Abstract
Tissue engineering strategies have shown promise in promoting formation of healing and regeneration after spinal cord injury (SCI); however, these strategies are limited by inflammation and the immune response. Infiltration of cells of the innate and adaptive immune responses and the inflammation that follows cause secondary damage adjacent to the injury, increased scarring, and a potently inhibitory environment for regeneration of damaged neurons. While typically the inflammation that ensues is associated with limited regeneration, the immune response is a crucial element in the closing of the blood brain barrier, minimizing the spread of injury, and initiating healing. This review summarizes the strategies that have been developed to modulate the immune response towards an anti-inflammatory environment that is permissive to regeneration of neurons, glia, and parenchyma. We focus on the use of biomaterials, biologically active molecules, gene therapy, nanoparticles, and stem cells to modulate the immune response, and illustrate concepts for future therapies. Current clinical treatments for SCI are limited to systemic hypothermia or methylprednisolone, which both act by systemically mitigating the effects immune response but have marginal efficacy. Herein we discuss emerging research strategies to further enhance these clinical treatments by directly targeting specific aspects of the immune response.
I. Introduction
Paralysis due spinal cord injury (SCI) has long lasting socioeconomic impacts, and results in profound physical deficits due to limited treatment options. Treatments for SCI have minimal efficacy due to limited regeneration in the spinal cord, which results from a number of factors such as the intrinsic potential of the neurons and a non-permissive environment (e.g., glial scar) that develops from the highly inflammatory milieu generated by local and infiltrating leukocytes. In an effort to address these limitations to nerve regeneration following SCI, tissue engineering approaches are being employed, such as scaffolds to guide regenerating axons and limit leukocyte infiltration, gene and drug delivery techniques to promote a less inflammatory milieu, and cell-mediated therapies to promote alternatively activated immune cell populations that support regeneration. This review will highlight therapeutic strategies that can shift the injury microenvironment from highly inflammatory towards a more anti-inflammatory milieu to facilitate regeneration of the injured nerve tissue.
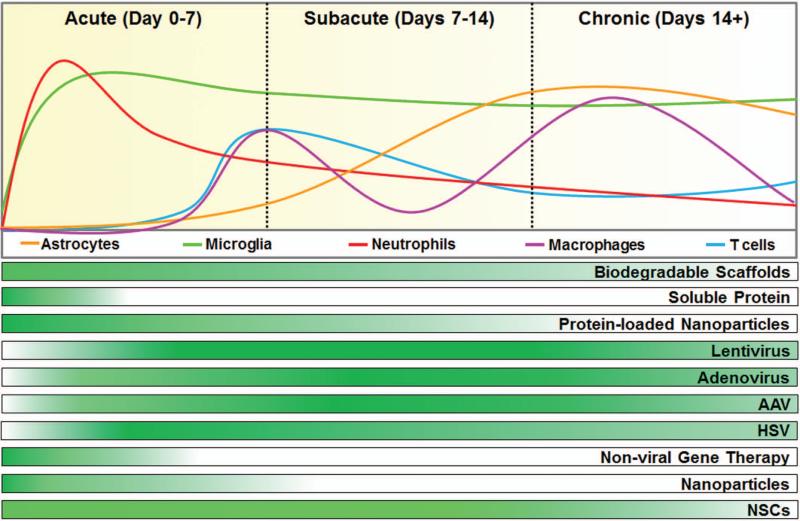
An understanding of the underlying biology has contributed to the development of strategies to modulate the inflammatory response that ensues after SCI. Figure 1 summarizes the peak infiltration time for each cell population infiltrating the injury throughout the acute, subacute, and chronic inflammatory phases in a rat SCI model. Furthermore, the figure indicates the duration of efficacy for potential therapies, and will be discussed in greater detail as they become relevant. Immediately following SCI, astrocytes and microglia become activated and secrete cytokines that initiate recruitment of leukocytes. The first leukocytes to infiltrate the injured tissue as part of the innate immune response are neutrophils, which are followed by monocytes that mature into macrophages or dendritic cells upon entering the tissue. Neutrophils and macrophages are essential for preventing infection at the injury by producing reactive oxygen species (ROS) that kill foreign pathogens. Macrophages, in particular, phagocytose foreign antigens and cellular debris left behind by necrotic or apoptotic cells, including myelin debris which contains inhibitory proteins that prevent axons from regenerating [Filbin, 2003; Yiu and He, 2006]. In addition to ROS, macrophages and neutrophils produce a wide array of inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α), which induce apoptosis of neurons and oligodendrocytes, resulting in secondary damage. These cytokines also cause astrocytes to undergo astrogliosis, a process that involves aberrant morphology and proliferation in an effort to limit excitotoxicity and oxidative stress followed by the development of the glial scar [Klusman and Schwab, 1997]. Glial scar formation is integral for closing of the blood brain barrier, but is largely composed of highly neuroinhibitory chondroitin sulfate proteoglycans (CSPGs) [Fitch et al., 1999; Busch and Silver, 2007]. T cells are the last of the immune cells to infiltrate the tissue, and they target antigens remaining within the injury. The inflammatory cascade that ensues after SCI in humans and rats results in cavitation at the epicenter of the injury that is associated with reduced factors such as laminin, matrix metalloproteinase (MMP)-1, and vascular endothelial growth factor A (VEGF-A) [Surey et al., 2014]. Finally, in the event of foreign material entering the spinal cord either during injury or implantation of a biomaterial, foreign body giant cells (FBGC) may form via macrophage fusion, though this is poorly characterized in the spinal cord. The contribution of each of these leukocyte populations has generally been considered injurious to regeneration, though few studies have investigated their impact or modulation.
Figure 1.
Design considerations of the onset and duration of immunomodulatory benefits of therapeutics to correspond with specific phases of inflammation after SCI can target specific inflammatory cell populations. Local inflammatory cell proliferation and leukocyte infiltration fluctuates within the spinal cord after injury, as indicated by the relative peaks and valleys for each population over time. In the mouse model of SCI, neutrophil infiltration peaks within the first 24 hours of the acute phase of inflammation. Microglia numbers rapidly increase during the acute phase and remain elevated into late stages of inflammation. Macrophages and T cells attain peak infiltration after one week and initiate the transition from acute to subacute phases of inflammation. Macrophages undergo a second population expansion during chronic inflammation. While astrocytes are present throughout inflammation, astrogliosis peaks between 7-14 days after injury. Efficacy of a variety of therapeutics throughout the phases of inflammation assuming immediate delivery to the spinal cord after injury is demonstrated below the cell infiltration timeline. Darker green color indicates greater immunomodulatory potential, while lighter colors indicate the therapeutic benefits are diminishing. Timelines for cell infiltration and therapeutic benefits were obtained from the following sources: [Popovich et al., 1997; Blits et al., 2003; Blits and Bunge, 2006; Donnelly and Popovich, 2008; Nguyen et al., 2011; Wang et al., 2012; Thomas and Shea, 2013; Gao et al., 2014; Papa et al., 2014].
