Abstract
The +3 and +2 oxidation states of europium have drastically different magnetic and spectroscopic properties. Electrochemical measurements are often used to probe EuIII/II oxidation state changes, but a full suite of spectroscopic characterization is necessary to demonstrate conversion between these two oxidation states in solution. Here, we report the facile conversion of a EuIII tetraglycinate complex into its EuII analogue. We present electrochemical, luminescence, electron paramagnetic resonance, UV–visible, and NMR spectroscopic data demonstrating complete reversibility from the reduction and oxidation of the +3 and +2 oxidation states, respectively. The EuII-containing analogue has kinetic stability within the range of clinically approved GdIII-containing complexes using an acid-catalyzed dissociation experiment. Additionally, we demonstrate that the +3 and +2 oxidation states provide redox-responsive behavior through chemical exchange saturation transfer or proton relaxation, respectively. These results will be applicable to a wide range of redox-responsive contrast agents and Eu-containing complexes.
Graphical abstract
The conversion of a EuIII tetraglycinate complex into its EuII analogue is presented with electrochemical, luminescence, electron paramagnetic resonance, UV–visible, and NMR spectroscopic data demonstrating complete reversibility from the reduction and oxidation of the +3 and +2 oxidation states, respectively.
INTRODUCTION
The majority of lanthanide chemistry occurs in the +3 oxidation state, where the relative stability of this valency makes metal-based redox chemistry somewhat uncommon. However, there is growing interest in the formation, characterization, stabilization, and application of complexes containing lanthanides outside of the +3 oxidation state.1–10 For example, it was only in 2013 that the full series of divalent lanthanides was reported.2a The +2 oxidation state for all lanthanides is air sensitive, but the half-filled 4f7 electronic configuration of EuII makes it the most stable divalent lanthanide with respect to oxidation. This stability permits the study of EuII-containing complexes in aqueous media. We are interested in studying aqueous EuII-containing complexes because they are redox-active complexes relevant to responsive magnetic resonance imaging (MRI).8–10
MRI permits non-invasive imaging at high spatial resolution and nearly unlimited depth penetration.11 Images acquired using MRI can be enhanced through the use of paramagnetic complexes (contrast agents) that influence the properties of the nuclei being imaged, which are often the protons on water molecules. In addition to highlighting spatial information using paramagnetic complexes, contrast agents have been designed to relay molecular information via changes in ligand structure or metal oxidation state.8–10,12,13 However, the majority of responsive contrast agents are limited by concentration-dependent responses that make distinguishing pre- and post-response states difficult in vivo.14a A promising platform for concentration-independent responsive imaging is the EuIII/II redox couple. EuII is analogous to the commonly used GdIII ion in providing longitudinal-relaxation-time (T1)-shortening (positive) contrast enhancement.8,9,15–17 However, unlike GdIII, EuII is redox active under physiologically relevant conditions. The EuII ion can undergo a one-electron oxidation to the +3 oxidation state that does not noticeably influence positive contrast enhancement,8,9 and we recently reported the first use of EuII-containing complexes as redox-active contrast agents in vivo.9,10
In one of those reports, a 4-fluorobenzo-functionalized EuII-containing cryptate persisted in the +2 oxidation state in necrotic (oxygen-deficient) tumor tissue of a mammary carcinoma after an intratumoral injection.9 The persistence of EuII within the necrotic tissue allowed the visualization of necrotic tissue using positive contrast enhancement. To highlight the positive contrast enhancement provided by EuII in vivo, we have assembled previously unpublished data from these studies to create web-enhanced objects for this forum article (Figure 1). Our data suggest that EuII was oxidized and cleared from non-necrotic tissue that permitted differentiation of necrotic tissue by 120 min post injection. However, the products of oxidation were not detectable by MRI in our studies. Accordingly, we turned our attention to the synthesis and characterization of a complex detectable in both the +3 and +2 oxidation states of Eu. To enable visualization of the Eu-based contrast agent after oxidation, we combined EuII with a ligand used for chemical exchange saturation transfer (CEST) contrast in MRI. Here, we report a full suite of spectroscopic characterization of a EuIII/II tetraglycinate complex demonstrating the conversion from the +3 to +2 and +2 to +3 oxidation states. Furthermore, we demonstrate the selective suppression of T1 or CEST contrast based on the oxidation state of Eu.
Figure 1.
(Left) Animated sagittal plane flythrough with the tumor highlighted by a red rectangle. (Right) Animated 3-dimensional rendering of a mouse with positive contrast enhancement colored green. Both sets of data are from the same mouse 120 min after an intratumoral injection (50 µL of 19.4 mM solution) of a EuII-containing complex.
We hypothesized that a one-electron reduction of a EuIII-containing MRI contrast agent would produce a EuII-containing complex that provides positive contrast enhancement in T1-weighted MRI, but could be oxidized to the original complex that provides CEST contrast in MRI. Demonstrating conversion between oxidation state changes is necessary for a thorough understanding of the solution-phase behavior of each oxidation state. Accordingly, we selected luminescence, electron paramagnetic resonance (EPR), nuclear magnetic resonance (NMR), and UV–visible spectroscopies as a full suite characterization of both oxidation states in solution. Because of the potential applications of these complexes is in aqueous solution, our particular focus was solution characterization. Accordingly, we selected not to pursue gas- or solid-phase analyses as they do not convey the stability or solution-phase properties of complexes.
We chose EuIII-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(acetamidoacetate) (the ligand is referred to as DOTA-4AmC by the manufacturer) because it contains chelated EuIII, is commercially available, and is among the tetraamide group of contrast agents that are well-characterized with respect to CEST imaging.13,18–20 CEST imaging is an MRI technique that produces contrast via selective radiofrequency presaturation of exchangeable protons. During the saturation event, saturated protons on the contrast agent exchange with non-saturated water protons (commonly referred to as bulk water protons). The saturation event results in a decrease in the bulk water proton signal intensity, and this decrease is used to produce contrast in the resulting images. The ability to detect CEST contrast is affected by the difference in bound- and bulk-water proton resonance frequencies that are often expressed as chemical shifts (units of ppm). Under certain circumstances, relatively small diamagnetic chemical shift differences (<3 ppm) can be imaged in vivo;21 however, magnetization transfer effects between lipids and other macromolecules broaden the bulk water signal in vivo, making contrast agents with relatively large chemical shift offsets desirable.
