Summary
Mitochondrial transfer from donor cells to cells of injured tissues is a promising cell-based therapy for effectively bringing about recovery of tissue bioenergetics. Here, we review recent studies on intercellular mitochondrial transfer in organs and cells. We also review studies that shed light on our current understanding of the known mechanisms and conditions that lead to intercellular mitochondrial transfer.
1. Introduction
It is believed that cells intercommunicate by mechanisms such as paracrine signaling, electrical coupling, and gap junctional transport. However, the last decade has seen burgeoning interest in a new mode of cell-cell communication – namely, intercellular organelle transfer (Rogers and Bhattacharya 2013). The field was established by several key findings. Rustom et al showed that PC12 cells support intercellular organelle transfer by nanotube formation (Rustom, Saffrich et al. 2004). Spees et al found that intercellular mitochondrial transfer increases aerobic capacity in mitochondria depleted cells (Spees, Olson et al. 2006). Islam et al reported that intercellular mitochondrial transfer occurs in vivo from bone-marrow-derived mesenchymal stromal cells (BM-MSCs) to alveolar epithelium of endotoxin-treated lungs, and that the transfer protects against the high mortality of endotoxin-induced acute lung injury (Islam, Das et al. 2012). Here we review the recent literature, noting particularly the impact of disease as an instigator of mitochondrial transfer.
2. Mitochondrial transfer in organs
Islam et al's findings were extended by studies by Li et al (Li, Zhang et al. 2014) and Ahmad et al (Ahmad, Mukherjee et al. 2014) who report protective effects of intercellular mitochondrial transfer in other lung diseases. In an in vivo cigarette smoke-induced injury model in rats, Li et al report that intravenous injection of BM-MSCs decreased alveolar wall destruction and fibrosis (Li, Zhang et al. 2014). Interestingly, the protective effect was more pronounced when Li et al injected induced pluripotential cell-derived MSCs (iPS-MSCs). Further, Li et al showed that mitochondrial transfer to co-cultured bronchial epithelial cells was greater for iPS- than BM-MSCs. These findings are notable as first, they point to therapeutic benefit of mitochondrial transfer in a disease characterized by chronic inflammation, suggesting that loss of mitochondrial function also occurs when inflammation is prolonged. Second, the better efficacy of iPS-MSCs suggests that mitochondrial quality control is an issue to be considered when designing studies for efficacy of mitochondrial transfer.
Ahmad et al considered the therapeutic efficacy of mitochondrial transfer from BM-MSCs in the context of asthma (Ahmad, Mukherjee et al. 2014). Their findings indicate that in a mouse model of asthma, airway installation of BM-MSCs attenuates airway hyperreactivity as well as apoptosis of airway epithelium. To determine mechanism, these authors co-cultured BM-MSCs with bronchial epithelial cells (BECs) injured by rotenone treatment. Interestingly, mitochondrial transfer occurred from BM-MSCs to rotenone-treated, but not untreated BECs, reducing apoptosis and improving mitochondrial function. These findings support Spees et al's proposal that mitochondrial transfer from healthy cells targets cells with mitochondrial dysfunction (Spees, Olson et al. 2006).
Some investigators have turned to mitochondrial transplantation as an alternative to intercellular mitochondrial transfer as a strategy for mitochondrial replenishment in injured tissue. Masuzawa et al studied the effects of intravenous injections of autologous mitochondria in an ischemia-perfusion model of cardiac injury (Masuzawa, Black et al. 2013). This procedure enabled good uptake of functionally active mitochondria in the heart, protecting against cardiac injury as indicated by reduced apoptosis of heart muscle and better-preserved cardiac function. In a model of ischemia-reperfusion injury in liver, direct infusion of intact mitochondria into rat spleens protected against loss of liver function and hepatocellular apoptosis (Lin, Liu et al. 2013). More recently, Kitani et al reported uptake of isolated mitochondria by endometrial cells (Kitani, Kami et al. 2014). Evidently, direct cellular uptake of purified mitochondria occurs sufficiently well to afford tissue protection in disease. However, mechanisms remain unclear as to how intravenously injected mitochondria stabilize against injured tissue and are then imbibed by cells.