Though the immune response is largely responsible for the creation of an unfavorable environment for tissue regeneration, the initial inflammatory response is important in initiating closing of the blood brain barrier, clearing debris, and preventing infection [Donnelly and Popovich, 2008]. While initial inflammation is essential for recovery from injury, the persistence of neutrophils and macrophages into the chronic phase of injury is believed to worsen outcomes. [Waxman, 1989; Beck et al., 2010]. In peripheral tissues, a shift in macrophage phenotype along a spectrum of activation states from inflammatory (M1 or classically activated) to anti-inflammatory (M2 or alternatively activated) is typically observed, and this shift is essential for the resolution of the immune response [Mosser, 2003; Murray and Wynn, 2011; Sindrilaru et al., 2011]. However, this transition does not occur on a sufficient scale after SCI with M1 macrophages chronically causing the degeneration of axons and inflammation [Popovich et al., 2002; Horn et al., 2008; Busch et al., 2009; Kigerl et al., 2009; Kitayama et al., 2011; Pruss et al., 2011]. Similarly, cytotoxic T cells that target myelin for removal are abundant during chronic SCI resulting in trauma induced autoimmunity, conversely therapeutics that recruit regulatory T cells can be used to modulate the T cell response to favor regeneration [Jones, 2014]. Additionally, depletion of potentially harmful leukocyte populations has resulted in seemingly contradictory results ranging from improved to a worsening of regenerative and functional outcomes following SCI. LY6G/Gr-1+ neutrophil depletion leads to higher ROS in a SCI lesion and impaired functional outcomes [Stirling et al., 2009]. In contrast, reducing neutrophil and macrophage infiltration using CD11d/CD18 antibodies reduces ROS and apoptosis, resulting in functional improvements [Saville et al., 2004; Bao et al., 2005; Geremia et al., 2012]. Macrophage depletion with dichloromethylene bisphosphonate improved partial hindlimb recovery and tissue repair [Popovich et al., 1999], while CD11c+ monocyte/macrophage depletion reduced functional outcomes [Shechter et al., 2009]. These seemingly contradictory results further support the idea that more sophisticated strategies that promote shifts within immune cell populations towards anti-inflammatory phenotypes, such as M2 macrophages and regulatory T cells will prove more advantageous to regeneration than simply eliminating select leukocyte populations. Additionally, there may be unintended consequences when depleting immune cell populations, particularly the increased risk of infection or disease due to being systemically immunocompromised. The following sections will focus on strategies that shift the inflammatory response toward anti-inflammatory cell phenotypes, such as M2 macrophages and regulatory T cells, that limit secondary injury and foster regeneration of the injured spinal cord.
II. Biomaterial
Scaffolds
Neurons in the adult central nervous system (CNS) maintain a capacity for regeneration that is limited by an intensely neuroinhibitory microenvironment that limits endogenous regeneration. This response is most clearly demonstrated by substantial regeneration of CNS axons through peripheral nerve grafts [David and Aguayo, 1981; Cote et al., 2011]. Biomaterial approaches to SCI have been designed to replicate the salient features of these peripheral nerve grafts: an architecture that simultaneously provides physical guidance, promotes cell infiltration to secrete regeneration factors, and mitigates inhibitory byproducts of primary and secondary damage. Biomaterial scaffolds have demonstrated the ability to effectively provide physical guidance and support cell infiltration. Biomaterial interactions with immune cells, however, are highly complex and affected by both the physical and chemical properties of scaffolds in ways that may be beneficial and/or deleterious and are described below. Creation of an ideal immunomodulatory scaffold for SCI has been a formidable challenge, and the following parameters and features are essential considerations for design and are summarized in Table 1.
Table 1.
Immunomodulation for SCI
| Regenerative Consequence | Design Parameters | References |
|---|---|---|
| Reduced infiltration and attachment | Porosity, nanotopography, hydrophobicity | [Dadsetan et al., 2008; Hezi-Yamit et al., 2009; Lee et al., 2011; Thomas et al., 2013] |
| Improved integration | Porosity | [Dadsetan et al., 2008; Thomas et al., 2013; Almeida et al., 2014] |
| Increased M2 polarization | Porosity, grooves, nanotopography, low stiffness | [Lee et al., 2011; Saino et al., 2011; Blakney et al., 2012; McWhorter et al., 2013; Almeida et al., 2014; Sussman et al., 2014] |
| Reduced FBGC | Porosity, aligned fibers, hydrophobicity, low stiffness | [Jones et al., 2007; Cao et al., 2010; Blakney et al., 2012; Sussman et al., 2014] |
| Reduced glial scar thickness | General biomaterials, porosity | [Wong et al., 2008; Yang et al., 2009; Khaing et al., 2011] |
| Limit T cells | Anti-Fas antibody incorporation, nanotopogrpahy | [Hume and Anseth, 2010; Kwon et al., 2012] |
The spinal cord is a complicated structure with a large number of ascending and descending neural tracts that connect specific brain structures to precise locations on the body. When approaching neural regrowth after SCI, to augment the functional improvements associated with plasticity it is imperative to maintain the spatial organization of the spinal cord in order to increase the likelihood that a regenerating axon will find a suitable target after regenerating past the injury site [Weidner and Tuszynski, 2002]. For minor contusive injuries, strategies such as cellular grafts that reduce secondary injury, facilitate sparing of intact tracts, and promote regrowth may be the best option [Haggerty and Oudega, 2013]. However, for more severe contusions and penetrating SCI, even if tissue architecture is initially preserved, secondary damage results in cavitation, atrophic parenchyma, and glial scarring. These scenarios require a biomaterial bridge that provides a permissive pathway for regenerating neurons to bypass or cross the injury site [Straley et al., 2010]. Therefore, in order to bridge the injury site and give neurons the best chance of reaching appropriate targets, the optimal biomaterial scaffold design for SCI will most likely incorporate either channels or a guidance system parallel to the spinal cord tracts [Sakiyama-Elbert et al., 2012].
While a conduit that guides neurons seems to be a logical scaffold design, direct experimental evidence to verify this claim is sparse, as are studies on its role in modulating the immune response. In 2008, Wong et al. completed the most comprehensive analysis to date that tests how overall scaffold design facilitates the regrowth of sensory and motor tracts. Using salt leached porous PCL cast in cylinder, tube, channel, and open path configurations, they observed that open path designs were superior to all other designs in being permissive to axon regeneration into the injury and reducing the extent of secondary damage and glial scarring [Wong et al., 2008]. Despite the reported result, the authors noted that the regenerating nerves could have been either regenerating white matter tracts or nerve roots entering from the side. Additionally, the lack of regeneration they report from the multichannel design are in direct contrast to other reports [Olson et al., 2009; Tuinstra et al., 2014].
When designing biomaterial scaffolds for the spinal cord, the mechanical properties of scaffolds and the changes that occur to them during degradation are essential considerations. Scaffolds must have appropriately matched stiffness while still being strong enough not to collapse and cause obstruction of nerve regeneration. When implanted in vivo, degradation, repetitive compression from movement, and immune cell related breakdown and clearance can all reduce the lifetime of biomaterial scaffolds and can cause graft failure if it happens too soon [Belkas et al., 2005]. On the other hand, appropriately timed degradation such that the biomaterial can be replaced by ECM deposition can result in axon bundles surviving completed degradation of the biomaterial [Tuinstra et al., 2014]. Strategies to alter scaffold mechanical properties may also alter wound healing and the immune response. For example, PEG hydrogels with lower stiffness lead to reduced macrophage activation and a less severe foreign body response [Blakney et al., 2012].