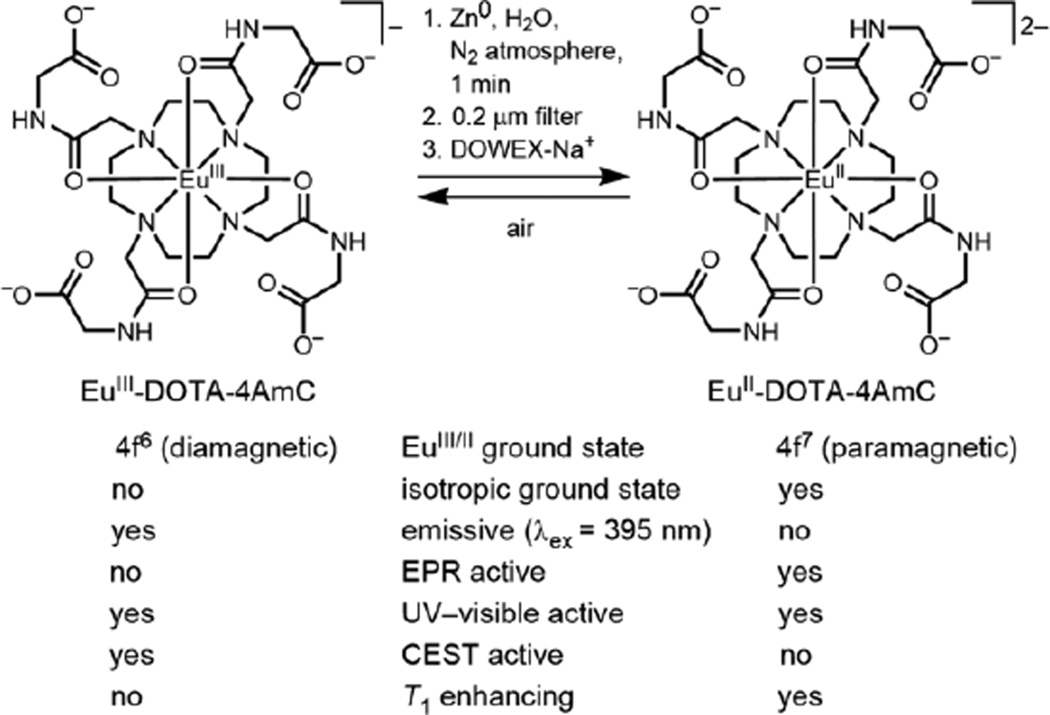
EuIII-DOTA-4AmC paramagnetically shifts bound-water protons downfield from bulk water by 55 ppm, and similar shifts have been imaged in vivo.20 We hypothesized that upon reduction of EuIII-DOTA-4AmC to EuII-DOTA-4AmC, the EuII ion would relax the nuclear dipole moments of nearby protons resulting in the complete quenching of saturation transfer effects. Instead of CEST contrast, we expected EuII-DOTA-4AmC to enhance contrast in T1-weighted MRI in accordance with previously reported EuII-based complexes:8–10,15–17 EuII-DOTA-4AmC would provide positive contrast enhancement until it was oxidized to EuIII-DOTA-4AmC, at which point it would display CEST contrast (Figure 2). Here, we present luminescence, electron paramagnetic resonance (EPR), NMR, and UV–visible spectroscopic data characterizing the conversion from the +3 to +2 and +2 to +3 oxidation states of EuIII/II-DOTA-4AmC.
Figure 2.
Reduction of a commercially available CEST contrast agent (EuIII-DOTA-4AmC) to the T1-shortening EuII-containing analogue (EuII-DOTA-4AmC) using Zn0 under an atmosphere of N2. Oxidation to the +3 oxidation state yields the original complex. The table compares magnetic and spectroscopic properties between EuIII and EuII-containing complexes in the ground state.
RESULTS AND DISCUSSION
Monitoring EuIII/II oxidation state changes and reversibility
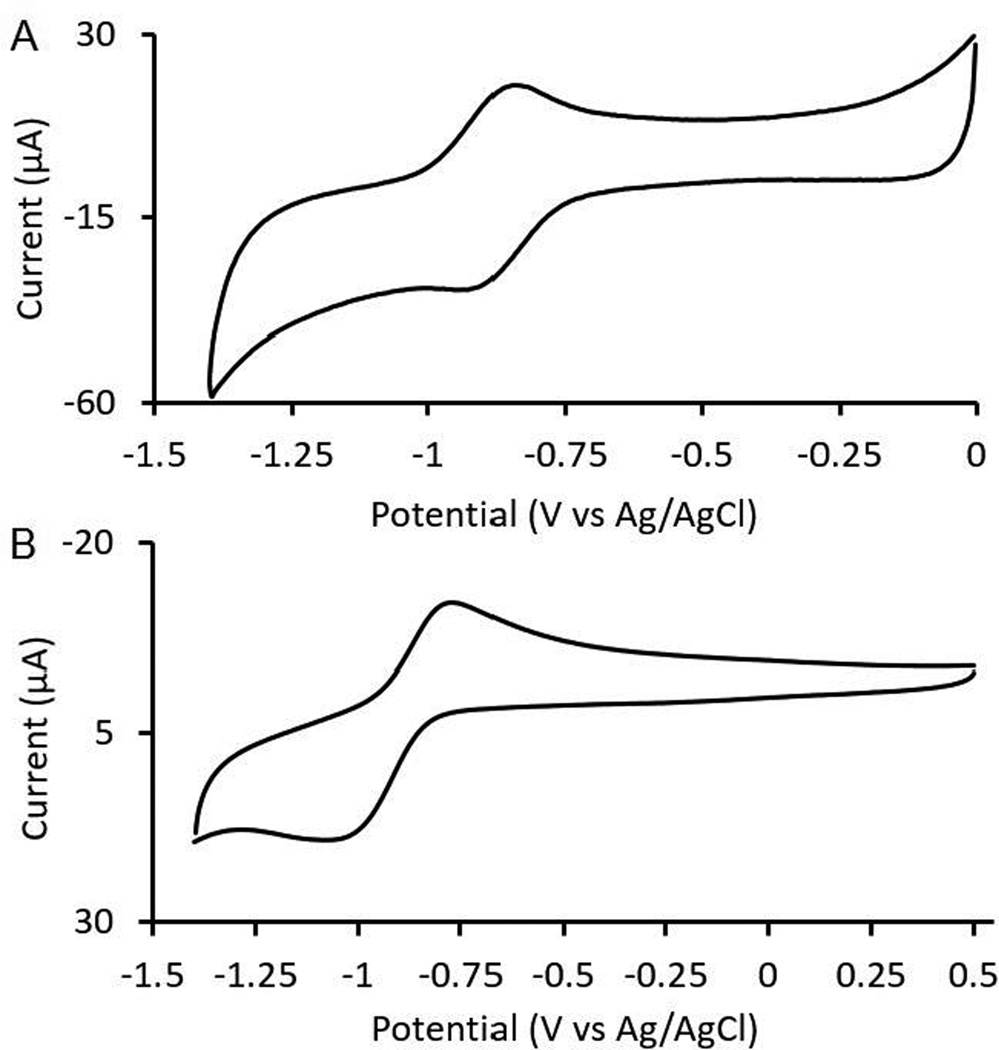
To characterize the electrochemical potential of EuIII-DOTA-4AmC, we performed cyclic voltammetry (Figure 3) and observed a reversible one-electron redox couple with an E1/2 of −0.879 ± 0.001 V vs Ag/AgCl (Epa − Epc = 57 mV). Our value is inconsistent with a report published during the preparation of this manuscript.23 The other report claimed the reduction potential of EuII-DOTA-4AmC to be more positive than the reduction potential of the EuII aqua ion. The observation in the other report is unexpected based on the presence of four amide functional groups that are known to exhibit delocalization of negative charge onto the amide carbonyl oxygens through resonance, but the cyclic voltammograms in that manuscript did not scan to a negative enough potential to observe the same E1/2 observed in Figure 3. To match the conditions of the other report, we acquired a separate cyclic voltammogram using potassium chloride as the supporting electrolyte at pH 7. Under these conditions, we measured an E1/2 of −0.903 V vs Ag/AgCl. The similar E1/2 values at pH 4 and 7 are not surprising given that the electron transfer is not proton coupled. Furthermore, our measured potentials are consistent with electrochemical studies of EuIII/II-based complexes.17,22 In those reports, there is a correlation between increasing numbers of anionic donor functional groups, such as carboxylates, and increasingly more negative reduction potentials. This trend is consistent with what would be expected for electron-rich metal ions like EuII complexed by a ligand with four amide donors.
Figure 3.
Cyclic voltammograms of (A) EuII/III-DOTA-4AmC (3 mM) with tetraethylammonium perchlorate (50 mM) as the supporting electrolyte (pH = 4) and (B) EuII/III-DOTA-4AmC (10 mM) with potassium chloride (100 mM) as the supporting electrolyte (pH = 7).