Although these findings exemplify the protective effects of exogenous mitochondria, recent studies support previous observations (Otsu, Das et al. 2009; Zhang, Raoof et al. 2010) that transferred mitochondria can be proinflammatory. In a study by Boudreau et al (Boudreau, Duchez et al. 2014), activated platelets released respiration competent mitochondria. Given intravenously, the mitochondria transferred to neutrophils, causing cytokine production and neutrophil rolling on endothelium. An additional inflammatory role is evident in a mouse arthritis model, in which there is lipid mediator-dependent neutrophil uptake of mitochondria from intravenously delivered platelet microparticles (Duchez, Boudreau et al. 2015). Other cell types, such as macrophages and dendritic cells that engulf lymphoblast-derived mitochondria also reveal inflammatory responses, since they modulate cytokine production (Maeda and Fadeel 2014). The extent to which naked mitochondria activate damage-associated proinflammatory mechanisms (Zhang, Raoof et al. 2010) versus protective immune responses requires evaluation.
3. Novel modes of mitochondrial transfer
Davis et al report an interesting form of mitochondrial transfer in retinal ganglion cells, which form mitochondria-rich axonal protrusions. In a process of extracellular evulsion, the mitochondria-containing protrusions exit the ganglion cells and are phagocytosed by local astrocytes (Davis, Kim et al. 2014). This phenomenon of ‘transmitophagy’ appears to be a novel mechanism of intercellular mitochondrial transfer that may have implications in other cell types. The extent to which intercellular mitochondrial expulsion occurs in different cell types requires further consideration.
Intercellular mitochondrial transfer has been addressed in the context of development. In cultured fetal mouse ovaries, the maturing oocyte receives mitochondria and Golgi bodies from sister cyst germ cells in an aggregate called the Balbiani body (Lei and Spradling 2016). This mitochondrial transfer is essential for oocyte development. The bioenergetics of the developing oocyte in the context of the ongoing mitochondrial transfer remains to be understood.
Intercellular transfer of mitochondria and mitochondrial contents has also been addressed in cancer. By means of phylogenetic analysis of canine transmissible venereal tumor in feral dog populations, Rebbeck et al showed that over the last 10,000 years tumor cells acquired mitochondrial DNA (mtDNA) from host cells (Rebbeck, Leroi et al. 2011). The authors suggest that since tumor cells have high turnover, tumor mitochondria are prone to mutations and instability. Acquisition of the more ‘fit’ host mitochondria may have facilitated restoration of tumor mitochondrial function.
Pasquier et al, co-cultured breast cancer cells, MCF7, with BM-MSCs or endothelial cells (ECs) (Pasquier, Guerrouahen et al. 2013). They found that ECs, but not BM-MSCs transferred mitochondria to MCF7 cells. In vitro transfer of mitochondria elicited a chemoresistant phenotype in MCF7 cells. Other investigators show that mitochondrial transfer occurs between cultured human mesothelioma cells (Ady, Desir et al. 2014) and human laryngeal squamous carcinoma cells (Antanaviciute, Rysevaite et al. 2014). Antanavicuite et al suggest that the mitochondrial transfer might contribute to tumor proliferation. Cytotoxicity of BM-MSCs was also evident in a tumor model in that high concentration of BM-MSCs abrogated angiogenesis and shrank tumor size (Otsu, Das et al. 2009). To the extent that BM-MSCs support mitochondrial transfer, it is possible that transfer of a high concentration of mitochondria might block tumor angiogenesis.
A role for mtDNA transfer in tumor viability is evident in the study by Tan et al (Tan, Baty et al. 2015). These authors report that intravenous injection of mtDNA deficient tumor cells delayed tumor growth in mice. Interestingly, cells recovered from primary tumors and metastatic cells derived from mtDNA-deficient carcinoma cells contained mtDNA from host cells and showed evidence of restored bioenergetics and mitochondrial function. These data highlight a role for intercellular mitochondrial transfer in tumor growth and metastases and point to new mechanisms that may offer clues to improved chemotherapy.
Mitochondrial transfer also accompanies infection with invasive pathogens. Fascinating findings by Ralston et al indicate that by a process called trogocytosis, the pathogen Entameba histolytica engulfs mitochondria-containing fragments of lymphoid cells and intestinal epithelium (Ralston, Solga et al. 2014). Although amebic trogocytosis promotes death of host cells, the functional role of the engulfed mitochondria in amebic invasion remains unclear. In another example of trogocytosis, the organisms, Francisella tularensis and Salmonella enterica transfer intracellular contents from infected to uninfected macrophages along with cytosolic materials such as plasma membrane components and functional major histocompatibility complex I (MHC-I) (Steele, Radlinski et al. 2016). Presumably, trogocytosis energizes the invading microorganism, although direct evidence of this is lacking.
