Abstract
Although the most notable clinical symptoms of Huntington’s disease (HD) are motor disturbances and brain atrophy, other symptoms include cognitive dysfunction, emotional and hormonal dysregulation. Emotional dysregulation (irritability, anger/aggression, and anxiety) and increased inflammation are early emerging symptoms which can be detected decades before the onset of motor symptoms in HD patients. Despite the advances in understanding the genetic causes of HD there is still no cure or preventative treatment. Thus, to better understand the pathogenesis of HD and develop effective treatments, a holistic understanding of HD is needed, as well as animal models that replicate the full spectrum of HD symptoms. The current study examined the emotional, hormonal, and gene expression responses to an acute stressor of adult male transgenic HD rhesus monkeys (HD, n=2) as compared to wild-type controls (n=2). Results revealed that HD monkeys expressed increased anxiety and irritability/aggression as compared to controls. Reactive cortisol response to the stressor was similar between groups. However, HD monkeys exhibited increased pro-inflammatory cytokines and higher induction of immune pathway genes as compared to controls. Overall, results reveal that HD monkeys exhibit these early emerging symptoms of HD and may be an effective animal model to facilitate the development of new therapeutics for HD patients.
Keywords: emotion, cytokines, inflammation, IL-6, TNF-α, ISGs
Huntington disease (HD) is an autosomal dominant, progressive neurodegenerative and eventually fatal disease. There is considerable opportunity to identify a therapy or cure for HD given that it is a monogenic illness, with a known gene and gene product. However, more than 20 years after identifying the huntingtin gene (HTT, there is still no cure or treatment for this devastating disease (Huntington’s Disease Collaborative Research Group, 1993). To date, rodent models of HD have unveiled a wealth of pharmacological targets, however, no agent that has been beneficial in an HD mouse model has also proven to be beneficial in human patients (Wild & Tabrizi, 2014). Additionally, even though 15,000 Americans are affected by HD, it is still a rather rare disease, with a preclinical population that is too small to sustain the number of clinical trials needed to narrow down these pharmacological targets (Shannon & Fraint, 2015). Therefore, to facilitate the development of effective treatments an animal model that embodies the full array of HD symptoms is greatly needed.
Although the most notable clinical symptoms of HD are motor disturbances and brain atrophy, other symptoms include cognitive dysfunction, emotional and hormonal dysregulation. Additionally, symptoms of emotional dysregulation and increased inflammation can be detected decades before the onset of clinical symptoms in HD patients (Duff et al, 2007; Bjorkqvist et al, 2008; Politis et al, 2015). In fact, up to 60% of HD patients symptoms first begin with mental and emotional dysfunctions, which are often not immediately attributed to the disease (Di Maio et al, 1993; Pascu et al, 2015). Additionally, symptoms of irritability, anger/aggression, and anxiety are rated among the top 10 symptoms that interfere with daily functioning among HD patients and caregivers (Vaccarino et al, 2011). Thus, alterations in emotional behavior are not only an early symptom, but also a good behavioral biomarker of HD. An additional early emerging feature of HD is the appearance of inflammation prior to clinical symptom onset, such as increased cortisol, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) detected in saliva, blood, cerebrospinal fluid, and brain tissue of persons carrying the mutant HTT gene (Bjorkqvist et al, 2008; van Duijn, et al, 2010; Wang et al, 2014). This hyperactivity of the innate immune system has been identified as a potential therapeutic target for HD (Wild & Tabrizi, 2014; Shannon & Fraint, 2015). Therefore, to facilitate the development of new HD therapies, animal models of HD should exhibit these early emerging symptoms of emotional and immune dysregulation.
Unlike rodents, humans and nonhuman primates have similar brain structure and circuitry which underlies complex cognitive processes (i.e. decision making) and emotional behavior (Neubert, et al, 2015), and they primarily use visual cues to extract information from others in their dynamic social environments (Watson & Platt, 2012). Considering these similarities between human and nonhuman primates, the transgenic HD rhesus monkey (HD monkey) model holds great promise for future HD treatment development because nonhuman primates may be able to model the full spectrum of HD patient symptoms. Recently we have shown that HD monkeys exhibit subtle cognitive deficits (Chan et al, 2014, 2015), difficulty with fine motor tasks (Chan et al, 2015), as well as volume reduction and nuclear inclusions of mutant HTT aggregates in brain areas to similar human HD patients (Chan et al, 2015). However, little is known about whether HD monkeys exhibit these pre-manifest/early HD symptoms of emotional dysregulation and innate immune hyperactivity. Thus, a better understanding of these behavioral and hormonal changes in HD monkeys will provide valuable information about this animal model and broaden its potential for identifying effective therapies for HD patients. The present study used an acute stressor to compare the emotional behavior and hormonal responses in an established group of HD monkeys and age-matched non-transgenic controls. The HTT gene of rhesus monkeys (macacca mulatta) normally carries 10–11 polyQ repeats (Putkhao, et al., 2013), while healthy humans normally carry 9–35 CAG repeats (Rubinsztein, et al., 1996; Groen, et al., 2010; Squitieri, et al., 2012). In our study, the HD monkeys carried exons 1–11 of the human HTT gene coding N-terminal 508 amino acids with approximately 67–72Q under the control of the human HTT promoter. As a result of the regulation by human HTT promoter and a larger HTT fragment, we anticipated a slower HD progression similar to adult-form HD. In fact, the first signs of dystonia did not appear until after 2 years of age and difficulty with a fine motor task was not evident until 3 years of age. Furthermore, decreased striatal functioning (as indicated by decreased NAA levels using in vivo proton magnetic resonance spectroscopy) was not detected until 4 years of age (Chan, et al., 2015). This report describes the emotional and hormonal changes between HD monkeys and controls at 5 years of age (young adulthood).
Methods
Subjects
Four male transgenic HD rhesus macaques (rHD1, rHD6, rHD7, rHD8) were created by transfection of mature oocytes by using a lentiviral vector carrying a mutant form of the HTT gene. Unfortunately, two HD monkeys (rHD1 and rHD7) had to be euthanized prior to the current study (Chan et al, 2015). Therefore, the current study included only four adult (5 years old) male rhesus monkeys (Macaca Mulatta) which were tested at the Yerkes National Primate Research Center (YNPRC) at Emory University, Atlanta GA. HD monkeys (n=2; rHD6 and rHD8) carry exons 1–11 of the human HTT gene coding N-terminal 509 amino acids with approximately 67–72Q under the control of the human HTT promoter as described previously (Chan et al, 2015). Due to innate sex differences in emotional behavior and cortisol stress reactivity (Mason et al, 1960; Hadidian 1980; Troisi et al, 1990; Raper, et al, 2013a), two age-, sex-, and rearing-matched wild-type rhesus monkeys were used as controls for comparison to the HD monkeys. Animals were surrogate-peer reared in a socially enriched environment that promoted species-specific socio-emotional skills (Sackett, et al., 2002; Rommeck et al., 2011; Raper, et al, 2013b). Animals were housed under a 12 hour light/dark cycle and all procedures were approved by the Institutional Animal Care and Use Committees of Emory University in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council, 2011) and the United States of Department of Agriculture (USDA) Animal Welfare Act.