The macro-architecture of biomaterial scaffolds facilitates the wound healing process by acting as a framework for cell infiltration and remodeling and, in doing so, altering the immune response. Cellular infiltration within a porous material network allows for integration of host tissue that reduces cavitation as well as the glial scar. While mice typically exhibit minimal cavitation in SCI, larger animals such as rats exhibit prominent cyst formation at the injury epicenter, similar to what occurs in human SCI. In contrast to the macrophage filled cysts in rats, mouse lesion sites are rich in the extracellular matrix (ECM) proteins laminin and collagen, pro angiogenic factors, and pro-wound-healing factors [Surey et al., 2014]. These characteristics are associated with delayed T cell entry and a lack of dendritic cell infiltration in mice relative to rats [Sroga et al., 2003]. Though the mechanism is incomplete, biomaterial scaffolds and hydrogels reduce the thickness of the glial scar while concomitantly reducing the macrophage population [Teng et al., 2002; Yang et al., 2009]. Whether this effect results from space filling or a material property is unclear, but the introduction of a scaffold profoundly affects immune cell infiltration while replacing cavitation and the thick glial scar with regenerating tissue as observed by the absence of significant cyst formation after spinal cord bridge implantation in highly porous or open designs [Wong et al., 2008; De Laporte et al., 2009b].
In order to promote and support regeneration, biomaterial scaffolds must be permissive to cellular infiltration, biomolecule diffusion, and vascular infiltration at the microstructural level. These properties are achieved by utilizing pores, grooves, or polymer fibers and can have profound effects on the immune response. Porosity allows for scaffold vascularization, alters cell migration and phenotype into and around the scaffold, and improves implant stability at the tissue-implant interface [Dadsetan et al., 2008; Thomas et al., 2013]. Pore size has been shown to alter macrophage phenotype in a size dependent fashion with 160 μm pores pushing macrophage population towards an M2 phenotype in comparison to 34 μm pores in subcutaneous implants [Sussman et al., 2014]. While the smaller pores in this example supported greater vascular infiltration, both pore sizes reduced the FBGC response and promoted healing. Whether this effect would be observed in the spinal cord is unknown; however, design parameters that promote formation of M2 macrophages and resolution of inflammation are essential for SCI. Pore geometry has been observed to alter macrophage phenotype with diagonal geometries resulting in less FBGCs and elongated (M2) macrophages in the context of 3D printed chitosan and polylactic acid (PLA) scaffolds [Almeida et al., 2014]. Grooves have also been shown to interact with macrophages in particular. Recently, McWhorter et al. demonstrated that cell elongation with grooves can induce M2 phenotypic marker expression and enhance the effects of M2 inducing cytokines, though this has not yet been demonstrated in vivo [McWhorter et al., 2013]. In a similar manner, aligned polycaprolactone (PCL) electrospun nanofibers minimize the immune response by reducing the thickness of the fibrous capsule surrounding the scaffold, reducing monocyte adhesion, and increasing cell infiltration compared to random fiber and films [Cao et al., 2010]. Additionally, the aligned orientation of fibronectin coated poly-l-lactic acid (PLLA) fibers has been shown to enhance GLT-1 mediated glutamate uptake and astrocyte migration in vitro [Zuidema et al., 2014]. Finally, nanofibrous scaffolds result in reduced macrophage-mediated pro-inflammatory cytokine release including granulocyte colony stimulating factor (G-CSF), (chemokine C-C ligand (CCL-5), and TNF-α relative to scaffolds made of micron scale fibers [Saino et al., 2011].
Though nanotopography effects can be challenging to isolate from changes in surface chemistry as they can be interrelated, both nanoscale surface features and chemistry can alter the function and migration patterns of immune cells. Few studies have investigated the interactions between nanotopography and the immune response; however, both the innate and adaptive immune response have been demonstrated to be affected. On nanostructured titanium, macrophage motility was restricted and reduced M1 macrophage polarization on titanium substrates was observed [Lee et al., 2011]. In a similar way, nanopatterned (grooved) polyurethane acrylate surfaces caused directional topography-guided migration of T cells [Kwon et al., 2012]. The effects of surface chemistry have been studied in much greater detail; in particular, the relationship between the chemical properties of a surface and protein adsorption has been thoroughly characterized as protein adsorption interacts and directly activates immune cells. Immediately following blood-material contact, a layer of protein adsorbs to the surface resulting in formation of a blood clot that recruits the cells of the innate immune system to the injury. The severity of the ensuing inflammation is highly dependent on the biochemical properties of the material and how it denatures adsorbed proteins. Natural materials such as collagen [De Laporte et al., 2009a], hyaluronic acid [Kang et al., 2009], and fibrin [Taylor et al., 2006] are often considered biocompatible being that they are naturally found in the ECM of many tissues including the spinal cord, but they can be immunogenic and their properties can change substantially during processing. Synthetic materials such as poly(lactide-co-glycolide) (PLG) [Tuinstra et al., 2014], poly (ethylene glycol) (PEG) [Luo and Shi, 2007], and poly(2-hydroxyethyl methacralate) (PMMA) [Hejcl et al., 2008], though not native to the spinal cord, also have minimal immune responses that can be further modulated. The degree of hydrophilicity/hydrophobicity is particularly important as hydrophobic materials are more likely to denature adsorbed proteins and are associated with decreased monocyte adhesion [Hezi-Yamit et al., 2009] as well as decreased macrophage adhesion and FBGC formation in vitro [Jones et al., 2007].
Particle Delivery
Nanoparticles have been used to deliver a therapeutic to the injured spinal cord, however, a growing number of researchers are investigating the inherent ability of polymeric particles to module the immune cell response. Local delivery of both PMMA and PCL–PEG composite nanoparticles have been shown to be selectively internalized by pro-inflammatory macrophages and microglia following SCI [Papa et al., 2013; Papa et al., 2014]. Similarly, monocyte uptake of PLG and polystyrene (PS) nanoparticles containing antigen peptides has been shown to induce antigen-specific tolerance for the prevention and treatment of experimental autoimmune encephalomyelitis (EAE), the murine multiple sclerosis model [Getts et al., 2012; Hunter et al., 2014; Maldonado et al., 2015]. Tolerance was dependent on particle uptake by the macrophage receptor with collagenous structures (MARCO) scavenger receptor, in part due to the anionically charged surface of the nanoparticles [Kanno et al., 2007]. As uptake of these particles was independent of the antigen, it is likely that naked PLG and PS nanoparticles can also be used to target and modify macrophages following SCI. Additionally, nanoparticles with antigen capable of inducing myelin-specific tolerance could be used to alleviate the chronic inflammatory phase of SCI, which is characterized by unfettered demyelination comparable to EAE models [Jones, 2014]. Other particles, including iron oxide [Pal et al., 2013], gold [Hutter et al., 2010], and quantum dots [Minami et al., 2012; Moquin et al., 2015] have also been used to target microglia and reducing inflammation using in vitro models.
Therapeutic Delivery Considerations
Local and systemic injection of biologically active molecules, including protein, gene, and cellular therapies, can be used to modulate the immune response at the site of injury. Systemic injection of these molecules can redirect immune cell trafficking and promote anti-inflammatory immune phenotypes throughout the body and within the spinal cord. Unfortunately, many molecules used have pleotropic effects and can cause off-target effects. Additionally, a larger amount of the protein needs to be delivered systemically to elicit changes in inflammation within the spinal cord. Moreover, while the blood brain barrier is temporarily open during the acute stage of SCI, and it begins to close after 3 days [Popovich et al., 1996; Whetstone et al., 2003], limiting the window that systemic treatments are effective [Jeffery et al., 2009]. Local injection of these molecules with osmotic pumps directly into the cerebrospinal fluid can be utilized to avoid systemic side effects and limit the amount of protein needed, though osmotic pumps tend to clog, may require a second surgery for removal, and can cause further tissue damage [Jones and Tuszynski, 2001]. The use of direct injection methods, whether systemic or local, can exhibit limited protein bioactivity due to rapid degradation within hours to days depending on the molecule, thus limiting the therapeutic window.