4. Mechanisms of mitochondrial transfer
Rustom et al's proposal that intercellular organelle transfer occurs through nanotubes (Rustom, Saffrich et al. 2004) has gained momentum. Nanotubes are filopodial protrusions from the donor cell that make contact with the recipient cell membrane, presumably to deliver cargo to the recipient cell's cytosol. BM-MSCs spontaneously generate nanotubes (Fig. 1). Recent studies addressed issues of directionality and molecular mechanisms underlying nanotube transport.
Figure 1. In vitro formation of nanotubes containing mitochondria in bone-marrow derived mesenchymal stem cells (BM-MSC).
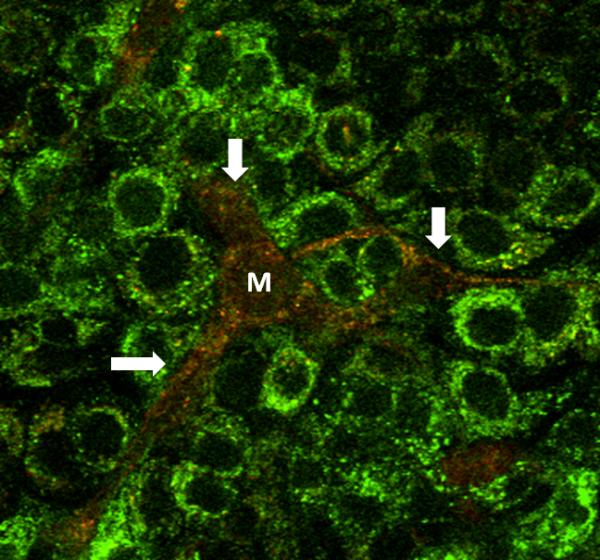
Image of mouse BM-MSC (M) co-cultured with A549 cells. A549 cells (green) were stained with calcein green and BM-MSC (red) are expressing dsRed-Mito. The image shows formation of multiple nanotubes (white arrows) containing mitochondria.
Liu et al, co-cultured hMSCs with human umbilical vein epithelial cells (HUVECs) in an in vitro ischemia-reperfusion injury model (Liu, Ji et al. 2014). These investigators found that MSCs transferred mitochondria via nanotubes and rescued aerobic respiration in HUVECs. They showed inhibiting F-actin polymerization and shielding phosphatidylserines on apoptotic HUVECs abrogated nanotube formation. Although previous studies suggest that nanotubes transport is unidirectional from host to donor cells, Liu et al noted equal bidirectional mitochondria transfer between MSCs and uninjured HUVECs. However, the transfer was unidirectional for injured HUVECs.
Wang et al, co-cultured healthy and UV-injured PC12 cells. These investigators found that mitochondria were donated from healthy cells to injured cells via nanotubes (Wang and Gerdes 2015). Moreover, they also found that apoptotic signals were also passed from injured cells to healthy cells through nanotubes. However, these nanotubes had smaller capacity and were structurally different from those carrying mitochondria. They also found that disrupting nanotube formation or co-culturing injured cells with PC12 cells with defective mitochondria negated protection from apoptosis.
Ahmad et al evaluated the role of the protein, Miro1 (mitochondrial Rho GTPase1) in nanotube transport (Ahmad, Mukherjee et al. 2014). Miro1 is a calcium-sensitive adaptor protein critical for mitochondrial motility along microtubules. In co-cultured BM-MSCs and alveolar epithelial cells, overexpression as opposed to knockdown of Miro1 led to more efficient mitochondrial transfer. Miro1 overexpressing MSCs also had enhanced rescue potential leading to greater epithelial repair.
In a report of nanotube formation in vivo, Islam et al determined that BM-MSCs generate mitochondria-containing nanotubes in the endotoxin injured mouse lung (Fig. 2) (Islam, Das et al. 2012). Mitochondrial transfer from the nanotubes took place through a multi-step process. BM-MSCs stabilized on the alveolar epithelium by establishing connexin-43 (Cx43)-containing gap junctional channels (GJCs). Subsequently, BM-MSCs and nanotubes released microvesicles which alveolar epithelial cells engulfed by clathrin-dependent endocytosis. Consequently, alveolar epithelia acquired mitochondria of BM-MSC origin resulting in increased epithelial ATP production. Formation of the Cx43 GJCs was essential for mitochondrial transfer, since BM-MSCs expressing dysfunctional Cx43 failed to adhere to alveolar epithelium and also failed to transfer mitochondria. Others also report Cx43 to be essential in forming gap junctions with intercellular nanotubes (Antanaviciute, Rysevaite et al. 2014). These findings highlight the importance of understanding mechanisms that stabilize the mitochondria donor cell at the site of mitochondrial transfer to better understand the transfer mechanism.
