Abstract
Despite its prevalence in modern society, little is known about the long-term impact of restricting sleep during the week and ‘catching up’ on weekends. This common sleep pattern was experimentally modeled with three weeks of 5 nights of sleep restricted to 4 hours followed by two nights of 8-hour recovery sleep. In an intra-individual design, 14 healthy adults completed both the sleep deprivation and an 8-hour control condition, and the subjective impact and the effects on physiological markers of stress (cortisol, the inflammatory marker IL-6, glucocorticoid receptor sensitivity) were assessed. Sleep restriction was not perceived to be subjectively stressful and some degree of resilience or resistance to the effects of sleep restriction was observed in subjective domains. In contrast, physiological stress response systems remain activated with repeated exposures to sleep loss and limited recovery opportunity. Morning IL-6 expression in monocytes was significantly increased during week 2 and 3 of sleep restriction, and remained increased after recovery sleep in week 2 (p<0.05) and week 3 (p<0.09). Serum cortisol showed a significantly dysregulated 24h-rhythm during weeks 1, 2, and 3 of sleep restriction, with elevated morning cortisol, and decreased cortisol in the second half of the night. Glucocorticoid sensitivity of monocytes was increased, rather than decreased, during the sleep restriction and sleep recovery portion of each week. These results suggest a disrupted interplay between the hypothalamic-pituitary-adrenal and inflammatory systems in the context of repeated exposure to sleep restriction and recovery. The observed dissociation between subjective and physiological responses may help explain why many individuals continue with the behavior pattern of restricting and recovering sleep over long time periods, despite a cumulative deleterious physiological effect.
Keywords: sleep restriction, stress, inflammation, cortisol, subjective
1. INTRODUCTION
Patterns of restricting sleep during the week and ‘catching up’ over the weekend are prevalent in modern society (Hansen et al., 2005; Monk et al., 2000; National Sleep Foundation, 2010; Tsui and Wing, 2009; Wing et al., 2009). These sleep patterns are not commonly though of as deleterious; however, there is limited empirical evidence to support this belief. Given the wealth of accumulated evidence that insufficient sleep is associated with elevated health risks (e.g., cardiovascular disorders (Grandner et al., 2013), metabolic disorders (Knutson et al., 2007), and chronic pain conditions (Finan et al., 2013)), gaining a better understanding of the impact of these common sleep patterns is essential.
Sleep loss can be conceptualized a physiological stressor, with both subjective (psychological) and physiological effects (described further below). The multiple systems involved in the physiologic stress response are homeostatic and tightly inter-related (Almawi et al., 1996; de Kloet, 2000) and include the sympatho-adrenal, the hypothalamic-pituitary-adrenal (HPA), as well as the inflammatory system. Inflammatory cytokines serve as chemical messengers and are negatively controlled by cortisol, a glucocorticoid (GC) that is the main output hormone of the HPA axis (reviewed in (Chrousos, 2009)). Impaired GC sensitivity has been reported in response to various acute and chronic stressors (Herman et al., 1995; Miller et al., 2002; Stark et al., 2001), and GC sensitivity is one possible mechanism by which observed increases in inflammation can be explained.
The HPA system is perhaps the most studied stress response system, and is known to typically habituate when faced with repeated or ongoing stressors (Grissom and Bhatnagar, 2009). However, sleep loss is a unique stressor because it is a biological resource necessary for regulation of multiple physiological systems, including the stress response system (Hamilton et al., 2007; McEwen, 2006). Further, in extreme cases, sleep is necessary for survival itself (Everson et al., 1989; Montagna et al., 1995). No prior research has examined whether humans can adapt to chronic patterns of insufficient sleep and limited recovery, or studied the impact of this common real-world pattern on stress-response systems. As described below, the impact of single episodes of sleep loss and (to a lesser extent) recovery sleep has been tested, however it remains unknown whether these results remain true when patterns of restricted sleep and recovery become chronic.
Within single episodes of experimental sleep loss, subjective ratings of sleepiness, positive mood, and self-reported physical functioning appear to show response stabilization, or acclimation. For example, subjective experiences of pain (Haack and Mullington, 2005) and sleepiness (Van Dongen et al., 2003) stabilize after a few days of sleep restriction or sleep deprivation (or deteriorate more slowly), despite ongoing sleep loss. On a physiological level, multiple markers of the stress system have been found to increase following a single episode of sleep loss, including cortisol (Balbo et al., 2009; Guyon et al., 2014) and the inflammatory marker interleukin [IL]-6 (Haack et al., 2007; Irwin et al., 2006; Pejovic et al., 2013; van Leeuwen et al., 2009; Vgontzas et al., 2004). Although habituation to acute stressors is a key feature of the HPA system (Grissom et al., 2009), it is unknown whether this classic pattern of habituation can be applied to the physiological stress of repeated exposures to sleep loss with limited recovery sleep, given that sleep loss is a unique physiological stressor.
Little is known about the impact of repeated episodes of sleep loss or the role of recovery sleep. To our knowledge, the current study protocol tests the longest model of chronic sleep restriction to date. Everson and colleagues have conducted studies of repeated exposure to sleep loss and recovery in an animal model, and have documented changes in metabolic indices (weight, food intake), and pathological organ and bone changes (Everson, 2009; Everson et al., 2009). Recovery from sleep loss has been rarely studied, but using a five night sleep restriction/two night recovery protocol, van Leeuwen and colleagues showed that IL-6 mRNA levels remained elevated after two nights of recovery sleep (van Leeuwen et al., 2009). These data provide preliminary support that ‘catching-up’ on sleep over the weekend might be insufficient to restore stress-response systems, and contribute to ongoing responses to repeated exposure to sleep loss over time. These limited data highlight a critical gap in our understanding of consequences of insufficient sleep, as it is the real-world experiences of repeated episodes of sleep loss and limited recovery sleep that are most likely to have a long term impact on health.
This study modeled real-world sleep-wake patterns of sleep restriction and recovery in the laboratory environment to investigate effects on multiple stress system markers, using an intensified model of sleep restricted to four hours of sleep on weekdays and extended to eight hours on weekends. This amplification of the magnitude of difference between weekdays/weekends was chosen in part due to the aim of assessing the impact of these patterns under highly controlled experimental conditions that can be maintained for a period of weeks, rather than the months or years that adults often will maintain these milder patterns of sleep restriction and recovery in the real world.
Based on previous research, we hypothesized that there would be a response stabilization or habituation across repeated episodes of sleep loss in subjective domains, but poor habituation and an incomplete recovery in physiological domains. If true, these findings could help explain why patterns of inadequate sleep persist, namely, because there would be no perceived negative impact of these behavior patterns. Additionally, this study was specifically designed to extend previous research demonstrating that sleep loss results in increases in serum or plasma IL-6 (Haack et al., 2007; Irwin et al., 2006; Pejovic et al., 2013; van Leeuwen et al., 2009; Vgontzas et al., 2004) by focusing on monocytes, and whether the expected increased expression of inflammatory mediators can be explained by changes in the sensitivity of monocytes to cortisol.