In addition to having E1/2 values that are reasonable for a tetraamide complex of EuIII/II, the reversible nature of the cyclic voltammograms in our report are consistent with a complex exhibiting reversible electron transfer characteristics. This observation is of fundamental importance when studying cyclen-based ligands that contain carboxylate groups because of the potential for incomplete chelation with these types of complexes. This phenomenon is well-documented with trivalent lanthanide ions and ligands bearing acetate functional groups on cyclen rings.24 The rate of chelation is determined, in part, by the protonation state of the ligand.24 While the addition of hydroxide accelerates the rate of chelation, the rate decreases with decreasing charge density, which is inversely proportional to ionic radius, of the lanthanide being used.24 EuII is larger (ionic radius of ~117 pm) than the largest trivalent lanthanide ion, LaIII (ionic radius of ~103 pm),25 and EuII also has a lower charge than LaIII. The relatively large radius and low charge would be expected to cause EuII to form a complex with cyclen-based ligands much more slowly than any trivalent lanthanide ion. Therefore, we suggest that starting from a EuIII-containing precursor is beneficial when studying the electron transfer chemistry of poly(amino carboxylate) or poly(amino glycinate) complexes.
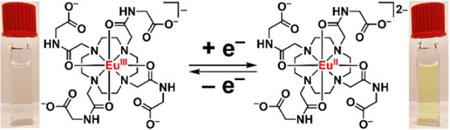
Based on our measured E1/2 of EuIII/II-DOTA-4AmC, we expected Zn0 (E1/2 = −0.960 V vs Ag/AgCl)26 to be a suitable reductant for the EuIII ion in this complex. Upon stirring aqueous EuIII-DOTA-4AmC in the presence of Zn0 under an atmosphere of N2, we observed the rapid (<1 min) formation of a yellow color from the previously colorless solution.
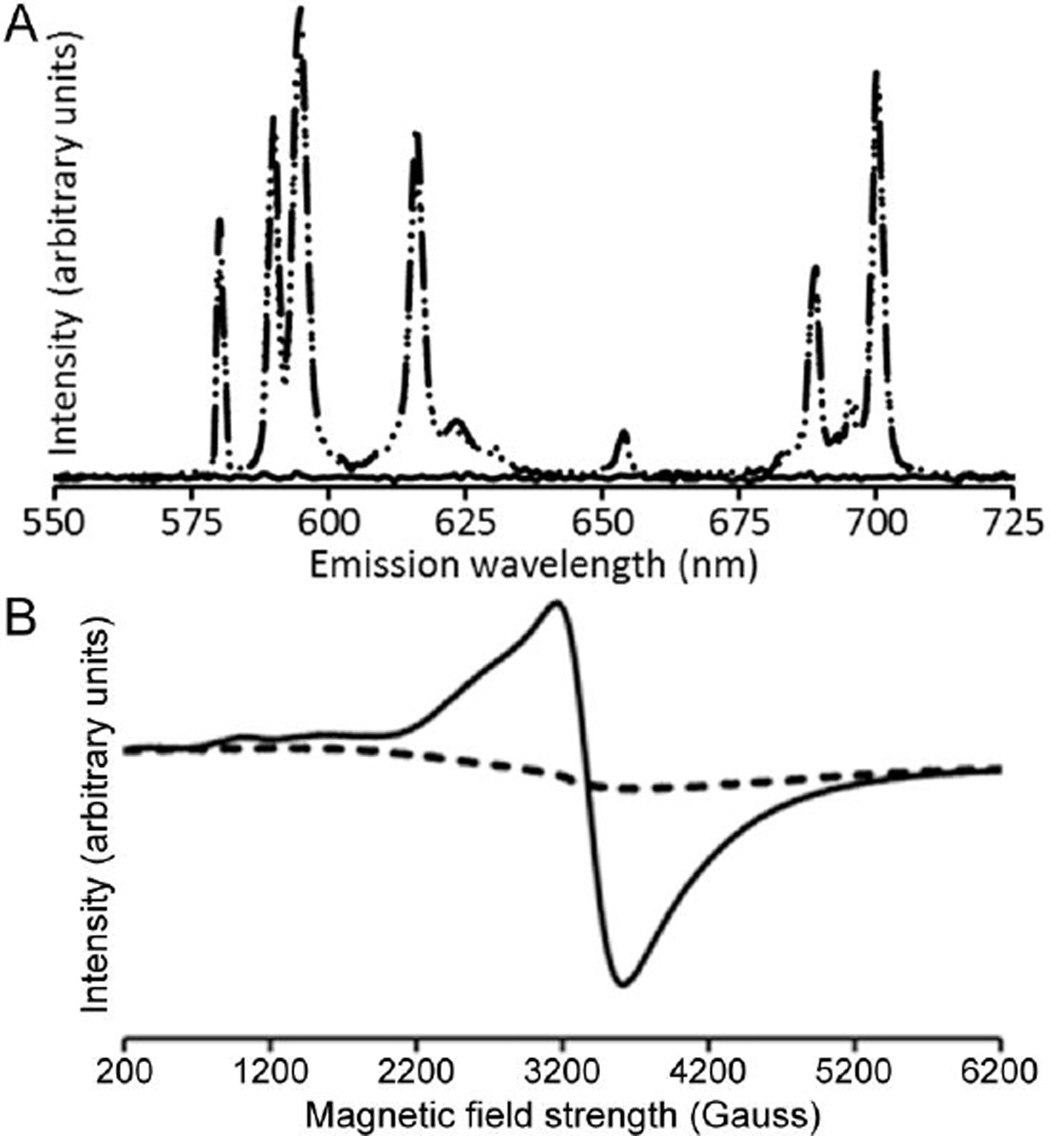
To probe for the presence of EuIII in the yellow solution, we performed luminescence spectroscopy. The emission spectrum of EuIII-DOTA-4AmC contained the characteristic emission bands from the radiative decay of the 5D0 excited state of EuIII to the 7F manifold (Figure 4A), and the yellow solution did not produce observable EuIII-based emissions despite having the same concentration of Eu. To quantify the loss of EuIII, we measured the minimum detectable concentration of EuIII using the emission of EuIII-DOTA-4AmC at 595 nm. The minimum detectable concentration of EuIII-DOTA-4AmC was 20.9 µM, indicating that the reduction reaction removed ≥99.0% of the initial EuIII. However, bubbling air into the yellow solution resulted in a colorless solution that produced an emission spectrum identical to EuIII-DOTA-4AmC in both emission wavelength and absolute intensity. These data suggest that the reaction with Zn0 was responsible for the absence of EuIII-based emissions in the yellow solution, and that the species responsible for the yellow color reacts with air to reform EuIII-DOTA-4AmC. Additionally, the complete recovery of the 5D0 to 7F2 emission at 615 nm, which is hypersensitive to coordination environment, strongly suggests that the EuIII coordination environment of the EuIII-DOTA-4AmC starting material and the complex formed after oxidation of the reduced species are the same. Although luminescence experiments offered evidence for the absence and quantitative recovery of EuIII, we turned to EPR spectroscopy for evidence of the presence of EuII.
Figure 4.
(A) Emission spectra (395 nm excitation) of EuIII-DOTA-4AmC (– –), EuII-DOTA-4AmC (—), and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure to air (· ·). The dashed and dotted lines overlap exactly. All solutions were 2 mM in degassed water under an atmosphere of N2 for EuII or air for the oxidized sample. (B) EPR spectra of EuIII-DOTA-4AmC (– –) and EuII-DOTA-4AmC (—) in H2O at 110 K under an atmosphere of N2.