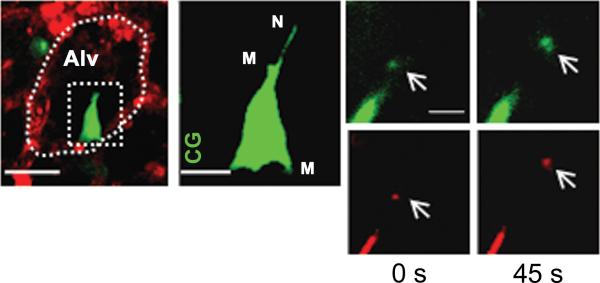
Figure 2. In vivo formation of tunneling nanotubes and microvesicles in bone-marrow derived stem cells (BM-MSC).
Mice were instilled with intranasal LPS and 4 hours after LPS, BM-MSCs were administered by intratracheal installation. We next isolated and blood-perfused the lung and imaged using confocal microscopy. (A) Image shows a mouse alveolus (red) and a mouse BM-MSC (green). The dotted oval structure represents an alveolar unit and the dotted rectangular structure demarcates a BM-MSC. Line , 10 μm. (B) Magnified image of BM-MSC as depicted in the rectangular box in figure (A). The image shows formation of nanotubes (N) and microvesicles (M). CG, calcein green. Line, 5 μm. (C) Panels show microvesicle (arrows) being released from BM-MSc at different time points in green (calcein green) and red (DsRed) renditions. Line, 3 μm. (Islam, Das et al. 2012)
5. Conclusions
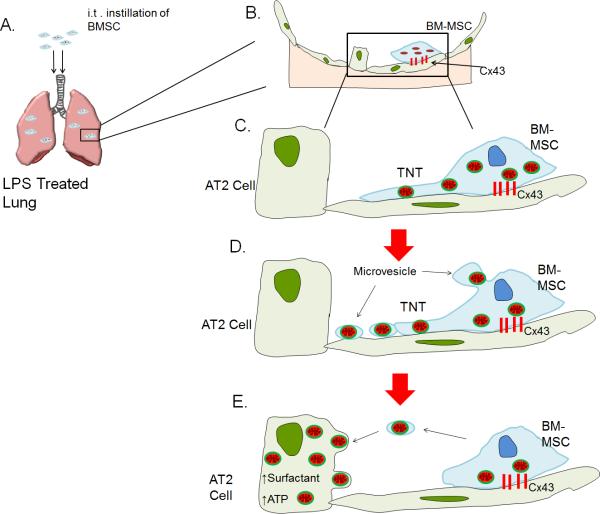
Intercellular mitochondrial transfer represents an exciting new mechanism for restitution of bioenergetics in tissues undergoing pathologic stress. The studies reviewed here have clarified some of the essential components of the mitochondrial transfer process (Fig. 3). These include processes by which donor cells identify and package mitochondria destined for transfer, stabilize against recipient cells, generate cellular processes – nanotubes or microvesicles – that carry mitochondrial cargo to the recipient cell, and processes by which mitochondria are internalized in the recipient cell, then protected degradation in the disease milieu. A better understanding of these mechanisms will present opportunities for meaningful intervention in a variety of diseases in which mitochondrial dysfunction contributes to pathology.
Figure 3. Schematic depiction of intercellular mitochondrial transfer from bone marrow-derived mesenchymal stem cells (BM-MSC) to alveolar type 2 (AT2) epithelial cell in an LPS-treated lung injury model in mice.
(A) Intracheal (i.t.) instillation of BM-MSCs in LPS treated mice leads to migration of BM-MSCs to the alveoli. (B) BM-MSCs stabilize themselves to the alveolar epithelium by forming connexin-43 (Cx43) containing gap junctional channels. (C) BM-MSC form nanotubes (TNTs) containing mitochondria that protrude towards epithelial cells (D) Microvesicles containing mitochondria are released from the end of TNTs and from the body of BM-MSC. (E) Microvesicles are internalized by AT2 cells leading to increased ATP production and surfactant secretion.
