Abstract
Evidence for immune/neuroimmune disturbances as a possible root cause of a range of disorders, including neurodevelopmental disorders, is growing. Although prenatal alcohol exposure (PAE) impacts immune function, few studies to date have examined immune function in relation to long-term negative health outcomes following PAE, and most have focused on males. To fill this gap, we utilized a rat model to examine the effects of PAE on immune/neuroimmune function during early-life [birth (postnatal day 1; P1), P8, and weaning (P22)] in PAE and control females. Due to the extensive interplay between the immune and endocrine systems, we also measured levels of corticosterone and corticosterone binding globulin (CBG). While corticosterone levels were not different among groups, CBG levels were lower in PAE offspring from P1–P8, suggesting a lower corticosterone reservoir that may underlie susceptibility to inflammation. Spleen weights were increased in PAE rats on P22, a marker of altered immune function. Moreover, we detected a unique cytokine profile in PAE compared to control offspring on P8 – higher levels in the PFC and hippocampus, and lower levels in the hypothalamus and spleen. The finding of a specific immune signature in PAE offspring during a sensitive developmental period has important implications for understanding the basis of long-term immune alterations and health outcomes in children with Fetal Alcohol Spectrum Disorder (FASD). Our findings also highlight the future possibility that immune-based intervention strategies could be considered as an adjunctive novel therapeutic approach for individuals with FASD.
Keywords: Prenatal alcohol exposure, Fetal Alcohol Spectrum Disorder (FASD), cytokines, spleen, brain, corticosteroid binding globulin (CBG)
1. Introduction
There is increasing evidence for immune and neuroimmune abnormalities in the etiology and pathophysiology of numerous neurodevelopmental disorders including schizophrenia (Noto et al., 2015) and autism spectrum disorder (ASD) (Krakowiak et al., 2015). Evidence for underlying immune/neuroimmune abnormalities in Fetal Alcohol Spectrum Disorder (FASD), which includes the broad range of deficits/disorders that arise following in utero alcohol exposure, is also emerging [reviewed in (Drew and Kane, 2014)]. Children with FASD have a higher incidence of both minor (e.g., recurrent otitis media, respiratory infections), and major, (e.g., sepsis) infections compared to non-exposed children (Gauthier et al., 2004; Johnson et al., 1981) as well as an increased incidence of malignancies, including cancers of embryonic origin (neuroblastoma, ganglioneuroblastoma, medulloblastoma) [reviewed in (Gottesfeld and Abel, 1991)], and leukemias (Latino-Martel et al., 2010). Animal models of prenatal alcohol exposure (PAE) support and extend the clinical findings. Increased susceptibility to infections (Grossmann et al., 1993; McGill et al., 2009) and malignancies (Gottesfeld and Abel, 1991), deficits in immune organ development (Bray et al., 1993; Ewald and Frost, 1987; Ewald and Walden, 1988; Redei et al., 1989), decreased splenic lymphocyte, T lymphoblast, and B cell proliferative responses to stimulation (Gottesfeld et al., 1990; Jerrells and Weinberg, 1998; Weinberg and Jerrells, 1991; Wolcott et al., 1995), blunted LPS-induced febrile responses (Taylor et al., 1999), dampened cytokine responses to immune challenge (Chiappelli et al., 1997; Kim et al., 1999; Lee and Rivier, 1993), and a more severe and prolonged course of inflammation in an adjuvant-induced arthritis model (Zhang et al., 2012) have been reported in models of in utero alcohol exposure [reviewed in Bodnar and Weinberg 2013].
It is well established that chronic alcohol consumption increases proinflammatory cytokine levels (Crews et al., 2006; He and Crews, 2008). In this context, if alcohol is consumed during pregnancy, the developing fetus is likely exposed not only to alcohol but also to heightened cytokine signals. In addition to their function in the immune response, cytokines are essential to brain development. Cytokine receptors are expressed on neuronal cells in the fetal brain (Gilmore et al., 2004) and play important roles in key neuronal processes, such as neurogenesis (Smith et al., 2007), myelination (Jakovcevski et al., 2009), synaptogenesis, synaptic pruning, and modulation of synapse strength (Stephan et al., 2012) [reviewed in (Deverman and Patterson, 2009)]. As such, deviations in the normal cytokine balance have been shown to alter the course of normal brain development (Cai et al., 2000). Further support of this notion comes from the “prenatal cytokine hypothesis”, put forth in the field of schizophrenia, which links increased cytokine exposure during the prenatal period and alterations in the trajectory of brain development (Howard, 2013).
Only a few studies to date have focused on neuroimmune function/inflammation following prenatal alcohol exposure, and of these, most have focused primarily on males or have pooled the data from males and females (Drew et al., 2015; Topper et al., 2015). Of note, in relation to neuroimmune function in general, females often show an increased incidence of autoimmune/inflammatory diseases or disorders compared to males (Whitacre, 2001), and in many cases, increased rates of inflammatory mediated depressive-like behaviors (Tonelli et al., 2008).
In the present study, we utilized our well-established animal model of prenatal alcohol exposure to investigate whether PAE affects development of neuroimmune function during early life. In the context of the discussion above, and as a first step in examining neuroimmune/inflammatory changes in our model, we conducted the present study in females. Specifically, we examined levels of key pro- and anti-inflammatory cytokines in peripheral and central compartments, at a number of key developmental ages [birth (postnatal day 1 [P1], P8 and weaning (P22)] in order to probe for a unique, immune-based signature of alcohol exposure. Furthermore, due to the extensive interplay between the immune and endocrine systems, with shared ligands, receptors, and regulatory feedback (Haddad et al., 2002), we also examined hypothalamic-pituitary-adrenal (HPA) parameters (plasma corticosterone and corticosterone binding globulin [CBG]) to determine whether they might have a modulatory role on immune function.
We hypothesized that in utero alcohol exposure would alter the developmental immune profile, as indexed by alterations in pro- and anti-inflammatory cytokine levels. Based on recent findings of increased cytokine levels in the hippocampus, cortex, and cerebellum following alcohol administration during the early postnatal period (third trimester equivalent model) (Drew et al., 2015; Topper et al., 2015), we predicted that PAE would result in overall increased cytokine levels within the brain and blood.
2. Materials and methods
2.1 Breeding
Male and female Sprague-Dawley (SD) rats (Charles River Laboratories, St. Constant, Quebec, Canada) were pair-housed by sex in clear polycarbonate cages with corn-cob bedding, and handled daily for a one week habituation period. Colony rooms were maintained on a 12:12 h light/dark cycle (lights on at 0700 hr), at 20 – 23°C, and rats were given ad libitum access to water and standard laboratory chow (18% Protein Extruded Rodent Diet, #2018, Teklad Global).
Nulliparous females (250 – 325 g; n=43) were pair-housed with a male and vaginal lavage samples were collected daily for estrous cycle staging and to check for the presence of sperm, indicating gestation day 1 (GD1). All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of British Columbia Animal Care Committee.