Utilization of a delivery vehicle, such as scaffolds and nanoparticle carriers, for biologically active molecules can extend their bioactivity by providing protection from proteolysis that commonly affects unbound soluble proteins. Proteins or enzymes can be incorporated into a biomaterial, adsorbed onto the surface, or covalently bound to a material. Inclusion of proteins into hydrogels or polymeric scaffolds can be used to localize the proteins at the injury site to modulate the local astrocyte and microglia response, as well as that of the infiltrating leukocytes following injury [Hayashi et al., 2009; Wilems and Sakiyama-Elbert, 2015]. Protein loaded scaffolds used in penetrating SCI models perform several functions by not only delivering proteins and increasing their bioavailability but also fill the tissue void and reduce glial scar formation. Micro and nanoparticles have been developed to deliver specific proteins to modulate immune cell infiltration and phenotype following SCI and can be used in both contusion and penetrating SCI models. Therapeutic proteins can be encapsulated within or incorporated throughout the particle depending on the desired release profile relative to leukocyte infiltration and duration of bioactivity. Particles can be administered locally via the cerebrospinal fluid or by incorporation into a hydrogel. Alternatively, systemically administered particles can include a targeting peptide that will traffic the particles with the therapeutic to the site of inflammation [Ruoslahti, 2012]. This method allows for safer administration at a more easily accessible injection site outside of the CNS but offers opportunities that mirror local delivery strategies. Encapsulation of the therapeutic is appropriate for systemically delivered particles as a precaution to limit pleotropic effects prior to arrival in the spinal cord. Zuidema et. al. provide further information regarding nanoparticle design for therapeutic and diagnostic delivery to the CNS [Zuidema, 2016].
III. Biologically Active Molecules
Biologically active molecules, including matrix proteins, growth factors, cytokines, enzymes, and antibodies, have been utilized to reduce inflammation and promote regeneration after SCI. Non-biologic pharmaceutical agents, such as methylprednisolone and dexamethasone, have been used to systemically suppress the immune response, and have been reviewed elsewhere [Ilinskaya and Dobrovolskaia, 2014]. Modulating the immune response to promote a more anti-inflammatory phenotype among immune cells at the site of SCI offers greater regenerative capacity than systemic immune suppression. This phenomenon has been documented with neutrophil and macrophage depletion studies that exacerbated damage after SCI, thus demonstrating the importance of the immune cells in promoting a microenvironment primed for regeneration [Shechter et al., 2009; Stirling et al., 2009]. To ensure a more regenerative immune cell phenotype, a number of local and systemic delivery strategies have been utilized to modulate the immune response with biologically active molecules. These molecules can be used to target specific events or cell populations within each phase of the inflammatory response with the goal of promoting an anti-inflammatory milieu, as the highly complex and precisely timed coordination of leukocytes and local cell populations that ensue after SCI are well documented. Delivering appropriately timed biologically active molecules that target one or more specific phase of inflammation after SCI can limit secondary damage and promote regeneration. While biologically active molecules hold promise in modulating the inflammatory milieu, these factors can degrade rapidly reducing their bioavailability. These challenges can be addressed through the use of more robust molecules, inclusion of a delivery vehicle, or considerations into timing and location of delivery that must be designed specifically for each protein. The following sections will explore therapeutic options for each phase of inflammation as well as an overview of considerations for delivery of these biologically active molecules. Table 2 summarizes the anti-inflammatory benefits and regenerative consequences garnered from proteins previously delivered to the injured spinal cord, as well as cellular and gene therapy approaches in subsequent sections of this review.
Table 2.
Immunomodulation for SCI
| Regenerative Consequence | Therapeutic Intervention | References |
|---|---|---|
| Reduced infiltration and recruitment | G-CSF,PPAR-γ, IL-33, MSCs | [Ifergan et al., 2006; Lee et al., 2009; Chu et al., 2014; Pomeshchik et al., 2015; Watanabe et al., 2015] |
| Increased M2 polarization | IL-4, IL-10, IL-13, IL-33, aFGF, galectin-1, G-CSF, M-CSF, BDNF, chABC, MSCs | [Vannier et al., 1992; Bethea et al., 1999; Martinez et al., 2008; Zhou et al., 2008; Lee et al., 2010; Kuo et al., 2011; Nakajima et al., 2012; Guo et al., 2013; Didangelos et al., 2014; Fenn et al., 2014; Hamilton et al., 2014; Gaudet et al., 2015; Ji et al., 2015; Pomeshchik et al., 2015; Watanabe et al., 2015] |
| Reduced glial scar thickness | IL-33, GM-CSF, C-CSF, chABC, miR-21, miR-145, HGF | [Bhalala et al., 2012; Jeong et al., 2012; Bartus et al., 2014; Chung et al., 2014; Didangelos et al., 2014; Pomeshchik et al., 2015; Wang et al., 2015] |
| Increased regulatory T cells | HGF, EPO, IL-33, NSCs | [Yuan et al., 2008; Wang et al., 2009; Benkhoucha et al., 2010; Bonnamain et al., 2012; Gao et al., 2014; Pomeshchik et al., 2015] |
Leukocyte Infiltration
Limiting the initial infiltration of these cells in the acute phase of inflammation can reduce the secondary damage that occurs due to the highly inflammatory microenvironment caused by infiltrating immune cells. Systemic administration of G-CSF and statins have been shown to reduce vascular permeability through increased expression of aquaporin 4 and VEGF [Ifergan et al., 2006; Chu et al., 2014]. Peroxisome proliferator-activated receptor gamma (PPARγ) can also reduce vascular permeability through an inhibition of MMP-9 [Lee et al., 2009], which is responsible for the digestion of the vascular matrix [Noble et al., 2002]. These approaches offer a first defense against excessive pro-inflammatory leukocyte infiltration into the spinal cord. Subsequent use of biologically active molecules that promote an anti-inflammatory phenotype of the resident microglia and the infiltrating leukocytes would provide the next line of defense against secondary injury.
Modulating Innate Immunity
The innate immune response following SCI is characterized by the infiltration of neutrophils, monocytes, macrophages, and dendritic cells that release pro-inflammatory cytokines that leads to secondary damage of the neurons and oligodendrocytes that survived the initial injury. It has been well documented that these leukocytes can undergo a phenotypic change resulting in a more anti-inflammatory phenotype that supports tissue regeneration. Typically, macrophages and microglia are targeted through the use of biological active molecules to convert them from a pro-inflammatory M1 phenotype towards an anti-inflammatory M2 phenotype. A number of interleukins have been used to facilitate M2 polarization including IL-4, IL-10, IL-13, and IL-33 [Bethea et al., 1999; Martinez et al., 2008; Lee et al., 2010; Fenn et al., 2014; Pomeshchik et al., 2015]. Similarly, acidic fibroblast growth factor (aFGF), galectin-1, G-CSF, and myeloid colony stimulating factor (M-CSF) have also been used to promote M2 macrophages and/or microglia following SCI resulting in modest improvements to axon growth and functional recovery [Kuo et al., 2011; Guo et al., 2013; Hamilton et al., 2014; Gaudet et al., 2015]. G-CSF has also been shown to reduce oligodendrocyte death suggesting that the reduction in pro-inflammatory cytokines leads to sparing of the remaining tissue. Interestingly granulocyte macrophage colony stimulating factor (GM-CSF) promotes M1 macrophage polarization and activation of dendritic cells, and leads to increased expression of brain derived neurotrophic factor (BDNF) and early recovery of locomotor function after SCI [Hayashi et al., 2009; Hamilton et al., 2014]. It should be noted that BDNF can promote M2 polarization leading to increased expression of IL-10 and IL-13 with a subsequent decrease in the pro-inflammatory cytokines TNF-α and IL-1β [Ji et al., 2015]. The downstream signaling of BDNF leading to M2 polarization may provide a rational for how M1-promoting GM-CSF can promote a regenerative milieu.
