Abstract
In spite of high prevalence and disease burden, scientific consensus on the etiology and treatment of Alcohol Use Disorder (AUD) has yet to be reached. The development and utilization of experimental psychopathology paradigms in the human laboratory represents a cornerstone of AUD research. In this review, we describe and critically evaluate the major experimental psychopathology paradigms developed for AUD, with an emphasis on their implications, strengths, weaknesses, and methodological considerations. Specifically we review alcohol administration, self-administration, cue-reactivity, and stress-reactivity paradigms. We also provide an introduction to the application of experimental psychopathology methods to translational research including genetics, neuroimaging, pharmacological and behavioral treatment development, and translational science. Through refining and manipulating key phenotypes of interest, these experimental paradigms have the potential to elucidate AUD etiological factors, improve the efficiency of treatment developments, and refine treatment targets thus advancing precision medicine.
Keywords: Experimental psychopathology, alcohol use disorder, alcohol administration, self-administration, cue-reactivity, stress
1. Experimental Psychopathology Paradigms for Alcohol Use Disorders
Alcohol use disorder (AUD) is characterized by a cluster of behavioral and physical symptoms involving prolonged and maladaptive alcohol use (American Psychiatric Association, 1994, 2013). Despite high prevalence rates and disease burden (Grant et al., 2015; Harwood, 2000), scientific consensus regarding the etiology of AUD has yet to be reached. Furthermore, while several treatment approaches have been developed for AUD, effect sizes are only modest (Anton et al., 2006; Ferri, Amato, & Davoli, 2006; Litten et al., 2012; Magill & Ray, 2009; Morgenstern & Longabaugh, 2000; Rubak, Sandbæk, Lauritzen, & Christensen, 2005).
Multiple experimental psychopathology paradigms in the human laboratory have been developed to address distinct aspects of AUD phenomenology. Controlled alcohol administration in the laboratory permits the fine-grained assessment of pharmacokinetic and pharmacodynamic responses to alcohol, which have long been proposed as important AUD risk factors (King, de Wit, McNamara, & Cao, 2011; Newlin & Thomson, 1990; Schuckit, 1984). Furthermore, self-administration paradigms permit objective behavioral assessments of alcohol consumption, motivation, and compulsive use which are central to addiction phenomenology (Koob & Volkow, 2009; Zimmermann, O’Connor, & Ramchandani, 2013). Bolstered in part by the assessment of cue-reactivity in the human laboratory, the importance of drug-paired cues has been advanced in several well-validated addiction theories (B. L. Carter & Tiffany, 1999; Robinson & Berridge, 1993). Lastly, the assessment of stress-reactivity in AUD aligns with important etiological models in both human and animal research which advance stress as both a risk and maintenance factor in addictive disorders (Cappell & Peter, 1972; Koob & Kreek, 2007; Levenson, Sher, Grossman, Newman, & Newlin, 1980).
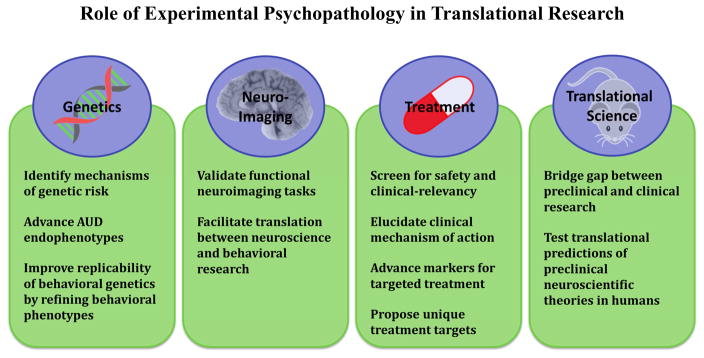
In this article, we describe and critically review the major experimental psychopathology paradigms for AUD, with an emphasis on relative strengths, weaknesses, and methodological concerns. We then provide a discussion of the application of these paradigms to translational research including genetics, neuroimaging, treatment, and translational science.
1.1. Alcohol Administration Paradigms
Individuals vary dramatically in their subjective response to alcohol administration and these differences are related to alcoholism risk (King, de Wit, et al., 2011; King, McNamara, Hasin, & Cao, 2014; Ray, Mackillop, & Monti, 2010; Schuckit, 1984; Schuckit & Smith, 1996). Though many outcome variables can be measured in these alcohol administration studies with varying degrees of objectivity (e.g. static ataxia [body sway]), the subjective experience of alcohol intoxication, often termed subjective response to alcohol (SR) has emerged a primary predictive factor in human etiological models (e.g. Bujarski, Hutchison, Prause, & Ray, 2015; Bujarski & Ray, 2014; King, Roche, & Rueger, 2011; Morean & Corbin, 2010; Quinn & Fromme, 2011; Ray, Bujarski, & Roche, 2016; Ray, Mackillop, et al., 2010; Schuckit, Risch, & Gold, 1988; Schuckit & Smith, 1996).
Though no studies to date have observed increased drinking from participation in alcohol administration studies (Drobes & Anton, 2000; Pratt & Davidson, 2005; Sommer et al., 2015), alcohol administration studies, particularly those recruiting AUD patients, raise important ethical questions (Enoch et al., 2009). Consequently the National Advisory Council on Alcohol Abuse and Alcoholism has published research practice guidelines (2005). To that end, the inclusion of a brief concluding motivational interviewing session has been shown to reduce participants’ alcohol consumption (Bacio, Lunny, Webb, & Ray, 2014; W. R. Miller & Rollnick, 2002; Rubak et al., 2005).
1.1.1. Oral Alcohol Administration
The most common alcohol challenge paradigm involves the administration of controlled oral doses of alcohol. The earliest oral alcohol administration studies used predefined dosing based on body weight and sex (e.g. 0.75 ml of ethanol/kg body weight; Schuckit, 1984). While easily implemented, these paradigms produce wide variability in observed blood alcohol concentrations (BAC), introducing considerable noise in the alcohol response data. BAC is known to be affected by numerous individual difference and state factors including sex (Cole-Harding & Wilson, 1987), weight (Devgun & Dunbar, 1990), body water content (Goist Jr. & Sutker, 1985), and recent food consumption (Jones & Neri, 1991). Thus, researchers have developed mathematical models of alcohol pharmacokinetics resulting in more precise alcohol dosing algorithms (Brick, 2006; Friel, Logan, O’Malley, & Baer, 1999).
Ecological validity and relative ease of implementation represent the primary advantages of oral alcohol challenges. Target doses for these studies should be selected after weighing the reliability of alcohol responses against safety and time concerns. An intoxicating dose of 0.08 g/dl (or 80 mg%) is typical of oral alcohol challenge studies as this dose is commensurate with high-risk, or binge drinking (NIAAA, 2004). The wide variability in observed BACs, even when utilizing pharmacokinetic algorithms, represents a primary weakness. This methodological noise is then reflected in the outcome data, thus harming statistical power and reliability. Relatedly there is considerable variability in the time to peak BAC. Alcohol responses are biphasic in response profile, with stimulation more prevalent on the ascending limb and sedation on the descending limb (Newlin & Thomson, 1990). Thus inter-subject variability in time to peak BAC can result in limb-level variability that further contributes to methodological noise.
1.1.2. Intravenous Alcohol Administration
To precisely control BAC and to dissociate pharmacological effects from responses to alcohol cues, alcohol can be administered intravenously (Bujarski, Hutchison, Roche, & Ray, 2015; Li, Yin, Crabb, O’Connor, & Ramchandani, 2001; Ramchandani et al., 2002, 2006; Ray et al., 2013; Ray & Hutchison, 2004; Ray, Kahler, Young, Chelminski, & Zimmerman, 2008). In these paradigms, alcohol is prepared in a diluted saline solution (typical concentrations of 5–6% EtOH). Studies conducted in our research laboratory have implemented an infusion rate nomogram taking into account participant sex and weight (e.g. infusion rate of 0.166 ml/min × weight in kilograms for males, or 0.126 ml/min × weight for females). A major advantage of intravenous alcohol paradigms is the precise control over observed BAC. Participants are repeatedly breathalyzed, and upon reaching each target BAC, the infusion rate is halved to maintain relatively stable BAC during testing.
Recently, researchers have developed a physiologically-based pharmacokinetic (PBPK) model which precisely estimates BAC based on participant characteristics including height, weight, age, and sex while incorporating self-correcting model parameters based on observed BAC values (Plawecki, Han, Doerschuk, Ramchandani, & O’Connor, 2008). This model, implemented in the Computerized Alcohol Infusion System (CAIS; Zimmermann et al., 2008; Zimmermann, O’Connor, & Ramchandani, 2013), permits fine-grained control of ascending limb BAC trajectories and, to a lesser extent, descending limb trajectories thus permitting systematic examination of limb effects and rate sensitivity (Wetherill et al., 2012). Furthermore, a recent extension of the PBPK model has shown that IV administration using CAIS is able to closely mimic subject-specific oral BrAC curves (Ramchandani, Plawecki, Li, & O’Connor, 2009).
Intravenous alcohol administration studies are thought to sacrifice ecological validity for greater experimental control and the elimination of visual, olfactory, and taste cues. Intravenous paradigms are significantly more expensive and require nursing support to place the IV catheter, a research pharmacy to prepare the EtOH-saline solution, and infusion supplies (IV pumps and lines). Implementing the CAIS system incurs additional costs as the infusion system requires a particular IV pump (iMed Gemini PC2-TX) and a dedicated computer to run the CAIS software.
1.1.3. Alcohol Clamp
The alcohol clamp paradigm involves maintaining steady state BAC over long periods of time through IV alcohol administration. Clamping permits the assessment of acute tolerance to alcohol’s effects (Morzorati, Ramchandani, Flury, Li, & O’Connor, 2002; O’Connor, Morzorati, Christian, & Li, 1998; Ramchandani et al., 2006; Ramchandani, Bolane, Li, & O’Connor, 1999). Implemented in CAIS (Zimmermann et al., 2008), the alcohol clamp procedure has demonstrated remarkably stable BAC over time courses of hours.