To verify the formation of EuII, we acquired EPR spectra of EuIII-DOTA-4AmC and the yellow solution at equal concentrations of Eu. The EPR spectrum of aqueous EuIII-DOTA-4AmC was consistent with that of a diamagnetic sample (Figure 4B). The diamagnetic behavior of EuIII is expected because of its 4f6 ground state electronic configuration where the total angular momentum quantum number is 0, causing the effective magnetic moment to be 0 Bohr magnetons despite the presence of 6 unpaired electrons. The reason EuIII exhibits paramagnetic behavior at non-cryogenic temperatures is because excited states with nonzero effective magnetic moments are thermally accessible. The EPR spectrum of the yellow solution contained a broad resonance (g-factor = 1.99) consistent with the presence of EuII.16 Additionally, a lack of precipitation upon the addition of phosphate indicates that the EuII species remains chelated in solution. The luminescence and EPR data combined with the lack of a precipitate in the presence of phosphate indicate that the yellow solution contained EuII-DOTA-4AmC.
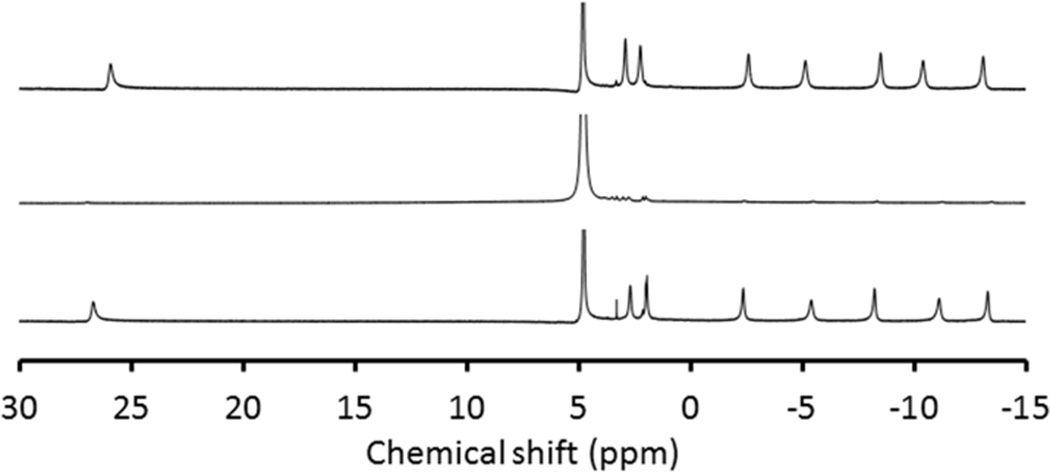
We further tested whether EuII remained chelated within DOTA-4AmC by acquiring 1H-NMR spectra of EuIII-DOTA-4AmC, EuII-DOTA-4AmC, and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure to air (Figure 5). To better our chances of observing free ligand in the NMR experiment, we increased the concentration of the complex by roughly an order of magnitude relative to all of the other experiments (from 2 to 23 mM). We observed signals for the EuIII-containing samples as expected based on chemical shifts of a previously reported ester analogue of EuIII-DOTA-4AmC.18a In the reduced sample, we observed extremely broad peaks in the range of 0 to 5 ppm that we assign to the EuII-containing complex, as would be expected for protons of ligands bound to the isotropic EuII ion. Isotropic ions, such as EuII and GdIII, are known to shorten relaxation times of nearby nuclei leading to a broadening effect. Anisotropic ions, such as EuIII and TbIII, shift the resonance of nearby nuclei without the degree of line broadening observed with isotropic ions. The absence of free ligand18b in the EuIII-DOTA-4AmC spectrum after oxidation of the reduced sample provides further evidence that no detectable dissociation occurred during the reduction or oxidation. This study corroborated the absence of EuII-DOTA-4AmC dissociation indicated by cyclic voltammetry, emission spectroscopy, and phosphate solubility observations. The apparent lack of dissociation upon producing EuII-DOTA-4AmC motivated us to pursue dissociation kinetics of the complex, and the yellow color suggested that UV–visible spectroscopy could be used to monitor dissociation rates.
Figure 5.
1H-NMR spectra of EuIII-DOTA-4AmC (top), EuII-DOTA-4AmC (middle), and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure to air (bottom). All solutions contained 23 mM of complex in degassed D2O under an atmosphere of N2 for EuII or air for the oxidized sample.
Conditional kinetic stability of EuII-DOTA-4AmC in acid
The kinetic stability of a Ln-containing contrast agent is a critical piece of characterization because low kinetic stability can lead to undesired dissociation of the metal ion from the complex. The dissociation rate of EuIII-DOTA-4AmC (8.1 × 10−6 s−1 corresponding to a dissociation half-life of 237 h) has been reported at pH 1;27 therefore, we characterized the UV–visible spectrum of EuIII-DOTA-4AmC and EuII-DOTA-4AmC (Figure 6A) at pH 1 to enable comparisons between the kinetic stabilities of these two complexes. It should be noted that acid-catalyzed dissociation kinetics are not representative of kinetic stability under physiological conditions, but these kinetics are of use when comparing complexes whose dissociation was measured under the same conditions. The UV–visible spectrum of EuIII-DOTA-4AmC contained a small charge-transfer band centered at 250 nm (extinction coefficient of 5.16 × 102 M−1 cm−1) that has been reported for this complex.18b However, the UV–visible spectrum of the EuII-DOTA-4AmC at pH 1 at the initial time point contained two broad absorptions centered at 250 and 353 nm (extinction coefficients of 1.77 × 103 and 9.36 × 102 M−1 cm−1, respectively). These absorptions are consistent with EuII-based transitions.28 A 33 nm bathochromic shift of the longer wavelength absorption maximum of EuII-DOTA-4AmC relative to EuCl2(aq) (353 vs 320 nm, respectively) caused the absorption profile of EuII-DOTA-4AmC to extend to 485 nm (imparting a yellow color to the solution), while the absorption of EuCl2 only extended to 415 nm. Therefore, to measure the kinetic stability of EuII-DOTA-4AmC, we monitored absorption at 425 nm as a function of time (Figure 6B).
Figure 6.
(A) UV–visible absorption spectra of EuIII-DOTA-4AmC (– –), EuII-DOTA-4AmC at t = 0 s (—) and t = 5400 s (— · —), and EuCl2 (— · · —). All samples were measured in 0.1 M HCl. (B) EuII-DOTA-4AmC absorption at 425 nm as a function of time in 0.1 M HCl. Error bars represent the standard error of the mean of three independent measurements.
The dissociation rate of EuII-DOTA-4AmC was (9.2 ± 0.5) × 10−4 s−1, which corresponds to a dissociation half-life of 13 min. Our measured dissociation rate of EuII-DOTA-4AmC is ~110× faster than the dissociation rate of EuIII-DOTA-4AmC. A faster dissociation is not surprising upon reduction of EuIII to EuII: The EuII ion is a relatively soft Lewis acid compared to EuIII; consequently, a relatively weaker binding interaction is expected between the oxygen atoms of the amide functional groups and EuII compared to EuIII. However, despite a decrease in kinetic stability, the dissociation rate of EuII-DOTA-4AmC is between the dissociation rates of two clinically approved contrast agents, GdIII-diethylenetriaminepentaacetate and GdIII-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate, that have dissociation rates of 1.2 × 10−3 and 2.1 × 10−5 s−1, respectively.27 These data suggest that the kinetic stability of EuII-DOTA-4AmC is not a hindrance for in vitro solution-phase characterization or preclinical in vivo imaging, but further investigation is warranted.