2. METHODS
2.1 Experimental Model
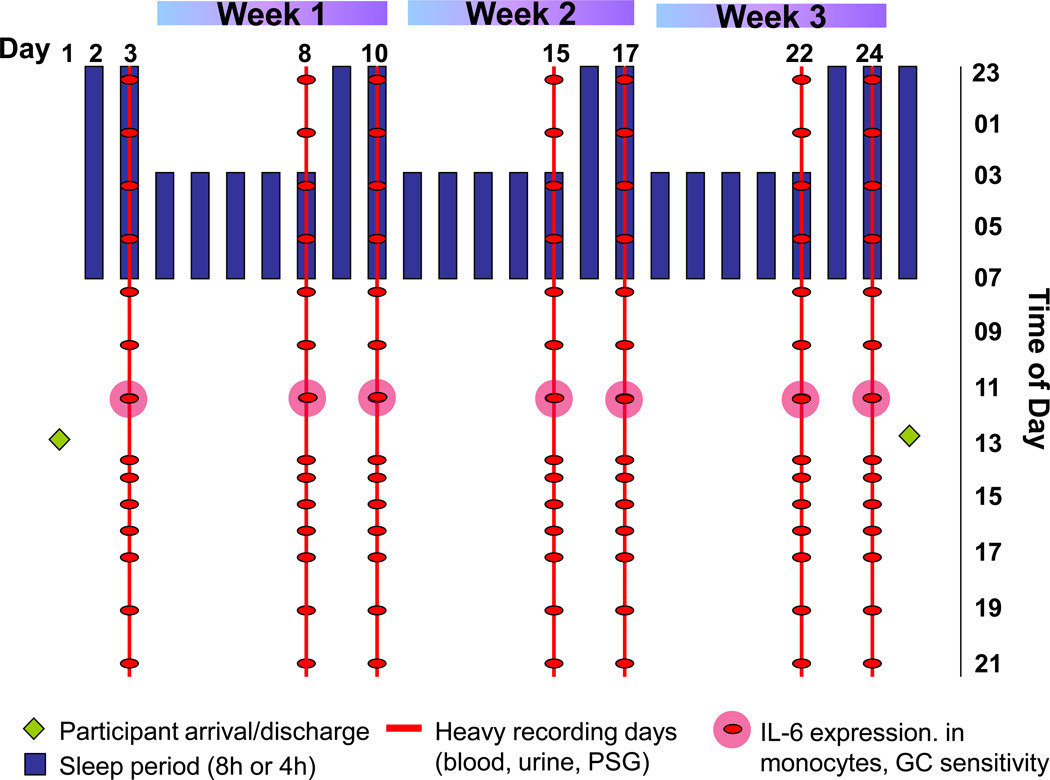
The hypothesis was tested using a sleep restriction condition consisting of three weeks of a repeating pattern of five nights of sleep restricted to 4h/night (0300–0700h) followed by two nights of recovery sleep with 8h/night (2300–0700h). This model was designed to mirror commonly observed patterns of moderately restricting on weeknights and recovering sleep on weekend nights that often occur in the general population for periods of months or years (National Sleep Foundation, 2010), albeit with an amplified sleep restriction pattern on weeknights (see Figure 1). This amplified sleep restriction period was designed to maximize the potential that the effects of what are often much longer periods of milder sleep restriction and recovery that occur in the real world could be captured in a relatively short three-week in-laboratory experimental protocol. The sleep control condition consisted of three weeks with a nightly sleep opportunity of 8 hours. In an intra-individual randomized balanced design, participants underwent two 25-day in-hospital stays (restricted sleep condition and sleep control condition) separated by more than two months. Each 25-day stay started with an adaptation and a baseline day, followed by three weeks of either the repeated exposure to sleep restriction/recovery or control sleep, and ended with an additional night of full sleep (totaling 25 days).
Figure 1.
Study protocol: Repeated exposure to sleep restriction-recovery patterns. In the control condition, participants had a sleep opportunity of 8 hour every day.
2.2 Participants
This study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center (BIDMC). Participants were recruited via community advertisements. Seventeen healthy young women and men were studied. Fourteen participants completed both 25-day-in-hospital conditions; three participants could only complete one of the two 25-day-in-hospital conditions due to change in work/family-related requirements (see Figure 2).
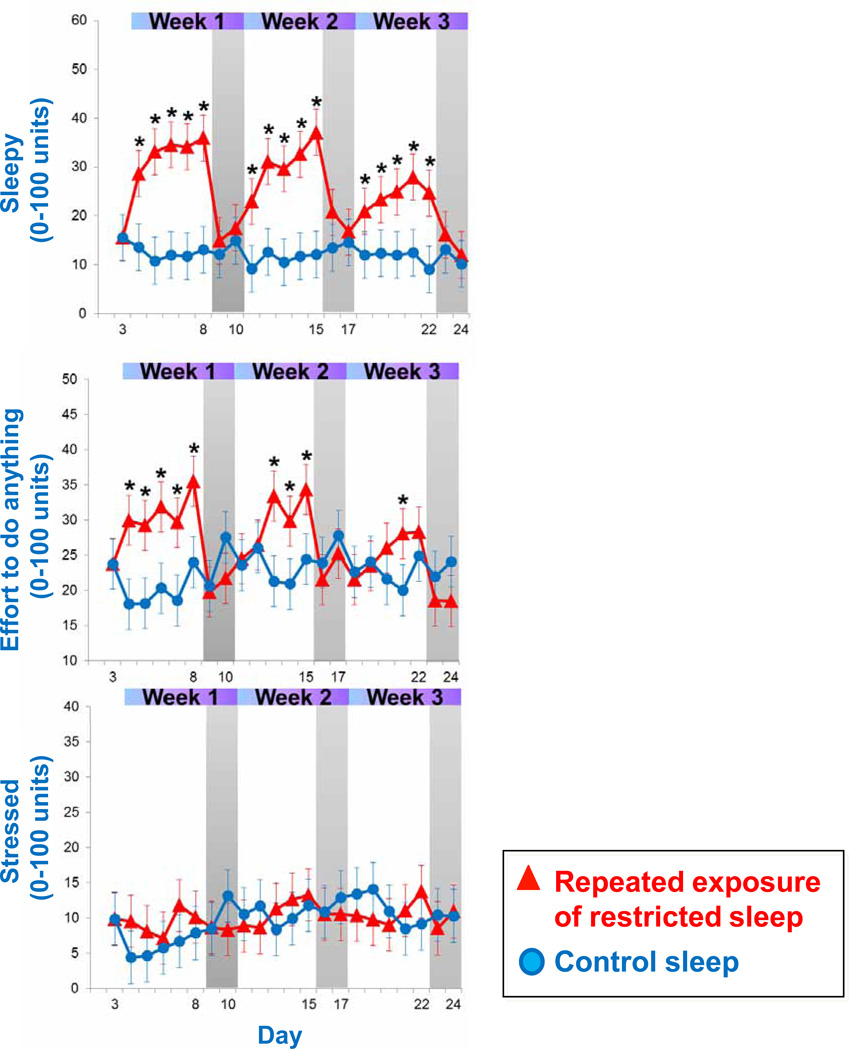
Figure 2.