Acknowledgments
PS was supported by a Visiting Fellow Award from the Westminster Medical School Research Trust. MNI was supported by an award from the Parker B. Francis Fellowship Program. JB was supported by NIH grants HL36024, HL57556, and HL122730.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ady JW, Desir S, et al. Intercellular communication in malignant pleural mesothelioma: properties of tunneling nanotubes. Frontiers in physiology. 2014;5:400. doi: 10.3389/fphys.2014.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Ahmad T, Mukherjee S, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO journal. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [This study shows the role of MIRO1 in regulating intercellular mitochondrial trafficking. In allergen-induced asthma model they show attenuation of airway responsiveness and epithelial injury with MSC therapy that was enhance with increased MIRO1 expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanaviciute I, Rysevaite K, et al. Long-distance communication between laryngeal carcinoma cells. PloS one. 2014;9(6):e99196. doi: 10.1371/journal.pone.0099196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau LH, Duchez AC, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Davis CH, Kim KY, et al. Transcellular degradation of axonal mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9633–9638. doi: 10.1073/pnas.1404651111. [This is the first study to describe mitochondria shed by retinal ganglion cell axons at the optic nerve head are taken up and degraded by neighbouring astocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchez AC, Boudreau LH, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(27):E3564–3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Islam MN, Das SR, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature medicine. 2012;18(5):759–765. doi: 10.1038/nm.2736. [This is the only study to date showing in vivo real time transfer of mitochondria from BMMSCs to endotoxin injured alveolar epithelial cells via nanotubes and microvesicles. The transfer of mitochondria led to restoration of mitochondrial function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani T, Kami D, et al. Direct human mitochondrial transfer: a novel concept based on the endosymbiotic theory. Transplantation proceedings. 2014;46(4):1233–1236. doi: 10.1016/j.transproceed.2013.11.133. [DOI] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016;352(6281):95–99. doi: 10.1126/science.aad2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Li X, Zhang Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. American journal of respiratory cell and molecular biology. 2014;51(3):455–465. doi: 10.1165/rcmb.2013-0529OC. [MSC therapy led to attentuation of alveolar destruction in an in vivo cigarette smoke-induced chronic lung injury model. MSCs derived from induced pluripotent stem cells were more effective in rescuing mitochondrial function than bone marrow derived MSCs.] [DOI] [PubMed] [Google Scholar]
- Lin HC, Liu SY, et al. Isolated mitochondria infusion mitigates ischemiareperfusion injury of the liver in rats. Shock. 2013;39(3):304–310. doi: 10.1097/SHK.0b013e318283035f. [DOI] [PubMed] [Google Scholar]
- Liu K, Ji K, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvascular research. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-alpha-induced necroptosis act as danger signals. Cell death & disease. 2014;5:e1312. doi: 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Masuzawa A, Black KM, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. American journal of physiology. Heart and circulatory physiology. 2013;304(7):H966–982. doi: 10.1152/ajpheart.00883.2012. [In an rabbit model of regional ischemia, naked mitochondria transplanted in ischaemic regions of the heart were successfully taken up by cardiac myocytes and resulted in rescuing cardiac bioenergetics and preserved function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K, Das S, et al. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier J, Guerrouahen BS, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. Journal of translational medicine. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Solga MD, et al. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508(7497):526–530. doi: 10.1038/nature13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck CA, Leroi AM, et al. Mitochondrial capture by a transmissible cancer. Science. 2011;331(6015):303. doi: 10.1126/science.1197696. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology. 2013;28(6):414–422. doi: 10.1152/physiol.00032.2013. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- **.Spees JL, Olson SD, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [This was the first study to show mitochondrial transfer from MSCs to somatic cells leads to rescue of aerobic function in recipient cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele S, Radlinski L, et al. Trogocytosis-associated cell to cell spread of intracellular bacterial pathogens. eLife. 2016;5 doi: 10.7554/eLife.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Tan AS, Baty JW, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell metabolism. 2015;21(1):81–94. doi: 10.1016/j.cmet.2014.12.003. [Delay in tumor growth and progression due to lack of mitochondrial DNA is reversed by horizontal transfer of mitochondrial DNA from host cells to tumor cells leading to restoration of respiratory and proliferative efficiency in the latter.] [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell death and differentiation. 2015;22(7):1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Zhang Q, Raoof M, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [This paper shows naked mitochondria can induce inflammatory responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]