.[7] Given that extended group contact is beneficial, a “continuous care model” for long-term weight control has gained popularity.[45] However, one of the major drawbacks of this model is that the provision of constant care is expensive and unrealistic in the current healthcare system. Findings from this trial and our previous work[17] suggest that a new approach – peer coaching – may be an excellent model to provide continuous support for weight control. Moreover, unlike professional support, peer coaches have the inherent capacity to provide support and reinforcement indefinitely. Thus, given that the Affordable Care Act provides reimbursement for peer supporters,[46] peer coaching may represent a unique and practical model that allows for sustainable, effective, scalable support for long-term weight control. Moreover, peer support may be particularly cost-effective; however, a formal cost analysis comparing peer support to other types of support (professional) or alternative maintenance interventions is needed.
While social and monetary reinforcement were used to increase “benefits” for weight maintenance, behavioral variety and novelty were used to reduce boredom and thus response “costs.” Findings suggest that such an approach may improve engagement and weight outcomes, which is consistent with results from an earlier study showing that greater exercise variety is associated with better long-term weight control.[47] However, there are also some conflicting findings. An earlier trial examined the effects of behavioral variety on weight outcomes and showed limited benefit.[12] Thus, additional research is needed to determine whether prescribing behavioral variety during obesity treatment improves outcomes.
Lastly, it is important to note that Internet delivery of our cost-benefit intervention yielded promising weight loss maintenance outcomes and high levels of engagement. Previous Internet delivered interventions for long-term weight control have shown mixed effects. Some trials found that Internet approaches outperformed minimal contact control conditions and yielded results similar to in-person interventions.[48, 49] However, others found that Internet treatment had no benefit above and beyond minimal contact and was not as efficacious as in-person intervention for maintenance of lost weight.[50–52] One of the major reasons that Internet approaches are not as effective as in-person for maintenance is poor long-term utilization rates.[53] Our previous work showed that adding small financial incentives improved utilization of a web-based weight loss program.[19] Similarly, results from this trial suggest that modest incentives (average maximum payout of $4/week) coupled with ongoing social reinforcement via professional or peers also facilitate engagement in an Internet program.
This study has some limitations. We aimed to address the entire cost-benefit ratio associated with engaging in behavior change long-term, thus, our intervention involved a treatment package that included multiple reinforcers to maximize benefits plus strategies to reduce response-cost. As such, the impact of the individual intervention components on weight outcomes is unknown. A dismantling study may help to elucidate which components are most important for weight loss maintenance. The sample was predominantly female and White. To understand how this cost-benefit approach works in more diverse populations, additional research is needed. Finally, the intervention was just 10-months in length. A larger, longer trial is needed to determine the long-term efficacy of this approach, including the relative efficacy of peer support vs. professional support for weight control.
This study has several important strengths. The trial focused on weight loss maintenance, a current major challenge in the field of obesity treatment.[4] In addition, it used a rigorous randomized design to test the proposed intervention and included objective measures of primary outcomes and adherence. Retention and engagement were excellent. And, lastly, for decades obesity researchers have theorized that the problematic cost-benefit ratio for engaging in weight management behaviors long-term may explain weight regain following treatment;[3, 5, 6, 8, 12] yet, this is the first trial to test whether targeting these factors actually improves outcomes. Results were promising.
Conclusion
Findings from this randomized trial showed that targeting the problematic cost-benefit ratio associated with weight loss maintenance may be an effective strategy for long-term weight control.
Figure 2.
Phase I weight loss and Phase II weight regain trajectories in kg for CB Pro, CB Peer, and Standard (Providence, RI, 2013–2014).
Highlights.
A cost-benefit intervention promotes excellent weight loss maintenance outcomes
Peer provided support may be just as effective as professional support for weight loss maintenance
Ongoing social and monetary reinforcement and behavioral variety may improve adherence to internet-based interventions
Acknowledgments
This trial was funded by the National Institutes of Health (NIH; R18 DK083248). The NIH had no role in the design, execution, or interpretation of study results.