Limiting Glial Scar Formation
The primary endogenous means to spare the remaining nerve tissue is through the formation of the glial scar by astrocytes, however, if left unchecked the glial scar can greatly exacerbate the amount of secondary damage. Delivery of IL-33, GM-CSF, and G-CSF can reduce glial scar formation although it is unclear as to whether this is through direct action on the astrocytes or due to the modulation of the leukocyte population leading to altered cytokine expression [Chung et al., 2014; Pomeshchik et al., 2015]. Astrocytes have been documented to produce IL-33, GM-CSF, and G-CSF in an effort to reduce inflammation on their own, thus exogenous delivery may further reduce inflammation and the development of the glial scar [Aloisi et al., 1992; Yasuoka et al., 2011]. The astrocyte-derived CSPGs within the glial scar are thought to be the primary barrier to axonal elongation via physical blockade and a set of cellular and biochemical cues that instruct axonal growth cones to turn away or become dystrophic and stop regenerating [Cregg et al., 2014]. Chondroitinase ABC (chABC) can reduce the presence of the glial scar through enzymatic digestion of the CSPGs, but can also promote M2 macrophages that may reduce glial scar formation [Didangelos et al., 2014]. The inherent benefits of chABC delivery to the glial scar, including CSPG digestion, reduced cavitation, and altered macrophage polarization, can be further enhanced through the extended presence of the enzyme via lentiviral delivery of the chABC vector [Bartus et al., 2014].
Modulating Adaptive Immunity
The adaptive immune response consists of natural killer and lymphocyte populations. Many researchers have reported high levels of T cell activation towards myelin peptides during the chronic inflammation following SCI, as reviewed by [Jones, 2014]. This response is presumably due to the presence of a large amount of myelin debris following the primary and secondary damage that was phagocytosed by antigen presenting cells, such as macrophages and dendritic cells, for presentation to T cells. Though the adaptive immune response has been infrequently targeted in SCI. IL-33 has been reported to limit T cell infiltration into the spinal cord [Pomeshchik et al., 2015]. While effective, it is unclear if this cytokine acts directly on the T cells or if it is an artifact of the reduced oligodendrocyte death that accompanies IL-33 delivery to the spinal cord. Other therapeutic interventions aim to convert cytotoxic T cells to more regeneration Th2 or regulatory T cell phenotypes to spare the remaining myelin and reduce the pro-inflammatory cytokines IL-6 and interferon gamma (IFN-γ). The presence of regulatory T cells would also confer tolerance of foreign cells and biomaterials. Hepatocyte growth factor (HGF), erythropoietin (EPO), and IL-33 have been used independently to limit or modulate the aberrant T cell response [Yuan et al., 2008; Benkhoucha et al., 2010; Pomeshchik et al., 2015]. In addition to limiting inflammation, HGF and EPO are potent chemoattractant factors for endogenous stem cell populations that could repair the damaged tissue [Li et al., 2012; Merino et al., 2015]. In addition to cytokines, ligands or antibodies can be used to directly influence the adaptive immune response. Although it has not been used within the spinal cord, anti-Fas antibodies effectively promote apoptosis of infiltrating cytotoxic T cell to prevent rejection of implanted cells or scaffolds [Kim et al., 2006; Pearl-Yafe et al., 2007; Hume and Anseth, 2010].
IV. Gene Therapy
While direct protein delivery can be effective for short term dosage, gene therapy provides an opportunity to achieve sustained expression. Gene therapy vectors can be broadly classified as viral and nonviral carriers. In order to substantially alter protein expression levels at biologically relevant concentrations, gene therapy must be efficient. Although great effort has been put into developing nonviral gene delivery methods such as naked DNA or RNA, liposomes, and nanoparticles, nonviral gene delivery is limited in its ability to efficiently transfect cells, particularly in vivo. Viral vectors bypass these limitations by utilizing highly evolved mechanisms to incorporate their genetic material into cells, resulting in greater transduction efficiency and therefore expression levels. Moreover, some vectors, such as non-lentiviral retroviruses and adenovirus are unable to infect dividing cells, reducing their efficiency and utility. Cells such as neurons, in particular, are not typically transduced by these types of viruses. For further discussion on gene therapy for SCI, please see the review by Walthers et. al. [Walthers and Seidlits, 2015].
When approaching immunomodulation with gene therapy, there are a several important considerations when choosing an appropriate vector. The timing of gene expression can be important when specifically targeting either the innate or adaptive immune response. An overview of the delayed onset and duration of gene expression is represented in Figure 1. Nonviral therapy offers lower level of expression, but does feature the quickest expression. Onset of transgene expression delivered by herpes simplex virus (HSV) or adenovirus is faster than other viral vectors, occurring within 24 hours after administration [Blits and Bunge, 2006]. Lentivirus typically exhibits expression within 48 hours after expression and maximal expression after 3-4 days [Thomas and Shea, 2013]. Finally, onset of expression for adeno-associated virus (AAV) occurs by day 3 and peaks at day 7 [Boulis et al., 1999]. Vector size may also be important consideration as AAV has a capacity less than 5 kb of ssDNA, retrovirus can contain a maximum of 8-10 kb of ssRNA, and adenovirus and HSV have substantially higher transgene capacities. Finally, viral vectors are immunogenic on their own, which can complicate immunomodulation. AAV and lentivirus are generally considered to have a low level of transient immunogenicity, while adenovirus is highly inflammatory. For more information, see the review by Nayak et. al. [Nayak and Herzog, 2010].
Leukocyte Response
The role of neutrophils and lymphocytes in regeneration following SCI is not well understood, thus hindering the development of gene therapies to mechanistically target these cells. Conversely, macrophages have been frequently targeted with gene therapy for SCI as recent research has defined a spectrum of pro/anti-inflammatory phenotypes that active macrophages can exist within. IL-10, an anti-inflammatory cytokine, is known to inhibit the synthesis and release of pro-inflammatory mediators such as TNFα, IL-1β, IL-6, IL-8 and IL-12, and has been shown to activate signaling pathways involved in neuronal survival and growth [Zhou et al., 2009b]. Lentiviral delivery of IL-10 to macrophages in vitro can sustain macrophage polarization towards an M2 phenotype, though this research has not yet been demonstrated in vivo in SCI [Boehler et al., 2014]. Delivery of IL-10 poliovirus replicons intramuscularly or intrathecally elevated IL-10 for 4 days with a peak at 24 hours and improved functional injury when given immediately after injury [Jackson et al., 2005]. Intrathecal injection of HSV encoding IL-10 after a hemisection injury demonstrated improved axonal sparing, more functional recovery, and direct trophic support for neurons by IL-10 [Zhou et al., 2009a, b].However, neither viral IL-10 study investigated the effects on the immune response or how those effects alter regeneration. IL-4 is another prototypical anti-inflammatory cytokine that antagonizes and suppresses the effects of IL-1, leading to M2 macrophage phenotypes [Vannier et al., 1992]. It has been shown that HSV mediated expression of IL-4 can reduce neuropathic pain, but, like IL-10, it has not been clearly shown to promote M2 macrophage mediated healing in vivo [Hao et al., 2006]. Finally, BDNF, a factor that provides neuroprotective benefits, promotes axon regeneration, and increases synaptic plasticity has ancillary effects in that it promotes M2 macrophage polarization that may enhance neuroprotective effects and partially contribute to the locomotor functional recovery after SCI [Ji et al., 2015].