2.2 Prenatal diets and feeding
On GD1, females were single housed and assigned to one of three treatment groups: (1) Prenatal alcohol exposure (PAE) – ad libitum access to an alcohol-containing liquid diet with 36% of total calories derived from ethanol, 6.37% v/v, n=13; (2) Pair-fed (PF) – liquid control diet with maltose dextrin isocalorically substituted for ethanol, in the amount consumed by a PAE partner (g/kg/body wt/day of gestation, n=15); (3) Control (C) – pelleted version of the liquid control diet, ad libitum, n=15. All diets were formulated to provide optimal nutrition (Weinberg/Keiver High Protein Experimental Diet #710324, Control (PF) Diet #710109, and Pelleted Control Diet #102698, Dyets Inc. Bethlehem, PA, USA) and were presented daily, one hour prior to lights off (1800-1900 hr) in order to maintain the normal corticosterone circadian rhythm in PF dams, which are fed a restricted ration (Gallo and Weinberg, 1981; Krieger, 1974). Rats in all groups had ad libitum access to water and were weighed weekly throughout gestation. On GD17 a blood sample was collected from the tail vein from a subset of PAE, PF, and C dams (n=3 – 6) at lights on (0700 hr). Blood alcohol levels were measured as previously reported (Hellemans et al., 2010; Uban et al., 2010) and ranged from ∼80 – 150 mg/dl in PAE rats. On GD21, diets were replaced with standard laboratory chow (19% Protein Extruded Rodent Diet, #2019, Teklad Global), ad libitum, and rats were continued on this diet throughout lactation.
2.3 Tissue collection
On the day of birth (postnatal day 1; P1), litters were culled to 6 males and 6 females, when possible. Female pups from each prenatal group were randomly assigned to one of three termination ages, with tissue collected on P1, P8, or P22 (n=7-11/group). Litters were maintained at a minimum of 8 pups (4 males, 4 females), with a balanced sex ratio, through the end of the experiment (P22), in order to control for changes in maternal behaviour, which can occur with small litters or litters with an unbalanced sex ratio (Alleva et al., 1989; Moore and Morelli, 1979). The dam and remaining pups were weighed weekly during the lactation period and any pup deaths or abnormalities were noted. On the day of tissue collection (1400-1700 hr), female rats were quickly removed from the home cage and decapitated. Trunk blood was collected, serum allowed to separate for 2 hours, and samples centrifuged at 2,190 g for 10 min at 4°C. Serum samples were stored at -80°C until assayed for hormone and cytokine levels. Due to the small brain size on P1, the whole brain was removed from the skull, the cerebellum and olfactory bulbs removed, and the brain weighed and quickly frozen on dry ice. On P8 and 22, the whole brain was again removed and weighed following removal of the cerebellum and olfactory bulbs, and the hypothalamus, PFC, and hippocampus were dissected on ice and frozen on dry ice. At all ages, the spleen was removed, weighed, and frozen on dry ice. To control for litter effects, only one rat from each litter was used per age.
2.4 Corticosterone radioimmunoassay
Total serum corticosterone levels were measured using the ImmuChem Double Antibody Corticosterone 125I radioimmunoassay kit (MP Biomedicals, LLC, Orangeburg, NY, USA, according the manufacturer's instructions with one modification – the lowest standard was further diluted to detect lower corticosterone concentrations (Taves et al., 2015). The minimum detectable corticosterone concentration was approximately 7.7 ng/mL, and the intra- and inter-assay coefficients of variation were <10 % and <7 %, respectively. All samples were measured in duplicate.
2.5 CBG measurement
The plasma corticosterone-binding capacity of CBG was measured using an established ligand-saturation assay (Smith and Hammond, 1991). Briefly, serum samples were diluted (1:1000 for P1 and P22, 1:200 for P8) in phosphate buffered saline (PBS) and stripped of endogenous steroids by incubation with dextran coated charcoal (DCC) for 30 minutes at room temperature. Samples were then incubated with ∼10 nM of [3H]-corticosterone (PerkinElmer Lifer Sciences, Waltham, MA) in the absence or presence of excess corticosterone, to monitor non-specific binding. After the adsorption of free steroid with DCC for 10 minutes and centrifugation at 0°C, CBG-bound [3H]-corticosterone was measured in the supernatants using a scintillation photospectrometer.
2.6 Tissue homogenization
Lysis buffer was prepared containing 150 mM NaCl, 20 mM Tris pH 7.5, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100, and immediately prior to homogenization the following were added (per 10 mL lysis buffer): 1 cOmplete mini protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN), 100 μl phosphate inhibitor 2 & 3 (Sigma-Aldrich, St. Louis, MO), 100 μl 1 M NaF, and 40 μl PMSF (from 500 mM stock in DMSO). Brain samples (whole brain on P1; hypothalamus, PFC, and hippocampus on P8, 22) were added to 1.6 ml tubes containing 8 zirconium oxide beads and lysis buffer (whole brain – 550 μl; hypothalamus – 100 μl; PFC & hippocampus – 250 μl). Brain samples were homogenized using the Omni Bead Ruptor 24 (Omni Internation, Kennesaw, GA) in 4 – 5 cycles (speed: 2.10 – 3.10, time: 5 sec), with 1 min on ice in between cycles. Spleen samples were homogenized as above, with modifications – 1.3 g garnet was added to the tubes prior to homogenization, 250 – 1,000 μl lysis buffer was used based on spleen size (P1: 250 μl; P8: 500 μl; P22: 1,000 μl), and samples were homogenized in 3 – 4 cycles (speed: 6.0, time: 10 sec). In addition, spleen samples were sonicated (sonicator model 4C15; Fisher Scientific, Pittsburgh, PA) three times (5 sec/cycles, amplitude 50%), on ice. Following homogenization/sonication, tissue samples were centrifuged at 1,400 g for 10 min at 4°C. Separate aliquots of supernatant were removed for protein quantification and cytokine analysis, and stored at -20°C until assayed.
2.7 Multiplex cytokine and CRP measurements
Multiplex cytokine assays were performed using the Meso Scale Discovery (MSD) proinflammatory panel 1 rat V-PLEX kit. This cytokine panel allows for the simultaneous measurement of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IFN-γ, KC/GRO (CXCL1), and TNF-α (catalog #: K15044D-1, MSD, Rockville, MD). Samples were diluted (1:4 for serum, 1:2 for tissue) in diluent 42 and assays were performed using the standard MSD protocol. The plate was read using a Sector Imager 2400 (MSD, Rockville, MD) and data were analyzed using the MSD Discovery Workbench software v. 4.0 (MSD, Rockville, MD). The lower limit of detection (LLOD) for the assays varied by plate and analyte. The following LLOD ranges were observed (pg/mL) – IL-1β: 7.76 – 17.8; IL-2: 21.50 – 55.30; IL-4: 0.15 – 0.36; IL-5: 9.23 – 15.90; IL-6: 5.66 – 11.40; IL-10: 2.10 – 5.79; IL-13: 0.85 – 1.43; IFN-γ: 0.31 – 0.82; TNF-α: 0.31 – 0.748; KC/GRO: 0.55 – 6.45.