Ratings of ‘Sleepy’, ‘Effort to do anything’, and ‘Stressed’ across the repeated exposure to sleep restriction-recovery patterns. Data present estimated mean±SEM based on mixed model analysis. *p<.05 between conditions
Participants were between the ages of 18–35 years, had a body mass index (BMI) between 18.5 and 30 kg/m2, a daily sleep duration between 7–9 hours (verified by sleep diary data over a 2-week period), began their habitual sleep period within one hour of 11pm (to ensure normal entrainment) and had blood chemistry levels within the normal range. Female participants were eligible if they had regular menstrual cycles and no significant discomfort during pre-menses/menses. Exclusion criteria included presence or past history of major medical problems, psychiatric disorders or sleep disorders. Additional exclusion criterion included pregnant/nursing status, regular medication use other than oral contraceptives, and donation of blood or platelets three month prior to or in-between study stays.
2.3 Study Protocol
2.3.1 Screening & randomization
Participants were initially screened over two visits to the hospital and were evaluated by a study physician for the exclusion criteria described above. Two weeks before entering each 25-day in-hospital stay, participants were asked to follow the study sleep schedule (11pm–7am), which was verified by sleep log data. The week prior to the second 25-day visit, blood tests used at the initial screening were repeated to ensure values are in the normal range. Participants were randomized to the order of experimental conditions (sleep restriction or control) on the first day of the first 25-day hospital stay. An independent statistician prepared envelopes with randomization codes, one of which was opened by a senior staff member prior the first hospital stay.
2.3.2 Research environment
During the two 25-day in-hospital stays (Figure 1), participants stayed in a private or semi-private room in the Clinical Research Center (CRC) at BIDMC. Intensive physiological recordings were conducted on seven out of the 25 days: on the baseline night, every fifth day of restricted/control sleep and every second night of recovery/control sleep in each of the three weeks. These intensive measurement periods included PSG recordings and frequent blood sampling though an intravenous catheter across 24 hours. Subjective well-being assessments were also collected on computerized visual analog scales every four hours throughout waking periods.
During the sleep restriction nights, the sleep opportunity was from 0300 to 0700h; however, participants had to remain in bed in a semi-supine position during the wakeful nighttime periods (2300–0300h) in order to limit differences in postural and physical activity inputs across all study nights and conditions. Light levels were less than 40 lux during wakeful nighttime periods (2300–0300h). During daytime periods (0700–2300h), participants had access to both artificial and natural light sources. Throughout both 25-day stays, participants were maintained on a balanced diet (NA+ and K+ controlled) and regimented fluid intake in order to maintain body weight/composition throughout the study. Meals and fluids were served at standardized hours. To prevent sedentary conditions and maintain constant activity levels, participants had scheduled walks (5–10 minutes each) within the CRC every other hour throughout the daytime periods of the protocol (between 0700 and 2300h). On non-intensive recording days, participants had an additional longer walk of approximately 30 minutes that could take place outdoors. Participants were also encouraged to follow their pre-study exercise habits through an opportunity on the non-intensive recording days to visit the hospital gym facilities. Room temperature was adjusted to each participant’s comfort level during the first two adaptation days, and the same daytime temperature was kept throughout the remaining days of the protocol. Nighttime temperature (2300–0700h) was set 2°C lower than daytime temperature. Participants were allowed to have visitors during daytime periods, as well as have access to email and phone, in order to minimize disruptions to their social networks and prevent social isolation. During all waking periods a research assistant accompanied participants in order to ensure adherence to the study protocol and procedures, as well as to engage participants in social activities such as board/video games or talking, as needed.
2.3.3 Measurements
2.3.3.1 Polysomnographic recording (PSG)
Sleep was recorded using the Embla system N7000 (Medcare US, Buffalo) on seven intensive recording days of each 25-day study run (at baseline, every fifth and seventh day of each of the three weeks). The PSG montage followed standard criteria and sleep electroencephalography was manually stage-scored on a 30 second epoch basis (American Academy of Sleep Medicine, 2007). All recordings were scored by the same sleep technician.
2.3.3.2 Blood Sampling
On the seven intensive recording days of each 25-day study run, blood was drawn at 2-hourly intervals using an indwelling 20-gauge forearm catheter. During sleep opportunities, a long line was attached to the catheter and blood collection was performed from an adjacent anti-chamber without disrupting the participant’s sleep. The total amount of blood taken over each 25-day protocol did not exceed 550ml.
2.4. Stress response system measures
2.4.1 Cortisol
was measured in serum collected every two hours on the seven intensive recording days using an electrochemiluminescence immunoassay (ECLIA, Labcorp.com). According to the company, intra-run and inter-run precision are 1.2% and 1.6%, respectively.
2.4.2 Stimulated IL-6
was measured in vitro as the capacity of monocytes to express IL-6, using the 1130h blood sample on each of the seven intensive recording days. Whole blood was stimulated with lipopolysaccharide (LPS) from Escherichia coli 0127-B8 (LPS 100pg/ml, Sigma-Aldrich), and then brefeldin A (10 ug/ml, Sigma-Aldrich) was added to the sample, which was incubated for 4 hours at 37 °C in a 5% CO2 atmosphere. Following fixation and permeabilization procedures (Intraprep™ Permeabilization reagents [Beckman Coulter]), fluorescence-conjugated antibodies were added (CD14 APC, CD45 KrO [both Beckman Coulter], IL-6 PE [BD Bioscience]) and samples incubated for 15 min at room temperature in the dark. Samples were vortexed, washed with phosphate-buffered saline solution (PBS 1X, Sigma Aldrich), and stored at 2–8 °C in the dark after re-suspension in 500 ul of PBS containing 0.5% formaldehyde. Preparations were analyzed within 24 hours using a Gallios™ flow cytometer (Beckmann-Coulter) at the Flow Cytometry Core at BIDMC, and 100,000 events were acquired. Percentage of IL6-positive monocytes (LPS-stimulated and spontaneous) were quantified using Kaluza® Flow Analysis software (Beckmann Coulter).
2.4.3 Unstimulated IL-6
The same procedures were applied to a whole blood sample that was not stimulated with LPS.
2.4.4 Glucocorticoid (GC) sensitivity of monocytes
was determined by the capacity of the synthetic glucocorticoid dexamethasone (DEX) to suppress IL-6 expression in monocytes, using the 1130h blood sample on each of the seven intensive recordings days. Whole blood was stimulated with LPS (see above), and then different concentrations of DEX (25, 50, 100, 200, and 400 nM; Sigma-Aldrich) as well as brefeldin A were added to the samples, which then underwent the same procedures as described above. For statistical purposes, IL-6 suppression curves were calculated as change from monocytic IL-6 expression without DEX. In addition, area under the curve (AUC) was computed for each IL-6 suppression curve according to methods described by Pruessner and colleagues (Pruessner et al., 2003). For this analysis, samples with different DEX concentrations were first calculated as change from baseline (i.e., sample without DEX), and then computed as AUC.
2.4.5 Subjective measures
were assessed every four hours during the waking periods of the protocol. Participants were asked to rate the intensity of various well-being items using computerized visual analogue scales (AsWin, programmed by Martin Rivers & Associates). The VAS set used in the current study contained items from the Deactivation Activation Check List (Thayer 1978) and scales have been used in our previous studies (Haack et al., 2009; Haack et al., 2005). The test battery required approximately five minutes per administration. Ratings of ‘Sleepy’, ‘Effort to do anything’, and ‘Stressed’ were aggregated across the daytime periods of each study day for statistical analysis.