The principle benefits of alcohol clamp paradigms lie in the unique parameters they are able to assess namely acute tolerance or sensitization and pharmacokinetic parameters including alcohol elimination rate (O’Connor et al., 1998; Ramchandani, Kwo, & Li, 2001). Long-term alcohol clamp procedures also allow for more robust assessments including behavioral tasks while at stable BAC. The limitations of intravenous alcohol administration are exaggerated in alcohol clamp paradigms insofar as the administration route and the BAC profile in clamp paradigms are quite different than those observed in the naturalistic setting.
1.2. Alcohol Self-Administration Paradigms
AUD involve the pathological consumption of alcohol and recurrent failure to limit use. Thus paradigms assessing self-administration benefit from strong face validity. Early studies recruited alcohol dependent subjects in an in-patient treatment context and permitted subjects to consume alcohol ad libitum over the course of days (Mello, 1972; Mello & Mendelson, 1965).
Self-administration paradigms examine operant conditioning factors such as reinforcement schedules, priming doses, alternative reinforcers, progressive reinforcement schedules, and punishment (e.g. monetary cost). Given the highly social nature of drinking, the presence of others was thought to influence self-administration in ways that were outside of experimental control, thus an early review recommended against testing participants in groups (Bigelow, Griffiths, & Liebson, 1975). Methodological advances in the observation and coding of social interactions however have renewed interest in social factors that influence alcohol consumption (Fairbairn, Sayette, Levine, Cohn, & Creswell, 2013; Sayette et al., 2012).
1.2.1. Oral Alcohol Self-Administration
Modern self-administration paradigms can be grouped into two categories: the “de Wit Choice against Placebo Paradigm” and the “O’Malley Choice Against Money Paradigm” (Zimmermann, O’Connor, & Ramchandani, 2011). In the de Wit paradigm, participants initially sample various placebo or alcohol beverages over four separate sessions on different days and subsequently choose between the sampled beverages over three sessions (de Wit, Pierri, & Johanson, 1989; de Wit, Söderpalm, Nikolayev, & Young, 2003; de Wit, Svenson, & York, 1999; Duka, Stephens, Russell, & Tasker, 1998). The alcohol beverages consumed in this paradigm are laboratory prepared mixed drinks consisting of 10% ethanol in tonic water and/or fruit juice, and placebo beverages are identical but for a 1% ethanol concentration taste mask. Access to beverages in this paradigm is restricted to one drink per 10–15 minutes. The primary outcome is the number of alcoholic beverages chosen and the percent of participants who consumed any alcohol. BAC levels observed in this paradigm are typically quite low (~0.05 g/dl). The second and more widely used paradigm is the O’Malley paradigm (Anton, Drobes, Voronin, Durazo-Avizu, & Moak, 2004; Drobes, Anton, Thomas, & Voronin, 2003; McKee et al., 2009; McKee, O’Malley, Shi, Mase, & Krishnan-Sarin, 2008; O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002). After a priming dose, subjects are offered a tray of four prepared drinks standardized to body weight/sex and are instructed that they may consume as many drinks as they like over the next hour, or receive monetary compensation for the drinks not consumed (e.g. $3 per drinks). This procedure is repeated for a second hour with a second tray of drinks. Primary outcomes include the number of drinks consumed, observed BAC levels and the percent of subjects who abstained.
The disincentives in both paradigms are implemented to ensure that operant responding is for alcohol rather than general reward, or thirst. Settings vary dramatically including hospital rooms, clinical research labs, labs designed to look like a bar, and actual bars. Most self-administration studies include a priming drink with typical target BAC doses ranging from 0.02 – 0.06 g/dl, though larger priming doses were found to increase the percentage of participants who abstained (Zimmermann et al., 2011).
The benefits of oral alcohol self-administration paradigms include strong external and ecological validity, relative implementation ease and low cost. Weaknesses include the wide variability in BAC profiles and the small number of total drinks available to participants which in turn reduces outcome variance and can induce a response demand characteristic (Orne, 2009).
1.2.2. Computerized Alcohol Infusion System
The development of CAIS (originally termed Computer-Assisted Self-infusion of Ethanol [CASE]) permits intravenous alcohol self-administration. The most widely utilized CAIS self-administration paradigm is a free-access (i.e. FR1) paradigm at a dose of 0.0075 g/dl BAC per administration delivered over 2.5 minutes. A priming dose of four administrations ordered over 10 minutes followed by a 15-minute waiting period is typical. Outcome measures include the number of drinks ordered and maximum BAC. Though FR1 is most common, CAIS can flexibly implement various reinforcement schedules including progressive schedules. Relatedly, Plawecki et al. (2013) have developed a response input system for CAIS that utilizes a sustained attention task to self-administer alcohol. Rather than simply pressing a button, participants are required to press and hold the response button and release within a small window of time to order an alcohol dose. By varying the duration of the release window this task is able to flexibly adapt to alcohol-related impairment in motor performance.
The CAIS self-administration paradigm has several distinct advantages. First, it produces highly controlled BrACs and controls for alcohol cues. Second, it delivers smaller doses of alcohol per administration, allowing participants to order many more “drinks” per session. Third, it implements a BAC safety limit temporarily inactivating the response button at pre-set BAC levels. Relatedly, intravenous administration is able to instantaneously halt the delivery of alcohol such that BAC immediately declines which is not possible with oral administration. Limitations of the CAIS paradigm include low ecological validity, as well as cost and complexity of implementation.
1.3. Cue-Reactivity Paradigms
The ability of drug-paired cues to elicit craving and approach behavior is a staple of multiple theories of addiction etiology (B. L. Carter & Tiffany, 1999; Robinson & Berridge, 1993, 2000, 2001; Tiffany, 1990). In the human lab, cue-reactivity is typically studied through the systematic presentation of drug-paired cues. Alcohol cue paradigms have been developed utilizing various modalities including in vivo and pictorial exposures, as well as imaginal, audio, video and virtual reality. For the purposes of this review, we will focus on in vivo and pictorial cue paradigms as they are the most widely implemented (Reynolds & Monti, 2013). Of note, initial evidence has suggested that laboratory cue-reactivity may prospectively predict treatment outcomes (Braus et al., 2001; Grüsser et al., 2004; Rohsenow et al., 1994). Lastly, similar ethical concerns to alcohol administration have been raised with respect to cue-reactivity paradigms (A. Carter & Hall, 2013) necessitating careful study design to minimize participant risk.
1.3.1. Pictorial Cue Paradigms
The most basic pictorial alcohol cue paradigm involves the presentation of alcohol related images (typically in blocks) followed by brief assessments of subjective state (e.g. craving). These paradigms are typically employed in neuroimaging studies of alcohol cue-reactivity (Courtney, Schacht, Hutchison, Roche, & Ray, 2015; Heinz et al., 2004; Mann et al., 2014; Schacht, Anton, Voronin, et al., 2013), where the primary outcomes are indicators of brain activation, especially in “reward regions” and top-down executive control regions (Courtney et al., 2015).
A widely-used behavioral task utilizing pictorial alcohol cues is the alcohol dot-probe task (Duka & Townshend, 2004; M. A. Miller & Fillmore, 2010; Townshend & Duka, 2001). The alcohol dot-probe measures attentional bias to alcohol-related images. The task involves presenting alcohol-related images and matched control images on a screen followed by a visual probe (e.g. an ‘X’) with the participant quickly identifying the location of the probe (i.e. left or right) by pressing a computer key. Reaction time (RT) to the visual probe is measured and the attentional bias outcome is indexed by the difference between alcohol and neutral mean RTs. To reduce habituation, the task also contains filler trials with control images (Townshend & Duka, 2001).
Pictorial cues are extremely flexible in their combination with various research methods. The primary limitation is the relatively low salience of the cues. Generally, the alcohol-related images are selected in order to include all alcohol beverage types (e.g. beer, wine, liquor, and mixed drinks), and to include a range of preparations (e.g. bottled and prepared). As a result, the salience of the individual images may vary substantially within a subject based on their preferred beverage. Furthermore, the presentation of a generic alcohol-related picture without the potential for actual alcohol consumption may not elicit craving or physiological responses to the same extent as in-vivo cues.
1.3.2. In Vivo Alcohol Cue Paradigms
Pioneered by Monti (1987), the widely used in vivo cue paradigm begins with singly-tested participants completing baseline assessments followed by the presented of a neutral water cue. Participants listen to an audio recording instructing them to handle, smell, and manipulate the water beverage for 3 minutes. Following this, participants repeat the assessment battery and complete a brief relaxation exercise. Then participants are presented with their preferred alcoholic beverage including a prepared drink in the appropriate glassware and a branded bottle and listen to an analogous instructions set.
Order of presentation is a central methodological issue for this paradigm. While some have utilized counterbalanced orders (Sayette, Griffin, & Sayers, 2010), the presentation of drug-cues first produces strong carryover effects (Monti et al., 1987). Thus most studies utilizing in vivo alcohol cues forgo counterbalancing (Hutchison et al., 2001, 2005; Monti et al., 1999; Reynolds & Monti, 2013).
In vivo alcohol cue paradigms benefit from strong ecological validity as the participants are presented with their preferred alcoholic beverage, and are exposed to naturalistic drinking cues. The salience of the cue is high and utilizes multiple sensory inputs. Weaknesses include the variability in the drinking cue being presented which may produce noise in the data. The high potential for carryover effects also represents a significant limitation. Lastly, it has been noted that not all subjects are significantly reactive to alcohol cues, including some with alcohol dependence (Mason, Light, Williams, & Drobes, 2009; Rubonis et al., 1994)
1.4. Stress-Reactivity Paradigms
Several prominent etiological models suggest that stress serves as a risk factor for AUD (Cappell & Peter, 1972; Greeley & Oei, 1999; Koob, 2009; Koob & Kreek, 2007; Koob & Le Moal, 1997; Levenson et al., 1980). The causal role of stress in AUD is bolstered by the consistent finding that hypothalamic-pituitary-adrenal axis activity promotes alcohol seeking behavior in both the preclinical and clinical experiments (Thomas & Bacon, 2013). In the laboratory, two predominant paradigms assessing psychological stress (as opposed to physical or pharmacological stress which are less common to date) have been widely utilized: the Trier Social Stress Test (TSST: Thomas & Bacon, 2013) and the Guided Stress Imagery Task (Sinha, Fuse, Aubin, & O’Malley, 2000).