CRP levels were detected using antibody pairs (catalog #: DY1744, R&D Systems, Minneapolis, MN), with capture antibodies printed in the wells of standard-bind multiplex assay plates (MSD proprietary printing service). Serum samples were diluted in PBS + 1% BSA (1:50,000 dilution), followed by a 1:2 dilution in diluent 42 (MSD, Rockville, MD). Detection antibodies were derivatized with electrochemiluminescent SULFO-Tag NHS-ester by MSD and 2.88 μl SULFO-TAG labeled Streptavidin was added, with the detection antibody, according to standard MSD protocol. The plate was read and analyzed as above. The LLOD for CRP was: 3.92 – 4.62 pg/ml.
2.8 Protein quantification
Total protein levels were quantified in tissue homogenates using the Pierce Microplate BCA Protein Assay Kit (reduction agent compatible; Pierce Biotechnology, Rockford, IL). Tissue homogenates were diluted (1:41) and the standard BCA protocol was followed with one modification: 5 μl of compatibility reagent solution was added to each sample. Tissue homogenate samples were run in quadruplicate to determine the average protein concentration. Tissue cytokine levels were then adjusted and values reported as pg cytokine/mg of protein.
2.9 Statistical analyses
Maternal data during gestation and lactation were analyzed using repeated measures analyses of variance (ANOVA) (IBM SPSS Statistics), with prenatal treatment as the between-subjects factor, and day of gestation or lactation as the within-subjects factor. As separate cohorts of offspring from each prenatal group were terminated on P1, P8, or P22 (n=7-11/group), body, brain, and spleen weight, and hormone data were analyzed by ANOVAs for the factor of prenatal treatment at each age. Significant main effects were further analyzed by Fisher post hoc tests. Cytokine data were initially analyzed using ANOVAs for the factors of prenatal treatment and age. Consistent age effects were detected for the majority of cytokines (see supplementary table S2), as expected (Giulian et al, 1988; Zhao and Schwartz, 1998), and there were no differential patterns of change in PAE compared to control offspring across development. Separate ANOVAs were then run each age to examine prenatal treatment effects. Tissue (brain, spleen, serum) cytokine levels were also examined by independent ANOVAs as cytokine levels have previously established to vary by tissue type (Deverman and Patterson, 2009). In line with our hypotheses, planned pairwise comparisons were carried out, as indicated. Outliers (±2.5 SD > mean) were removed from cytokine analyses, when appropriate. Corticosterone, CRP, and cytokine levels were not normally distributed and were Blom transformed (Blom, 1958) for statistical analysis. Untransformed data are presented in Fig. 2 – 5, for clarity. Note: Significant effects of KC/GRO were not detected at any age and data were omitted from all graphs.
Fig. 2. Corticosterone, corticosteroid binding globulin, and C-reactive protein levels.

Bar represent mean levels of corticosterone (A – C), corticosteroid binding globulin (D – F), or C-reactive protein (G – I) ± SEM. Levels of corticosterone and C-reactive protein are presented as nM and μg/ml, respectively, with statistical analysis of Blom transformed (normalized) data. CBG levels (nM) were not transformed for statistical analysis. In the case of a main effect of prenatal treatment, the asterisk (*) indicates a significant post hoc. The “×” indicates a significant planned pairwise comparison following a trend for a main effect of prenatal treatment. For post hocs/planned pairwise comparisons, the symbol denotes a comparison with the control group, unless otherwise indicated.
Post hoc: * p < 0.05; ** p < 0.01; *** p < 0.001; Planned pairwise comparison: × p < 0.05; P1: n = 8 – 11/prenatal treatment group; P8, 22: n = 7 – 8/prenatal treatment group; CBG: corticosteroid binding globulin; CRP: C-reactive protein; P1, 8, 22: postnatal day 1, 8, 22; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
Fig. 5. Cytokine levels in blood, hypothalamus, prefrontal cortex, hippocampus, and spleen on P22.

Bars represent mean cytokine level ± SEM. Data are presented as pg cytokine/mg protein for brain (hypothalamus, PFC, hippocampus) and spleen or pg cytokine/ml for serum, with the difference in unit denoted by the double line intersecting the x-axis. Cytokine levels within each tissue were analyzed by separate analyses of variance (ANOVAs) using Blom transformed (normalized) data. Serum levels of IL-1β and IL-5 and hippocampal levels of IL-13 were below the lower limit of detection of the assay. In the case of a main effect of prenatal treatment, the asterisk (*) indicates a significant post hoc and the symbol denotes a comparison with the control group, unless otherwise indicated.
Post hoc: * p < 0.05; ** p < 0.01; *** p < 0.001; n = 7 – 8/prenatal treatment group; P22: postnatal day 22; HYPO: hypothalamus; PFC: prefrontal cortex; HPX: hippocampus; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
Pearson's correlations between ethanol consumption during the second half of gestation (GD12 – 21) and both spleen weight and cytokine levels were also performed. The relationship between the variables was first examined by scatterplot and regression in order to confirm a linear relationship. Data met the assumptions of the Pearson's correlation.
Differences were considered significant at p ≤ 0.05, and trends (p > 0.05 and < 0.085) were examined, by planned pairwise comparisons, according to our a prior hypotheses. Nonsignificant effects are not reported. Significant ANOVA F statistics and p values are reported in the text; post hoc p values are reported in figure legends. Significant p-values for main effects and post-hoc comparisons were reported according to the following range: p < 0.05 (*); 0.05 > p > 0.01 (**); p < 0.001 (***).
3. Results
3.1 Pregnancy outcome
Analysis of maternal weight throughout gestation revealed, as expected, a significant interaction between prenatal group and gestation day [F(6, 120) = 12.80, p < 0.001], with PAE and PF dams weighing less than C dams from GD 7 through GD 21 (Table 1). Total intake of liquid ethanol diet is shown in supplementary figure 1 (S1). There were no prenatal group effects on maternal weight during lactation, and no prenatal treatment effects on gestation length, number of pups per litter, or number of pup deaths (Table 1).
Table 1. Pregnancy outcomes and maternal body weights during gestation and lactation.