2.5 Statistics
Linear mixed models were used to analyze the data, with condition (repeated sleep restriction vs. control sleep) and study day (baseline, fifth and seventh day of each of the three weeks) as fixed factors, and participants and participants × day as random factors. For variables that were also repeated within a study day (e.g., cortisol measured every two hours, IL-6 suppression measured at various concentrations of DEX at each recording day), time of day/concentration were also entered as additional fixed factors. The baseline day was used as a covariate in order to account for differences at study start. Accordingly, data in graphs are depicted as estimated means from mixed model analysis. Since baseline day was used as covariate, interpretation of the interaction as well as main condition effects is considered appropriate and are presented if significant. Physiological stress outcome measures were: (1) serum cortisol assessed every two hours during intensive recording days, (2) IL-6 positive monocytes (LPS-stimulated and unstimulated), assessed once per intensive recording day at 11:30h, and (3) GC sensitivity of monocytes, measured as IL-6 suppression curves at various doses of DEX once per intensive recording day at 11:30h, and calculated as AUC (Pruessner et al., 2003). Subjective outcome measures were ratings of ‘Sleepy’, ‘Effort to do anything’, and ‘Stressed’, which were aggregated to a single daytime mean (0700–2300) across each of the seven intensive recording days.
3. RESULTS
Of the 17 participants randomized, 14 completed both sleep restriction and sleep-control laboratory stays. Table 1 presents baseline characteristics of the participants who were randomized and are included in analyses. On average, there were 144±23 days (4.8 ±0.8 months) between laboratory stays. Data and statistical analyses are described below; Supplemental Table 1 presents summary data from subjective and physiological stress markers across repeated patterns of sleep restriction and recovery.
Table 1.
Participant characteristics
| Control Sleep | Restricted Sleep | ||
|---|---|---|---|
| N* | 16 | 15 | |
| Sex | Female/Male | 8/8 | 7/8 |
| Age (yrs) | Mean±SEM | 24.9±1.1 | 24.9±1.2 |
| Screening BMI (kg/m2) | Mean±SEM | 24.8±0.8 | 24.6±0.7 |
| Habitual sleep duration (hours)** | Mean±SEM | 8.1±0.2 | 8.4±0.1 |
14 of the participants completed both 25-day stays (control sleep and restricted sleep; 3 participants completed only 1 stay.
Based on 10–14 day recording period through diary
3.1 Subjective well-being responses
Figure 2 presents the subjective well-being responses to the repeated exposure of sleep restriction-recovery patterns. Mixed model revealed a significant condition effect (p<.05) for ratings of ‘Sleepy’ and ‘Effort to do anything’, but not for ‘Stressed’. Values for ‘Sleepy’ and ‘Effort to do anything’ significantly increased during the sleep restriction days of each week, and almost completely returned to baseline values during intermittent recovery sleep nights (no significant difference compared to baseline). When comparing ratings of ‘Sleepy’ across