1.4.1. Trier Social Stress Test
Social stress tasks include public speaking tasks (Matthew Field & Quigley, 2009), social interaction tasks (Higgins & Marlatt, 1975), and mental arithmetic tasks (Pratt & Davidson, 2009). The TSST, which involves all three of these stressors is the most common (Kirschbaum, Pirke, & Hellhammer, 1993; Thomas & Bacon, 2013). First the participant is instructed to prepare for a mock job interview. Second, the participant is placed in a room in front of non-responsive confederates without their prepared notes. Third, the participant completes serial subtractions to the same unresponsive audience who notes each error. The TSST has been shown to produce robust increases in subjective and objective measures of stress response including dramatic increases in cortisol, heart rate, and blood pressure (Dickerson & Kemeny, 2004; Kirschbaum et al., 1993; Singh, Petrides, Gold, Chrousos, & Deuster, 1999). Despite these robust stress responses, increases in alcohol craving and self-administration have not been consistently observed (de Wit et al., 2003; Nesic & Duka, 2006; Söderpalm & de Wit, 2002; Thomas, Bacon, Randall, Brady, & See, 2011; Thomas, Randall, Brady, See, & Drobes, 2011); although variability in alcohol use among the samples may contribute to these inconsistent findings.
Strengths of the TSST include the salience and intensity of the stressor which results in robust increases in physiological stress response. Weaknesses include the reliance on social stress which may be of varying salience across individuals. The TSST is also relatively difficult to implement and time intensive in that it requires at least 15 minutes of participant time and multiple highly trained research assistants to serve as confederates.
1.4.2. Guided Imagery Task
In the addiction literature, the guided imagery task developed by Sinha and colleagues has become increasingly popular (Fox, Bergquist, Hong, & Sinha, 2007; Sinha, 2009, 2011; Sinha et al., 2008; Sinha, Catapano, & O’Malley, 1999; Sinha et al., 2000). In this paradigm, participants are asked to provide details about a current unresolved stressful situation to a trained research assistant. These details are then turned into an audio recorded script that the participant listens to during testing. Typically, this paradigm also includes a neutral script and alcohol-related scripts can be developed to serve as a cue condition. Generally both stress and alcohol imagery increase measures of craving, negative affect, and physiological reactivity (Sinha, 2011). The most rigorously controlled of these paradigms involve additional training in mental imagery to ensure the fidelity of the guided imagery task (Sinha, 2011).
Outcome variables in this paradigm include mood and craving measures, as well as psychophysiology and stress hormones. The guided imagery task has been shown to reliably induce negative emotionality and craving, though effects on objective measures are mixed (Thomas & Bacon, 2013). Initial reports of the predictive utility of stress imagery on treatment outcomes have been positive (Higley et al., 2011).
Strengths of the guided imagery task include the personalized nature of the stress, and the relative ease of implementation which allows this task to be combined with other novel assessments and laboratory paradigms. Weaknesses include the generally weak effects on physiological and hormonal stress outcomes and the inherent variability in stressor salience and type with personalized scripts.
1.5. Other Laboratory Approaches
These nine paradigms represent the predominant experimental approaches to cutting-edge human experimental AUD research. Other approaches such as pharmacologically-induced craving paradigms (Umhau et al., 2011), behavioral economic analysis (Bickel, Madden, & Petry, 1998; MacKillop & Murphy, 2007), and impulsivity measures (Courtney et al., 2012; Jentsch et al., 2014) have also contributed to the AUD literature tremendously, though we are unable to review them in this paper. Next we will provide a brief introduction to the application of these paradigms in translational research aiming to elucidate the etiology and treatment of AUD.
2. Experimental Psychopathology and Alcoholism Genetics
Twin studies reliably demonstrate that AUD is highly influenced by genetic factors (Verhulst, Neale, & Kendler, 2015). However, these broad disease-phenotype approaches are unable to elucidate the biobehavioral mechanism of risk conferred by specific gene variants. Consistent with an endophenotype approach in psychiatric genetics (Gottesman & Gould, 2003; Salvatore, Gottesman, & Dick, 2015), the utilization of experimental psychopathology paradigms in combination with genetic analysis uniquely allows researchers to map genetic factors onto specific, reliable, and clinically relevant laboratory phenotypes.
For example, alcohol administration studies using twins and sibling pairs have demonstrated that alcohol responses are highly heritable (Heath & Martin, 1991; Viken, Rose, Morzorati, Christian, & Li, 2003). Controlled alcohol administration has been used to identify pharmacokinetic variants in alcohol metabolizing genes encoding the alcohol dehydrogenase and aldehyde dehydrogenase enzymes (Luczak, Glatt, & Wall, 2006) which are associated with greater aversive responses to alcohol administration (Cook et al., 2005; Wall, Thomasson, Schuckit, & Ehlers, 1992). Genetic loci affecting pharmacodynamic alcohol responses span multiple neurotransmitter systems including GABA (Pierucci-Lagha et al., 2005; Ray & Hutchison, 2009; Roh et al., 2011), acetylcholine (Joslyn et al., 2008), serotonin (Schuckit et al., 1999), and endogenous opioids (e.g. OPRM1; Hendershot, Claus, & Ramchandani, 2014; Ray, Barr, et al., 2012; Ray et al., 2013; Ray & Hutchison, 2004). Early evidence of gene × gene interactions have also been observed suggesting that alcohol challenge paradigms may be sensitive to epistatic genetic effects (Ray, Bujarski, Squeglia, Ashenhurst, & Anton, 2014). Consistent with enhanced hedonic responses among G-allele carriers of the OPRM1 A118G locus (Ray & Hutchison, 2004), a recent study utilizing the CAIS demonstrated greater self-administration among G-allele carriers (Hendershot et al., 2014), though other studies utilizing oral alcohol self-administration were null (Anton, Voronin, Randall, Myrick, & Tiffany, 2012; Setiawan et al., 2011). These studies together have advanced subjective responses to alcohol as an endophenotype for AUD, thus providing much needed clarity regarding the mechanism of genetic risk (Ray, Mackillop, et al., 2010).
Though less numerous, similar empirical and theoretical research has been conducted with cue- and stress-reactivity paradigms. For example, cue-reactivity paradigms have shown sensitivity to genetic factors including OPRM1 (Ray, 2011; Van Den Wildenberg, Wiers, et al., 2007), corticotrophin-releasing hormone binding protein (Ray, 2011), and D4 dopamine receptors (DRD4) (McGeary et al., 2006), though some null findings exist (Van Den Wildenberg, Janssen, Hutchison, Van Breukelen, & Wiers, 2007). Pictorial cue-reactivity paradigms implemented in the context of neuroimaging have also revealed genetic factors impacting alcohol cue-reactivity including Cannabinoid Receptor 1 (Hutchison et al., 2008), DRD4, and OPRM1 (Filbey et al., 2008; McClernon, Hutchison, Rose, & Kozink, 2007). Twin studies with the TSST have also demonstrated significant heritability (Kirschbaum, Wüst, Faig, & Hellhammer, 1992; Kudielka, Hellhammer, Kirschbaum, Harmon-Jones, & Winkielman, 2007). Driven by the well understood neurobiology of stress, candidate gene studies of stress-reactivity have focused on HPA-axis genetic variants including the Glucocorticoid Receptor and neuropeptide Y genes (Wüst et al., 2004).
Taken together, these studies suggest that the application of experimental psychopathology paradigms in genetics research may help elucidate biobehavioral mechanisms through which genetic variants confer risk for AUD. The utilization of multiple experimental psychopathology paradigms in future research would shed light on whether given genetic variants predict multiple behavioral phenotypes, or whether the variant is uniquely associated with a particular mechanism of risk. However, it is important to note that the behavioral genetics literature broadly has been plagued by publication bias which may result in inflated genetic effect sizes (Duncan & Keller, 2011; Hart, de Wit, & Palmer, 2013). Though speculative, by advancing candidate endophenotypes it is possible that experimental psychopathology paradigms may improve the reproducibility of behavioral genetics research.
3. Experimental Psychopathology and Neuroimaging
Addiction neuroscience research has benefit greatly from functional neuroimaging including both positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Many high-quality imaging studies utilize paradigms developed and refined in the human behavioral laboratory and thus experimental psychopathology informs the development of functional neuroimaging tasks which in turn interrogate the biological bases of behavioral phenotypes. For example, in their systematic review and meta-analysis, Schacht et al. (2013) showed that presentation of alcohol cues robustly activated limbic and prefrontal regions of the brain including ventral striatum, anterior cingulate, and ventromedial prefrontal cortex. Their meta-analysis also identified several regions more active in AUD cases relative to controls including posterior cingulate, precuneus, and superior temporal gyrus. Furthermore, some early evidence suggests that functional neuroimaging cue-reactivity outcomes may predict relapse propensity (e.g. Bach et al., 2015; Beck et al., 2012; Grüsser et al., 2004; Schacht, Anton, Randall, et al., 2013; Schuckit et al., 2016; Seo et al., 2015).
While cue-reactivity paradigms represent the predominant approach in the neuroimaging literature, alcohol administration paradigms have also been utilized to examine the neurobiological correlates SR. For example, one study combined oral alcohol administration with PET suggesting that endogenous opioid activity in the nucleus accumbens and orbitofrontal cortex may contribute to the subjective rewarding effects of alcohol (Mitchell et al., 2012). Additionally, Ramchandani et al. (2011) demonstrated that intravenous alcohol administration via CAIS induced dopamine release in the striatum, and that the magnitude of this dopaminergic response was modulated by OPRM1. FMRI studies have also observed alcohol effects on cerebral blood flow (Strang et al., 2014). In sum, by bridging the gap between well-validated behavioral phenotypes and measures of biological function, the application of experimental psychopathology paradigms to functional neuroimaging is poised to generate considerable insight into the clinical neuroscience of AUD.