Footnotes
Conflict of interest statement. Dr. Leahey is currently Chief Scientist and a paid consultant at WayBetter, Inc.; however, the work presented herein is not at all related to her relationship with WayBetter. All remaining authors declare that there are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Look ARG, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery RW, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 4.MacLean PS, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23(1):7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foreyt JP, Goodrick GK, Gotto AM. Limitations of behavioral treatment of obesity: review and analysis. J Behav Med. 1981;4(2):159–74. doi: 10.1007/BF00844268. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery RW, et al. The weight loss experience: a descriptive analysis. Ann Behav Med. 2004;27(2):100–6. doi: 10.1207/s15324796abm2702_4. [DOI] [PubMed] [Google Scholar]
- 7.Perri MG, et al. Effects of peer support and therapist contact on long-term weight loss. J Consult Clin Psychol. 1987;55(4):615–7. doi: 10.1037/0022-006X.55.4.615. [DOI] [PubMed] [Google Scholar]
- 8.Anton SD, Foreyt JP, Peeri MG. Preventing weight regain after weight loss. In: Bray GA, Bouchard C, editors. Handbook of Obesity. Taylor & Francis Group; Boca Raton, FL: 2014. pp. 145–166. [Google Scholar]
- 9.Curtis B, et al. Assessing the effect of weight and weight loss in obese persons with type 2 diabetes. Diabetes Metab Syndr Obes. 2008;1:13–23. doi: 10.2147/dmso.s4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulconbridge LF, et al. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity (Silver Spring) 2012;20(4):783–93. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray G, Bouchard C, editors. Handbook of Obesity Treatment. Taylor and Francis Group; Boca Raton: 2014. pp. 131–144. [Google Scholar]
- 12.Jeffery RW, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Prev Med. 2009;49(5):384–9. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Befort CA, et al. Weight maintenance, behaviors and barriers among previous participants of a university-based weight control program. Int J Obes (Lond) 2008;32(3):519–26. doi: 10.1038/sj.ijo.0803769. [DOI] [PubMed] [Google Scholar]
- 14.Davis EM, et al. Racial and socioeconomic differences in the weight-loss experiences of obese women. Am J Public Health. 2005;95(9):1539–43. doi: 10.2105/AJPH.2004.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HR . Judgment, Decision, and Choice: A Cognitive/Behavioral Synthesis. New York, NY: Freeman; 1989. [Google Scholar]
- 16.JBK . Behavioral Economics and Health. In: Detels, et al., editors. Oxford Textbook of Public Health. Oxford University Press; 2009. [Google Scholar]
- 17.Leahey TM, Wing RR. A randomized controlled pilot study testing three types of health coaches for obesity treatment: Professional, peer, and mentor. Obesity (Silver Spring) 2013;21(5):928–34. doi: 10.1038/oby.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leahey TM, et al. Benefits of adding small financial incentives or optional group meetings to a web-based statewide obesity initiative. Obesity (Silver Spring) 2015;23(1):70–6. doi: 10.1002/oby.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leahey TM, et al. Adding evidence-based behavioral weight loss strategies to a statewide wellness campaign: a randomized clinical trial. Am J Public Health. 2014;104(7):1300–6. doi: 10.2105/AJPH.2014.301870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas JG, Leahey TM, Wing RR. An automated Internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care. 2015;38(1):9–15. doi: 10.2337/dc14-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIH. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 23.Wing RR, et al. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly JE, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 25.Butryn ML, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring) 2007;15(12):3091–6. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 26.Herman KM, et al. Keeping the weight off: physical activity, sitting time, and weight loss maintenance in bariatric surgery patients 2 to 16 years postsurgery. Obes Surg. 2014;24(7):1064–72. doi: 10.1007/s11695-014-1212-3. [DOI] [PubMed] [Google Scholar]