In vitro CEST and T1 measurements of EuIII/II-DOTA-4AmC
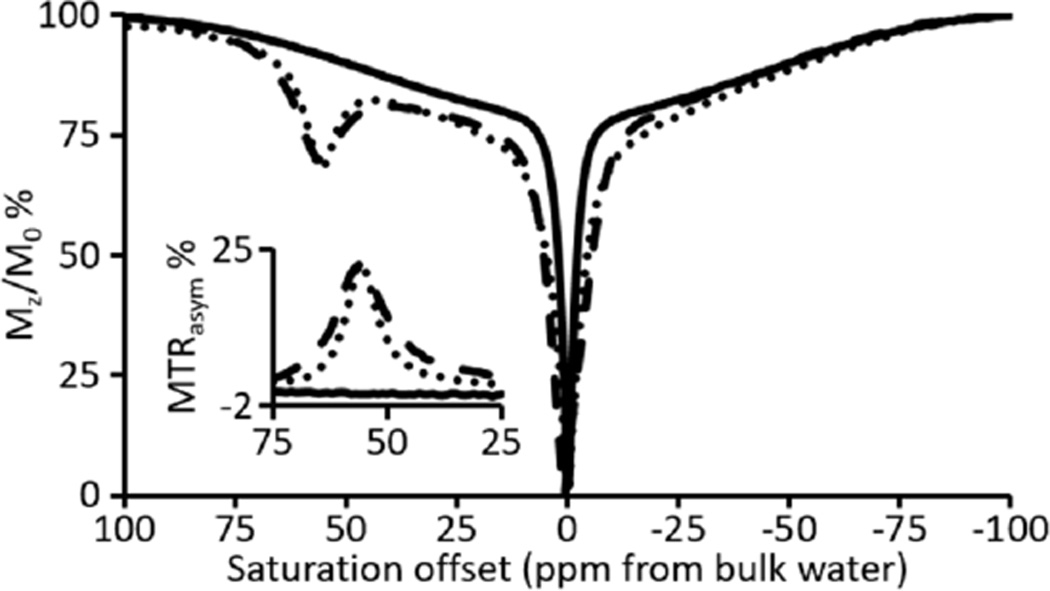
To evaluate the effect of the +2 oxidation state of Eu on CEST, we acquired CEST spectra of EuIII-DOTA-4AmC, EuII-DOTA-4AmC, and EuIII-DOTA-4AmC produced from the oxidation of EuII-DOTA-4AmC (Figure 7). The CEST spectrum of EuIII-DOTA-4AmC matched the reported CEST spectrum with a signal 55 ppm downfield from bulk water.29 Upon reduction to EuII-DOTA-4AmC, the CEST spectrum was devoid of signal other than from the direct saturation of bulk water. Oxidization of EuII-DOTA-4AmC to EuIII-DOTA-4AmC resulted in the restoration of the original CEST signal at 55 ppm, which is consistent with the reversibility observed in the emission spectra. Magnetization-transfer ratio asymmetry analyses (MTRasym) revealed the net asymmetry between upfield and downfield regions of the CEST spectra (inset of Figure 7). The MTRasym for EuIII-DOTA-4AmC and EuII-DOTA-4AmC revealed asymmetries of 22 and 0%, respectively. The lack of CEST contrast for EuII-DOTA-4AmC is likely due to the ability of the EuII ion to shorten the T1 of nearby protons resulting in the complete quenching of saturation transfer effects. CEST modulation was recently reported using silica nanoparticle-,30 p-nitrophenylamide-,19 N-methylquinolinium-,13 and nitrone-functionalized20 analogues of EuIII-DOTA-4AmC in which a change in proton- or water-exchange rate or the presence of unpaired electrons on the ligand resulted in diminished CEST contrast. Our results, however, demonstrate that the +2 oxidation state of europium can suppress CEST contrast below a detectable threshold (inset of Figure 7) and that oxidation to the +3 oxidation state results in the recovery of the original CEST signal. Furthermore, the complete recovery of the CEST signal, which is specific to EuIII-DOTA-4AmC, indicates that dissociation of europium ions did not occur during or after the electron transfer events.
Figure 7.
CEST spectra (pH 7.4) and MTRasym plots (inset) of EuIII-DOTA-4AmC (– –), EuII-DOTA-4AmC (—), and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure to air (· ·).
Before performing phantom imaging experiments, we measured the relaxivity (r1) of EuIII-DOTA-4AmC and EuII-DOTA-4AmC using inversion recovery experiments. EuIII-DOTA-4AmC did not display T1 shortening at imaging-relevant concentrations, while the relaxivity (1.4 T, 37 °C) of EuII-DOTA-4AmC was 3.72 ± 0.04 mM−1 s−1. The lack of detectable relaxivity for EuIII-DOTA-4AmC is expected because, despite EuIII exhibiting a small amount of paramagnetic behavior at 37 °C from thermally accessible excited states, the anisotropic nature of the +3 oxidation state is not conducive with expediting the relaxation of nearby nuclei.31 The +2 oxidation state of europium is isotropic. Accordingly, the relatively efficient relaxation of proton nuclei that we observed is expected. The relaxivity of EuII-DOTA-4AmC is similar to clinically approved GdIII-containing contrast agents, but lack of positive contrast enhancement for EuIII-DOTA-4AmC demonstrates the lack of T1 shortening by the anisotropic +3 oxidation state of Eu.
CEST and T1-weighted imaging of EuIII/II-DOTA-4AmC at 7 T
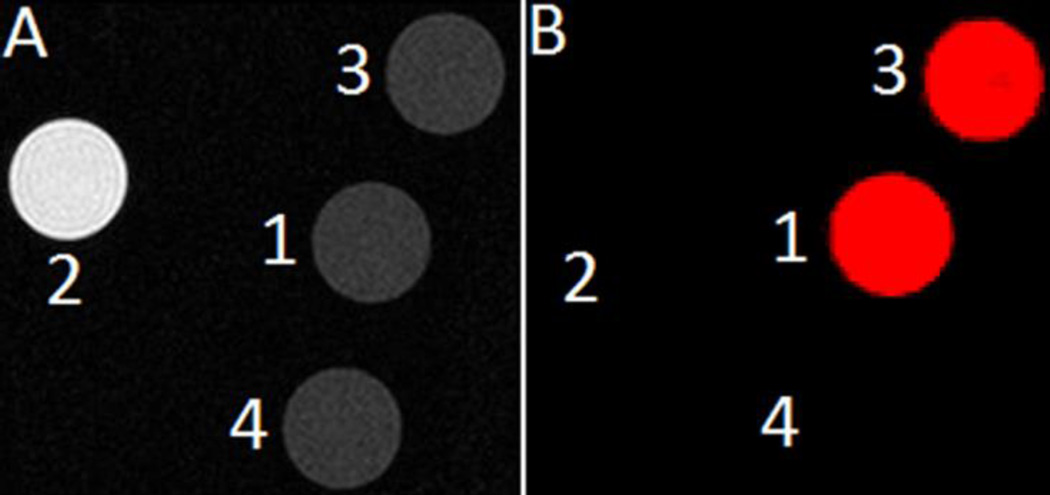
To visualize the responsive behavior of EuIII-DOTA-4AmC and EuII-DOTA-4AmC, we acquired T1-weighted and CEST phantom images (7 T, ambient temperature). The T1-weighted image revealed positive contrast enhancement only from EuII-DOTA-4AmC, but not from EuIII-DOTA-4AmC, EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC, or water (Figure 8). The CEST difference image (colored red) revealed CEST contrast from EuIII-DOTA-4AmC and EuIII-DOTA-4AmC from the oxidation of EuII-DOTA-4AmC. EuII-DOTA-4AmC and water exhibited no CEST contrast. These images are consistent with our observations regarding the formation of EuII-DOTA-4AmC and its ability to completely quench CEST effects. The images in Figure 8 demonstrate the ability to provide selective suppression of T1 shortening or CEST contrast with the EuIII/II couple.
Figure 8.
(A) T1-weighted image and (B) CEST difference image (55 ppm image subtracted from the −55 ppm image) of 5 mm diameter NMR tubes containing (1) EuIII-DOTA-4AmC, (2) EuII-DOTA-4AmC, (3) EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC, and (4) water.