| Pregnancy outcome variable | Prenatal treatment group | ||
|---|---|---|---|
| C | PF | PAE | |
| Number of pregnant dams | 15 | 15 | 13 |
| Length of gestation (d) | 22.9 ± 0.1 | 22.7 ± 0.1 | 22.7 ± 0.2 |
| Number of pups | 13.1 ± 0.08 | 14.5 ± 0.06 | 12.5 ± 0.08 |
| Number of pups deaths (P1-22) | 5 | 3 | 4 |
| Dam weight (g) | |||
| GD1 | 292.9 ± 6.7 | 289.1 ± 6.7 | 293.0 ± 7.2 |
| GD7 | 334.5 ± 6.8 | 309.7 ± 6.8 | 312.5 ± 7.3+ |
| GD14 | 388.7 ± 6.6 | 350.4 ± 6.6 | 350.5 ± 7.1+++ |
| GD21 | 486.5 ± 9.3 | 441.4 ± 9.3 | 423.4 ± 10.0++ |
| LD1 | 402.5 ± 11.9 | 380.0 ± 9.7 | 359.5 ± 9.7 |
| LD8 | 372.8 ± 10.2 | 366.0 ± 8.3 | 360.5 ± 8.3 |
| LD15 | 352.8 ± 7.5 | 365.2 ± 6.1 | 362.8 ± 6.1 |
| LD22 | 355.8 ± 5.1 | 347.8 ± 4.2 | 341.7 ± 4.2 |
Data are presented as mean ± SEM; Post hoc :
PAE = PF < C;
p < 0.05;
p < 0.01;
p < 0.001;
GD: gestational day; LD: lactation day; d: day; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
3.2 Offspring body, brain, and spleen weight
On P1, both PAE and PF pups had lower body weight than C pups [F(2, 26) = 4.92, p < 0.05] (Fig 1A), with catch up growth such that there were no differences in weight by P8 (Fig. 1B). Similarly, PAE and PF pups had lower brain weight than C pups on P1 [F(2, 26) = 8.76, p < 0.01] (Fig 1D), with catch up growth by P8 (Fig 1E). However, on P22, despite no significant differences in body weight, brain weight was increased in PAE and PF compared to C pups [Fig. 2F; F(2, 22) = 5.08, p < 0.05] (Fig 1F). Of note, when corrected for body weight, brain weight was not different among prenatal groups on P22 (data not shown).
Fig. 1. Body, brain, and spleen weight on P1, 8, and 22.

Bars represent mean body (A – C), brain (D – F) or spleen (G – I) weight ± SEM. In the case of a main effect of prenatal treatment, the asterisk (*) indicates a significant post hoc, with the comparison being made to the control group, unless otherwise indicated.
Post hoc: * p < 0.05; ** p < 0.01; *** p < 0.001; P1: n = 8 – 10/prenatal treatment group; P8, 22: n = 7 – 8/prenatal treatment group; P1, 8, 22: postnatal day 1, 8, 22; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
Spleen weight was significantly increased in PAE compared to both C and PF pups on P22 [F(2, 23) = 13.06, p < 0.001] (Fig 1I) but not on P1 or P8 (Fig 1G, H). There was, however, a strong positive correlation between spleen weight on P8 and maternal alcohol consumption during the second half of pregnancy (GD12 – 21; r = 0.73, R2 = 0.533; p < 0.05).
3.3 Serum measures of corticosterone, CBG and CRP
There were no differences in corticosterone levels among prenatal treatment groups at any age (Fig. 2 A – C). CBG levels, however, were lower in PAE compared to C and PF pups at birth and P8 [P1: F(2, 24) = 5.13, p < 0.05; P8: F(2, 23) = 6.11, p < 0.01] (Fig 2D, E].
Examination of CRP levels revealed a trend for a main effect of prenatal treatment on P1 [F(2, 22) = 3.18, p = 0.06], with planned pairwise comparisons showing lower CRP levels in PAE compared to C pups (Fig 1G). CRP levels did not differ among groups on P8 or P22 (Fig. 2H – I).
3.4 Cytokine levels in serum, whole brain, and spleen at birth (P1)
On P1, serum cytokine levels were low overall, with detectable levels of TNF-α, IFN-γ, IL-4, IL-10, and IL-13 only (Fig 3), and no significant effects of prenatal treatment. In whole brain on P1, however, lower levels of IFN-γ were found in PAE compared to C pups [F(2, 21) = 4.13, p < 0.05; Fig.3D). By contrast, in the spleen we found specific effects of pair-feeding: IL-2 and IL-5 levels were higher in the spleen of PF compared to C and PAE pups [IL-2: F(2, 20) = 3.96, p < 0.05; IL-5: F(2, 19) = 4.55, p < 0.05; Fig 3B, G].
Fig. 3. Cytokine levels in blood, whole brain, and spleen at birth (P1).
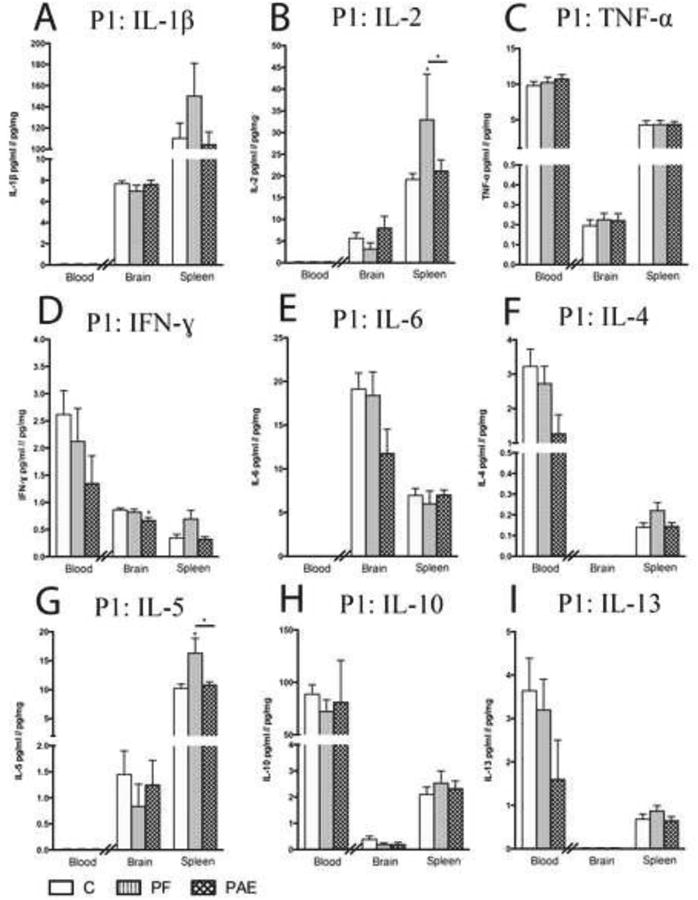
Bars represent mean cytokine level ± SEM. Data are presented as pg cytokine/mg protein for brain and spleen or pg cytokine/ml for serum, with the difference in unit denoted by the double line intersecting the x-axis. Cytokine levels within each tissue were analyzed by separate analyses of variance (ANOVAs) using Blom transformed (normalized) data. Serum levels of IL-1β, IL-2, IL-6, and IL-5 and whole brain levels of IL-4 and IL-13 were below the lower limit of detection of the assay. In the case of a main effect of prenatal treatment, the asterisk (*) indicates a significant post hoc. For post hocs, the symbol denotes a comparison with the control group, unless otherwise indicated.
Post hoc: * p < 0.05; ** p < 0.01; *** p < 0.001; n = 7 – 11/prenatal treatment group; P1: postnatal day 1; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
3.5 Cytokine levels in serum, brain (hypothalamus, PFC, hippocampus), and spleen on P8
On P8 we detected generally elevated serum cytokine levels in PAE pups. TNF-α levels were higher in PAE compared to both PF and C pups [F(2, 23) = 5.13, p < 0.05], and IL-13 and IFN-γ levels were higher in PAE compared to PF pups [IL-13: F(2, 23) = 4.49, p < 0.05; IFN-γ: F(2, 23) = 3.64, p < 0.05] (Fig 4 C, I, D).