- 27.Phelan S, et al. Holiday weight management by successful weight losers and normal weight individuals. J Consult Clin Psychol. 2008;76(3):442–8. doi: 10.1037/0022-006X.76.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt HR, et al. Long-term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res. 2002;10(2):78–82. doi: 10.1038/oby.2002.13. [DOI] [PubMed] [Google Scholar]
- 29.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 30.Epstein LH, et al. Long-term habituation to food in obese and nonobese women. Am J Clin Nutr. 2011;94(2):371–6. doi: 10.3945/ajcn.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temple JL, et al. Dietary variety impairs habituation in children. Health Psychol. 2008;27(1 Suppl):S10–9. doi: 10.1037/0278-6133.27.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, et al. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161(2):218–27. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 34.Blom G. Statistical estimates and transformed beta-variables. New York: Wiley; 1958. [Google Scholar]
- 35.Westfall P, Young S. On adjusting p-values for multiplicity. Biometrics. 1993;49:941–945. [Google Scholar]
- 36.Westfall P, Wolfinger R. Closed multiple testing procedures and PROC MULTTEST. SAS Observations. 2000 Jul [Google Scholar]
- 37.Stevens J, et al. The definition of weight maintenance. Int J Obes (Lond) 2006;30(3):391–9. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 38.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285(9):1172–7. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 39.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833–6. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 40.Jeffery RW. Financial incentives and weight control. Prev Med. 2012;55(Suppl):S61–7. doi: 10.1016/j.ypmed.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffery RW, Thompson PD, Wing RR. Effects on weight reduction of strong monetary contracts for calorie restriction or weight loss. Behav Res Ther. 1978;16(5):363–9. doi: 10.1016/0005-7967(78)90005-0. [DOI] [PubMed] [Google Scholar]
- 42.Jeffery RW, et al. Monetary contracts in weight control: effectiveness of group and individual contracts of varying size. J Consult Clin Psychol. 1983;51(2):242–8. doi: 10.1037//0022-006x.51.2.242. [DOI] [PubMed] [Google Scholar]
- 43.Volpp KG, et al. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kullgren JT, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013;158(7):505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perri MG, Sears SF, Jr, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care. 1993;16(1):200–9. doi: 10.2337/diacare.16.1.200. [DOI] [PubMed] [Google Scholar]
- 46.Government, U.S; Health, editor. Patient Protection and Affordable Care Act. 2010. pp. 111–148. [Google Scholar]
- 47.Bond DS, et al. The Relationship between Physical Activity Variety and Objectively Measured Moderate-to-Vigorous Physical Activity Levels in Weight Loss Maintainers and Normal-Weight Individuals. J Obes. 2012;2012:812414. doi: 10.1155/2012/812414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey-Berino J, Pintauro SJ, Gold EC. The feasibility of using Internet support for the maintenance of weight loss. Behav Modif. 2002;26(1):103–16. doi: 10.1177/0145445502026001006. [DOI] [PubMed] [Google Scholar]
- 49.Harvey-Berino J, et al. Effect of Internet support on the long-term maintenance of weight loss. Obes Res. 2004;12(2):320–9. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 50.Harvey-Berino J, et al. Does using the Internet facilitate the maintenance of weight loss? Int J Obes Relat Metab Disord. 2002;26(9):1254–60. doi: 10.1038/sj.ijo.0802051. [DOI] [PubMed] [Google Scholar]
- 51.Svetkey LP, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 52.Cussler EC, et al. Maintenance of weight loss in overweight middle-aged women through the Internet. Obesity (Silver Spring) 2008;16(5):1052–60. doi: 10.1038/oby.2008.19. [DOI] [PubMed] [Google Scholar]
- 53.Glasgow RE, et al. Engagement in a diabetes self-management website: usage patterns and generalizability of program use. J Med Internet Res. 2011;13(1):e9. doi: 10.2196/jmir.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]