4. Experimental Psychopathology and Alcoholism Treatment
Only three FDA approved pharmacotherapies currently exist for the primary treatment of AUD (Disulfiram, Acamprosate, and Naltrexone [oral and injectable]) and these medications have demonstrated only moderate efficacy (Jørgensen, Pedersen, & Tønnesen, 2011; Maisel, Blodgett, Wilbourne, Humphreys, & Finney, 2013). Similarly there are several empirically supported behavioral therapies for AUD including cognitive behavioral therapy, 12-step facilitation, and motivational interviewing, though the efficacy of existing behavioral treatments is only modest, particularly at more severe stages of alcoholism (Anton et al., 2006; Ferri et al., 2006; Magill & Ray, 2009; Rubak et al., 2005).
Relative to randomized clinical trials (RCTs), experimental psychopathology approaches represent an efficient strategy for AUD treatment development in terms of both expense and time (Litten et al., 2012; Ray, Hutchison, & Tartter, 2010). In addition to testing drug safety and tolerability, experimental psychopathology paradigms allow for the testing of clinically meaningful endpoints theoretically providing information about treatment efficacy and paving the way for RCTs. At present it is not known which paradigms are most effective for screening novel treatments, thus studies may employ multiple experimental paradigms to maximize the potential for detecting clinically-relevant effects. In addition to providing efficient screening of novel treatments, experimental psychopathology paradigms allow for the advancement of personalized treatment through elucidating the mechanism of action of efficacious treatments and identifying moderators of treatment response.
4.1. Experimental Psychopathology and Pharmacology
One proposed mechanism of action for AUD medication is the ability to “block the buzz” or potentiate the aversive aspects of alcohol intoxication might be effective treatment options (Heilig et al., 2010). Several medications with known clinical efficacy have been shown to blunt the rewarding effects of alcohol including naltrexone (King, Volpicelli, Frazer, & O’Brien, 1997; Ray & Hutchison, 2007), nalmefene (Drobes, Anton, Thomas, & Voronin, 2004), and topiramate (Miranda Jr et al., 2008). Medications have also been shown to increase aversive alcohol responses including varenicline (Childs, Roche, King, & de Wit, 2012; Fucito et al., 2011) and naltrexone (McCaul, Wand, Eissenberg, Rohde, & Cheskin, 2000; Ray, Hutchison, et al., 2008; Swift, Whelihan, Kuznetsov, Buongiorno, & Hsuing, 1994). Disulfiram represents an extreme example of this aversive mechanism; however, compliance concerns are substantial (Jørgensen et al., 2011). Though several medications have shown consistent effects in RCTs and alcohol challenge studies, other medications suggest these approaches are not perfectly correlated (Bisaga & Evans, 2006; Brasser, McCaul, & Houtsmuller, 2004; Leggio et al., 2013).
Targeting alcohol reinforcement and compulsivity, several AUD medications have also been shown to reduce oral self-administration, including naltrexone (Davidson, Palfai, Bird, & Swift, 1999; O’Malley et al., 2002; Setiawan et al., 2011) and varenicline (McKee et al., 2009). Furthermore, initial studies have suggested that naltrexone and acamprosate may owe some of their clinical efficacy to their ability to blunt reactivity to drug-paired cues (Miranda et al., 2014; Ooteman, Koeter, Verheul, Schippers, & van den Brink, 2007).
Through refining the phenotype of interest, experimental psychopathology has contributed to the development of personalized treatment. One salient example is the pharmacogenetics of naltrexone. Guided by the pharmacology of naltrexone, alcohol challenge studies have identified genetic moderators of naltrexone, most notably the A118G SNP of OPRM1 (Bond et al., 1998; Ray, Barr, et al., 2012; Ray, Bujarski, Chin, & Miotto, 2012; Ray & Hutchison, 2007). This pharmacogenetic effect has been translated to clinical trials, however with somewhat mixed results (Anton et al., 2008; Gelernter et al., 2007; Oslin et al., 2003, 2015). To explain these mixed results it has been argued on the basis of other experimental psychopathology research that OPRM1-naltrexone pharmacogenetics might be most salient in the earlier stages of AUD where reward drinking is more salient (Bujarski, Hutchison, Prause, et al., 2015; Bujarski & Ray, 2014; Koob & Le Moal, 1997; Ray, Barr, et al., 2012; Ray, Courtney, Bujarski, & Squeglia, 2012). Beyond naltrexone and OPRM1, the pharmacogenetics literature is expanding rapidly and continues to benefit from an experimental psychopathology approach (for review see Roche & Ray, 2015).
4.2. Experimental Psychopathology and Behavioral Treatment
While the pharmacological treatment literature has extensively utilized experimental psychopathology methods, the behavioral treatment literature is considerably less well developed. Merging experimental psychopathology paradigms and behavioral treatment, Wiers et al. (2009) have developed a paradigm to measure and retrain alcohol approach tendencies termed the alcohol approach/avoidance task (alcohol-AAT). Using this paradigm, Wiers et al (2011) conducted a randomized controlled trial in alcohol dependent patients of an adjunctive cognitive-bias modification intervention. In a brief intervention Wiers et al (2011) demonstrated improved treatment outcomes at one year follow-up thus demonstrating the potential to pivot experimental psychopathology paradigms to behavioral treatment. Furthermore, there is a growing interest in using experimental psyhcopathology methods coupled with neuroimaging to elucidate mechanisms of change in behavioral interventions for AUD (DeVito et al., 2012; Feldstein Ewing & Chung, 2013; Feldstein Ewing, Filbey, Hendershot, McEachern, & Hutchison, 2011).
5. Experimental Psychopathology and Translational Science
Ethical considerations restrict the level of experimental control and neurobiological precision of human research. Conversely, preclinical research benefits from strong neurobiological precision, yet the translational applicability of preclinical research is often merely assumed through face validity. Studies aiming to translate preclinical theories to human populations stand to bridge this gap with experimental psychopathology research representing a promising modality for these translational efforts.
Our laboratory has recently published a series of studies utilizing an intravenous alcohol challenge to test predictions derived from well supported preclinical models of alcoholism etiology. Specifically we explored the transition from positive to negative reinforcement, posited by the allostatic model of addiction (Koob & Kreek, 2007; Koob & Le Moal, 1997, 2005, 2008). In these data the coupling between alcohol-induced stimulation and alcohol craving (indexing positive reinforcement) was significantly greater in heavy drinkers, as compared to alcohol dependent participants (Bujarski & Ray, 2014). This diminished salience of the stimulant/hedonic effects of alcohol among alcohol dependent participants has since been replicated and extended in a larger sample including light-to-moderate drinkers (Bujarski, Hutchison, Prause, et al., 2015).
Experimental psychopathology paradigms have also tested translational predictions of the incentive sensitization theory (IST; Robinson & Berridge, 1993, 2000, 2001). IST suggests neurobiological dissociation between reward (‘liking’), and motivational salience (‘wanting’) and suggests that cues acquire pathological incentive motivation properties such that the cue “grabs attention, becomes attractive and ‘wanted,’ and thus guides behavior to the incentive” (Robinson & Berridge, 1993, p. 261). Alcohol challenge research in humans has experimentally demonstrated the dissociability of ‘liking’ and ‘wanting’ in light and heavy drinkers (Hobbs, Remington, & Glautier, 2005). Furthermore, an alcohol cue dot-probe task has demonstrated greater attentional bias to alcohol cues among heavy drinkers as compared to non-heavy drinkers (Duka & Townshend, 2004; Matt Field, Mogg, Zetteler, & Bradley, 2004; Townshend & Duka, 2001). In sum, the thoughtful application of experimental psychopathology methods represents a promising avenue for the much needed translation of preclinical neurobiological theories to human clinical populations.
6. Conclusion and Future Directions
In this systematic review we provided a review of the major experimental psychopathology paradigms developed to understand the etiology of AUD and develop more effective treatments. These methods include alcohol challenge, self-administration, cue-reactivity, and stress induction paradigms. In reviewing each paradigm, we provided a description of their implication and discussed their relative strengths and weaknesses. The trade-offs endemic to experimental research between internal and ecological validity appear throughout alcohol experimental psychopathology (e.g. between oral and intravenous administration). Thus future studies utilizing multiple paradigms with complementary strengths and weaknesses are warranted. Furthermore, efforts to standardize experimental procedures would advance the field by establishing testing guidelines and procedures, which in turn can promote greater consilience in the literature.
Relatedly, in the context of treatment development, studies should strive to utilize multiple paradigms assessing complementary mechanisms of action. By utilizing multiple paradigms, treatment development studies stand a better chance to determine therapeutic mechanisms of action and more effectively screen medications (Ray, Hutchison, et al., 2010). Though this strategy increases the likelihood of effective treatment screening, further research is needed to determine the degree to which these experimental psychopathology paradigms translate to treatment outcomes (Yardley & Ray, 2016).
In sum, the utilization of robust experimental psychopathology paradigms represents an integral and active area within AUD research. We contend that the effective utilization of these experimental paradigms and practices will permit the elucidation of AUD etiological factors, will improve the efficiency of treatment development, and will refine treatment targets permitting personalized medicine that will dramatically reduce the human and economic cost of AUD.
Figure 1.
The contribution of experimental psychopathology approaches to translational alcohol use disorder research.
Table 1.