consecutive weeks in the sleep restriction group, mixed model analyses indicated a significant week effect. Ratings were progressively lower from week to week, indicating that the subjective experience of feeling sleepy habituated across the repeated exposure of sleep restriction-recovery patterns. Similarly, ratings of “Effort to do anything’ trended towards a significant week effect (p<0.07), indicating that the subjective experience of ‘Effort to do anything’ somewhat habituates to the repeated exposure of sleep restriction-recovery patterns. Ratings of ‘Stressed’ did not show any significant increases between sleep restriction periods.
3.2 Physiological stress responses
3.2.1 Diurnal cortisol rhythm
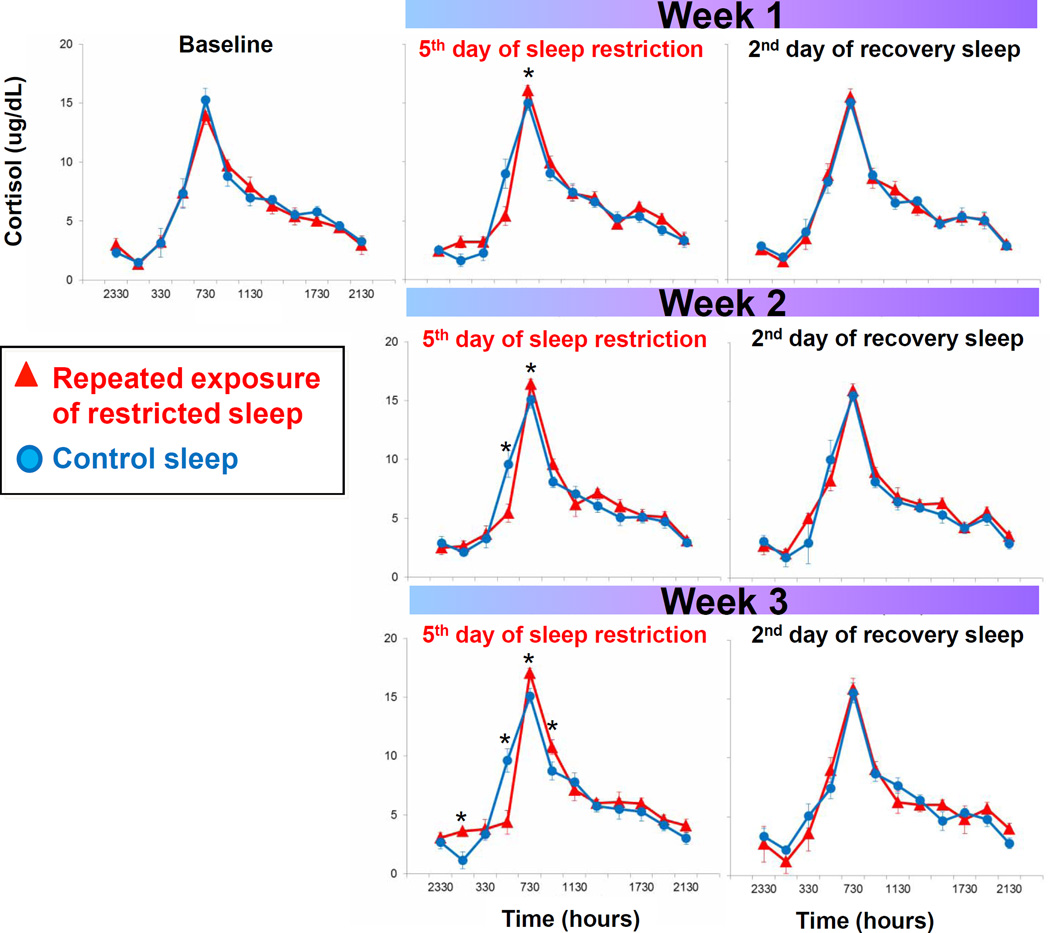
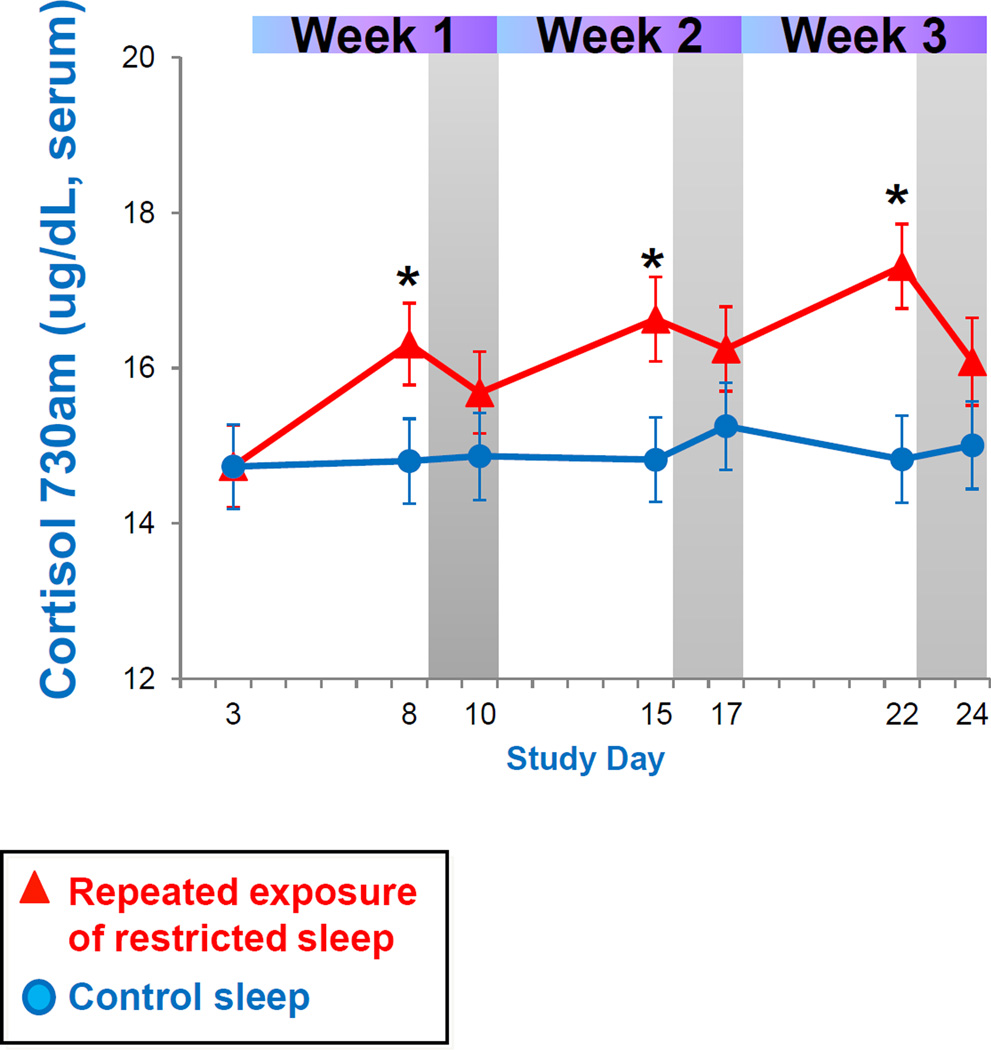
Figure 3 presents daily serum cortisol levels across the repeated exposure to sleep restriction and recovery patterns. Mixed model analysis indicated a significant interaction effect between condition by day by time of day. As seen in Figure 3, cortisol levels are increasingly dysregulated across repeated exposure to sleep restriction, as indicated by the increasing number of significant time point differences in Figure 3. Most consistently, fasted cortisol levels shortly after awakening (0730) are increasingly higher during the repeated sleep restriction exposure compared to control sleep participants, as indicated by a significant week effect in mixed model analysis (Figure 4). Though not significantly different, morning cortisol levels did not completely return to baseline levels after two nights with an 8 hour sleep opportunity (see Figure 4).
Figure 3.
Diurnal cortisol rhythms across the repeated exposure sleep restriction-recovery patterns. Data present mean±SEM. *p<.05 between conditions.
Figure 4.
Cortisol levels after awakening across repeated sleep restriction-recovery patterns. Data present estimated mean±SEM based on mixed model analysis. *p<.05 between conditions.
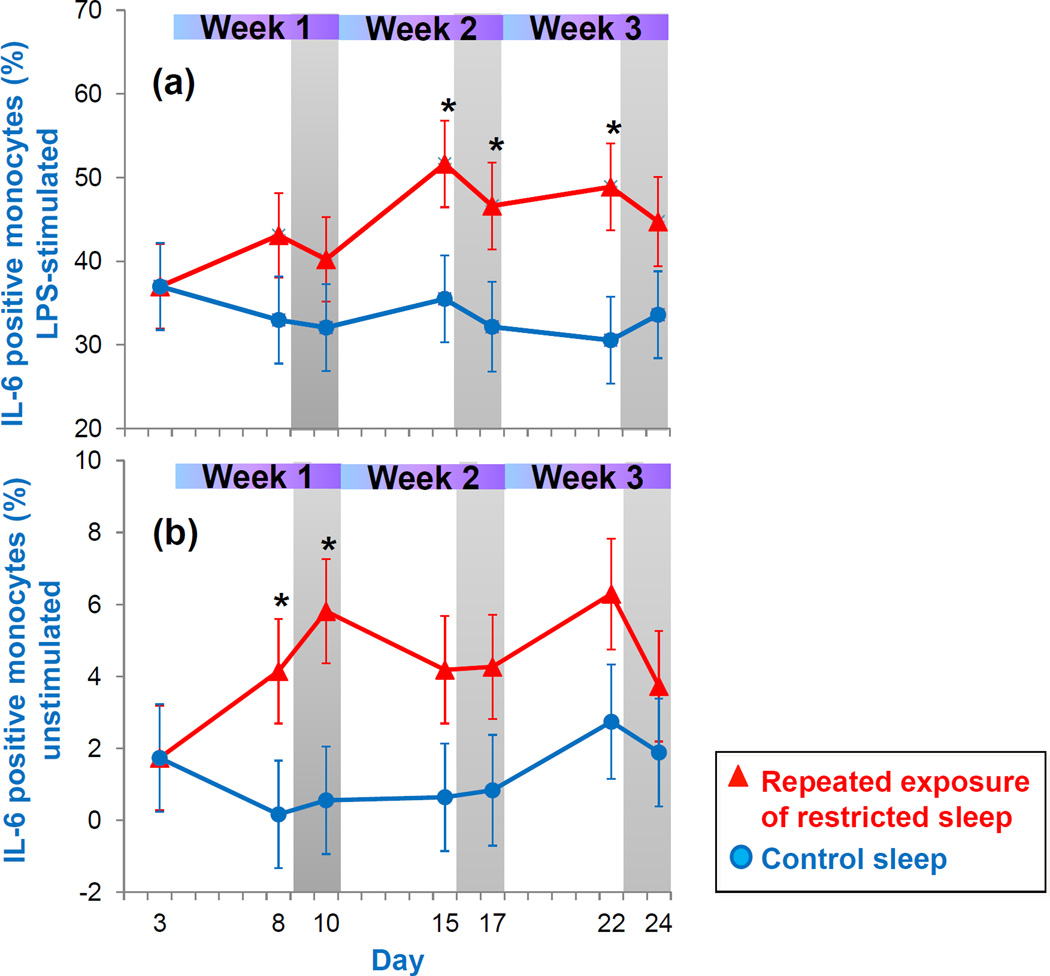
3.2.2 IL-6 positive monocytes
Figure 5a presents the percentage of LPS-stimulated IL-6 positive monocytes throughout the repeated exposure to sleep restriction and recovery. Mixed model analysis indicated a significant condition effect. Compared to the control sleep condition, values were significantly higher during the second and third week of sleep restriction. Values remained higher after two nights of recovery sleep following the second week (p<0.05) and third week (p=0.09) of restricted sleep. Figure 5b presents percentage of non-stimulated IL-6 positive monocytes. Mixed model analysis indicated a significant condition effect. When compared to the control sleep condition, non-stimulated IL-6 levels were significantly higher during the first sleep restriction week (p<0.05) and trended to be higher during the second and third sleep restriction week (both p=0.06). Levels stayed significantly higher after two nights of recovery sleep following the first sleep restriction exposure (p<0.05) and trended to be higher after the second sleep restriction exposure (p= 0.07).