While our measured reduction potential is negative relative to the EuII aqua ion and to the more oxidatively stable EuII-containing cryptates,22d we envisioned using EuII-DOTA-4AmC in a similar manner to our in vivo studies (Figure 1).9 The oxygen-sensitive nature of the complex is expected to permit the differentiation of necrotic tissue while oxidizing elsewhere. Additionally, we recently demonstrated the transitory behavior of EuII in vivo in a report evaluating EuII after intravenous, intraperitoneal, and subcutaneous injections.10 We observed that tissues associated with rapid clearance and high oxygenation lead to rapid oxidation of EuII, while EuII persists in tissues with relatively slow clearance and low oxygenation.
CONCLUSIONS
In summary, we used luminescence, NMR, EPR, and UV–visible spectroscopies to monitor changes in oxidation state between the commercially available contrast agent, EuIII-DOTA-4AmC, and its EuII analogue. The hypersensitive emissions, reversible electrochemistry, reversible CEST signal at 55 ppm, and stability in the presence of phosphate indicate that Eu remains chelated in both oxidation states. The kinetic stability of EuII-DOTA-4AmC was lower than that of EuIII-DOTA-4AmC but in the range of the kinetic stabilities of clinically approved contrast agents. Our assignment of EuII-DOTA-4AmC is based on our rigorous solution-phase characterization and use of EuIII-DOTA-4AmC as our starting material. Our data do not provide insight into the coordination number or geometry of EuII-DOTA-4AmC, but our data do unambiguously defend the assignment of a complex with a molecular form that can be described as EuII-DOTA-4AmC. This report highlights the unique magnetic and spectroscopic properties of the +3 and +2 oxidation states of Eu within the ligand framework of DOTA-4AmC. We expect these results to be applicable to a wide range of chemists interested in expanding the scope of lanthanide redox chemistry.
EXPERIMENTAL METHODS
General methods
Commercially available chemicals were of reagent-grade purity or better and were used without further purification unless otherwise noted. Phosphate-buffered saline (PBS, 10×) was purchased from Fisher BioReagents. EuIII-DOTA-4AmC (DOTA-4AmC = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(acetamidoacetate)) was purchased from Macrocyclics. Water was purified using a PURELAB Ultra Mk2 water purification system (ELGA) and degassed under reduced pressure prior to use.
DOWEX 50Wx8 sulfonic acid resin was converted to the sodium form (DOWEX-Na+) by swirling the resin (3 g) in aqueous NaOH (1 M, 10 mL) for 5 min before allowing the resin to settle and decanting the supernatant. Excess NaOH was removed by washing the resin with water (10 × 10 mL) until the pH of the washings was 7. Residual solvent was removed from the resin under reduced pressure before the resin was brought into a wet glovebox with an atmosphere of N2 at which point it was swelled with degassed water.
Cyclic voltammetry
Cyclic voltammetry was performed using a three electrode setup composed of a glassy carbon working electrode, a platinum wire auxiliary electrode, and a Ag/AgCl reference electrode coupled with a Pine Wavenow USB or a BAS 50W potentiostats. Acquisition parameters for the cyclic voltammogram of EuII/III-DOTA-4AmC (Figure 3A) were 8 segments, initial potential of −1.4 V (rising), upper potential of 0 V, lower potential of −1.4 V, final potential of −1.4 V, and a sweep rate of 150 mV/s. Acquisition parameters for the cyclic voltammogram of EuII/III-DOTA-4AmC (Figure 3B) were 8 segments, initial potential of 0.5 V (falling), lower potential of −1.4 V, upper potential of 0.5 V, final potential of 0.5 V, and a sweep rate of 100 mV/s. Samples were prepared by dissolving EuIII-DOTA-4AmC and either tetraethylammonium perchlorate or potassium chloride in water. The pH of the resulting solution was 4 and was adjusted to 7 using an aqueous solution of NaOH (1 M) for the potassium chloride solution. The solutions were sparged with Ar while stirring for 5 min prior to measurements, but were not sparged or stirred during the measurements. The standard potential is reported as mean ± standard error of 3 independently prepared samples.
Reduction of EuIII-DOTA-4AmC with Zn0
EuII-DOTA-4AmC was prepared by vigorously stirring EuIII-DOTA-4AmC (4.0 mg, 4.2 µmol, 1 equiv) with Zn dust (50.0 mg, 760 µmol, 181 mol equiv, 362 electron equiv) in water (0.700 mL) for 1 min to produce a yellow supernatant. Excess Zn dust was removed by filtering the mixture through a 0.2 µm hydrophilic filter. The yellow filtrate was mixed with DOWEX-Na+ (0.1 g) for 1 min before resin was removed by filtering through a 0.2 µm hydrophilic filter, and this process was repeated twice more. This cation-exchange step reduced the concentration of Zn2+ to below the detection limit (<1 ppb) of inductively coupled plasma mass spectrometry (ICP–MS). To the remaining yellow filtrate was added 10× PBS and water to bring the final concentration to 4 mM Eu in 1× PBS (pH = 7.4 ± 0.2). Subsequent dilutions were performed with degassed 1× PBS under an atmosphere of N2.
Elemental concentration measurements
The concentrations of Eu and Zn were determined using ICP–MS or energy dispersive X-ray fluorescence (EDXF) spectroscopy. ICP–MS measurements were acquired on an Agilent Technologies 7700 series ICP–MS instrument at the Lumigen Instrument Center in the Department of Chemistry at Wayne State University. All dilutions were performed with aqueous 2% HNO3, which was also used for blank samples during calibration. Calibration curves were created using the 153Eu isotope ion count for a 1–200 ppb concentration range (diluted from Fluka ICP standard solution, 1000 mg Eu/L) or the 64Zn isotope ion count for a 1–200 ppb concentration range (diluted from Fluka ICP standard solution, 1000 mg Zn/L). EDXF measurements were performed with a Shimadzu EDX-7000 spectrometer (Shimadzu Scientific Instruments). Calibration curves were created using the Eu fluorescence intensity at 5.845 keV for a 250–1000 ppm concentration range (diluted from Fluka ICP standard solution, 1000 mg Eu/L).
Luminescence spectroscopy
Emission spectra were acquired on a HORIBA Jobin Yvon FluoroMax-4 spectrofluorometer and samples were loaded into quartz cuvettes and sealed with air-tight caps under an atmosphere of N2. An emission range of 550–725 nm was recorded with excitation at 395 nm (1 nm slit widths and 1 nm resolution).
Minimum detectable concentration of EuIII-DOTA-4AmC
The minimum detectable concentration of EuIII-DOTA-4AmC was determined by a textbook procedure.32 The absolute emission intensity at 595 nm was measured after excitation at 395 nm (1 nm slit widths and 1 nm resolution) as a function of concentration of EuIII-DOTA-4AmC (Figure S1). The emission intensity of EuIII-DOTA-4AmC (0.0662 mM) was measured using 7 independently prepared samples, and the standard deviation of the emission intensities was 2200 units. Using eq 1,32 where σ and m [(3.15 ± 0.01) × 105 units mM−1] are the standard deviation and slope of emission intensity vs concentration, respectively, the minimum detectable concentration of EuIII-DOTA-4AmC was 20.9 µM.
| eq 1 |
EPR spectroscopy
EPR spectroscopy was performed on a Bruker EMX X-band spectrometer equipped with an Oxford variable-temperature cryostat. EPR samples were prepared and sealed with wax under an atmosphere of N2 and were a total volume of 0.3 mL in Norell SEPR250S EPR tubes. Aqueous solutions in EPR tubes were frozen in liquid nitrogen prior to loading into the sample cavity. EPR spectra were acquired of EuIII-DOTA-4AmC and EuII-DOTA-4AmC in water. Acquisition parameters included a temperature of 110 K, microwave frequency of 9.378654 GHz, microwave power of 1.992 mW, modulation frequency of 100 kHz, receiver gain of 30 dB, and modulation amplitude of 4.0 G.