Surrogate Peer-rearing Conditions
After delivery, infants were housed individually in cages (40 cm X 30 cm X 40 cm) under open radiant incubators with contact comfort provided by a synthetic plush surrogate (30 cm in length) and visual, auditory, and olfactory contact with other infants in the nursery until 1 month of age. Daily care of animals was provided by a principal human caregiver 6 h daily, 5 days a week. On weekends, familiar human caregivers fed, handled and played with the infants 2–4 h both days. Beginning at 1 month of age, infants also received daily socialization with three other age-and sex-matched peers (3–4h, 5 days/week) in a large play cage, containing toys and towels, located in the primate nursery. At 3 months of age, animals were transferred to larger quad rack of cages and housed individually, but visual and physical contact was possible between pairs of infants through the large central mesh separating two adjacent cages. At approximately 6–7 months of age, animals were housed in quads (4 animals per large enclosures) until approximately 12 months of age and then were housed in pairs thereafter.
Human Intruder Paradigm
At approximately 5 years of age, animals were transported to a novel testing room, and then transferred to a stainless steel cage (53 cm x 53 cm x 55 cm) with one side made of clear lexan plastic allowing for video recording. The Human Intruder paradigm lasted 30-min and consisted of three conditions (Alone, Profile, Stare) presented in the same order for all animals. First, the monkey remained alone in the cage for 9 min (Alone) to acclimate to the environment and obtain a baseline level of behavior. Then, the intruder (experimenter wearing a human rubber mask) entered the room without making eye contact and sat two meters from the test cage while presenting his/her profile to the animal for 9 min (Profile condition). The intruder then left the room while the animal remained in the cage alone for a 3-min period, after which the intruder re-entered the room and made direct eye-contact with the animal for 9 min (Stare condition). Emotional reactivity to the intruder was assessed via videotape recording for later coding using the Observer XT 10 software (Noldus Inc., Netherlands) and a detailed ethogram (see Table 1). One experimenter coded all of the videotapes, but had a high degree of inter-rater reliability (Cohen’s Kappa=0.86) with other trained experimenters who coded the four videos. The experimenter also had an average intra-rater reliability of Cohen’s Kappa=0.98.
Table 1.
Behavioral Ethogram
| Category and specific behavior | Measurement | Brief Definition |
|---|---|---|
| Coo Vocalization | Frequency | Clear soft, moderate in pitch and intensity, usually “oooooh” sounding |
| Freezing | Duration | Rigid, tense, motionless posture except slight head movement |
| Hostile Behaviors | Cumulative Frequency | |
| Threat Bark Vocalization | frequency | Low pitch, high intensity, rasping, guttural |
| Threat (facial expression) | frequency | Any of the following: open mouth (no teeth exposed), head-bobbing, or ear flapping |
| Cage Aggression | frequency | Vigorously slaps, shakes or slams body against cage |
| Lunge | frequency | A quick, jerky movement toward the intruder |
| Motor Stereotypies | Frequency | Abnormal involuntary motor patterns (primarily involuntary leg movements) |
| aGrunt Vocalization | Frequency | Deep, muffled, low intensity, almost gurgling sound |
| aLipsmack | Frequency | Rapid movement of pursed lips, accompanied by a smacking sound |
| aFear grimace | Frequency | Refracted lips, exposed clenched teeth |
List of all behaviors scored, how they are measured and a brief definitions.
Indicates that the behavior was never seen.
Blood Sampling
Stress reactivity is readily detected in plasma, as measured by elevations in cortisol, within 10 minutes of accessing the monkey from their home cage; therefore animals were trained using positive reinforcement to voluntarily present a leg for awake blood collection using established protocols at the YNPRC demonstrating that under these conditions hormonal levels reliably reflect basal levels (Blank, et al., 1983; Raper, et al., 2013a,b). All animals were tested at the same time of day (Lights-On: 0700) and blood samples were collected immediately before (0700), immediately after (0730), and 24 hours after the acute stressor (Human Intruder paradigm). All blood samples were collected in 8ml cell preparation vacutainer tubes containing sodium citrate (1ml of 0.1M sodium citrate solution). Samples were centrifuged at 1,800 RCF for 20 minutes, plasma was pipetted into sterile cryovials and stored at −80°C until assayed. The cell layer was pipetted into a microtube and spun at 2,000 RCF for 5 min, the remaining plasma was then poured off, and 1ml of QIAzol lysis reagent was added to microtube. The microtube was then briefly vortexed to mix the cells and lysis reagent and allowed to sit for 5 minute at room temperature before being flash frozen and stored at −80°C until RNA extraction.
Plasma Assays
Cortisol was measured by immunoassay produced by DRG International for the Hybrid XL platform (catalog #HYE-5343) and performed by the Yerkes Biomarker Core (YNPRC, Emory University, Atlanta GA). Samples and standards were analyzed in duplicates with a maximum tolerated coefficient of variation (CV) of 6%. The lower level of detection limit was 2µg/dL. No inter-assay CV was determined as all samples were run at the same time to minimize between-assay variability.
Inflammatory response factor IL-6, TNF-α, and CRP were measured by an electrochemiluminescence detection technique using the manufacturer’s protocols. 96-well plates measuring nonhuman primate IL-6, nonhuman primate TNF-α, and human CRP were analyzed on a Sector Imager 2400 (Mesoscale Discovery [MSD], Gaithusburg, MD, USA) by the Emory Multiplexed Immunoassay Core (Emory University, Atlanta GA). Samples and standards were analyzed in duplicates with a maximum tolerated coefficient of variation (CV) of 20%. The lower level of detection limit for IL-6, TNF-α, and CRP was 0.064 pg/mL, 8.89 pg/ml, and 0.008ng/ml, respectively. No inter-assay CV was determined as all samples were run at the same time to minimize between-assay variability.
RNA-Sequencing
The Nonhuman Primate Genomics Core at the YNPRC performed the RNA-Sequencing and analysis for this study. Total RNA was prepared using the QIAGEN RNEasy Micro Kit. Libraries were generated from 5ng of Total RNA using the CLONTECH SMARTer HV kit, barcoding and sequencing primers were added using NexteraXT DNA kit. Libraries were validated by microelectrophoresis, quantified, pooled, and clustered on Illumina TruSeq v3 flowecell. Clustered flowcell was sequenced on an Illumina HiSeq 1000 in 100-base single-read reactions.
Data Analysis
Emotional Behavior
Prior to analysis, the behavioral data were examined for normality using the Kolmogorov-Smirnov (K–S) tests. When behaviors were not normally distributed they were transformed using a natural log plus 1 constant. Group differences in emotional behaviors were examined with repeated measures ANOVA with Group (control, HD) as the between subjects factor and Condition (Alone, Profile, Stare) as the within subjects repeated measures. Due to the small sample size, which can cause imprecise calculations of standard error, a bootstrap technique was also applied to the dataset. Bootstrapping is a resampling method, which relies on random sampling with replacement, thus it can adjust for bias and calculate more precise standard errors (Chernick, 2008). Therefore, a bootstrap with 200 replications was conducted on the repeated measures ANOVA with a Kenward-Roger Degrees of Freedom Approximation corrected p-value. See Supplementary Table 1 for comparison of original and bootstrap adjusted standard error of the mean.
Physiological Stress Response
To examine changes in plasma levels of cortisol and cytokines in response to the acute stressor, repeated measures ANOVA were used with Group (control, HD) as between subjects factor and Time (pre-, post-stressor, and 24hr post-stressor) as the within subjects repeated measures for each measure separately (cortisol, IL-6, TNF-α, CRP). Additionally, a bootstrap with 200 replications was conducted on the repeated measures ANOVA with a Kenward-Roger Degrees of Freedom Approximation corrected p-value. Change in IL-6 concentrations from pre-stress to post-stress was also calculated (post-stress – pre-stress) for each animal and analyzed by General Linear Model ANOVA with Group (2) as the between subjects factor and difference in IL-6 level as the dependent variable. A bootstrap with 200 replications was also conducted on the GLM ANOVA for the difference in IL-6 level with a Kenward-Roger Degrees of Freedom Approximation corrected p-value. Exploratory analyses of CRP level at 24hr post-stressor were conducted using One-way ANOVA with Group as the between subjects factor. A bootstrap with 200 replications was conducted on the One-way ANOVA for CRP level at 24hr post-stressor with a Kenward-Roger Degrees of Freedom Approximation corrected p-value. See Supplementary Table 2 for comparison of original and bootstrap adjusted standard error of the mean.