Figure 5.
IL-6 positive monocytes assessed in (a) LPS-stimulated and (b) unstimulated whole blood across repeated exposure to sleep restriction-recovery patterns. Data present estimated mean±SEM based on mixed model analysis. *p<.05 between conditions.
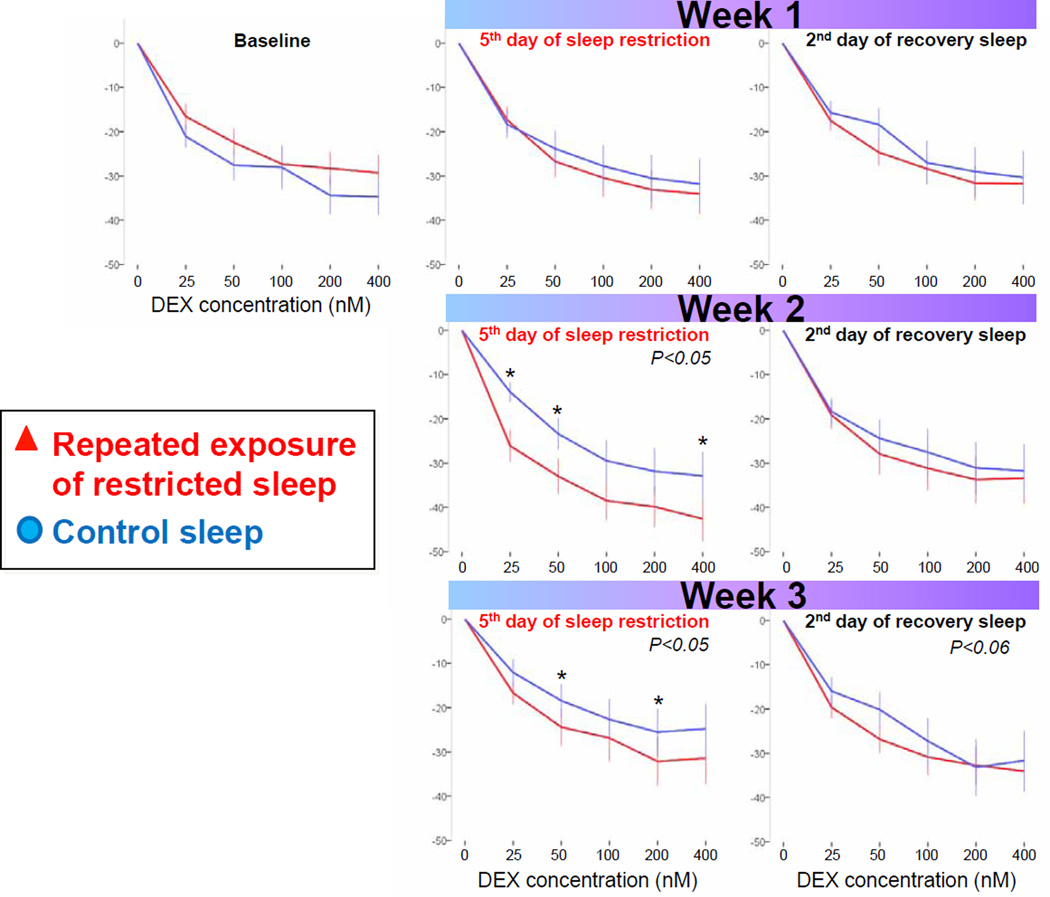
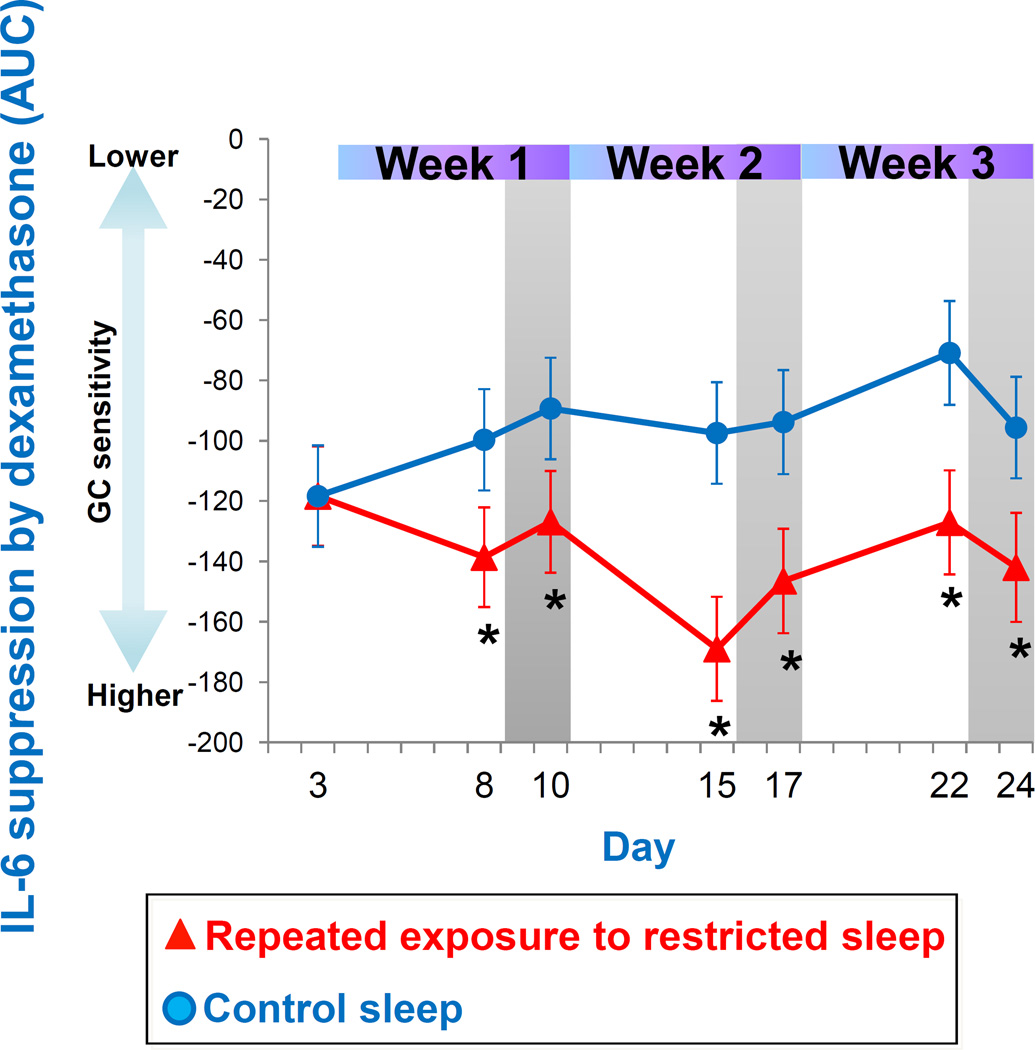
3.2.3 Glucocorticoid (GC) sensitivity of monocytes
Figure 6 presents the GC sensitivity determined by the ability of dexamethasone (DEX) to suppress IL-6 expression in monocytes across repeated exposure to sleep restriction-recovery patterns. Mixed model analysis indicated a significant interaction effect between condition and day. While GC sensitivity was not significantly affected during the first sleep restriction exposure, it was significantly higher during the second and third sleep restriction exposures when compared to control sleep. GC sensitivity trended to remain higher after two nights of recovery sleep following the third exposure to restricted sleep (p<0.06). Figure 7 depicts the area under the curve (AUC) for the IL-6 suppression curves. Mixed model analyses revealed a significant condition effect, due to higher GC sensitivity throughout the three weeks of sleep restriction-recovery exposure, when compared to control sleep.