Overview of experimental psychopathology paradigms developed for alcohol use disorder research.
| Class | Variant | Description | Dependent Variables | Strengths | Weaknesses | Key Citations |
|---|---|---|---|---|---|---|
| Alcohol Administration | Oral Administration | Participants are administered experimenter controlled doses of alcohol to target BrAC levels (doses often computed based on participant characteristics e.g. sex and weight) | Subjective Responses to Alcohol (e.g. BAES, SHAS, DEQ) Objective Responses (e.g. static ataxia, grooved pegboard) |
|
|
Schuckit, 1984 King et. al. 2011 Brick 2006 |
| Intravenous Administration | Ethanol-Saline solution infused into patents’ veins to target BrAC’s (infusion rate computed based on participant characteristics e.g. sex and weight) | Subjective Responses to Alcohol (e.g. BAES, SHAS, DEQ, POMS) Objective Responses (e.g. grooved pegboard) |
|
|
Ray & Hutchison 2004 Ray et al., 2009 Bujarski et al. 2015 |
|
| Alcohol Clamp | Alcohol administered intravenously in order to maintain stable BrAC over extended periods of time (e.g. > 1 hour) | Subjective Responses to Alcohol (e.g. BAES, SHAS, DEQ) Objective Responses (e.g. static ataxia, EtOH elimination rate) |
|
|
Ramchandani et al. 1999 Ramchandani et al. 2006 |
|
| Alcohol Self-Administration | Oral Self-Administration | Participant permitted to consume prepared mini-drinks often with a financial disincentive. Typically a priming dose of alcohol is administered prior to self-administration. | Indices of motivation for alcohol (e.g. number of drinks consumed, consumption rate, reinforcement breakpoint) |
|
|
de Wit et al. 1989 O’Malley et al. 2002 McKee et al. 2009 |
| Computerized Alcohol Infusion System (CAIS) | Computer controlled alcohol infusion system that allows participants to self-administer IV mini-drinks. Typically a priming dose of alcohol is administered prior to self-administration. | Indices of motivation for alcohol (e.g. number of mini-drinks administered, peak BrAC, total alcohol infused, reinforcement breakpoint) |
|
|
Zimmermann et al. 2008 Zimmermann et al. 2009 Hendershot et al. 2014 |
|
| Alcohol Cue-Reactivity | In Vivo Alcohol Cues | Participants are presented with and asked to hold, smell, and manipulate their preferred alcoholic beverage. Cue reactivity is compared to a non-alcoholic beverage control. | Subjective craving (e.g. AUQ) Mood (e.g. POMS) Psychophysiology |
|
|
Monti et al. 1987 McGeary et al. 2006 |
| Pictorial Alcohol Cues | Computerized presentation of alcohol-related cues versus control cues matched on multiple factors (e.g. beverages, color, and visual salience). | Subjective craving (single items) Reaction time to alcohol-paired target stimuli Psychophysiology |
|
|
Townshend & Duka 2001 Schact et al. 2013 |
|
| Stress-Reactivity | Trier Social Stress Task | Participants perform socially stressful activities in front of confederates (e.g. mental arithmetic and public speaking) | Self-reported affect Subjective craving Cortisol Psychophysiological measures (e.g. HR, BP) |
|
|
Söderpalm & de Wit 2002 de Wit et al. 2003 |
| Guided Imagery Task | Participants generate scripts of unresolved stressors, which are turned into an auditory stimulus presented to the participant. | Self-reported affect Subjective craving Cortisol Psychophysiological measures (e.g. HR, BP) |
|
|
Sinha et al. 2000 Fox et al. 2007 |
Highlights.
Experimental psychopathology methods represent a cornerstone of alcoholism research
We review major paradigms emphasizing implications, pros/cons and challenges
We review the application of experimental psychopathology to translational research
Acknowledgments
SB was supported as a pre-doctoral trainee from the National Institutes on Alcohol Abuse and Alcoholism (F31 AA022569). The authors would also like to thank ReJoyce Green, Meghan Yardley, Daniel Roche, Anita Cservenka, Emily Hartwell, Jonathan Westman, and Aaron Lim for their input on manuscript preparation. LAR has consulted for GSK and has received research support from Pfizer and Medicinova.
Footnotes
SB does not have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Amer Psychiatric Pub Inc; 1994. [Google Scholar]
- American Psychiatric Association. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173(1–2):32–40. doi: 10.1007/s00213-003-1720-7. http://doi.org/10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM … COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. http://doi.org/10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. http://doi.org/10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Naltrexone Modification of Drinking Effects in a Subacute Treatment and Bar-Lab Paradigm: Influence of OPRM1 and Dopamine Transporter (SLC6A3) Genes. Alcoholism: Clinical and Experimental Research. 2012;36(11):2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Vollsta Dt-Klein S, Kirsch M, Hoffmann S, Jorde A, Frank J, … Kiefer F. Increased mesolimbic cue-reactivity in carriers of the mu-opioid-receptor gene OPRM1 A118G polymorphism predicts drinking outcome: a functional imaging study in alcohol dependent subjects. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2015;25(8):1128–1135. doi: 10.1016/j.euroneuro.2015.04.013. http://doi.org/10.1016/j.euroneuro.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Bacio GA, Lunny KF, Webb JN, Ray LA. Alcohol Use Following an Alcohol Challenge and a Brief Intervention among Alcohol-Dependent Individuals. The American Journal on Addictions. 2014;23(1):96–101. doi: 10.1111/j.1521-0391.2013.12071.x. http://doi.org/10.1111/j.1521-0391.2013.12071.x. [DOI] [PubMed] [Google Scholar]
- Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, … Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Archives of General Psychiatry. 2012;69(8):842–852. doi: 10.1001/archgenpsychiatry.2011.2026. http://doi.org/10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ, Petry NM. The price of change: The behavioral economics of drug dependence. Behavior Therapy. 1998;29(4):545–565. [Google Scholar]
- Bigelow G, Griffiths R, Liebson I. Experimental models for the modification of human drug self-administration. In: Weiss B, Laties VG, editors. Behavioral Pharmacology. Springer; US: 1975. pp. 61–75. Retrieved from http://link.springer.com/chapter/10.1007/978-1-4684-2634-2_6. [Google Scholar]
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug and Alcohol Dependence. 2006;83(1):25–32. doi: 10.1016/j.drugalcdep.2005.10.008. http://doi.org/10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, … Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol Effects During Acamprosate Treatment: A Dose-Response Study in Humans. Alcoholism: Clinical and Experimental Research. 2004;28(7):1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. http://doi.org/10.1097/01.ALC.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, … Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. Journal of Neural Transmission. 2001;108(7):887–894. doi: 10.1007/s007020170038. http://doi.org/10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brick J. Standardization of Alcohol Calculations in Research. Alcoholism: Clinical and Experimental Research. 2006;30(8):1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. http://doi.org/10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Prause N, Ray LA. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addiction Biology. 2015:n/a–n/a. doi: 10.1111/adb.12293. http://doi.org/10.1111/adb.12293. [DOI] [PubMed]
- Bujarski S, Hutchison KE, Roche DJO, Ray LA. Factor Structure of Subjective Responses to Alcohol in Light and Heavy Drinkers. Alcoholism: Clinical and Experimental Research. 2015;39(7):1193–1202. doi: 10.1111/acer.12737. http://doi.org/10.1111/acer.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: An examination of Koob’s allostatic model in humans. Drug and Alcohol Dependence. 2014;140:161–167. doi: 10.1016/j.drugalcdep.2014.04.015. http://doi.org/10.1016/j.drugalcdep.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, Peter C. Alcohol and tension reduction: A review. Quarterly Journal of Studies on Alcohol. 1972;33(1–A):33–64. [PubMed] [Google Scholar]
- Carter A, Hall W. Ethical implications of research on craving. Addictive Behaviors. 2013;38(2):1593–1599. doi: 10.1016/j.addbeh.2012.07.002. http://doi.org/10.1016/j.addbeh.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Childs E, Roche DJO, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcoholism, Clinical and Experimental Research. 2012;36(5):906–914. doi: 10.1111/j.1530-0277.2011.01675.x. http://doi.org/10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Harding S, Wilson JR. Ethanol metabolism in men and women. Journal of Studies on Alcohol. 1987;48(4):380–387. doi: 10.15288/jsa.1987.48.380. http://doi.org/10.15288/jsa.1987.48.380. [DOI] [PubMed] [Google Scholar]
- Cook TA, Luczak SE, Shea SH, Ehlers CL, Carr LG, Wall TL. Associations of ALDH2 and ADH1B genotypes with response to alcohol in Asian Americans. Journal of Studies on Alcohol and Drugs. 2005;66(2):196–204. doi: 10.15288/jsa.2005.66.196. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Gálvan A, Poldrack RA, MacKillop J, … Ray LA. The Relationship Between Measures of Impulsivity and Alcohol Misuse: An Integrative Structural Equation Modeling Approach: IMPULSIVITY AND ALCOHOL MISUSE. Alcoholism: Clinical and Experimental Research. 2012;36(6):923–931. doi: 10.1111/j.1530-0277.2011.01635.x. http://doi.org/10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addiction Biology. 2015 doi: 10.1111/adb.12314. n/a-n/a. http://doi.org/10.1111/adb.12314. [DOI] [PMC free article] [PubMed]
- Davidson D, Palfai T, Bird C, Swift R. Effects of Naltrexone on Alcohol Self-Administration in Heavy Drinkers. Alcoholism: Clinical and Experimental Research. 1999;23(2):195–203. http://doi.org/10.1111/j.1530-0277.1999.tb04099.x. [PubMed] [Google Scholar]
- de Wit H, Pierri J, Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology. 1989;98(1):113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- de Wit H, Söderpalm AHV, Nikolayev L, Young E. Effects of Acute Social Stress on Alcohol Consumption in Healthy Subjects. Alcoholism: Clinical and Experimental Research. 2003;27(8):1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. http://doi.org/10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- de Wit H, Svenson J, York A. Non-specific effect of naltrexone on ethanol consumption in social drinkers. Psychopharmacology. 1999;146(1):33–41. doi: 10.1007/s002130051085. http://doi.org/10.1007/s002130051085. [DOI] [PubMed] [Google Scholar]