Behavior and physiological response analyses were conducted with SAS 9.4 for Windows (SAS Institute Inc, North Carolina) and significance level was set at p < 0.05. Effect sizes were calculated using partial eta squared (ηp2). Partial eta squared is the proportion of variance accounted for by an effect plus its associated error of variance within an ANOVA model, and thus is bounded by 0 and 1.
Gene Expression Response
To examine potential gene expression differences between HD and control monkeys, RNA-seq read data were analyzed by alignment to the provisional assembly of the Indian rhesus macaque genome (MuSuRCA v7, Zimin et al, 2014, assembly available at: http://www.unmc.edu/rhesusgenechip/index.htm#NewRhesusGenome). Alignment was performed using STAR version 2.3.0e (Dobin et al, 2013); parameters were set using the annotation as a splice junction reference. Transcript abundance estimates were calculated with htseq-count version 0.6.1p1 (Simon Anders, et al, 2014). Normalization was performed using DESeq2 version 1.6.3 (Love, et al, 2014) producing a normalized read count table and a regularized log expression table.
Results
Emotional Behavior Response
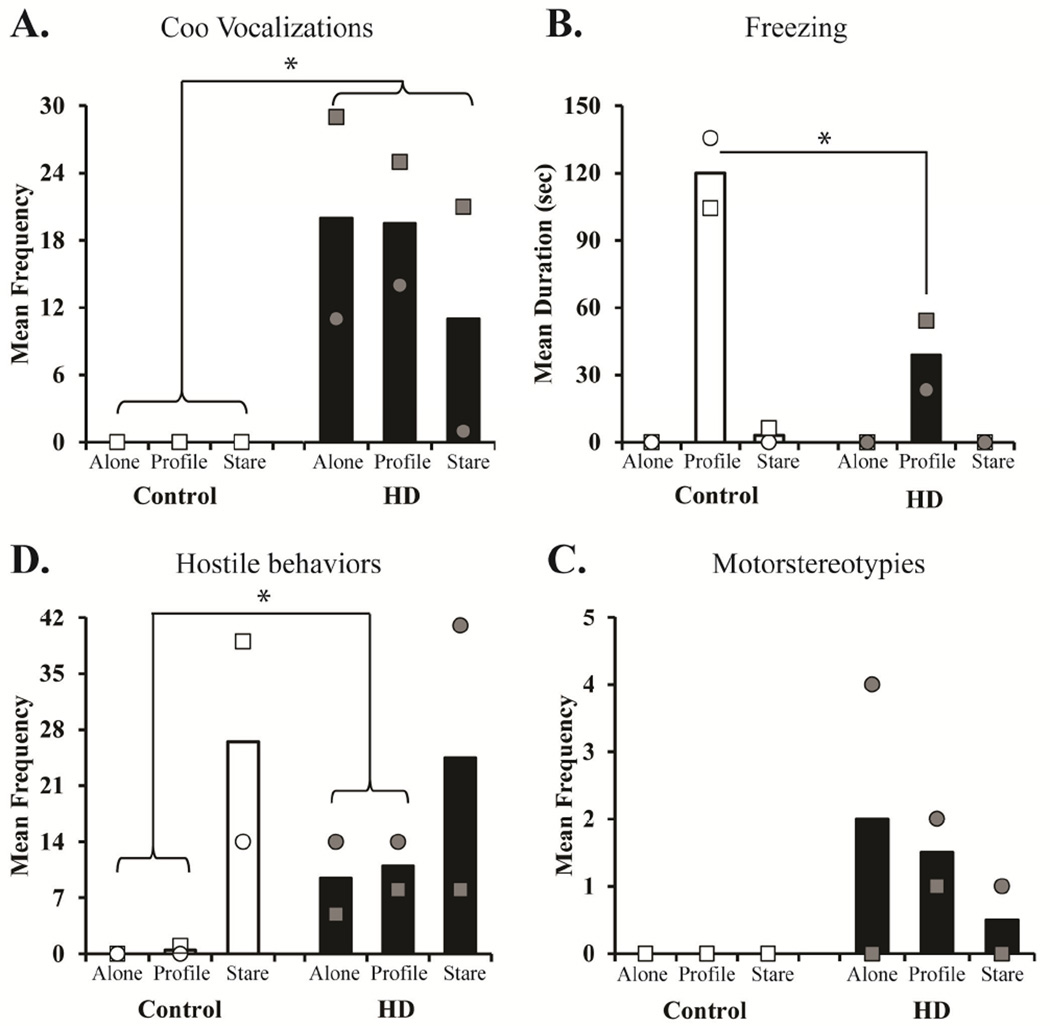
Data from the Human Intruder task revealed that while some behavioral responses were normal, others were increased in the HD monkeys. When placed alone in a novel environment, young monkeys (infants and juveniles) often emit coo vocalization in an attempt to re-connect with their mother and/or cagemate, however adult monkeys typically emit less coo vocalizations on this task (Kalin & Shelton, 1998). Interesting, HD monkeys emitted more coo vocalizations throughout the task as compared to controls (Group: F[1,2]=16.37, p=0.05, ηp2=0.89; 200 bootstrap F[1,1.89]=21.41, p=0.05; see Figure 1A). When faced with the mild threat of the intruder’s profile, both groups displayed the species typical increase in freezing compared to other conditions (Alone and Stare), however the HD monkeys exhibited significantly less freezing than the controls (Condition X Group: F[2,4]=11.73, p=0.021, ηp2=0.85; 200 bootstrap F[2,3.88]=20.93, p=0.017; see Figure 1B). When faced with the salient threat of the intruder’s direct eye-contact (Stare condition), both groups displayed species typical increased hostile behaviors. Interestingly, HD monkeys expressed more hostile behaviors than controls during the no threat (Alone) and mild threat (Profile) conditions (Condition X Group: F[2,4]=14.83, p=0.014, ηp2=0.881; 200 bootstrap F[2,3.11]=35.18, p=0.008; see Figure 1C). Lastly, no Group differences or interactions were found for motor stereotypies, although a few chorea-like HD motor stereotypies were exhibited by the HD monkeys during the human intruder test (Group: F[1,2]=1.78, p=0.31, ηp2=0.47; 200 bootstrap F[1,1.18]= 1.18, p=0.47; see Figure 1D).
Figure 1. Emotional Behaivor Response to the Human Intruder task.
Bars represent the mean for (A) coo vocalizations, (B) freezing, (C) hostile, and (D) motorstereotypies during the Alone, Profile, and Stare conditions for animals with wild-type controls (open bars & open shapes represent individuals) and HD monkeys (black bars & grey shapes represent individuals). Coo vocalizations and hostile behavior expression was transformed (LN x + 1) for data analysis, and nontransformed data are graphed. * indicates significant group differences (p < 0.05).