Fig. 4. Cytokine levels in blood, hypothalamus, prefrontal cortex, hippocampus, and spleen on P8.

Bars represent mean cytokine level ± SEM. Data are presented as pg cytokine/mg protein for brain (hypothalamus, PFC, hippocampus) and spleen or pg cytokine/ml for serum, with the difference in unit denoted by the double line intersecting the x-axis. Cytokine levels within each tissue were analyzed by separate analyses of variance (ANOVAs) using Blom transformed (normalized) data. Serum levels of IL-1β and IL-5 were below the lower limit of detection of the assay. In the case of a main effect of prenatal treatment, the asterisk (*) indicates a significant post hoc. The “×” indicates a significant planned pairwise comparison. For post hocs/planned pairwise comparisons, the symbol denotes a comparison with the control group, unless otherwise indicated.
Post hoc: * p < 0.05; ** p < 0.01; *** p < 0.001; Planned pairwise comparisons: × p < 0.05; n = 7 – 8/prenatal treatment group; P8: postnatal day 8; HYPO: hypothalamus; PFC: prefrontal cortex; HPX: hippocampus; C: control; PF: pair-fed; PAE: prenatal alcohol exposure.
Increased levels of cytokines were also observed in the hippocampus and PFC of PAE pups. In the hippocampus, levels of IFN-γ and IL-1β were higher in PAE compared to both PF and C pups [IFN-γ: F(2, 21) = 4.36, p < 0.05; IL-1β: F(2, 21) = 5.28, p < 0.05], and IL-4 was increased in PAE compared to C pups [F(2, 21) = 3.76, p < 0.05] (Fig 4 D, A, F). As well, while main effects failed to reach significance for IL-2, IL-5, and TNF-α [IL-2: F(2, 21) = 3.28, p = 0.058; IL-5: F(2, 21) = 3.20, p = 0.061; TNF-α: F(2, 21) = 3.23, p = 0.060], planned pairwise comparisons revealed increased cytokine expression in PAE compared to C pups (p < 0.05) (Fig 4 B, G, C). In the PFC, levels of IL-5 were increased in PAE compared to C pups [F(2, 22) = 3.85, p < 0.05], and IL-6 levels were higher in both PAE and PF compared to C pups [F(2, 21) = 4.90, p < 0.05] (Fig 4G, E).
By contrast, in the hypothalamus we detected lower levels of cytokines in PAE pups. Specifically, IL-2 levels were lower in PAE compared to C pups [F(2, 22) = 4.56, p < 0.05] (Fig 4 B). Furthermore, while main effects for IL-1β and TNF-α failed to reach significance [IL-1β: F(2, 22) = 3.09, p = 0.068; TNF-α: F(2, 22) = 2.51, p = 0.107], planned pairwise comparisons revealed lower IL-1β and TNF-α levels (p < 0.05) in PAE compared to C pups.
Similarly, cytokines levels were lower in the spleen of PAE pups, with decreased levels of IL-13, IL-5, IL-10, and IL-6 in PAE compared to C and PF pups and decreased IL-4 in PAE compared to C pups [IL-13: F(2, 23) = 11.41, p < 0.001; IL-5: F(2, 23) = 8.66, p < 0.01; IL-10: F(2, 23) = 4.56, p < 0.05; IL-6: F(2, 23) = 6.53, p < 0.01; IL-4: F(2, 23) = 3.67, p < 0.05] (Fig 4E – I). In addition, while a main effect or prenatal treatment was not detected for IL-2 [F(2, 23) = 3.09, p = 0.067], planned pairwise comparisons revealed lower IL-2 levels in PAE compared to C pups (Fig 4 B).
3.6 Cytokine levels in serum, brain (hypothalamus, PFC, hippocampus), and spleen at weaning (P22)
Serum cytokines returned to control levels in PAE pups by weaning. In PF pups, however, circulating cytokine levels were low on P22, with lower levels of IL-13 in PF compared to both C and PAE, and lower levels of IFN-γ, IL-10, and IL-4 in PF compared to C pups [IL-13: F(2, 23) = 6.97, p < 0.01; IFN-γ: F(2, 23) = 4.90, p < 0.05; IL-10: F(2, 23) = 4.47, p < 0.05; IL-4: F(2, 23) = 6.00, p < 0.01] (Fig 5I, D, H, F). Serum levels of IL-2 and IL-6 were undetectable in PF pups on P22 (Fig. 4B, E).
In the hypothalamus and hippocampus, cytokine levels normalized, with no prenatal treatment effects detected. Comparatively, in the PFC, PF had higher levels of L-4, compared to PAE pups, as well as lower levels of IL-6 and IL-1β compared to both C and PAE pups [IL-4: F(2, 23) = 3.53, p < 0.05; IL-6: F(2, 23) = 3.52, p < 0.05; IL-1β: F(2, 23) = 4.94, p < 0.05] (Fig 5F, E, A).
In the spleen, despite significant increases in spleen weight in PAE offspring on P22, levels of pro- and anti-inflammatory cytokines did not differ among prenatal treatment groups. However, significant correlations between maternal alcohol consumption during the second half of pregnancy (GD 12 – 21) and levels of three cytokine were identified: levels of IL-6, IL-4, and IL-2 (Fig 5E, F, B), which were lower in PAE compared to C/PF pups on P8, were all negatively correlated with maternal alcohol consumption on P22 (IL-6: r = -0.838, R2 = 0.702, p = 0.009; IL-4: r = -0.707, R2 = 0.500, p = 0.050; trend for IL-2: r = -0.648, R2 = 0.420, p = 0.082).
4. Discussion
Our data demonstrate significant effects of PAE on key developmental parameters, HPA axis mediators, and both central and peripheral immune measures, in female offspring. Importantly, despite some overlap between PAE and PF offspring in body and brain weights, we detected unique endocrine and immune signatures for each of these prenatal treatments. While corticosterone levels did not differ among prenatal treatment groups, CBG levels were lower in PAE compared to C and PF pups at birth and P8. CRP levels were also lower in PAE compared to C pups at birth. As well, spleen weight was significantly increased in PAE compared to both C and PF pups on P22 and there was a strong positive correlation between spleen weight on P8 and maternal alcohol consumption during the second half of pregnancy. On P8, PAE offspring also had higher cytokine levels in the PFC and hippocampus, and lower levels in the hypothalamus and spleen, compared to C pups, whereas PF offspring had increased splenic cytokine levels on P1, and lower circulating and PFC cytokine levels on P22. Altered cytokine levels together with altered endocrine-immune interplay during a sensitive early-life period can shape the developmental trajectory. Thus, these findings lend support to the hypothesis that pervasive alterations in immune function associated with PAE may have their roots in aberrant early-life cytokine activity. Finally, it will be important in future studies to determine whether similar changes in endocrine and immune function also occur in PAE male offspring.