Figure 6.
GC sensitivity determined by the ability of dexamethasone (DEX) to suppress IL-6 expression in monocytes across repeated exposure to sleep restriction-recovery patterns. Higher IL-6 suppression by DEX indicates higher GC sensitivity. For visual comparison of suppression curves within and between days, data are depicted as change from baseline (i.e., sample without DEX concentration). Presented are mean±SEM. *p<.05 between conditions.
Figure 7.
GC sensitivity calculated as AUC of IL-6 suppression. Higher IL-6 suppression by DEX indicates higher GC sensitivity. Data present estimated mean±SEM based on mixed model analysis.*p<0.05 between conditions.
4. DISCUSSION
This study, to the best of our knowledge, provides the first evidence for the impact of real-world sleep patterns of sleep restriction and recovery on stress responses systems. Consistent with our hypotheses, repeated episodes of restricted sleep and recovery were not experienced as subjectively stressful. While they were perceived more generally as ‘burdensome’ (as reflected by increasing subjective symptoms of sleepiness and effort), participants’ subjective responses acclimated to repeated exposure to sleep restriction and tended to fully recover after two nights of full sleep. In contrast, the physiological stress response systems assessed (cortisol and inflammatory) showed increased activity and did not habituate or fully recover when repeatedly exposed to this sleep restriction-recovery pattern. Further, the observed increased GC sensitivity of monocytes suggests that there was a disrupted interplay between the HPA and inflammatory system. The fact that these escalating physiological responses were dissociated from subjective impact suggests one reason that these behavior patterns persist despite accumulating physiological costs. Plainly stated, if the person does not ‘feel’ an accumulated negative impact of these sleep patterns, there is no internal motivation to change the behavior.
The current study furthers previous research that experimentally modeled ‘catching up’ after a single week of sleep restriction is insufficient to restore the homeostasis of the inflammatory response system (van Leeuwen et al., 2009) by demonstrating that when repeatedly exposed to such sleep restriction-recovery periods, LPS-stimulated IL-6 positive monocytes increase and do not appear to habituate to the repeated exposure to sleep restriction, and two nights of recovery sleep do not normalize levels. IL-6 expression in unstimulated monocytes were also significantly elevated during the first week of sleep restriction followed by sleep recovery (paralleling (Irwin et al., 2015b)), and tended to remain elevated while these sleep patterns continue.
These findings indicate that even in the absence of an exogenous activation of innate immune components (e.g., LPS), monocytes spontaneously produce more IL-6 in response to sleep loss, do not habituate with the repeated exposure to sleep loss, and do not fully recover even after a limited opportunity for recovery sleep. These findings contrast, at least to some extent, to the recent meta-analytic finding that sleep disruption, rather than habitually short sleep durations and experimentally modeled sleep loss are not associated with increased IL-6 (Irwin et al., 2015a). However, this meta-analysis also demonstrates the variability in IL-6 findings, which is likely introduced, at least in part, by the level of experimental control in each study, including as the impact of food composition, timing of meal intake relative to blood draws, and whether the participants were resting quietly in a seated period prior to blood sample collection. Perhaps more importantly, the reported IL-6 results may be more closely tied to the range of magnitudes and durations of sleep loss examined across studies. The current study is the first that closely models the chronicity of real-world patterns of sleep loss, is designed to take a more mechanistic approach by investigating whether changes in the sensitivity of monocytes to the counter-inflammatory signal cortisol may be responsible for increased IL-6 expression, and is highly controlled, leaving little room for confounds from experimental factors.
Sleep loss appears to be a somewhat unique physical stressor, in that the HPA response to sleep loss compared to other stressors is mild (Balbo et al., 2010; Guyon et al., 2014; Meerlo et al., 2008). Additionally results from this study demonstrates that chronic sleep loss does not produce the typical pattern of habituation with repeat exposure with respect to HPA (Grissom et al., 2009) and IL-6 responses (the latter when measured on a cellular level (McInnis et al., 2015)). While it is adaptive for the HPA axis to habituate to non-harmful stressors, sleep is a necessary biological resource (Everson et al., 2009; Hamilton et al., 2007; McEwen, 2006), so habituation to chronic sleep loss may be harmful rather than adaptive. While the changes in HPA axis functioning observed in the current study are small, as they have been in previous studies of experimental sleep loss, there may be a cumulative effect after months and potentially years of insufficient sleep. Additionally, there is increasing evidence that small changes in inflammatory and stress mediators are present in a variety of diseases, including cardiovascular, metabolic, neurodegenerative diseases, as well as some forms of cancer and pain conditions, which provides further support for the importance of the small changes observed in the current study (Medzhitov, 2010).
One possible explanation for the observed increase IL-6 is that IL-6 producing monocytes became less sensitive to the counter-inflammatory signal of cortisol (i.e., glucocorticoid sensitivity decreased). Stress-induced activation of the HPA and inflammatory systems is metabolically costly, with potential deleterious effects if these systems are overactive. Therefore, while it is adaptive for the HPA axis to habituate to non-harmful stressors, in this context it may be harmful to adjust to the stress of chronic sleep loss given that sleep is a necessary biological resource (Everson et al., 2009) and, more globally, sleep is thought to be required to adequately adapt to a stressor (Hamilton et al., 2007; McEwen, 2006). The process of habituation to repeated stress is, in part, regulated by cortisol negative feedback mechanisms, as demonstrated by inhibited habituation with blockage of the GC receptor (reviewed in (Grissom et al., 2009)). HPA and inflammatory systems are tightly regulated, and the GC cortisol is crucial for the appropriate termination of every stress response via inhibition of monocytes and other immune cell populations in the production of transcription factors (such as NF-kB) and downstream inflammatory cytokines, such as IL-6. However, in parallel to this IL-6 increase, we also observed an increase in GC sensitivity; one that did not appear to be sufficient to prevent IL-6 production by monocytes. Previous research has found contrasting results where decreased GC sensitivity is observed (along with increasing inflammation) under condition of chronic stress (e.g., Cohen et al., 2012); it is challenging to expand upon the discussion of how the mechanisms differ between those studies and ours without additional research. However this phenomenon of increased IL-6 production despite increased GC sensitivity observed in the current study suggests that monocytes are responding even more aggressively in the context of chronic sleep loss. The focus of their attack is unclear, but possible targets include metabolic toxins that may accumulate during insufficient sleep, possibly due to a less active glymphatic system (Jessen et al., 2015). The failure of even a change in receptor sensitivity to block the inflammatory response to sleep loss reflects the essential nature of this response system. Although speculative, it is possible that, historically, sleep loss only occurred during periods of extreme environmental danger. If true, it is likely that over time sleep loss became linked with a strong inflammatory response, which was adaptive for survival from potential tissue injury.