Physiological Stress Response
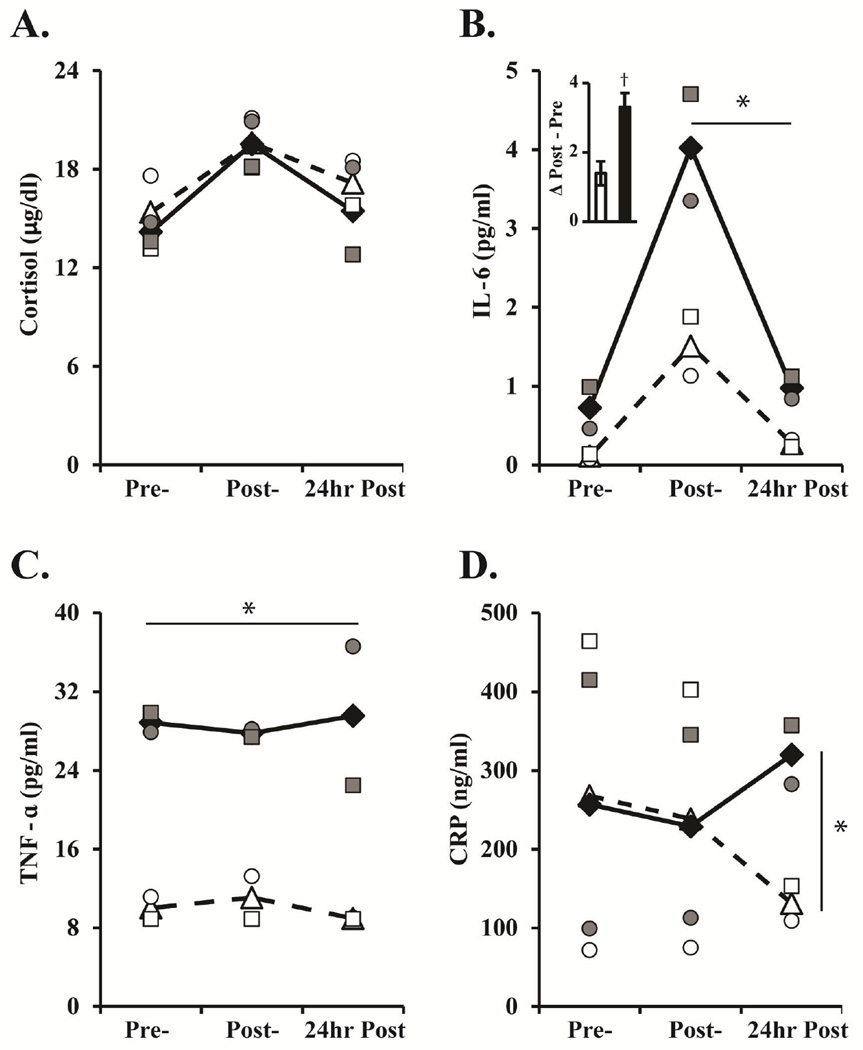
The cortisol response to the acute stressor was similar between groups (Group: F[1,2]=0.19, p=0.71, ηp2=0.09; 200 bootstrap F[1,1.36]=0.08, p=0.82), with both groups showing a significant increase in cortisol from pre- to post-stressor and a significant decline from post-stressor to 24hours post-stressor (Time: F[2,4]=18.21, p=0.01, ηp2=0.90; 200 bootstrap F[2,3.69]=28.06, p=0.01; see Figure 2A). In contrast, HD monkeys exhibited higher IL-6 levels at post-stressor and 24 hours post-stressor as compared to controls (Time X Group: F[2,4]=8.94, p=0.033, ηp2=0.82; 200 bootstrap F[2,3.32]=17.92, p=0.02; see Figure 2B). Additionally, the difference in IL-6 levels pre- to post-stressor demonstrates that HD monkeys had a steeper increase compared to controls (see Figure 2B bar graph insert). Although the difference in IL-6 from pre- to post-stressor was not statistically significant, the effect size accounted for 86% of the variance (Group: F[1,3]=12.65, p=0.07, ηp2=0.86; 200 bootstrap F[1,2.89]= 14.48, p=0.03). For TNF-α levels, there was no significant change in response to the acute stressor (Time: F[2,4]=0.02, p=0.96, ηp2=0.001; 200 bootstrap F[2,3.87]=6.37, p=0.08), yet there was a significant Group effect (F[1,2]=59.78, p=0.016, ηp2=0.96; 200 bootstrap F[1,1.99]=57.14, p=0.05; see Figure 2C) with HD monkeys exhibiting overall higher TNF-α levels compared to controls. No significant effects of Time, Group, nor interactions were found for CRP (Time: F[2,4]=0.12, p=0.89, ηp2=0.06; 200 bootstrap F[2,3.88]=2.28, p=0.25; Group: F[1,2]=0.12, p=0.76, ηp2=0.05; 200 bootstrap F[1,1.57]=0.13, p=0.78). However, exploratory analyses of CRP were conducted to directly examine the impact of the acute stressor on CRP secretions. Results revealed that HD monkeys secreted more CRP at 24 hours post-stressor as compared to controls (Group: F[1,3]=19.03, p=0.049, ηp2=0.91; 200 bootstrap F[1,2.57]=26.21, p=0.014; see Figure 2D).
Figure 2. Hormonal Response to the Human Intruder task.
Mean levels of (A) Cortisol, (B) IL-6, (C) TNF-α, and (D) CRP immediately before (Pre), immediately after (Post), and 24 hours after (24hr Post) the acute Human Intruder stressor. The change in IL-6 levels from Pre- to Post-stress is illustrated by a difference score, inserted in graph 2B. Controls are represented by open triangles with dashed lines and individuals are represented by open shapes. HD monkeys are represented by black diamonds with solid lines and individuals are represented by grey shapes. † indicates a group trend with large effect size.
Gene Expression Response
To identify potential pathways affecting the immune response toward an acute social stressor, we preformed mRNA-Seq on peripheral blood mononuclear cells (PBMCs) from both wild-type controls and HD monkeys immediately before and after the Human Intruder task. One of the key limitations of this study is the availability of only two HD monkeys with which to perform comparisons, which made analysis of the transcriptomic data using standard gene-by-gene statistical approaches (i.e. CuffDiff, DESEQ) unfeasible due to the power limitation. Instead, we used Gene Set Enrichment Analysis (GSEA) to explore transcriptional differences (Subramanian 2005). GSEA cumulatively tests enrichment of related genes between two sets of data, rather than the effect size of single genes, and thus provides greater sensitivity in situations with low number of replicates; moreover, significant work to establish immune-specific gene-sets have improved the utility of this tool for immunological analyses (Godec 2016; Haining 2010).