The finding that PAE and PF dams both weighed less than controls is not surprising, as we reduce the food ration of PF dams to match that of PAE dams, and supports previous data from our laboratory (Hellemans et al., 2008; Lan et al., 2009; Uban et al., 2010) and others (Abel, 1978; Thomas et al., 2000). Consistent with these findings were lower body and brain weights in PAE and PF compared to control pups at birth, with catch-up growth by P8. Interestingly, PAE and PF offspring had increased brain weights compared to controls on P22. Rapid compensatory brain growth has been linked to impairments in cognitive performance (Fisher et al., 2006). Similarly, accelerated growth of immune organs has been associated with impaired system development (Alonso-Alvarez et al., 2007; Pihlaja et al., 2006), with a likely impact on health (Metcalfe and Monaghan, 2001). This is in line with our finding of increased spleen weight in PAE pups (P22) and the positive correlation between spleen weight on P8 and maternal alcohol consumption during the second half of pregnancy, when spleen organogenesis occurs. While spleen size may be altered by infection (Roberts and Weidanz, 1978), autoimmune disorders (Garchow et al., 2011), cancers (Moloney et al., 1970), and prenatal cocaine exposure (Sobrian et al., 1990), to our knowledge, this is the first report of increased spleen weight following PAE. Furthermore, splenic levels of IL-13, IL-5, IL-6, IL-4 and IL-2 were markedly lower in PAE compared to PF and/or C offspring on P8. Interestingly, this dampening of splenic cytokine levels predated the increase in spleen weight on P22, which suggests that cytokine alterations may be an important factor in the mechanism underlying the increased immune organ weight. Additional support for this comes from the correlation between splenic cytokine (IL-6, IL-4, IL-2) levels on P22 and maternal alcohol consumption. Thus it appears that higher levels of gestational alcohol consumption are directly linked to changes in spleen weight and a dampening of splenic cytokine levels during the pre-weaning period. Early-life changes in the developmental trajectory of the spleen and immune function and could have long-lasting effects on adult immune cell populations and responses.
While there were no significant differences among groups in corticosterone levels, CBG, the major transport protein for glucocorticoids, was lower in PAE offspring during the first week of life, consistent with our previous findings (Weinberg, 1989). Lower CBG levels in the context of similar corticosterone levels implies higher free corticosterone levels in PAE offspring. This may be particularly detrimental during the stress hyporesponsive period when low corticosterone levels support normal brain development (Lupien et al., 2009) and likely normal immune system development (Buckingham et al., 1996). As well, CBG serves as a reservoir for glucocorticoids, allowing for targeted delivery of corticosterone in the context of an immune challenge (Hammond, 1990; Perogamvros et al, 2012). Thus, decreased CBG levels during early-life could be one of the underlying factors resulting in the previously reported increase in early-life major and minor infections (Gauthier et al, 2004; Johnson et al, 1981).
Overall, serum cytokine levels were low at birth and there were only modest increases in pro-inflammatory serum cytokine levels of PAE pups on P8. In the brain, by contrast, PAE pups exhibited increased hippocampal levels of TNF-α, IFN-γ, IL-1β, IL-2, IL-4 and IL-5, as well as increased levels of IL-5, and IL-6 in the PFC. Alterations in the fine cytokine balance during early-life may have significant consequences for brain development, including both structure and function [reviewed in (Deverman and Patterson, 2009). While it is not yet possible to elucidate the molecular and developmental effects of disturbances in this specific combination of cytokines, there is value in contextualizing our findings within the growing body of research on the effects overexpression of individual cytokines within the brain. For example, elevated levels of TNF-α have been shown to be neurotoxic to the developing brain, disrupting the blood-brain barrier in vivo (Megyeri et al, 1992), and inducing apoptosis of oligodendrocytes and impairments in myelination in vitro (Cammer and Zhang, 1999; Pang et al, 2005; Selmaj and Raine, 1988). Similarly, in vitro studies have linked both IL-1β and IL-2 to oligodendrocyte toxicity (Curatolo et al, 1997; Takahashi et al, 2003) and in vivo, IL-1β injection has been shown to impair myelination and induce apoptosis in the brain (Pang et al, 2005). On the other hand, in vitro work has linked IL-4 with neurogenesis and oligodendrogenesis (Butovsky et al, 2006) and enhanced survival of neurons (Araujo and Cotman, 1993). Moreover, studies have shown a link between elevated maternal and child IL-4 levels and an increased risk of an autism diagnosis (Abdallah et al, 2013; Goines et al, 2011; Krakowiak et al, 2015). In our model, however, it is currently unclear whether increased IL-4 is having positive compensatory effects, or potentially interfering with normal apoptosis that is critical for brain development. On the other hand, IL-6, which has both pro- and anti-inflammatory properties, is shown to affect neuronal survival and differentiation and modulate production of neurotrophins (Frei et al., 1989; Hama et al., 1989; Satoh et al., 1988). However, enhanced IL-6 may have negative impacts on brain development; Harding et al., (2004) showed that a single nucleotide polymorphism (SNP) in the IL-6 gene promoter leads to enhanced IL-6 production and is associated with hemorrhagic brain injuries and white mater damage in preterm infants (Harding et al., 2004). Finally, our findings of increased cytokine levels in the brain of PAE females are in line those from studies using other exposure models (third trimester equivalent model of alcohol vapor inhalation or exposure by gavage) and in different species (rats and mice) and sexes (Drew et al., 2015; Topper et al., 2015), which speaks to the robustness of these data and supports the suggestion that neuroinflammation may be a cross-cutting feature of FASD.
Importantly, the altered cytokine profile in PAE pups was only detected on P8, a time when microglia are more active and cytokine-producing (Schwarz, 2012), which would suggest an altered microglial activational state. This is in line with findings by Drew et al., (2015), showing increased numbers of primed/active microglia in alcohol-exposed offspring on P10 (Drew et al., 2015). While cytokine changes resolved by P22, it remains to be determined if this represents achievement of a normal cytokine balance or the transition of microglia to a quiescent state, which could mask underlying neuroimmune disturbances. Nevertheless, as cytokines play an important role in neurodevelopment, alterations in cytokine levels during sensitive developmental windows, may have long-lasting impacts on brain structure and function [reviewed in (Deverman and Patterson, 2009)].
Unlike the PFC and hippocampus, in the hypothalamus, cytokine levels (IL-2, IL-1β, TNF-α) were generally lower in PAE offspring compared to controls. As infection induces release of cytokines and other factors from immune cells, which act at the level of the hypothalamus to alert the nervous system to the infection, and initiates the febrile response, and sickness behaviors, it is possible that early-life alterations in hypothalamic cytokine production could underlie, at least in part, the decreased resistance to both major and minor infections in children with FASD. Whether there is a link between alterations in early-life cytokine levels and later-life cytokine dysregulation in the hypothalamus of PAE offspring, as suggested by previous work of Taylor and colleagues (Taylor et al, 1999; Yirmiya et al, 1993) remains to be determined.