The observation that physiological stress responses remain elevated in the face of ongoing sleep loss with limited recovery sleep is in stark contrast to the finding that the experience of sleep loss is not perceived as subjectively stressful, and that other well-being responses (e.g., perceived effort, sleepiness) show habituation in the face of ongoing sleep loss with limited recovery sleep. Further, while physiological stress responses do not appear to recover even after a limited amount of recovery sleep, subjective (or perceived) recovery from sleep loss is rapid. This phenomenon, observed experimentally for the first time (to our knowledge) in this study, may help explain why many individuals continue with the behavior pattern of restricting and recovering sleep over long time periods, despite a cumulative deleterious physiological effect.
While the current study is sufficiently powered to detect differences (at N=14) and uses an intra-individual design, the overall sample is still small and homogeneous. More research is needed to assess whether these findings will generalize to a more diverse population. For example, several studies have reported that women show higher inflammatory markers than men when exposed to sleep loss (Irwin et al., 2015a; Irwin et al., 2010; Prather et al., 2013). Mixed model analysis did not reveal a sex difference effect in response to the sleep restriction-recovery condition in this study (p=0.36 for main sex effect); however, this study was not powered to detect sex differences. Additionally, the goal of this study was to detect the effects of common patterns of sleep restriction during the week and extending sleep on the weekends in the laboratory environment. Laboratory experiments with human participants are currently feasible when conducted in the scope of weeks, rather than months or years and, as such, an amplified model of sleep restriction and recovery (e.g., sleep restricted to four hours during the week and eight hours on weekends) was utilized. The extent to which patterns of stress responses will change if milder patterns of restricted sleep and recovery were carried out for a longer period of time, (e.g., years or decades), as are often experienced in real life, will need to be addressed in future studies.
5. Conclusion
To our knowledge, this is the first study that has used an in-laboratory design to model patterns of repeated sleep restriction and recovery that are prevalent in modern society. It is also among the first that begins to map out a mechanistic path of multiple stress responses systems in the context of experimental sleep loss in humans. Despite habituation in subjective domains, we observed that physiological stress systems show patterns of continued elevated responses across repeated cycles of sleep restriction, even with limited opportunities for recovery sleep. The current study provides preliminary, yet powerful evidence that we cannot fully adjust to patterns of restricted sleep loss and recovery. Despite accumulating physiological impact, if the subjective experience to these sleep patterns is one of habituation, it can easily be seen why obtaining insufficient sleep on a chronic basis is experienced as benign and why motivation to change these behavior patterns remains low. The growing awareness of chronic low-grade inflammation as a basis for increasing rates of cardiovascular, metabolic, pain or mood related disorders (Medzhitov, 2010) suggests that these patterns of insufficient sleep may pose a significant health risk. Given its high prevalence in modern society, the impact of these patterns of chronically restricted sleep with limited recovery on long-term health cannot be ignored.
Supplementary Material
Highlights.
Three weeks of sleep restriction-recovery patterns studied in the laboratory was not perceived as subjectively stressful but did activate physiological stress response systems.
Limited recovery sleep did not restore these physiological stress response systems to baseline.
The dissociation between subjective and physiological stress responses may help explain why individuals continue with commonly observed patterns of restricting sleep during the work week and attempting to ‘catch up’ on weekends.
Acknowledgments
This work was funded by grants HL 105544 from the National Heart, Lung, and Blood Institute, and grants UL1 RR02758 and M01-RR-01032 from the National Center for Research Resources to the Harvard Clinical and Translational Science Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60:563–572. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. Rules, terminology and technical specifications. Westchester, IL, USA: 2007. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of Sleep and Its Disturbances on Hypothalamo-Pituitary-Adrenal Axis Activity. International Journal of Endocrinology. 2010 doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbo M, Morselli LL, Tasali E, Leproult R, Van Cauter E, Spiegel K. Effect of sleep loss on the hypothalamo-pituitary-adrenal (HPA) axis. Sleep. 2009;32:497. [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- Everson CA. Cumulative effects of repeated sleep restriction on the metabolic phenotype of rats. Sleep. 2008;31:324. [Google Scholar]
- Everson CA. Repeated sleep restriction induces morphological changes in internal organs in the rat. Sleep. 2009;32:391. [Google Scholar]
- Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2009;297:R1430–R1440. doi: 10.1152/ajpregu.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. Journal of Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and science of sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: Get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Balbo M, Morselli L, Tasali E, Leproult R, L'Hermite-Baleriaux M, Van Cauter E, Spiegel K. Adverse Effects of Two Nights of Sleep Restriction on the Hypothalamic-Pituitary-Adrenal Axis in Healthy Men. J Clin Endocrinol Metab. 2014;99:2861–2868. doi: 10.1210/jc.2013-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Lee E, Cohen D, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: Potential mediator of increased spontaneous pain. Pain. 2009;145:136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007;26:288–295. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory chanages in neuroendocrine stress integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry. 2015a doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang MG, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behavior and Immunity. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Witarama T, Caudill M, Olmstead R, Breen EC. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behavior and Immunity. 2015b;47:86–92. doi: 10.1016/j.bbi.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism-Clinical and Experimental. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Wang D, Gianferante D, Hanlin L, Chen X, Thoma MV, Rohleder N. Response and habituation of pro- and anti-inflammatory gene expression to repeated acute stress. Brain Behavior and Immunity. 2015;46:237–248. doi: 10.1016/j.bbi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people - A diary study. Chronobiol Int. 2000;17:49–60. doi: 10.1081/cbi-100101031. [DOI] [PubMed] [Google Scholar]
- Montagna P, Cortelli P, Gambetti P, Lugaresi E. Fatal familial insomnia: sleep, neuroendocrine and vegetative alterations. Adv Neuroimmunol. 1995;5:13–21. doi: 10.1016/0960-5428(94)00042-m. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Summary of Findings. Washington DC 20005: 2010. 2010 Sleep in America Poll. www.sleepfoundation.org. [Google Scholar]
- Pejovic S, Basta M, Vgontzas AN, Kritikou I, Shaffer ML, Tsaoussoglou M, Stiffler D, Stefanakis Z, Bixler EO, Chrousos GP. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. American Journal of Physiology-Endocrinology and Metabolism. 2013;305:E890–E896. doi: 10.1152/ajpendo.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the Heart and Soul Study. J Psychiatr Res. 2013;47:1228–1235. doi: 10.1016/j.jpsychires.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Tsui YY, Wing YK. A Study on the Sleep Patterns and Problems of University Business Students in Hong Kong. Journal of American College Health. 2009;58:167–176. doi: 10.1080/07448480903221418. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- van Leeuwen WMA, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. Plos One. 2009;4 doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Wing YK, Li SX, Li AM, Zhang JH, Kong APS. The Effect of Weekend and Holiday Sleep Compensation on Childhood Overweight and Obesity. Pediatrics. 2009;124:E994–E1000. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.