1H-NMR spectroscopy
NMR spectra were acquired on a Varian VNMRS-500 (499.48 MHz, 11.7 T) in the Lumigen Instrument Center in the Chemistry Department at Wayne State University.
UV–visible spectroscopy and dissociation kinetics
UV–visible spectra were acquired on a Shimadzu UVmini-1240 spectrophotometer and samples were loaded into quartz cuvettes and sealed with air-tight caps under an atmosphere of N2. Extinction coefficients were calculated by dividing the absorbance maxima by the concentration of Eu in the sample for a 1 cm path length.
The dissociation rate of EuII-DOTA-4AmC by monitoring absorbance at 425 nm as a function of time in 0.1 M HCl. This method was chosen based on reports that characterize the kinetic stability of similar complexes.27 The absorbance at 425 nm was chosen because it is within an absorbance range specific to the EuII-containing complex (415–485 nm), whereas the EuII aqua ion (a product of dissociation) does not absorb beyond 415 nm. The UV–visible spectra of EuII-DOTA-4AmC in the 415–485 nm range revealed a decrease in the concentration of EuII-DOTA-4AmC as a function of time. The concomitant formation of the EuII aqua ion absorbance profile ruled out the possibility of oxidation being responsible for the loss of EuII-DOTA-4AmC in our experiment. The absorbance values at 425 nm as a function of time were used to calculate the dissociation rate based on eq 2,18b where kd is the calculated first-order dissociation rate constant; t is time; and A0, At, and Ae are the absorbance values measured at the start of the reaction (t = 0 s), at time t, and at a final time of t = 5400 s, respectively.
| eq 2 |
CEST measurements
CEST spectra were acquired on the same spectrometer used to acquire 1H-NMR spectra with a sample temperature of 22 °C using a saturation frequency array with saturation time of 4 s, a saturation power of 24 µT, a 45 degree observe pulse, an initial frequency of 100 ppm (50,000 Hz), and a final frequency of −100 ppm (−50,000 Hz) in 1 ppm (500 Hz) increments. All samples were in 1× PBS (pH = 7.4 ± 0.2) and included EuIII-DOTA-4AmC, EuII-DOTA-4AmC, and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure to air. The concentration of Eu in all samples was 4 mM.
Relaxivity measurements
T1 measurements were performed on a Bruker Minispec mq60 NMR (1.4 T) spectrometer at 37 °C by measuring T1 with inversion recovery experiments. Relaxivity was obtained from the slope of 1/T1 vs concentration of either EuIII-DOTA-4AmC or EuII-DOTA-4AmC using a linear regression fitting (Figure S2). All samples were measured in 1× PBS (pH = 7.4 ± 0.2). Measurements were performed in triplicate with independently prepared solutions, and the relaxivity values are reported as the mean ± standard error of the mean of the independent measurements.
Magnetic resonance imaging
Details for the mouse imaging experiments have been described elsewhere.9 Web-enhanced objects were created using National Institutes of Health (NIH) ImageJ software. Magnetic resonance imaging was performed with a Varian small animal scanner (299.44 MHz, 7.0 T) at Henry Ford Hospital equipped with a 12 cm bore magnet and a 38 mm diameter homemade transmit–receive quadrature birdcage coil. All samples, with the exception of water, were in 1× PBS (pH = 7.4 ± 0.2) and included EuIII-DOTA-4AmC, EuII-DOTA-4AmC, and EuIII-DOTA-4AmC after oxidation of EuII-DOTA-4AmC by exposure air. The concentration of Eu in all samples was 4 mM, with the exception of water that was 0 mM. The T1-weighted images were acquired at ambient temperature with an echo time of 15.68 ms, repetition time of 800 ms, 13 slices at 1 mm thickness, 24 mm × 24 mm field of view, and 4 averages. CEST images were acquired of the exact same samples in the same instrument using a magnetization transfer pulse sequence with a 7 µs (500°) saturation pulse at 55 ppm (16,500 Hz) and −55 ppm (−16,500 Hz).
Supplementary Material
Acknowledgments
The authors acknowledge support from the NIH (R01EB013663) and are grateful to Wayne State University (WSU) for a Thomas C. Rumble Graduate Research Fellowship (L.A.E.) and to A. Paul and Carol Schaap for a Schaap Faculty Scholar Award (M.J.A.). The Animal Model and Therapeutics Evaluation Core is supported, in part, by an NIH Center grant (P30CA022453) to the Barbara Ann Karmanos Cancer Institute at WSU. The authors thank Dr. Derek J. Averill from Shimadzu Scientific Instruments for use of the EDXF spectrometer.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Minimum detectable concentration and relaxivity data. This material is available free of charge via the Internet at http://pubs.acs.org. Two web-enhanced objects are available in the HTML version of the paper.
REFERENCES
- 1.(a) Kim JE, Bogart JA, Carroll PJ, Schelter EJ. Inorg. Chem. 2016;55:775–784. doi: 10.1021/acs.inorgchem.5b02236. [DOI] [PubMed] [Google Scholar]; (b) Bogart JA, Lewis AJ, Medling SA, Piro NA, Carroll PJ, Booth CH, Schelter EJ. Inorg. Chem. 2013;52:11600–11607. doi: 10.1021/ic401974t. [DOI] [PubMed] [Google Scholar]
- 2.(a) MacDonald MR, Bates JE, Ziller JW, Furche F, Evans WJ. J. Am. Chem. Soc. 2013;135:9857–9868. doi: 10.1021/ja403753j. [DOI] [PubMed] [Google Scholar]; (b) Fieser ME, MacDonald MR, Krull BT, Bates JE, Ziller JW, Furche F, Evans WJ. J. Am. Chem. Soc. 2015;137:369–382. doi: 10.1021/ja510831n. [DOI] [PubMed] [Google Scholar]
- 3.Meihaus KR, Fieser ME, Corbey JF, Evans WJ, Long JR. J. Am. Chem. Soc. 2015;137:9855–9860. doi: 10.1021/jacs.5b03710. [DOI] [PubMed] [Google Scholar]
- 4.Kelly RP, Bell TDM, Cox RP, Daniels DP, Deacon GB, Jaroschik F, Junk PC, Le Goff XF, Lemercier G, Martinez A, Wang J, Werner D. Organometallics. 2015;34:5624–5636. [Google Scholar]
- 5.Chciuk TV, Flowers RA., II J. Am. Chem. Soc. 2015;137:11526–11531. doi: 10.1021/jacs.5b07518. [DOI] [PubMed] [Google Scholar]
- 6.(a) Kuda-Wedagedara ANW, Wang C, Martin PD, Allen MJ. J. Am. Chem. Soc. 2015;137:4960–4963. doi: 10.1021/jacs.5b02506. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Allen MJ. [accessed March 12, 2016];Synlett. [Online early access] [Google Scholar]
- 07.Mikulas TC, Chen M, Fang Z, Peterson KA, Andrews L, Dixon DA. J. Phys. Chem. A. 2016;120:793–804. doi: 10.1021/acs.jpca.5b11215. [DOI] [PubMed] [Google Scholar]