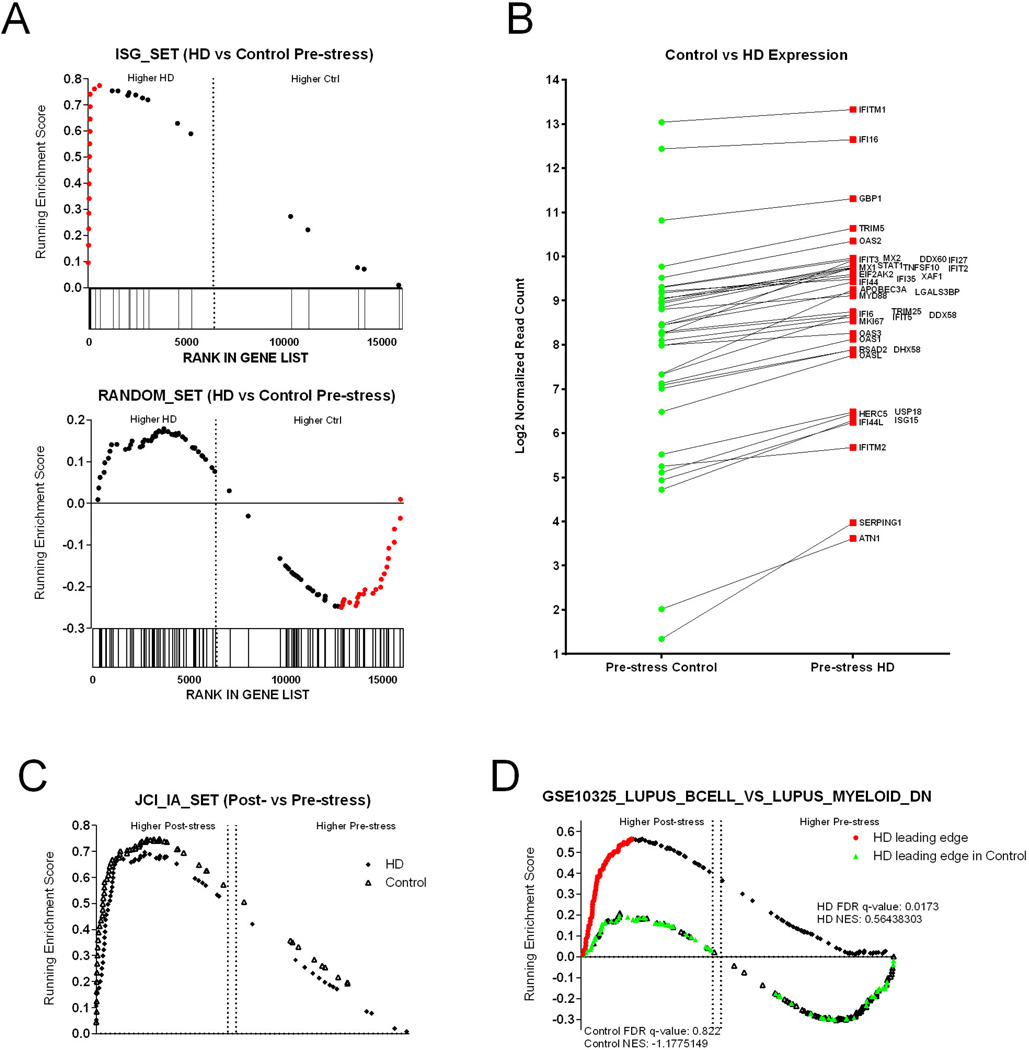
To identify families of genes that differ between the control and HD monkeys during baseline conditions, we directly contrasted the pre-stressor transcriptomic data using GSEA and tested for enrichment against gene-sets maintained in the Molecular Signatures Database (MSigDB) and our own custom gene sets. Interestingly, we found that there was significant enrichment (NES = 2.04, p < 0.001, FDR q-value < 0.001) for a previously defined (Rotger 2011) gene-set composed of Interferon Stimulated Genes (ISGs) in the HD monkeys (Figure 3A), and plotting the mean readcounts of individual ISGs revealed that the vast majority were elevated in the HD monkeys (Figure 3B). This analysis indicated that the mHTT gene may influence circulating immune cells similar to the antiviral state.
Figure 3. Gene Expression Changes on the Human Intruder task.
(A) Gene set enrichment plots (GSEA) of two gene families, Interferon Stimulated Genes (ISGs) showing overexpression in the HD samples and a random set of genes showing no enrichment in either phenotype. The running enrichment score (y axis) is plotted by each gene’s individual rank (x axis); bars below the x axis indicate individual gene ranks in the whole data set. The ranking is based on relative gene expression between HD and controls. Genes in the leading edge (contributing the most to the enrichment score) are shown in red. (B) The mean log2 normalized read counts of the leading-edge genes are shown between the control and HD monkeys. (C) GSEA plots of Pre- vs Post-stress for both controls and HD monkeys showing Post-stress enrichment for both groups. (D) GSEA plots of Pre- vs Post-stress for controls and HD showing Post-HI enrichment in only the HD group. The genes in the leading edge of the HD group are shown in red along the HD enrichment distribution and those same genes are shown in green along the control enrichment distribution showing no enrichment for the control animals.
To ameliorate the effect of individual variation in our analyses, we chose an analysis strategy focusing on the comparison of pre-stressor vs post-stressor datasets, and by performing the contrasts separately for the control and HD monkeys. In this manner, the variances of genes that vary due to individual variation, rather than the post-stressor effect are minimized. After establishing Pre- vs Post-stressor contrasts for the control and HD monkeys separately, we then screened for enrichment against the gene-sets in the C5 (gene ontology) and C7 (immunologic signatures) modules in the MSigDB, and against our own custom derived gene-sets. We then established tables of enrichment statistics for each group. Several immune processes were significantly enriched in the post-stressor samples for both the HD and controls groups, indicating that the acute social stress test was affecting the transcriptional response in the PBMCs (Supplementary Table 3). Of note, a gene-set we defined previously containing genes elevated in chronically SIV-infected macaques that were not known ISGs were highly and significantly enriched post-stressor (p < 0.001 for both comparisons) in both the HD and control animals (Figure 3C).
We then screened for specific pathways perturbed exclusively in the HD monkeys, but not in controls, after the acute social stressor exposure by screening our GSEA table to find gene-sets significantly enriched in the HD pre- versus post-stressor contrast alone. Interestingly, we found that gene-sets associated with Lupus (a chronic autoimmune disease) were enriched in the HD monkeys, but not the controls (Figure 3C). Several publications have established that Lupus is associated with an underlying ISG expression (Bennett 2003). However, the leading edge genes driving the enrichment were not ISGs, but were genes expressed at high levels in B-lymphocytes sorted from lupus patients relative to myeloid cells. Taken together, our observations that HD monkeys have (i) elevated levels of ISGs as compared to controls at baseline/pre-stressor conditions, and (ii) significant enrichment of non-ISG lupus genes after the stressor that are not seen in controls, provide an intriguing hypothesis that mHTT gene leads to higher induction of inflammatory pathways associated with autoimmunity. Given the under-powered nature of our analysis, however, these data will require further validation.
Discussion
Transgenic monkeys with Huntington’s disease (HD monkeys) exhibit increased anxiety, irritability/aggression, and inflammatory cytokines in adulthood. Additionally, HD monkeys have elevated ISGs and significant enrichment of non-ISF lupus genes as compared to controls, suggesting increased expression in inflammatory pathways and autoimmunity. Together these results demonstrate that HD rhesus monkeys exhibit some of the key behavioral and immune features found in HD patients. Therefore, these results, along with previous findings in these same animals, suggest that the HD monkey model embodies a full array of HD symptoms (Chan et al, 2014, 2015), making it ideal for narrowing down preclinical pharmacological targets and identifying promising new treatments for HD patients.