- Devgun MS, Dunbar JA. Alcohol consumption, blood alcohol level and the relevance of body weight in experimental design and analysis. Journal of Studies on Alcohol. 1990;51(1):24–28. doi: 10.15288/jsa.1990.51.24. http://doi.org/10.15288/jsa.1990.51.24. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;122(3):228–235. doi: 10.1016/j.drugalcdep.2011.10.002. http://doi.org/10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. http://doi.org/10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF. Drinking in alcoholics following an alcohol challenge research protocol. Journal of Studies on Alcohol. 2000;61(2):220–224. doi: 10.15288/jsa.2000.61.220. http://doi.org/10.15288/jsa.2000.61.220. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A Clinical Laboratory Paradigm for Evaluating Medication Effects on Alcohol Consumption: Naltrexone and Nalmefene. Neuropsychopharmacology. 2003;28(4):755–764. doi: 10.1038/sj.npp.1300101. http://doi.org/10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of Naltrexone and Nalmefene on Subjective Response to Alcohol Among Non-Treatment-Seeking Alcoholics and Social Drinkers. Alcoholism: Clinical and Experimental Research. 2004;28(9):1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. http://doi.org/10.1097/01.ALC.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Duka T, Stephens DN, Russell C, Tasker R. Discriminative stimulus properties of low doses of ethanol in humans. Psychopharmacology. 1998;136(4):379–389. doi: 10.1007/s002130050581. http://doi.org/10.1007/s002130050581. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176(3–4):353–361. doi: 10.1007/s00213-004-1906-7. http://doi.org/10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A Critical Review of the First 10 Years of Candidate Gene-by-Environment Interaction Research in Psychiatry. American Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. http://doi.org/10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M, Johnson K, George DT, Schumann G, Moss HB, Kranzler HR, Goldman D. Ethical Considerations for Administering Alcohol or Alcohol Cues to Treatment-Seeking Alcoholics in a Research Setting: Can the Benefits to Society Outweigh the Risks to the Individual? Alcoholism: Clinical and Experimental Research. 2009;33(9):1508–1512. doi: 10.1111/j.1530-0277.2009.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Sayette MA, Levine JM, Cohn JF, Creswell KG. The effects of alcohol on the emotional displays of Whites in interracial groups. Emotion. 2013;13(3):468–477. doi: 10.1037/a0030934. http://doi.org/10.1037/a0030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2013;27(2):329–335. doi: 10.1037/a0031491. http://doi.org/10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Hendershot CS, McEachern AD, Hutchison KE. Proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: the example of motivational interviewing. Journal of Studies on Alcohol and Drugs. 2011;72(6):903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Amato L, Davoli M. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2006. Alcoholics Anonymous and other 12-step programmes for alcohol dependence. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005032.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology. 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Quigley M. Mild stress increases attentional bias in social drinkers who drink to cope: A replication and extension. Experimental and Clinical Psychopharmacology. 2009;17(5):312–319. doi: 10.1037/a0017090. http://doi.org/10.1037/a0017090. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray LA, Smolen A, Claus ED, Audette A, Hutchison KE. Differential Neural Response to Alcohol Priming and Alcohol Taste Cues Is Associated With DRD4 VNTR and OPRM1 Genotypes. Alcoholism: Clinical and Experimental Research. 2008;32(7):1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. http://doi.org/10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-Induced and Alcohol Cue-Induced Craving in Recently Abstinent Alcohol-Dependent Individuals. Alcoholism: Clinical and Experimental Research. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. http://doi.org/10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Friel PN, Logan BK, O’Malley D, Baer JS. Development of dosing guidelines for reaching selected target breath alcohol concentrations. Journal of Studies on Alcohol. 1999;60(4):555–565. doi: 10.15288/jsa.1999.60.555. http://doi.org/10.15288/jsa.1999.60.555. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215(4):655–663. doi: 10.1007/s00213-010-2160-9. http://doi.org/10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R … VA Cooperative Study #425 Study Group. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcoholism, Clinical and Experimental Research. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. http://doi.org/10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Goist KC, Jr, Sutker PB. Acute alcohol intoxication and body composition in women and men. Pharmacology Biochemistry and Behavior. 1985;22(5):811–814. doi: 10.1016/0091-3057(85)90532-5. http://doi.org/10.1016/0091-3057(85)90532-5. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. http://doi.org/10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. Psychological Theories of Drinking and Alcoholism. 1999;2:14–53. [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, … Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. http://doi.org/10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate Gene Studies of a Promising Intermediate Phenotype: Failure to Replicate. Neuropsychopharmacology. 2013;38(5):802–816. doi: 10.1038/npp.2012.245. http://doi.org/10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ. Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods, and data. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 2000. [Google Scholar]
- Heath AC, Martin NG. Intoxication after an Acute Dose of Alcohol: An Assessment of Its Association with Alcohol Consumption Patterns By using Twin Data. Alcoholism: Clinical and Experimental Research. 1991;15(1):122–128. doi: 10.1111/j.1530-0277.1991.tb00529.x. http://doi.org/10.1111/j.1530-0277.1991.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, … Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neuroscience and Biobehavioral Reviews. 2010;35(2):334–344. doi: 10.1016/j.neubiorev.2009.11.018. http://doi.org/10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, … Bartenstein P. Correlation Between Dopamine D2 Receptors in the Ventral Striatum and Central Processing of Alcohol Cues and Craving. American Journal of Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. http://doi.org/10.1176/ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addiction Biology. 2014 doi: 10.1111/adb.12165. n/a-n/a. http://doi.org/10.1111/adb.12165. [DOI] [PMC free article] [PubMed]
- Higgins RL, Marlatt GA. Fear of interpersonal evaluation as a determinant of alcohol consumption in male social drinkers. Journal of Abnormal Psychology. 1975;84(6):644–651. doi: 10.1037//0021-843x.84.6.644. http://doi.org/10.1037/0021-843X.84.6.644. [DOI] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology. 2011;218(1):121–129. doi: 10.1007/s00213-011-2355-8. http://doi.org/10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitisation theory. Psychopharmacology. 2005;178(4):493–499. doi: 10.1007/s00213-004-2026-0. http://doi.org/10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, … Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Archives of General Psychiatry. 2008;65(7):841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Ray LA, Sandman E, Rutter M-C, Peters A, Davidson D, Swift R. The Effect of Olanzapine on Craving and Alcohol Consumption. Neuropsychopharmacology. 2005;31(6):1310–1317. doi: 10.1038/sj.npp.1300917. http://doi.org/10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology. 2001;155(1):27–34. doi: 10.1007/s002130000629. http://doi.org/10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Annals of the New York Academy of Sciences. 2014 doi: 10.1111/nyas.12388. n/a-n/a. http://doi.org/10.1111/nyas.12388. [DOI] [PMC free article] [PubMed]
- Jones AW, Neri A. Evaluation of Blood-Ethanol Profiles after Consumption of Alcohol Together with a Large Meal. Canadian Society of Forensic Science Journal. 1991;24(3):165–173. http://doi.org/10.1080/00085030.1991.10756993. [Google Scholar]
- Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcoholism, Clinical and Experimental Research. 2011;35(10):1749–1758. doi: 10.1111/j.1530-0277.2011.01523.x. http://doi.org/10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proceedings of the National Academy of Sciences. 2008;105(51):20368–20373. doi: 10.1073/pnas.0810970105. http://doi.org/10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, Stimulant, and Sedative Alcohol Responses and Relationship to Future Binge Drinking. Arch Gen Psychiatry. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biological Psychiatry. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. http://doi.org/10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Roche DJO, Rueger SY. Subjective Responses to Alcohol: A Paradigm Shift May Be Brewing. Alcoholism: Clinical and Experimental Research. 2011;35(10):1726–1728. doi: 10.1111/j.1530-0277.2011.01629.x. http://doi.org/10.1111/j.1530-0277.2011.01629. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129(1):15–22. doi: 10.1007/s002130050156. http://doi.org/10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The “Trier Social Stress Test” A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. http://doi.org/10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. The Journal of Clinical Endocrinology & Metabolism. 1992;75(6):1526–1530. doi: 10.1210/jcem.75.6.1464659. http://doi.org/10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Supplement 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. http://doi.org/10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence. The American Journal of Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. http://doi.org/10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. http://doi.org/10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nature Neuroscience. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. http://doi.org/10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the Brain Antireward System. Annual Review of Psychology. 2008;59(1):29–53. doi: 10.1146/annurev.psych.59.103006.093548. http://doi.org/10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009;35(1):217–238. doi: 10.1038/npp.2009.110. http://doi.org/10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, Harmon-Jones E, Winkielman P. Ten years of research with the Trier Social Stress Test—revisited. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. 2007:56–83. [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, … Kenna GA. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacology Biochemistry and Behavior. 2013;103(4):784–791. doi: 10.1016/j.pbb.2012.11.013. http://doi.org/10.1016/j.pbb.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: Pharmacological effects, expectancy, and tension reduction. Journal of Abnormal Psychology. 1980;89(4):528–538. doi: 10.1037//0021-843x.89.4.528. http://doi.org/10.1037/0021-843X.89.4.528. [DOI] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O’Connor S, Ramchandani VA. Genetic and Environmental Influences on Alcohol Metabolism in Humans. Alcoholism: Clinical and Experimental Research. 2001;25(1):136–144. http://doi.org/10.1111/j.1530-0277.2001.tb02138.x. [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, … Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addiction Biology. 2012;17(3):513–527. doi: 10.1111/j.1369-1600.2012.00454.x. http://doi.org/10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychological Bulletin. 2006;132(4):607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug and Alcohol Dependence. 2007;89(2):227–233. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Magill M, Ray LA. Cognitive-Behavioral Treatment With Adult Alcohol and Illicit Drug Users: A Meta-Analysis of Randomized Controlled Trials. Journal of Studies on Alcohol and Drugs. 2009;70(4):516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–293. doi: 10.1111/j.1360-0443.2012.04054.x. http://doi.org/10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Vollstädt-Klein S, Reinhard I, Leménager T, Fauth-Bühler M, Hermann D, … Smolka MN. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcoholism, Clinical and Experimental Research. 2014;38(11):2754–2762. doi: 10.1111/acer.12546. http://doi.org/10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addiction Biology. 2009;14(1):73–83. doi: 10.1111/j.1369-1600.2008.00133.x. http://doi.org/10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone Alters Subjective and Psychomotor Responses to Alcohol in Heavy Drinking Subjects. Neuropsychopharmacology. 2000;22(5):480–492. doi: 10.1016/S0893-133X(99)00147-5. http://doi.org/10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194(4):433–441. doi: 10.1007/s00213-007-0860-6. http://doi.org/10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R. Genetic Moderators of Naltrexone’s Effects on Alcohol Cue Reactivity. Alcoholism: Clinical and Experimental Research. 2006;30(8):1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. http://doi.org/10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, … Balchunas E. Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biological Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. http://doi.org/10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology. 2008;196(2):189–200. doi: 10.1007/s00213-007-0952-3. http://doi.org/10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. Behavioral Studies of Alcoholism. In: Kissin B, Begleiter H, editors. The Biology of Alcoholism. Springer; US: 1972. pp. 219–291. Retrieved from http://link.springer.com/chapter/10.1007/978-1-4684-0895-9_9. [Google Scholar]