- 08.Ekanger LA, Ali MM, Allen MJ. Chem. Commun. 2014;50:14835–14838. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 09.Ekanger LA, Polin LA, Shen Y, Haacke EM, Martin PD, Allen MJ. Angew. Chem. Int. Ed. 2015;54:14398–14401. doi: 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekanger LA, Polin LA, Shen Y, Haacke EM, Allen MJ. Contrast Media Mol. Imaging. 2016 doi: 10.1002/cmmi.1692. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Radecki G, Nargeot R, Jelescu IO, Le Bihan D, Ciobanu L. Proc. Natl. Acad. Sci. USA. 2014;111:8667–8672. doi: 10.1073/pnas.1403739111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) van Veluw SJ, Zwaneburg JJM, Engelen-Lee J, Spliet WGM, Hendrikse J, Luijten PR, Biessels GJ. J. Cereb. Blood Flow Metab. 2013;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Moats RA, Fraser SE, Meade TJ. Angew. Chem. Int. Engl. 1997;36:725–728. [Google Scholar]; (b) Aime S, Botta M, Gianolio E, Terreno E. Angew. Chem. Int. Ed. 2000;39:747–750. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]; (c) Woods M, Kiefer GE, Bott S, Castillo-Muzquiz A, Eshelbrenner C, Michaudet L, McMillan K, Mudigunda SDK, Ogrin D, Tircsó G, Zhang S, Zhao P, Sherry AD. J. Am. Chem. Soc. 2004;126:9248–9256. doi: 10.1021/ja048299z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu G, Li Y, Pagel MD. Magn. Reson. Med. 2007;58:1249–1256. doi: 10.1002/mrm.21428. [DOI] [PubMed] [Google Scholar]; (e) Tsitovich PB, Spernyak JA, Morrow JR. Angew. Chem. Int. Ed. 2013;52:13997–14000. doi: 10.1002/anie.201306394. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Loving GS, Mukherjee S, Caravan P. J. Am. Chem. Soc. 2013;135:4620–4623. doi: 10.1021/ja312610j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) He J, Bonnet CS, Eliseeva SV, Lacerda S, Chauvin T, Retailleau P, Szeremeta F, Badet B, Petoud S, Tóth É, Durand P. J. Am. Chem. Soc. 2016 doi: 10.1021/jacs.5b12084. in press. [DOI] [PubMed] [Google Scholar]
- 13.Ratnakar SJ, Viswanathan S, Kovacs Z, Jindal AK, Green KN, Sherry AD. J. Am. Chem. Soc. 2012;134:5798–5800. doi: 10.1021/ja211601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Ekanger LA, Allen MJ. Metallomics. 2015;7:405–421. doi: 10.1039/c4mt00289j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pierre VC, Allen MJ, Caravan P. >J. Biol. Inorg. Chem. 2014;19:127–131. doi: 10.1007/s00775-013-1074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Garcia J, Kuda-Wedagedara ANW, Allen MJ. Eur. J. Inorg. Chem. 2012;2012:2135–2140. doi: 10.1002/ejic.201101166. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Garcia J, Allen MJ. Inorg. Chim. Acta. 2012;393:324–327. doi: 10.1016/j.ica.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Garcia J, Neelavalli J, Haacke EM, Allen MJ. Chem. Commun. 2011;47:12858–12860. doi: 10.1039/c1cc15219j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Burai L, Scopelliti R, Tóth É. Chem. Commun. 2002:2366–2367. doi: 10.1039/b206709a. [DOI] [PubMed] [Google Scholar]
- 16.Caravan P, Tóth É, Rockenbauer A, Merbach AE. J. Am. Chem. Soc. 1999;121:10403–10409. [Google Scholar]
- 17.(a) Burai L, Tóth É, Moreau G, Sour A, Scopelliti R, Merbach AE. Chem. Eur. J. 2003;9:1394–1404. doi: 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]; (b) Seibig S, Tóth É, Merbach AE. J. Am. Chem. Soc. 2000;122:5822–5830. [Google Scholar]; (c) Burai L, Tóth É, Seibig S, Scopelliti R, Merbach AE. Chem. Eur. J. 2000;6:3761–3770. doi: 10.1002/1521-3765(20001016)6:20<3761::aid-chem3761>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.(a) Zhang S, Wu K, Biewer MC, Sherry AD. Inorg. Chem. 2001;40:4284–4290. doi: 10.1021/ic0003877. [DOI] [PubMed] [Google Scholar]; (b) Baranyai Z, Brücher E, Iványi T, Király R, Lázár I, Zékány L. Helv. Chim. Acta. 2005;88:604–616. [Google Scholar]; (c) Song B, Wu Y, Yu M, Zhao P, Zhou C, Kiefer GE, Sherry AD. Dalton Trans. 2013;42:8066–8069. doi: 10.1039/c3dt50194a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhang S, Trokowski R, Sherry AD. J. Am. Chem. Soc. 2003;125:15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]; (e) Aime S, Barge A, Castelli DD, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Magn. Reson. Med. 2002;47:639–648. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]; (f) Zhang S, Winter P, Wu K, Sherry AD. J. Am. Chem. Soc. 1998;123:1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]; (g) Aime S, Barge A, Botta M, De Sousa AS, Parker D. Angew. Chem. Int. Ed. 1998;37:2673–2675. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673::AID-ANIE2673>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Ratnakar SJ, Woods M, Lubag AJM, Kovács Z, Sherry AD. J. Am. Chem. Soc. 2008;130:6–7. doi: 10.1021/ja076325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratnakar SJ, Soesbe TC, Lumata LL, Do QN, Viswanathan S, Lin C-Y, Sherry AD, Kovacs Z. J. Am. Chem. Soc. 2013;135:14904–14907. doi: 10.1021/ja406738y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Moake M, Har-el Y, Long CM, Chan KWY, Cardona A, Jamil M, Walczak P, Gilad AA, Sgouros G, van Zijl PCM, Bulte JWM, McMahon MT. Magn. Reson. Med. 2012;67:1106–1113. doi: 10.1002/mrm.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Regueiro-Figueroa M, Barriada JL, Pallier A, Esteban-Gómez D, de Blas A, Rodríguez-Blas T, Tóth É, Platas-Iglesias C. Inorg. Chem. 2015;54:4940–4952. doi: 10.1021/acs.inorgchem.5b00548. [DOI] [PubMed] [Google Scholar]; (b) (c) Gál M, Kielar F, Sokolová R, Ramešová Š, Kolivoška V. Eur. J. Inorg. Chem. 2013:3217–3223. [Google Scholar]; (d) Gamage N-DH, Mei Y, Garcia J, Allen MJ. Angew. Chem. Int. Ed. 2010;49:8923–8925. doi: 10.1002/anie.201002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk AM, Jordan VC, Sherry AD, Ratnakar SJ, Kovacs Z. [accessed March 12, 2016];Angew. Chem. Int. Ed. [Online early access] [Google Scholar]
- 24.Tóth É, Brücher E, Lázár I, Tóth I. Inorg. Chem. 1994;33:4070–4076. [Google Scholar]
- 25.Shannon RD. Acta Cryst. 1976;32:751–767. [Google Scholar]
- 26.Calculated by subtracting 0.197 V from the standard reduction potential of ZnII (−0.763 V vs normal hydrogen electrode). Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd. Hoboken, NJ: John Wiley & Sons, Inc; 2001. pp. 3–810.
- 27.Sherry AD, Caravan P, Lenkinski RE. J. Magn. Reson. Imaging. 2009;30:1240–1248. doi: 10.1002/jmri.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashiyama N, Takemura K, Kimura K, Adachi G. Inorg. Chim. Acta. 1992;194:201–206. [Google Scholar]
- 29.Evbuomwan OM, Lee J, Woods M, Sherry AD. Inorg. Chem. 2014;53:10012–10014. doi: 10.1021/ic501290q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evbuomwan OM, Merritt ME, Kiefer GE, Sherry AD. Contrast Media Mol. Imaging. 2012;7:19–25. doi: 10.1002/cmmi.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 32.Harris DC. Quantitative Chemical Analysis. 8th. New York, NY: W. H. Freeman and Company; 2010. p. 103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.