Effects of pair-feeding were also detected in this study. Importantly, the cytokine signature of PF offspring was temporally and directionally different from that of PAE pups. Overall, pair-feeding was associated with a general pattern of increased splenic cytokines at birth and decreased circulating and PFC cytokine levels at weaning compared to C and/or PAE offspring, whereas PAE was generally associated with higher cytokine levels in the PFC and hippocampus, and lower cytokine levels in the hypothalamus and spleen compared to C and/or PF pups at P8. As one of the confounds associated with pair-feeding is the mild prenatal stress associated with reduced food intake of the dam, the prenatal stress literature may provide some insight into our findings, as there is substantial evidence that prenatal stress has an inhibitory effect on immune function of offspring [reviewed in (Merlot et al, 2008)]. For example, in a rhesus monkey model, prenatal stress impaired the in vitro cytokine (TNF-a and IL-6) release with LPS challenge (Coe et al, 2002), which is generally in line with our finding of decreased circulating cytokines in PF rats at weaning. Similarly, maternal food restriction also typically results in an impaired cytokine response to challenge (Desai et al., 2009). Taken as a whole, pair-feeding, while serving as a control for the reduced food intake of alcohol-consuming dams, is a treatment in itself, consisting of both food restriction and the mild prenatal stress of maternal hunger. The marked differences in the effects of PAE and pair-feeding in the present study, however, is suggestive of differential programming of offspring neuroendocrine/neuroimmune function by these early-life insults. Support for this comes from our previous findings (Glavas et al., 2007) showing that even when there is some overlap between PAE and pair-feeding effects, the mechanisms underlying these effects are different in PAE and PF offspring.
In summary, our findings of a unique cytokine profile in PAE rats, during a period when the brain is maturing and being remodeled, provide insight into factors that may underlie some of the long-term negative health consequence observed in children with FASD. As cytokines are key contributors to early brain development, changes to the delicate cytokine balance for even a short period during early development may have significant lifelong consequences for neuroimmune processes. Of particular clinical relevance, given the high rate of mental health disorders in children with FASD, and the growing evidence for immune disturbances as an underlying factor in depression and anxiety disorders (Lyall et al., 2014; Meyer, 2013), we suggest that the altered cytokines levels detected in the brain of PAE offspring could play a role, at least in part, in the increased vulnerability to later-life mental health disorders observed in FASD. Furthermore, it is possible that lower cytokines levels in the spleen, a critical immune organ, and the hypothalamus, a critical integrator between immune and endocrine responses, may underlie the increased susceptibility to infections following in utero alcohol exposure. Together, our findings highlight the future possibility that immune-based intervention strategies, particularly targeting early-life, could be considered as an adjunctive novel therapeutic approach for individuals with FASD.
Supplementary Material
Highlights.
Prenatal alcohol exposure alters cytokine levels in multiple compartments.
Alcohol-exposed pups had increased cytokines in serum, hippocampus, and PFC.
By contrast, cytokine levels were lower in the hypothalamus and spleen of PAE pups.
An altered immune signature during early developmental has long-term implications.
Findings highlight the possible utility of novel immune-based interventions for PAE.
Acknowledgments
The authors wish to acknowledge Dr. Geoffrey Hammond for his insight and expertise in the area of CBG and his assistance with the CBG measurements. Dr. Eric Sandberg from Meso Scale Discovery (MSD) provided excellent technical assistance and troubleshooting expertise for the MSD assays. We would also like to thank Dr. Edie Dullaghan, Head of Target Validation at the Centre for Drug Research and Development (CDRD), for generously providing access to their Sector Imager, and Leanna Yee, senior technician, Target Validation, at CDRD for her technical assistance. Finally, we would like to express our thanks to members of the Weinberg laboratory for their assistance: David Mak and Tessa Morin for their help compiling data, Wayne Yu for his technical assistance with the cytokine assays, and Wendy Comeau for her assistance with tissue collection.
Grant support: This work was supported by: NIH/NIAAA RO1 AA022460, R37 AA007789, NeuroDevNet (Canadian Networks of Centres of Excellence), and the Canadian Foundation on Fetal Alcohol Research to JW, and a Natural Sciences and Engineering Research Council of Canada (NSERC) CGS-D to T.S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2013;14:528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- Abel EL. Effects of ethanol on pregnant rats and their offspring. Psychopharmacology (Berl) 1978;57:5–11. doi: 10.1007/BF00426950. [DOI] [PubMed] [Google Scholar]
- Alleva E, Caprioli A, Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam (Mus musculus) J Comp Psychol. 1989;103:83–87. doi: 10.1037/0735-7036.103.1.83. [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G. Blackwell Publishing Ltd Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Functional Ecology. 2007;21:873–879. [Google Scholar]
- Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- Blom G. Statistical estimates and transformed beta-variables. John Wiley & Sons; NY: 1958. [Google Scholar]
- Bray LA, Shao H, Ewald SJ. Effect of ethanol on development of fetal mouse thymocytes in organ culture. Cell Immunol. 1993;151:12–23. doi: 10.1006/cimm.1993.1218. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Loxley HD, Christian HC, Philip JG. Activation of the HPA axis by immune insults: roles and interactions of cytokines, eicosanoids, glucocorticoids. Pharmacol Biochem Behav. 1996;54:285–298. doi: 10.1016/0091-3057(95)02127-2. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Cammer W, Zhang H. Maturation of oligodendrocytes is more sensitive to TNF alpha than is survival of precursors and immature oligodendrocytes. J Neuroimmunol. 1999;97:37–42. doi: 10.1016/s0165-5728(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Chiappelli F, Kung MA, Tio DL, Tritt SH, Yirmiya R, Taylor AN. Fetal alcohol exposure augments the blunting of tumor necrosis factor production in vitro resulting from in vivo priming with lipopolysaccharide in young adult male but not female rats. Alcohol Clin Exp Res. 1997;21:1542–1546. [PubMed] [Google Scholar]
- Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–681. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Curatolo L, Valsasina B, Caccia C, Raimondi GL, Orsini G, Bianchetti A. Recombinant human IL-2 is cytotoxic to oligodendrocytes after in vitro self aggregation. Cytokine. 1997;9:734–739. doi: 10.1006/cyto.1997.0228. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle DA, Casillas E, Boles J, Ross MG. Early undernutrition attenuates the inflammatory response in adult rat offspring. J Matern Fetal Neonatal Med. 2009;22:571–575. doi: 10.1080/14767050902874105. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:445–454. doi: 10.1111/acer.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJ. Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol. 2014;118:41–80. doi: 10.1016/B978-0-12-801284-0.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald SJ, Frost WW. Effect of prenatal exposure to ethanol on development of the thymus. Thymus. 1987;9:211–215. [PubMed] [Google Scholar]
- Ewald SJ, Walden SM. Flow cytometric and histological analysis of mouse thymus in fetal alcohol syndrome. J Leukoc Biol. 1988;44:434–440. doi: 10.1002/jlb.44.5.434. [DOI] [PubMed] [Google Scholar]