- Mello NK, Mendelson JH. OPERANT ANALYSIS OF DRINKING PATTERNS OF CHRONIC ALCOHOLICS. Nature. 1965;206:43–46. doi: 10.1038/206043a0. [DOI] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction. 2010;105(5):883–890. doi: 10.1111/j.1360-0443.2009.02860.x. http://doi.org/10.1111/j.1360-0443.2009.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Guilford press; 2002. Retrieved from http://psycnet.apa.org/psycinfo/2002-02948-000. [Google Scholar]
- Miranda R, Ray LA, Blanchard A, Reynolds EK, Monti PM, Chun T, … Ramirez J. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addiction Biology. 2014;19(5):941–954. doi: 10.1111/adb.12050. http://doi.org/10.1111/adb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, … McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcoholism: Clinical and Experimental Research. 2008;32(3):489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Translational Medicine. 2012;4(116):116ra6. doi: 10.1126/scitranslmed.3002902. http://doi.org/10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96(2):122. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, … Abrams DB. Naltrexone’s Effect on Cue-Elicited Craving Among Alcoholics in Treatment. Alcoholism: Clinical and Experimental Research. 1999;23(8):1386–1394. [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective Response to Alcohol: A Critical Review of the Literature. Alcoholism: Clinical and Experimental Research. 2010;34(3):385–395. doi: 10.1111/j.1530-0277.2009.01103.x. http://doi.org/10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R. Cognitive–behavioral treatment for alcohol dependence: a review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95(10):1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. http://doi.org/10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor SJ. Self-Reported Subjective Perception of Intoxication Reflects Family History of Alcoholism When Breath Alcohol Levels Are Constant. Alcoholism: Clinical and Experimental Research. 2002;26(8):1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. http://doi.org/10.1111/j.1530-0277.2002.tb02670.x. [DOI] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism. Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. NIAAA; 2005. Retrieved from http://www.niaaa.nih.gov/research/guidelines-and-resources/administering-alcohol-human-studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacology Biochemistry and Behavior. 2006;83(2):239–248. doi: 10.1016/j.pbb.2006.02.006. http://doi.org/10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. http://doi.org/10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA council approves definition of binge drinking. NIAAA Newsletter 2004 [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping Breath Alcohol Concentration Reduces Experimental Variance: Application to the Study of Acute Tolerance to Alcohol and Alcohol Elimination Rate. Alcoholism: Clinical and Experimental Research. 1998;22(1):202–210. http://doi.org/10.1111/j.1530-0277.1998.tb03639.x. [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek M. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MWJ, Verheul R, Schippers GM, van den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. European Neuropsychopharmacology. 2007;17(8):558–566. doi: 10.1016/j.euroneuro.2007.02.012. http://doi.org/10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Orne MT. Demand characteristics and the concept of quasi-controls. Artifacts in Behavioral Research: Robert Rosenthal and Ralph L. Rosnow’s Classic Books. 2009:110. [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. http://doi.org/10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs Placebo for the Treatment of Alcohol Dependence: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(5):430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, … Kranzler HR. GABRA2 Alleles Moderate the Subjective Effects of Alcohol, Which are Attenuated by Finasteride. Neuropsychopharmacology. 2005;30(6):1193–1203. doi: 10.1038/sj.npp.1300688. http://doi.org/10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O’Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. Biomedical Engineering, IEEE Transactions on. 2008;55(12):2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O’Connor S. Voluntary Intravenous Self-Administration of Alcohol Detects an Interaction Between GABAergic Manipulation and GABRG1 Polymorphism Genotype: A Pilot Study. Alcoholism: Clinical and Experimental Research. 2013;37(s1):E152–E160. doi: 10.1111/j.1530-0277.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol. 2005;37(3):135–141. doi: 10.1016/j.alcohol.2006.02.002. http://doi.org/10.1016/j.alcohol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA Axis and the A118G Polymorphism of the μ-Opioid Receptor in Stress-Induced Drinking Behavior. Alcohol and Alcoholism. 2009;44(4):358–365. doi: 10.1093/alcalc/agp007. http://doi.org/10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective Response to Alcohol Challenge: A Quantitative Review. Alcoholism: Clinical and Experimental Research. 2011;35(10):1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. http://doi.org/10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A Physiologically-Based Pharmacokinetic (PBPK) Model for Alcohol Facilitates Rapid BrAC Clamping. Alcoholism: Clinical and Experimental Research. 1999;23(4):617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, … O’Connor S. Recent Drinking History: Association with Family History of Alcoholism and the Acute Response to Alcohol during a 60 mg% Clamp. Journal of Studies on Alcohol and Drugs. 2002;63(6):734. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Kwo PY, Li TK. Effect of Food and Food Composition on Alcohol Elimination Rates in Healthy Men and Women. The Journal of Clinical Pharmacology. 2001;41(12):1345–1350. doi: 10.1177/00912700122012814. http://doi.org/10.1177/00912700122012814. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, de Wit H. The Alcohol Clamp: Applications, Challenges, and New Directions – An RSA 2004 Symposium Summary. Alcoholism: Clinical and Experimental Research. 2006;30(1):155–164. doi: 10.1111/j.1530-0277.2006.00017.x. http://doi.org/10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O’Connor S. Intravenous Ethanol Infusions Can Mimic the Time Course of Breath Alcohol Concentrations Following Oral Alcohol Administration in Healthy Volunteers. Alcoholism: Clinical and Experimental Research. 2009;33(5):938–944. doi: 10.1111/j.1530-0277.2009.00906.x. http://doi.org/10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, … Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. http://doi.org/10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA. Stress-Induced and Cue-Induced Craving for Alcohol in Heavy Drinkers: Preliminary Evidence of Genetic Moderation by the OPRM1 and CRH-BP Genes. Alcoholism: Clinical and Experimental Research. 2011;35(1):166–174. doi: 10.1111/j.1530-0277.2010.01333.x. http://doi.org/10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF. The Role of the Asn40Asp Polymorphism of the Mu Opioid Receptor Gene (OPRM1) on Alcoholism Etiology and Treatment: A Critical Review. Alcoholism: Clinical and Experimental Research. 2012;36(3):385–394. doi: 10.1111/j.1530-0277.2011.01633.x. http://doi.org/10.1111/j.1530-0277.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of Naltrexone in Asian Americans: A Randomized Placebo-Controlled Laboratory Study. Neuropsychopharmacology. 2012;37(2):445–455. doi: 10.1038/npp.2011.192. http://doi.org/10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, MacKillop J, Courtney KE, Monti PM, Miotto K. Subjective Response to Alcohol Among Alcohol-Dependent Individuals: Effects of the Mu-Opioid Receptor (OPRM1) Gene and Alcoholism Severity. Alcoholism: Clinical and Experimental Research. 2013;37:E116–E124. doi: 10.1111/j.1530-0277.2012.01916.x. http://doi.org/10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJO. Subjective Response to Alcohol as a Research Domain Criterion. Alcoholism: Clinical and Experimental Research. 2016;40(1):6–17. doi: 10.1111/acer.12927. http://doi.org/10.1111/acer.12927. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Squeglia LM, Ashenhurst JR, Anton RF. Interactive Effects of OPRM1 and DAT1 Genetic Variation on Subjective Responses to Alcohol. Alcohol and Alcoholism. 2014;49(3):261–270. doi: 10.1093/alcalc/agt183. http://doi.org/10.1093/alcalc/agt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Bujarski S, Squeglia LM. Pharmacogenetics of Alcoholism: A Clinical Neuroscience Perspective. Pharmacogenomics. 2012;13(2):129–132. doi: 10.2217/pgs.11.173. http://doi.org/10.2217/pgs.11.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A Polymorphism of the μ-Opioid Receptor Gene (OPRM1) and Sensitivity to the Effects of Alcohol in Humans. Alcoholism: Clinical and Experimental Research. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. http://doi.org/10.1097/01.ALC.0000148114.34000.B9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of Naltrexone on Alcohol Sensitivity and Genetic Moderators of Medication Response: A Double-blind Placebo-Controlled Study. Archives of General Psychiatry. 2007;64(9):1069. doi: 10.1001/archpsyc.64.9.1069. http://doi.org/10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Associations Among GABRG1, Level of Response to Alcohol, and Drinking Behaviors. Alcoholism: Clinical and Experimental Research. 2009;33(8):1382–1390. doi: 10.1111/j.1530-0277.2009.00968.x. http://doi.org/10.1111/j.1530-0277.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, MacKillop J, Miranda R, Audette A, Swift R, Monti PM. Effects of Naltrexone During the Descending Limb of the Blood Alcohol Curve. The American Journal on Addictions. 2008;17(4):257–264. doi: 10.1080/10550490802138400. http://doi.org/10.1080/10550490802138400. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of Human Laboratory Models to Pharmacotherapy Development for Alcohol Dependence. Current Pharmaceutical Design. 2010;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- Ray LA, Kahler CW, Young D, Chelminski I, Zimmerman M. The factor structure and severity of DSM-IV alcohol abuse and dependence symptoms in psychiatric outpatients. Journal of Studies on Alcohol and Drugs. 2008;69(4):496. doi: 10.15288/jsad.2008.69.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective Responses to Alcohol Consumption as Endophenotypes: Advancing Behavioral Genetics in Etiological and Treatment Models of Alcoholism. Substance Use & Misuse. 2010;45(11):1742–1765. doi: 10.3109/10826084.2010.482427. http://doi.org/10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EK, Monti PM. The Cue Reactivity Paradigm in Addiction Research. In: JamescKillop, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. Wiley-Blackwell; 2013. pp. 381–410. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/9781118384404.ch14/summary. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction. 2000;95(8s2):91–117. doi: 10.1080/09652140050111681. http://doi.org/10.1046/j.1360-0443.95.8s2.19.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. http://doi.org/10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Roche DJ, Ray LA. Subjective response as a consideration in the pharmacogenetics of alcoholism treatment. Pharmacogenomics. 2015;16(7):721–736. doi: 10.2217/pgs.14.143. http://doi.org/10.2217/pgs.14.143. [DOI] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, … Higuchi S. Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alcoholism: Clinical and Experimental Research. 2011;35(3):400–407. doi: 10.1111/j.1530-0277.2010.01357.x. http://doi.org/10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, … Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. Journal of Consulting and Clinical Psychology. 1994;62(3):620–626. doi: 10.1037//0022-006x.62.3.620. http://doi.org/10.1037/0022-006X.62.3.620. [DOI] [PubMed] [Google Scholar]