Astrogliosis and wound healing
One of the most common approaches to mitigating astrogliosis is to target CSPG breakdown using chondroitinase, which proteolytically inactivates CSPGs such that they no longer inhibit axon elongation. This approach has been successful in reducing cavitation, preserving of spinal neurons and axons, and improving sensorimotor function [Bartus et al., 2014]. Additionally, chondroitinase leads to IL-10 upregulation and subsequent increase in M2 macrophages [Didangelos et al., 2014]. While breakdown of CSPGs is an effective way to remove one of the key barriers to regeneration, preventing deposition is expected to be more effective. To this end, several microRNAs has been identified. In particular, microRNA-21 plays a prominent role in the initial hypertrophic stage of astrogliosis that is essential for wound healing as opposed to the later hyperplasia responsible for progression of the glial scar. Over-expression of miR-21 attenuates hypertrophy and knock down augments the hypertrophic phenotype even in chronic stages of SCI recovery [Bhalala et al., 2012]. Likewise, lentivirus-mediated pre-miRNA 145 delivery under the astrocyte specific glial fibrillary acidic protein (GFAP) promoter, reduced astrocytic cell density at the lesion border of the injured spinal cord, reduced the size of astrocytes and the number of related cell processes, as well as cell proliferation and migration [Wang et al., 2015]. miR-145 likely exhibits global control over astrogliosis, in contrast to miR-21, which specifically controls the hypertrophic stage of astrogliosis. Finally, ex vivo gene therapy with HGF reduces transforming growth factor beta (TGF-β) levels, limits astrocytic scar formation and promotes axonal regeneration beyond the glial scar after SCI [Jeong et al., 2012].
V. Stem Cell-Mediated
Cell-mediated therapies hold promise in both modulating the immune response and repopulating the injury site. Stem cells are widely used following SCI, as they can directly contribute to nerve regeneration by repopulating the injury site and differentiating into neurons and glia that will form the new tissue. Interestingly, these cells have the potential to either evade immune recognition or to locally modulate an immune response. Embryonic stem cells are less susceptible to immune rejection than adult cells due to an absence of major histocompatibility complex (MHC)-II and CD80/CD86 and very low levels of MHC-I expression [Drukker et al., 2002; Drukker, 2006], however, these cells cannot confer this tolerance to local cells through secretion of anti-inflammatory cytokines. Within neural stem cell (NSC) populations, the presentation of MHC molecules is variable due to the isolation source and the inflammatory milieu, suggesting subpopulations of NSCs offer improved survival capacity [Bonnamain et al., 2012b]. These cells that survive, along with endogenous NSCs in the spinal cord, have the ability to modulate the local immune response, such as macrophage polarization and T cell phenotype after SCI, a characteristic shared with mesenchymal stem cells (MSCs) [Wang et al., 2009; Bonnamain et al., 2012a; English, 2013; Barbeau et al., 2014; Gao et al., 2014; Yan et al., 2014].
MSCs and NSCs modulate the immune cells through numerous mechanisms, including direct cell-cell contact and indirect contact via cytokines and signaling molecules [Wang et al., 2009; Bonnamain et al., 2012a; English, 2013; Luz-Crawford et al., 2013; Barbeau et al., 2014; Gao et al., 2014; Nazmi et al., 2014; Obermajer et al., 2014; Yan et al., 2014]. NSCs release soluble factors such as TGF-β1, prostaglandin E, nitric oxide, and hemeoxygenase-1 that increase regulatory T cell populations at the expense of effector T cells [Wang et al., 2009; Bonnamain et al., 2012a; Nazmi et al., 2014]. NSCs can also effect these changes directly through contact with T cell populations using intracellular adhesion molecule (ICAM) and B7 cell surface proteins [Nazmi et al., 2014]. Through direct contact and local cytokine release, NSCs promoted an increase in regulatory T cells, increased expression of anti-inflammatory cytokines, decreased pro-inflammatory cytokines [Gao et al., 2014]. Similarly, MSCs injected into a contused spinal cord reduced macrophage infiltration, restored the blood spinal cord barrier, led to alternative polarization of macrophages and microglia, and improved hind-limb motor function [Watanabe et al., 2015]. A decrease in the pro-inflammatory cytokines (TNF-α, IL-6), mediators of vascular permeability (MMP-9), and macrophage recruitment factors CCL-2, CCL5, and chemokine C-X-C ligand (CXCL)10) coupled with an increase in GM-CSF within the first 24 hours after SCI likely contributed to the improved functional outcomes [Watanabe et al., 2015].
VI. Conclusions and Future Directions
Substantial strides have been made in individual approaches to immunomodulation after SCI, but these approaches only have the capacity to indirectly promote regeneration. Individually, these approaches effect changes at a specific time point or through a target cell population within the inflammatory cascade, as demonstrated in Figure 1. Each of these treatments has demonstrated modest effects on its own, but small improvements in rodent sensorimotor function do not correspond to clinically significant improvements in quality of life. Future therapies will need to investigate how biomaterials, biologically active molecules, gene therapy, nanoparticles, and/or cell transplants can be used in concert to directly promote regeneration while mitigating inflammation without impairing wound healing and closing of the blood brain barrier. Combination therapies will require further investigation as to the mechanism by which these therapies modulate the immune system to optimize their efficacy; however, in combination, these therapies may have additive or synergistic effects on regeneration that may transform the field.
Acknowledgements
Funding was provided by the National Institutes of Health (RO1EB005678).
List of abbreviations
- AAV
adeno-associated virus
- aFGF
acidic fibroblast growth factor
- BDNF
brain derived neurotrophic factor
- CCL
chemokine C-C ligand
- chABC
chondroitinase ABC
- CNS
central nervous system
- CSPG
chondroitin sulfate proteoglycan
- CXCL
chemokine CXC ligand
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- EPO
erythropoietin
- FBGC
foreign body giant cell
- G-CSF
granulocyte colony stimulating factor
- GFAP
glial fibrillary acidic protein
- GM-CSF
granulocyte macrophage colony stimulating factor
- HGF
hepatocyte growth factor
- HSV
herpes simplex virus
- ICAM
intracellular adhesion molecule
- IFN-γ
interferon gamma
- IL
interleukin
- MARCO
macrophage receptor with collagenous structures
- M-CSF
macrophage colony stimulating factor
- MHC
major histocompatibility complex
- MMP-9
matrix metallopeptidase 9
- MSC
mesenchymal stem cell
- NSC
neural stem cell
- PCL
polycaprolactone
- PLLA
poly-l-lactic acid
- PEG
poly(ethylene glycol)
- PLA
polylactic acid
- PMMA
poly(2-hydroxyethyl methacralate)
- PPARγ
peroxisome proliferator-activated receptor gamma
- PS
polystyrene
- SCI
spinal cord injury
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Footnotes
Competing Interests The authors declare no competing interests.