- Fisher MO, Nager RG, Monaghan P. Compensatory growth impairs adult cognitive performance. PLoS Biol. 2006;4:e251. doi: 10.1371/journal.pbio.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. The Journal of nutrition. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, Obad S, Petri A, Kauppinen S, Kiriakidou M. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3:605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Manar MH, Brown LA. Is maternal alcohol use a risk factor for early-onset sepsis in premature newborns? Alcohol. 2004;33:139–145. doi: 10.1016/j.alcohol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z, Abel EL. Maternal and paternal alcohol use: effects on the immune system of the offspring. Life Sci. 1991;48:1–8. doi: 10.1016/0024-3205(91)90419-c. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z, Christie R, Felten DL, LeGrue SJ. Prenatal ethanol exposure alters immune capacity and noradrenergic synaptic transmission in lymphoid organs of the adult mouse. Neuroscience. 1990;35:185–194. doi: 10.1016/0306-4522(90)90133-o. [DOI] [PubMed] [Google Scholar]
- Grossmann A, Astley SJ, Liggitt HD, Clarren SK, Shiota F, Kennedy B, Thouless ME, Maggio-Price L. Immune function in offspring of nonhuman primates (Macaca nemestrina) exposed weekly to 1.8 g/kg ethanol during pregnancy: preliminary observations. Alcohol Clin Exp Res. 1993;17:822–827. doi: 10.1111/j.1530-0277.1993.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hama T, Miyamoto M, Tsukui H, Nishio C, Hatanaka H. Interleukin-6 as a neurotrophic factor for promoting the survival of cultured basal forebrain cholinergic neurons from postnatal rats. Neurosci Lett. 1989;104:340–344. doi: 10.1016/0304-3940(89)90600-9. [DOI] [PubMed] [Google Scholar]
- Hammond GL. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev. 1990;11:65–79. doi: 10.1210/edrv-11-1-65. [DOI] [PubMed] [Google Scholar]
- Harding DR, Dhamrait S, Whitelaw A, Humphries SE, Marlow N, Montgomery HE. Does interleukin-6 genotype influence cerebral injury or developmental progress after preterm birth? Pediatrics. 2004;114:941–947. doi: 10.1542/peds.2003-0494-F. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. The cytokine hypothesis: A neurodevelopmental explanation for the emergence of schizophrenia later in life. Advances in Bioscience and Biotechnology. 2013;4:81–88. [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells TR, Weinberg J. Influence of ethanol consumption on immune competence of adult animals exposed to ethanol in utero. Alcohol Clin Exp Res. 1998;22:391–400. [PubMed] [Google Scholar]
- Johnson S, Knight R, Marmer DJ, Steele RW. Immune deficiency in fetal alcohol syndrome. Pediatr Res. 1981;15:908–911. doi: 10.1203/00006450-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Kim CK, Turnbull AV, Lee SY, Rivier CL. Effects of prenatal exposure to alcohol on the release of adenocorticotropic hormone, corticosterone, and proinflammatory cytokines. Alcohol Clin Exp Res. 1999;23:52–59. [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, Van de Water J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009;34:1314–1328. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latino-Martel P, Chan DS, Druesne-Pecollo N, Barrandon E, Hercberg S, Norat T. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1238–1260. doi: 10.1158/1055-9965.EPI-09-1110. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Prenatal alcohol exposure blunts interleukin-1-induced ACTH and beta-endorphin secretion by immature rats. Alcohol Clin Exp Res. 1993;17:940–945. doi: 10.1111/j.1530-0277.1993.tb05645.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. 2014;44:1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J, Meyerholz DK, Edsen-Moore M, Young B, Coleman RA, Schlueter AJ, Waldschmidt TJ, Cook RT, Legge KL. Fetal exposure to ethanol has long-term effects on the severity of influenza virus infections. J Immunol. 2009;182:7803–7808. doi: 10.4049/jimmunol.0803881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megyeri P, Abraham CS, Temesvari P, Kovacs J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148:137–140. doi: 10.1016/0304-3940(92)90823-p. [DOI] [PubMed] [Google Scholar]
- Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Moloney WC, Boschetti AE, King VP. Spontaneous leukemia in Fischer rats. Cancer Res. 1970;30:41–43. [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Noto C, Ota VK, Santoro ML, Gouvea ES, Silva PN, Spindola LM, Cordeiro Q, Bressan RA, Gadelha A, Brietzke E, Belangero SI, Maes M. Depression, Cytokine, and Cytokine by Treatment Interactions Modulate Gene Expression in Antipsychotic Naive First Episode Psychosis. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9489-3. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80:226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability--effects on hormone measurement and action. Nat Rev Endocrinol. 2012;8:717–727. doi: 10.1038/nrendo.2012.134. [DOI] [PubMed] [Google Scholar]
- Pihlaja M, Siitari H, Alatalo RV. Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J Anim Ecol. 2006;75:1154–1164. doi: 10.1111/j.1365-2656.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Redei E, Clark WR, McGivern RF. Alcohol exposure in utero results in diminished T-cell function and alterations in brain corticotropin-releasing factor and ACTH content. Alcohol Clin Exp Res. 1989;13:439–443. doi: 10.1111/j.1530-0277.1989.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Weidanz WP. Splenomegaly, enhanced phagocytosis, and anemia are thymus-dependent responses to malaria. Infect Immun. 1978;20:728–731. doi: 10.1128/iai.20.3.728-731.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Nakamura S, Taga T, Matsuda T, Hirano T, Kishimoto T, Kaziro Y. Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol. 1988;8:3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. The developing brain. Morgan & Claypool Life Sciences; 2012. The immune system and the developing brain; pp. 1–118. [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Smith CL, Hammond GL. An amino acid substitution in biobreeding rat corticosteroid binding globulin results in reduced steroid binding affinity. The Journal of biological chemistry. 1991;266:18555–18559. [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrian SK, Burton LE, Robinson NL, Ashe WK, James H, Stokes DL, Turner LM. Neurobehavioral and immunological effects of prenatal cocaine exposure in rat. Pharmacol Biochem Behav. 1990;35:617–629. doi: 10.1016/0091-3057(90)90299-w. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology. 2015;156:511–522. doi: 10.1210/en.2013-1606. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Yirmiya R. Fetal alcohol exposure attenuates interleukin-1beta-induced fever: neuroimmune mechanisms. J Neuroimmunol. 1999;99:44–52. doi: 10.1016/s0165-5728(99)00102-2. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alc ohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation. 2015;12:160. doi: 10.1186/s12974-015-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical development of offspring. Alcohol Clin Exp Res. 1989;13:73–83. doi: 10.1111/j.1530-0277.1989.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Jerrells TR. Suppression of immune responsiveness: sex differences in prenatal ethanol effects. Alcohol Clin Exp Res. 1991;15:525–531. doi: 10.1111/j.1530-0277.1991.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Wolcott RM, Jennings SR, Chervenak R. In utero exposure to ethanol affects postnatal development of T- and B-lymphocytes, but not natural killer cells. Alcohol Clin Exp Res. 1995;19:170–176. doi: 10.1111/j.1530-0277.1995.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pilati ML, Chiappelli F, Taylor AN. Fetal alcohol exposure attenuates lipopolysaccharide-induced fever in rats. Alcohol Clin Exp Res. 1993;17:906–910. doi: 10.1111/j.1530-0277.1993.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lan N, Bach P, Nordstokke D, Yu W, Ellis L, Meadows GG, Weinberg J. Prenatal alcohol exposure alters the course and severity of adjuvant-induced arthritis in female rats. Brain Behav Immun. 2012;26:439–450. doi: 10.1016/j.bbi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