- Rubak S, Sandbæk A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305–312. [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol. 1994;55(4):487–494. doi: 10.15288/jsa.1994.55.487. http://doi.org/10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II, Dick DM. Endophenotypes for Alcohol Use Disorder: An Update on the Field. Current Addiction Reports. 2015:1–15. doi: 10.1007/s40429-015-0046-y. http://doi.org/10.1007/s40429-015-0046-y. [DOI] [PMC free article] [PubMed]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, … Moreland RL. Alcohol and Group Formation. A Multimodal Investigation of the Effects of Alcohol on Emotion and Social Bonding. Psychological Science. 2012 doi: 10.1177/0956797611435134. 956797611435134. http://doi.org/10.1177/0956797611435134. [DOI] [PMC free article] [PubMed]
- Sayette MA, Griffin KM, Sayers WM. Counterbalancing in Smoking Cue Research: A Critical Analysis. Nicotine & Tobacco Research. 2010:ntq159. doi: 10.1093/ntr/ntq159. http://doi.org/10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology. 2013;18(1):121–133. doi: 10.1111/j.1369-1600.2012.00464.x. http://doi.org/10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology. 2013;227(4):627–637. doi: 10.1007/s00213-013-2996-x. http://doi.org/10.1007/s00213-013-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology. 2013;38(3):414–422. doi: 10.1038/npp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry. 1984;41(9):879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABAα6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biological Psychiatry. 1999;45(5):647–651. doi: 10.1016/s0006-3223(98)00248-0. http://doi.org/10.1016/S0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO. Alcohol consumption, ACTH level, and family history of alcoholism. The American Journal of Psychiatry. 1988 doi: 10.1176/ajp.145.11.1391. Retrieved from http://psycnet.apa.org/psycinfo/1989-15964-001. [DOI] [PubMed]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53(3):202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Paulus MP, Tapert SF, Simmons AN, Tolentino NJ, Shafir A. The Ability of Functional Magnetic Resonance Imaging to Predict Heavy Drinking and Alcohol Problems 5 Years Later. Alcoholism: Clinical and Experimental Research. 2016;40(1):206–213. doi: 10.1111/acer.12935. http://doi.org/10.1111/acer.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Mohr J, Beck A, Wüstenberg T, Heinz A, Obermayer K. Predicting the future relapse of alcohol-dependent patients from structural and functional brain images. Addiction Biology. 2015 doi: 10.1111/adb.12302. n/a-n/a. http://doi.org/10.1111/adb.12302. [DOI] [PubMed]
- Setiawan E, Pihl RO, Cox SML, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The Effect of Naltrexone on Alcohol’s Stimulant Properties and Self-Administration Behavior in Social Drinkers: Influence of Gender and Genotype. Alcoholism: Clinical and Experimental Research. 2011;35(6):1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. http://doi.org/10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential Hypothalamic-Pituitary-Adrenal Axis Reactivity to Psychological and Physical Stress. The Journal of Clinical Endocrinology & Metabolism. 1999;84(6):1944–1948. doi: 10.1210/jcem.84.6.5746. http://doi.org/10.1210/jcem.84.6.5746. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling Relapse Situations in the Human Laboratory. In: Sommer WH, Spanagel R, editors. Behavioral Neurobiology of Alcohol Addiction. Springer; Berlin Heidelberg: 2011. pp. 379–402. Retrieved from http://link.springer.com/chapter/10.1007/978-3-642-28720-6_150. [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142(4):343–351. doi: 10.1007/s002130050898. http://doi.org/10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology. 2008;34(5):1198–1208. doi: 10.1038/npp.2008.78. http://doi.org/10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152(2):140–148. doi: 10.1007/s002130000499. http://doi.org/10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Söderpalm AHV, de Wit H. Effects of Stress and Alcohol on Subjective State in Humans. Alcoholism: Clinical and Experimental Research. 2002;26(6):818–826. doi: 10.1097/00000374-200206000-00011. http://doi.org/10.1111/j.1530-0277.2002.tb02610.x. [DOI] [PubMed] [Google Scholar]
- Sommer C, Seipt C, Spreer M, Blümke T, Markovic A, Jünger E, … Zimmermann US. Laboratory Alcohol Self-Administration Experiments Do Not Increase Subsequent Real-Life Drinking in Young Adult Social Drinkers. Alcoholism: Clinical and Experimental Research. 2015;39(6):1057–1063. doi: 10.1111/acer.12716. http://doi.org/10.1111/acer.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, Hendershot CS. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology. 2014:1–12. doi: 10.1007/s00213-014-3706-z. http://doi.org/10.1007/s00213-014-3706-z. [DOI] [PubMed]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. The American Journal of Psychiatry. 1994;151(10):1463–1467. doi: 10.1176/ajp.151.10.1463. http://doi.org/10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Bacon A. Stress and Affective Inductions in Addiction Research. In: JamescKillop, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. Wiley-Blackwell; 2013. pp. 411–434. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/9781118384404.ch15/summary. [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology. 2011;218(1):19–28. doi: 10.1007/s00213-010-2163-6. http://doi.org/10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Brady K, See RE, Drobes DJ. An Acute Psychosocial Stressor Does Not Potentiate Alcohol Cue Reactivity in Non-Treatment-Seeking Alcoholics. Alcoholism: Clinical and Experimental Research. 2011;35(3):464–473. doi: 10.1111/j.1530-0277.2010.01363.x. http://doi.org/10.1111/j.1530-0277.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. http://doi.org/http://dx.doi.org/10.1037/0033-295X.97.2.147. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157(1):67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36(6):1178–1186. doi: 10.1038/npp.2010.253. http://doi.org/10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Wildenberg E, Janssen RGJH, Hutchison KE, Van Breukelen GJP, Wiers RW. GENETIC STUDY: Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addiction Biology. 2007;12(2):210–220. doi: 10.1111/j.1369-1600.2007.00064.x. http://doi.org/10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Van Den Wildenberg E, Wiers RW, Dessers J, Janssen RGJH, Lambrichs EH, Smeets HJM, Van Breukelen GJP. A Functional Polymorphism of the μ-Opioid Receptor Gene (OPRM1) Influences Cue-Induced Craving for Alcohol in Male Heavy Drinkers. Alcoholism: Clinical and Experimental Research. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. http://doi.org/10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine. 2015;45(5):1061–1072. doi: 10.1017/S0033291714002165. http://doi.org/10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective Intoxication in Response to Alcohol Challenge: Heritability and Covariation With Personality, Breath Alcohol Level, and Drinking History. Alcoholism: Clinical and Experimental Research. 2003;27(5):795–803. doi: 10.1097/01.ALC.0000067974.41160.95. http://doi.org/10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Wall TL, Thomasson HR, Schuckit MA, Ehlers CL. Subjective Feelings of Alcohol Intoxication in Asians with Genetic Variations of ALDH2 Alleles. Alcoholism: Clinical and Experimental Research. 1992;16(5):991–995. doi: 10.1111/j.1530-0277.1992.tb01907.x. http://doi.org/10.1111/j.1530-0277.1992.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T, Windisch K, Darlington T, Zimmerman US, … O’Connor SJ. Subjective Perceptions Associated with the Ascending and Descending Slopes of Breath Alcohol Exposure Vary with Recent Drinking History. Alcoholism: Clinical and Experimental Research. 2012;36(6):1050–1057. doi: 10.1111/j.1530-0277.2011.01642.x. http://doi.org/10.1111/j.1530-0277.2011.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining Automatic Action Tendencies Changes Alcoholic Patients’ Approach Bias for Alcohol and Improves Treatment Outcome. Psychological Science. 2011;22(4):490–497. doi: 10.1177/0956797611400615. http://doi.org/10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain, and Behavior. 2009;8(1):101–106. doi: 10.1111/j.1601-183X.2008.00454.x. http://doi.org/10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wüst S, van Rossum EFC, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common Polymorphisms in the Glucocorticoid Receptor Gene Are Associated with Adrenocortical Responses to Psychosocial Stress. The Journal of Clinical Endocrinology & Metabolism. 2004;89(2):565–573. doi: 10.1210/jc.2003-031148. http://doi.org/10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- Yardley MM, Ray LA. Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addiction Biology. 2016 doi: 10.1111/adb.12349. n/an/a. http://doi.org/10.1111/adb.12349. [DOI] [PMC free article] [PubMed]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. Development and Pilot Validation of Computer-Assisted Self-Infusion of Ethanol (CASE): A New Method to Study Alcohol Self-Administration in Humans. Alcoholism: Clinical and Experimental Research. 2008;32(7):1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA. Modeling Alcohol Self-Administration in the Human Laboratory. In: Sommer WH, Spanagel R, editors. Behavioral Neurobiology of Alcohol Addiction. Springer; Berlin Heidelberg: 2011. pp. 315–353. Retrieved from http://link.springer.com/chapter/10.1007/978-3-642-28720-6_149. [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA. Behavioral Neurobiology of Alcohol Addiction. Springer; 2013. Modeling alcohol self-administration in the human laboratory; pp. 315–353. Retrieved from http://link.springer.com/chapter/10.1007/978-3-642-28720-6_149. [DOI] [PubMed] [Google Scholar]