References
- Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014;10(2):613–622. doi: 10.1016/j.actbio.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149(7):2358–2366. [PubMed] [Google Scholar]
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. Journal of neurochemistry. 2005;94(5):1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- Barbeau DJ, La KT, Kim DS, Kerpedjieva SS, Shurin GV, Tamama K. Early growth response-2 signaling mediates immunomodulatory effects of human multipotential stromal cells. Stem Cells Dev. 2014;23(2):155–166. doi: 10.1089/scd.2013.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34(14):4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain : a journal of neurology. 2010;133(Pt 2):433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005;26(14):1741–1749. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2010;107(14):6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16(10):851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32(50):17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100(6):1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blits B, Bunge MB. Direct gene therapy for repair of the spinal cord. J Neurotrauma. 2006;23(3-4):508–520. doi: 10.1089/neu.2006.23.508. [DOI] [PubMed] [Google Scholar]
- Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Gower RM, Leonard JN, Shea LD. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111(6):1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnamain V, Mathieux E, Thinard R, Thebault P, Nerriere-Daguin V, Leveque X, Anegon I, Vanhove B, Neveu I, Naveilhan P. Expression of heme oxygenase-1 in neural stem/progenitor cells as a potential mechanism to evade host immune response. Stem Cells. 2012a;30(10):2342–2353. doi: 10.1002/stem.1199. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci. 2012b;6:17. doi: 10.3389/fncel.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulis NM, Bhatia V, Brindle TI, Holman HT, Krauss DJ, Blaivas M, Hoff JT. Adenoviral nerve growth factor and beta-galactosidase transfer to spinal cord: a behavioral and histological analysis. J Neurosurg. 1999;90(1 Suppl):99–108. doi: 10.3171/spi.1999.90.1.0099. [DOI] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(32):9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Current opinion in neurobiology. 2007;17(1):120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93(3):1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Tang Y, Dong Q. Protection of granulocyte-colony stimulating factor to hemorrhagic brain injuries and its involved mechanisms: effects of vascular endothelial growth factor and aquaporin-4. Neuroscience. 2014;260:59–72. doi: 10.1016/j.neuroscience.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Chung J, Kim MH, Yoon YJ, Kim KH, Park SR, Choi BH. Effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on glial scar formation after spinal cord injury in rats. J Neurosurg Spine. 2014;21(6):966–973. doi: 10.3171/2014.8.SPINE131090. [DOI] [PubMed] [Google Scholar]
- Cote MP, Amin AA, Tom VJ, Houle JD. Peripheral nerve grafts support regeneration after spinal cord injury. Neurotherapeutics. 2011;8(2):294–303. doi: 10.1007/s13311-011-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadsetan M, Hefferan TE, Szatkowski JP, Mishra PK, Macura SI, Lu L, Yaszemski MJ. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials. 2008;29(14):2193–2202. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-coglycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009a;30(12):2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009b;17(2):318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci. 2014;34(49):16424–16432. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M. Immunogenicity of embryonic stem cells and their progeny. Methods Enzymol. 2006;420:391–409. doi: 10.1016/S0076-6879(06)20019-3. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(15):9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91(1):19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci. 2014;34(26):8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature reviews Neuroscience. 2003;4(9):703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(19):8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL, ZhuGe QC. Transplanted neural stem cells modulate regulatory T, gammadelta T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15(3):4431–4441. doi: 10.3390/ijms15034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Sweet DR, Polinski NK, Guan Z, Popovich PG. Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol Cell Neurosci. 2015;64:84–94. doi: 10.1016/j.mcn.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Bao F, Rosenzweig TE, Hryciw T, Weaver L, Dekaban GA, Brown A. CD11d Antibody Treatment Improves Recovery in Spinal Cord-Injured Mice. Journal of neurotrauma. 2012;29(3):539–550. doi: 10.1089/neu.2011.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZH, Yap WT, Getts MT, Pleiss M, Luo X, King NJC, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature Biotechnology. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhang H, Yang J, Liu S, Bing L, Gao J, Hao A. Granulocyte colony-stimulating factor improves alternative activation of microglia under microenvironment of spinal cord injury. Neuroscience. 2013;238:1–10. doi: 10.1016/j.neuroscience.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Haggerty AE, Oudega M. Biomaterials for spinal cord repair. Neurosci Bull. 2013;29(4):445–459. doi: 10.1007/s12264-013-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TA, Zhao C, Pavicic PG, Jr., Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol. 2014;5:554. doi: 10.3389/fimmu.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ohta S, Kawakami Y, Toda M. Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci Res. 2009;64(1):96–103. doi: 10.1016/j.neures.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Hejcl A, Urdzikova L, Sedy J, Lesny P, Pradny M, Michalek J, Burian M, Hajek M, Zamecnik J, Jendelova P, Sykova E. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J Neurosurg Spine. 2008;8(1):67–73. doi: 10.3171/SPI-08/01/067. [DOI] [PubMed] [Google Scholar]
- Hezi-Yamit A, Sullivan C, Wong J, David L, Chen M, Cheng P, Shumaker D, Wilcox JN, Udipi K. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A. 2009;90(1):133–141. doi: 10.1002/jbm.a.32057. [DOI] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(38):9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume PS, Anseth KS. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials. 2010;31(12):3166–3174. doi: 10.1016/j.biomaterials.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014 doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter E, Boridy S, Labrecque S, Lalancette-Hebert M, Kriz J, Winnik FM, Maysinger D. Microglial response to gold nanoparticles. ACS Nano. 2010;4(5):2595–2606. doi: 10.1021/nn901869f. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Wosik K, Cayrol R, Kebir H, Auger C, Bernard M, Bouthillier A, Moumdjian R, Duquette P, Prat A. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol. 2006;60(1):45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171(17):3988–4000. doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336(2):173–183. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, McBain SC, Dobson J, Chari DM. Uptake of systemically administered magnetic nanoparticles (MNPs) in areas of experimental spinal cord injury (SCI). J Tissue Eng Regen Med. 2009;3(2):153–157. doi: 10.1002/term.139. [DOI] [PubMed] [Google Scholar]
- Jeong SR, Kwon MJ, Lee HG, Joe EH, Lee JH, Kim SS, Suh-Kim H, Kim BG. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp Neurol. 2012;233(1):312–322. doi: 10.1016/j.expneurol.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Ji XC, Dang YY, Gao HY, Wang ZT, Gao M, Yang Y, Zhang HT, Xu RX. Local Injection of Lenti-BDNF at the Lesion Site Promotes M2 Macrophage Polarization and Inhibits Inflammatory Response After Spinal Cord Injury in Mice. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83(3):585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Tech. 2001;54(5):317–324. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- Jones TB. Lymphocytes and autoimmunity after spinal cord injury. Exp Neurol. 2014;258:78–90. doi: 10.1016/j.expneurol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Kang CE, Poon PC, Tator CH, Shoichet MS. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng Part A. 2009;15(3):595–604. doi: 10.1089/ten.tea.2007.0349. [DOI] [PubMed] [Google Scholar]
- Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicological sciences : an official journal of the Society of Toxicology. 2007;97(2):398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13(2):289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Kitayama M, Ueno M, Itakura T, Yamashita T. Activated microglia inhibit axonal growth through RGMa. PloS one. 2011;6(9):e25234. doi: 10.1371/journal.pone.0025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762(1-2):173–184. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- Kuo HS, Tsai MJ, Huang MC, Chiu CW, Tsai CY, Lee MJ, Huang WC, Lin YL, Kuo WC, Cheng H. Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J Neurosci. 2011;31(11):4137–4147. doi: 10.1523/JNEUROSCI.2592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KW, Park H, Song KH, Choi JC, Ahn H, Park MJ, Suh KY, Doh J. Nanotopography-guided migration of T cells. J Immunol. 2012;189(5):2266–2273. doi: 10.4049/jimmunol.1102273. [DOI] [PubMed] [Google Scholar]