Persons with expanded polyQ repeats at the HTT gene exhibit emotional dysregulation such as depression, apathy, irritability, anger/aggression, and anxiety. Some emotional changes, such as depression and lack of interest, seem to emerge in stage 2 of HD (i.e. after the onset of motor impairments), suggesting that these symptoms are a side effect of the diagnosis or neurological symptoms of HD, but not caused by the disease itself (Epping & Paulsen, 2011). Alternatively, irritability, anger/aggression, and anxiety are present prior to motor symptom onset, indicating that these emotional changes are key features of the disease (Dewhurst, Oliver & Trick, 1969; Berrios et al, 2002; Kirkwood et al, 2002; Duff et al, 2007; Marshall et al, 2007; Vassos et al, 2007; Bouwens, et al, 2015). The current study revealed that HD monkeys exhibit increased coo vocalizations throughout the Human Intruder task, suggesting increased anxiety. When rhesus monkeys are separated from their social group, they use coo vocalizations to identify and reconnect with family and group members (Rowell & Hinde 1962; Hauser 1991; Pfefferle et al, 2014). Interestingly, the emission of coos during the Human Intruder task changes over development, such that older monkeys emit few (if any) coos (Kalin & Shelton, 1998). Therefore, the increased emission of coo vocalizations by HD monkeys compared to controls, suggest that they are exhibiting increased anxiety. While anxiety has not been a major focus of HD research, a recent review suggests that generalized anxiety disorders are highly prevalent in the HD population, and perhaps more prevalent than compared with the general population (Dale & van Duijn 2015). Considering that HD patients have a high prevalence of anxiety that is present prior to motor symptoms, anxiety could be considered a key behavioral phenotype of HD and an important characteristic for HD animal models to express. The fact that our HD monkey model expresses increased anxiety supports its use in the development of new HD treatments. Paulsen and colleagues (2005) found that among manifest HD patients, anxiety symptoms peaked during stage 2, which may correspond with pathological changes in the basal ganglia or other brain areas. A previous study in these HD monkeys have reported that fine motor impairments and changes in striatal growth trajectory appear at 3 and 4 years of age, respectively (Chan, et al, 2015). Therefore, it is possible that changes in the basal ganglia may explain the increased anxiety seen at 5 years of age in the current study. Although it should be noted that HD monkeys exhibited a few chorea-like HD motor stereotypies during the task, there was no significant difference from controls. Future studies will follow the emotional behavior development from infancy to adulthood in a new generation of HD monkeys to confirm whether increased anxiety is the result of brain or motor behavior changes.
Irritability can be characterized as a mood state predisposing toward anger, hostility, and overt aggression (Snaith & Taylor, 1985; Craig et al, 2015). Similar to anxiety, irritability has been seen prior to motor symptom onset in humans (Bouwens, et al, 2015; Van den Stock, et al, 2015). The current study used the Human Intruder task to examine the ability of HD monkeys to modulate their emotional response based on the salience of the threat present. Results demonstrated that HD monkeys exhibited increased hostile behaviors during the no threat (Alone) and mild threat (Profile) conditions, but exhibited similar amounts of hostility during the high threat (Stare) condition. This significant Group by Condition interaction demonstrates that HD monkeys exhibit the appropriate/species typical response to the high threat, but exhibit a high/unusual level of hostility during lower threat conditions. Interestingly, neonatal amygdala lesions in monkeys result in a similar pattern of increased hostility during the no threat and mild threat conditions (Raper, et al, 2013b). It is possible that the pattern of hostility exhibited by HD monkeys is due to alterations in amygdala activity. Recent studies in HD patients have shown decreased amygdala activity during emotional tasks lending support for this hypothesis (Mason et al, 2015; Van den Stock, et al, 2015). Additionally, HD patients have also been shown to exhibit lower fear and higher anger ratings of fearful pictures as compared to healthy controls (Eddy, et al, 2011). This decreased fear and increased anger toward fearful stimuli is mirrored by the reduced freezing and higher level of hostility exhibited by our HD monkeys during the mild threat (Profile) condition, in which control monkeys expressed significantly higher levels of freezing and less hostility.
Overall, the current data suggest that HD monkeys exhibit increased anxiety and irritability similar to that seen in HD patients. It has been suggested that these mood disturbances of irritability and anxiety may delay the appropriate diagnosis of HD (Pascu, et al, 2015). Additionally, irritability and anxiety can have devastating consequences for HD patients, causing considerable distress to the patient, family members, as well as the professionals involved in their care (Victorson et al, 2007; McCabe, et al, 2009; Nimmagadda et al, 2011). In fact, irritability and anxiety are rated among the top 10 symptoms that interfere with daily functioning among HD patients and caregivers (Vaccarino et al, 2011). Recent longitudinal studies have shown increased irritability and apathy in HD patients, which might reflect increased mood disturbances in response to a loss of normal functioning (Tabrizi, et al, 2013; Bouwens, et al, 2015). Considering that irritability and anxiety causes distress for patients and caregivers and may worsen overtime, these emotional characteristics are important for HD animal models to exhibit. This study presents evidence that HD monkeys exhibit these key features of emotional alterations (increased irritability and anxiety), which perhaps makes them an ideal animal model to further the development of new HD treatments.
Aside from emotional alterations, HD patients also exhibit hormonal alterations that include increased cortisol and inflammatory cytokines (Heuser, Chase & Mouradian, 1991; Leblhuber et al, 1995; Bjorkqvist et al, 2008; Aziz et al, 2009; Forrest et al, 2010; van Duijn et al, 2010; Wang et al, 2014). This is likely due to mutant HTT being highly expressed in both the central nervous system and peripheral immune cells of HD patients (Trottier et al, 1995; Ferrante et al, 1997; Bjorkqvist et al, 2008). Mutant HTT promotes cell-autonomous pro-inflammatory immune activation (Bjorkqvist et al, 2008; Crotti et al, 2014), thus HD patients can exhibit increased cytokines up to a decade prior to the first clinical symptoms (Bjorkqvist et al, 2008; van Duijn et al, 2010; Wang et al, 2014; Chang et al, 2015). Therefore, alterations in the immune system are also an early emerging feature of HD. Our study found no differences between HD and control monkeys in their reactive cortisol response to the acute stressor, however, HD monkeys did exhibit increased pro-inflammatory cytokines. Specifically, HD monkeys exhibited a greater increase in IL-6 immediately after and 24 hours after the stressor, and higher CRP levels than controls at 24 hours post. Interestingly, TNF-α levels did not change in response to the stressor in either controls or HD monkeys, yet HD monkeys exhibited an overall higher level of TNF-α compared to controls. Results from our study are consistent with those from pre-manifest and early-stage HD patients, demonstrating increased IL-6 and TNF-α levels in plasma (Bjorkqvist, et al, 2008). In fact, innate immune hyperactivity has been identified as a potential therapeutic target for HD (Wild & Tabrizi, 2014; Shannon & Fraint, 2015). The present results suggest that HD rhesus monkeys exhibit immune hyperactivity, and could be a valuable animal model to help narrow down pharmacological treatments for HD patients.
In addition to immune hyperactivity (increased cytokines) in HD monkeys, gene expression analysis also pointed to increased response in multiple inflammatory pathways and autoimmunity among HD monkeys as compared to controls. Unlike controls, under baseline (nonstress) conditions HD monkeys had elevated ISGs. There are several caveats, however to analyses based on direct comparison of the HD and control animals - since there are only two animals per group, we cannot rule out that the HD monkeys had elevated ISGs due to natural variation unrelated to the mutant HTT gene. Alternatively, other possible explanations such as low grade infections in the HD monkeys may also explain the elevated interferon genes. Additionally, in response to an acute social stressor, HD monkeys exhibited significant enrichment of non-ISF lupus genes compared to controls, suggesting autoimmunity. However, caution should be used in interpreting the gene expression results considering the small sample size available in this study. The primary purpose of the mRNA-Seq analysis was to investigate whether immune pathways were perturbed in the HD monkeys, and given the power limitations of the data, these analyses are best considered as a means to prioritize future studies.
As mentioned above, the major limitation of the current study is the small sample size. Future studies with additional animals are needed to confirm our findings, as well as conducting longitudinal assessments to examine the developmental emergence of the emotional, inflammatory, and gene expression changes seen here. An another important caveat inherent in this study is the potential for environmental influences to impact brain and behavior, such that rearing conditions can alter brain maturation and behavior (Sanchez, et al, 1998; Rommeck, et al, 2011). Therefore, it’s possible that rearing could have impacted the emotional behavior expression in the monkeys. However, this is unlikely because we have previously demonstrated that our surrogate-peer rearing techniques at the YNPRC (similar to continuous rotational peer rearing; Rommeck, et al, 2011) produces the same developmental pattern of emotional behavior on the Human Intruder task, cognitive skills, and hormone secretions similar to mother-reared monkeys (Kalin, et al, 1991; Zeamer, et al, 2010; Raper, et al, 2013b). Despite the limitations of this study, a major strength is combining the examination of emotional behavior, immune function, and gene expression responses within a single test of an acute social stressor. This multifaceted approach enabled us to fully characterize the early emerging symptoms of HD in our animal model.
Conclusions
In summary, the present findings inform our understanding of the pathogenesis of early emerging HD symptoms, emotional and immune system dysregulation. The majority of current HD animal models are rodents, which have allowed the identification of many pharmacological targets for treatments, with the added benefit that motor and cognitive symptoms of HD are easily examined in rodents. However, there is a critical unmet need for animal models that recapitulate the early emerging symptoms of emotional and immune dysregulation in HD patients. Humans and nonhuman primates share a deep homology in brain circuitry mediating socio-emotional behaviors, reliance on visual cues to extract information from others in their complex social environments, as well as genetic and endocrine similarities (Watson & Platt, 2012; Yue, et al, 0214). Our current findings demonstrate that HD monkeys exhibit similar emotional behavior (increased anxiety and irritability) and innate immune hyperactivity (increased pro-inflammatory cytokines and immune pathway genes) as human HD patients. Thus, nonhuman primates are able to model a full array of HD symptoms (e.g. cognitive, emotional, inflammatory, fine motor) and hold great promise for the development of new therapeutics.
Supplementary Material
Highlights.
Huntington’s disease patients exhibit increased irritability, anxiety and inflammation
Transgenic Huntington’s disease monkeys also express increased irritability and anxiety
Huntington’s disease monkeys have high levels of cytokine in the periphery
Huntington’s disease monkeys have increased expression of immune pathway genes
Acknowledgments
Authors are grateful to Dr. Arthur Merrill Sr and Mrs. Sarah Merrill (The Arthur and Sarah Merrill Foundation) for their generous donation which made the current study possible. Additional thanks goes to Malu Tansey, Ph.D. in the Emory Multiplexed Immunoassay Core for assistance with the cytokine assays and Jonathan Lowe, B.S. in the Yerkes Biomarker Core for assistance with the cortisol assay. Last, but certainly not least, special thanks Nancy Bliwise, Ph.D. and Donghai Liang, M.P.H. for their statistical advice and expertise.
Funding source: This research was supported by the Merrill Huntington’s Disease Pilot Grant Program at Emory University (00023988) and the Transgenic Huntington’s Disease Monkey Resource sponsored by the Office of Research Infrastructure Programs (ORIP) OD010930. Yerkes National Primate Research Center is supported by the National Institutes of Health, Office of Research Infrastructure Programs (ORIP/OD) P51-OD011132.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicts of interest.
Contributor Information
Jessica Raper, Email: jraper@emory.edu.
Steve Bosinger, Email: steven.bosinger@emory.edu.
Zachary Johnson, Email: zpjohns@emory.edu.
Gregory Tharp, Email: gktharp@emory.edu.
Sean Moran, Email: sean.p.moran@vanderbilt.edu.
Anthony W.S. Chan, Email: awchan@emory.edu.
References
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NA, Pijl H, Frolich M, Maurits AW, van der Graaf M, Roelfsema F, Roos RAC. Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios GE, Wagle AC, Markova IS, Wagle SA, Rosser A, Hodges JR. Psychiatric symptoms in neurologically asymptomatic Huntington’s disease gene carriers: a comparison with gene negative at risk subjects. Acta Psychiatr Scand. 2002;105:224–230. doi: 10.1034/j.1600-0447.2002.0o456.x. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Möller T, Tabrizi SJ. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of venipuncture on serum levels of prolactin, growth hormones, and cortisol in outdoor compound-housed female rhesus monkeys. Acta Endocrinol. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Bouwens JA, van Duijn E, van der Mast RC, Roos RAC, Giltay EJ. Irritability in a prospective cohort of Huntington’s disease mutation carriers. J Neuropsychiatry Clin Neurosci. 2015;27:206–212. doi: 10.1176/appi.neuropsych.14030051. [DOI] [PubMed] [Google Scholar]
- Chan AWS, Jiang J, Chen Y, Li C, Prucha MS, Hu Y, Chi T, Moran S, Rahim T, Li S, Li X, Zola SM, Testa CM, Mao H, Villalba R, Smith Y, Zhang X, Bachevalier J. Progressive cognitive deficit, motor impairment and striatal pathology in a transgenic Huntington Disease monkey model from infancy to adulthood. PLoS ONE. 2015;10:e0122335, 1–16. doi: 10.1371/journal.pone.0122335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AWS, Xu Y, Jiang J, Rahim T, Kocerha J, Zhao D, Chi T, Engelhardt H, Moran S, Larkin K, Neumann A, Cheng H, Li C, Nelson K, Banta H, Zola SM, Villinger F, Yang J, Testa CM, Mao H, Zhang X, Bachevalier J. A two years longitudinal study of a transgenic Huntington disease monkey. BMC Neuroscience. 2014;15(1):36. doi: 10.1186/1471-2202-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-H, Wu Y-R, Chen Y-C, Chen C-M. Plasma inflammatory biomarkers for Huntington’s disease patients and mouse model. Brain Beh Immun. 2015;44:121–127. doi: 10.1016/j.bbi.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Chernick MR. Bootstrap methods: A guide for practioners and researchers. 2nd. Hoboken, NJ: John Wiley & Sons Inc; 2008. [Google Scholar]
- Craig KJ, Hietanen H, Markova IS, Berrios GE. The Irritability Questionnaire: a new scale for the measurement of irritability. Psychiatry Res. 2008;159:367–375. doi: 10.1016/j.psychres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Crotti A, Benner C, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, Cattaneo E, Gage FH, Cleveland DW, Glass CK. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17:513–521. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale M, van Duijn E. Anxiety in Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2015;27:262–271. doi: 10.1176/appi.neuropsych.14100265. [DOI] [PubMed] [Google Scholar]
- Dewhurst K, Oliver J, Trick KLK. Neuropsychiatric aspects of Huntington’s chorea. Confinia Neurologica. 1969;31:254–268. doi: 10.1159/000103486. [DOI] [PubMed] [Google Scholar]