- Lee S, Choi J, Shin S, Im YM, Song J, Kang SS, Nam TH, Webster TJ, Kim SH, Khang D. Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta Biomater. 2011;7(5):2337–2344. doi: 10.1016/j.actbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Lee SI, Jeong SR, Kang YM, Han DH, Jin BK, Namgung U, Kim BG. Endogenous expression of interleukin-4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010;88(11):2409–2419. doi: 10.1002/jnr.22411. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Hong JS, Baek WK, Park JW. PPARgamma agonist pioglitazone reduces matrix metalloproteinase-9 activity and neuronal damage after focal cerebral ischemia. Biochem Biophys Res Commun. 2009;380(1):17–21. doi: 10.1016/j.bbrc.2008.12.181. [DOI] [PubMed] [Google Scholar]
- Li X, Liu X, Zhao W, Wen X, Zhang N. Manipulating neural-stem-cell mobilization and migration in vitro. Acta Biomater. 2012;8(6):2087–2095. doi: 10.1016/j.actbio.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi R. Polyethylene glycol inhibits apoptotic cell death following traumatic spinal cord injury. Brain Res. 2007;1155:10–16. doi: 10.1016/j.brainres.2007.03.091. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O'Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112(2):E156–165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110(43):17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JJ, Bellver-Landete V, Oset-Gasque MJ, Cubelos B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: focus in CNS repair. J Cell Physiol. 2015;230(1):27–42. doi: 10.1002/jcp.24695. [DOI] [PubMed] [Google Scholar]
- Minami SS, Sun B, Popat K, Kauppinen T, Pleiss M, Zhou Y, Ward ME, Floreancig P, Mucke L, Desai T, Gan L. Selective targeting of microglia by quantum dots. J Neuroinflammation. 2012;9:22. doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin A, Neibert KD, Maysinger D, Winnik FM. Quantum dot agglomerates in biological media and their characterization by asymmetrical flow field-flow fractionation. Eur J Pharm Biopharm. 2015;89:290–299. doi: 10.1016/j.ejpb.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Mohamed Arif I, Dutta K, Kundu K, Basu A. Neural stem/progenitor cells induce conversion of encephalitogenic T cells into CD4+-CD25+- FOXP3+ regulatory T cells. Viral Immunol. 2014;27(2):48–59. doi: 10.1089/vim.2013.0090. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J Immunol. 2014;193(10):4988–4999. doi: 10.4049/jimmunol.1401776. [DOI] [PubMed] [Google Scholar]
- Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15(7):1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Singh A, Nag TC, Chattopadhyay P, Mathur R, Jain S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int J Nanomedicine. 2013;8:2259–2272. doi: 10.2147/IJN.S44238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell'Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26. doi: 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Papa S, Rossi F, Ferrari R, Mariani A, De Paola M, Caron I, Fiordaliso F, Bisighini C, Sammali E, Colombo C, Gobbi M, Canovi M, Lucchetti J, Peviani M, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano. 2013;7(11):9881–9895. doi: 10.1021/nn4036014. [DOI] [PubMed] [Google Scholar]
- Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Yaniv I, Shirwan H, Askenasy N. Fas ligand enhances hematopoietic cell engraftment through abrogation of alloimmune responses and nonimmunogenic interactions. Stem Cells. 2007;25(6):1448–1455. doi: 10.1634/stemcells.2007-0013. [DOI] [PubMed] [Google Scholar]
- Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, Wojciechowski S, Kanninen K, Koistinaho J, Malm T. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun. 2015;44:68–81. doi: 10.1016/j.bbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. Journal of neuropathology and experimental neurology. 2002;61(7):623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158(2):351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142(2):258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, Schwab JM. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain pathology. 2011;21(6):652–660. doi: 10.1111/j.1750-3639.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24(28):3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12(5):1900–1911. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert S, Johnson PJ, Hodgetts SI, Plant GW, Harvey AR. Scaffolds to promote spinal cord regeneration. Handb Clin Neurol. 2012;109:575–594. doi: 10.1016/B978-0-444-52137-8.00036-X. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O'Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. Journal of neuroimmunology. 2004;156(1-2):42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of clinical investigation. 2011;121(3):985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. The Journal of comparative neurology. 2003;462(2):223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(3):753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27(1):1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surey S, Berry M, Logan A, Bicknell R, Ahmed Z. Differential cavitation, angiogenesis and wound-healing responses in injured mouse and rat spinal cords. Neuroscience. 2014;275:62–80. doi: 10.1016/j.neuroscience.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42(7):1508–1516. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113(3):226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Kubilius MB, Holland SJ, Seidlits SK, Boehler RM, Anderson AJ, Cummings BJ, Shea LD. Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials. 2013;34(9):2213–2220. doi: 10.1016/j.biomaterials.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Shea LD. Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. Journal of controlled release : official journal of the Controlled Release Society. 2013;170(3):421–429. doi: 10.1016/j.jconrel.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration. Tissue engineering Part A. 2014;20(5-6):1027–1037. doi: 10.1089/ten.tea.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992;89(9):4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthers CM, Seidlits SK. Gene delivery strategies to promote spinal cord repair. Biomark Insights. 2015;10(Suppl 1):11–29. doi: 10.4137/BMI.S20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Yang SH, Tzeng SF. MicroRNA-145 as one negative regulator of astrogliosis. Glia. 2015;63(2):194–205. doi: 10.1002/glia.22743. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009;216(1):177–183. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early Transplantation of Mesenchymal Stem Cells after Spinal Cord Injury Relieves Pain Hypersensitivity Through Suppression of Pain-Related Signaling Cascades and Reduced Inflammatory Cell Recruitment. Stem Cells. 2015 doi: 10.1002/stem.2006. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Demyelination in spinal cord injury. Journal of the neurological sciences. 1989;91(1-2):1–14. doi: 10.1016/0022-510x(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Weidner N, Tuszynski MH. Spontaneous plasticity in the injured spinal cord-implications for repair strategies. Mol Psychiatry. 2002;7(1):9–11. doi: 10.1038/sj.mp.4000983. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilems TS, Sakiyama-Elbert SE. Sustained dual drug delivery of anti-inhibitory molecules for treatment of spinal cord injury. J Control Release. 2015;213:103–111. doi: 10.1016/j.jconrel.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DY, Leveque JC, Brumblay H, Krebsbach PH, Hollister SJ, Lamarca F. Macro-architectures in spinal cord scaffold implants influence regeneration. J Neurotrauma. 2008;25(8):1027–1037. doi: 10.1089/neu.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res. 2014;324(1):65–74. doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Yang Y, De Laporte L, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, Shea LD. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue engineering Part A. 2009;15(11):3283–3295. doi: 10.1089/ten.tea.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011;1385:8–17. doi: 10.1016/j.brainres.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nature reviews Neuroscience. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P. Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS One. 2008;3(4):e1924. doi: 10.1371/journal.pone.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009a;220(1):183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. Journal of neurochemistry. 2009b;110(5):1617–1627. doi: 10.1111/j.1471-4159.2009.06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidema J, Gilbert R. Cells, Tissue, Organs. 2016.
- Zuidema JM, Hyzinski-Garcia MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-L-lactic acid fibers. Biomaterials. 2014;35(5):1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]