- Di Maio L, Squitieri F, Napolitano G, Campanella G, Trofatter JA, Conneally PM. Onset symptoms in 510 patients with Huntington’s disease. Journal of Medical Genetics. 1993;30:289–292. doi: 10.1136/jmg.30.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC the Predict-HD Ivestigators of the Huntington Study Group. Psychiatric symptoms in Huntington’s disease before diagnosis: The predict-HD study. Biol Psychi. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Mitchell IJ, Beck SR, Cavanna AE, Rickards HE. Altered subjective fear responses in Huntington’s disease. Parkinsonsim and Related Disorders. 2011;17:386–389. doi: 10.1016/j.parkreldis.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Epping EA, Paulsen JS. Depression in the early stages of Huntington disease. Neurodegener Dis Manag. 2011;1:407–414. doi: 10.2217/nmt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Gutekunst CA, Persichetti F, McNeil SM, Kowall NW, Gusella JF, MacDonald ME, Beal MF, Hersch SM. Heterogeneous topographic and cellular distribution of huntingtin expression in the normal human neostriatum. J Neurosci. 1997;17:3052–3063. doi: 10.1523/JNEUROSCI.17-09-03052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest CM, Mackay GM, Stoy N, Spiden SL, Taylor R, Stone TW, Darlington LG. Blood levels of kynurenines, interleukin-23 and soluble human leucocyte antigen-G at different stages of Huntington’s disease. J Neurochem. 2010;112:112–122. doi: 10.1111/j.1471-4159.2009.06442.x. [DOI] [PubMed] [Google Scholar]
- Godec J, Tan Y, Liberzon A, Tamayo P, Bhattacharya S, Butte AJ, Mesirov JP, Haining WN. Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation. Immunity. 2016;44:194–206. doi: 10.1016/j.immuni.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen JL, de Bie RM, Foncke EM, Roos RA, Leenders KL, Tijssen MA. Late-onset Huntington disease with intermediate CAG repeats: true or false? J Neurol Neurosurg Psychiatry. 2010;81:228–230. doi: 10.1136/jnnp.2008.170902. [DOI] [PubMed] [Google Scholar]
- Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hauser MD. Sources of acoustic variation in rhesus macaque (Macaca mulatta) vocalizations. Ethology. 1991;89:29–46. [Google Scholar]
- Heuser IJE, Chase TN, Mouradian MM. The limbic-hypothalamic-pituitary-adrenal axis in Huntington’s disease. Biol Psychi. 1991;30:943–952. doi: 10.1016/0006-3223(91)90007-9. [DOI] [PubMed] [Google Scholar]
- Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s Disease Chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. Am J Primatol. 1998;44:125–135. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kalin N, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kirkwood SC, Siemers E, Viken R, Hodes ME, Conneally PM, Christian JC, Foroud T. Longitudinal personality changes among presymptomatic Huntington disease gene carriers. Neuropsychi Neuropychol Behav Neurol. 2002;15:192–197. [PubMed] [Google Scholar]
- Leblhuber F, Peichl M, Neubauer C, Reisecker F, Steinparz FX, Windhager E, Maschek W. Serum dehydroepiandrosterone and cortisol measurements in Huntington’s chorea. J Neurol Sci. 1995;132:76–79. doi: 10.1016/0022-510x(95)00114-h. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, White K, Weaver M, Flury Wetherill L, Hui S, Stout JC, et al. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64:116–121. doi: 10.1001/archneur.64.1.116. [DOI] [PubMed] [Google Scholar]
- Mason SL, Zhang J, Begeti F, Guzman NV, Lazar AS, Rowe JB, Barker RA, Hampshire A. The role of the amygdala during emotional processing in Huntington’s disease: from pre-manifest to late stage disease. Neuropsychologia. 2015;70:80–89. doi: 10.1016/j.neuropsychologia.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MP, Firth L, O’Connor E. A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings. 2009;16:355–362. doi: 10.1007/s10880-009-9168-5. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8th. Washington, D.C: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Neubert F-X, Mars RB, Sallet J, Rushworth MFS. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci USA. 2015;112:E2695–E704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmagadda SR, Agrawal N, Worrall-Davies A, Markova I, Rickards H. Determinants of irritability in Huntington’s disease. Acta Neuropsychi. 2011;23:309–314. doi: 10.1111/j.1601-5215.2011.00563.x. [DOI] [PubMed] [Google Scholar]
- Pascu AM, Ifteni P, Teodorescu A, Burtea V, Correll CU. Delayed identification and diagnosis of Huntington’s disease due to psychiatric symptoms. Int J Ment Health Syst. 2015;9:e1–e4. doi: 10.1186/s13033-015-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Kazem AJN, Brockhausen RR, Ruiz-Lambides AV, Widdig A. Monkeys spontaneously discriminate their unfamiliar paternal kin under natural conditions using facial cues. Current Biology. 2014;24:1806–1810. doi: 10.1016/j.cub.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Lahiri N, Niccolini F, Su P, Wu K, Giannetti P, Scahill RI, Turkheimer FE, Tabrizi SJ, Piccini P. Increased central microglial activation associated with peripheral cytokine levels in premanifest Huntington’s disease gene carriers. Neurobio Dis. 2015;83:115–121. doi: 10.1016/j.nbd.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Putkhao K, Kocerha J, Cho IK, Yang J, Parnpai R, Chan AWS. Pathogenic cellular phenotypes are germline transmissible in a transgenic primate model of Huntington’s disease. Stem Cells Dev. 2013;22:1198–1205. doi: 10.1089/scd.2012.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SB, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Hormones and Behavior. 2013a;63:646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson ME, Sanchez M, Machado C, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinololgy. 2013b;38:1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommeck I, Capitanio JP, Strand SC, McCowan B. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta) Am J Primatol. 2011;73:692–701. doi: 10.1002/ajp.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, Bernasconi E, Descombes P, Erkizia I, Fellay J, Hirschel B, Miró JM, Palou E, Hoffmann M, Massanella M, Blanco J, Woods M, Günthard HF, de Bakker P, Douek DC, Silvestri G, Martinez-Picado J, Telenti A. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011;121:2391–400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TE, Hinde RA. Vocal communication by the rhesus monkey (Macaca mulatta) P Zoological Society of London. 1962;138:279–294. [Google Scholar]
- Rubinsztein DC, Leggo J, Coles R, Almqvist E, Biancalana V, Cassiman JJ, Chotai K, Connarty M, Crauford D, Curtis A, Curtis D, Davidson MJ, Differ AM, Dode C, Dodge A, Frontali M, Ranen NG, Stine OC, Sherr M, Abbott MH, Franz ML, Graham CA, Harper PS, Hedreen JC, Hayden MR, et al. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. Am J Hum Genet. 1996;59:16–22. [PMC free article] [PubMed] [Google Scholar]
- Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtail monkeys (Macaca nemestrina) Am J Primatol. 2002;56:165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Shannon KM, Fraint A. Therapeutic advances in Huntington’s Disease. Movement Disord. 2015;30:1539–1546. doi: 10.1002/mds.26331. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Taylor CM. Irritability: definition, assessment and associated factors. Br J Psychiatry. 1985;147:127–136. doi: 10.1192/bjp.147.2.127. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Jankovic J. Huntington’s disease: how intermediate are intermediate repeat lengths? Mov Disord. 2012;27:1714–1717. doi: 10.1002/mds.25172. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR, TRACK-HD Investigators Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Trottier Y, Devys D, Imbert G, Saudou F, An I, Lutz Y, Weber C, Aqid Y, Hirsch EC, Mandel JL. Cellular localization of the Huntington’s disease protein and discrimination of the normal and mutated form. Nat. Genet. 1995;10:104–110. doi: 10.1038/ng0595-104. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Sills T, Anderson KE, Borowsky B, Coccaro E, Craufurd D, Endicott J, Giuliano J, Groves M, Guttman M, Ho AK, Kupchak P, Paulsen JS, Stanford MS, van Kammen DP, Watson D, Wu KD, Evans K. Assessing behavioral manifestations prior to clinical diagnosis of Huntington Disease: “anger and irritability” and “obsessions and compulsions.”. PLOS Current Huntington Disease. 2011 doi: 10.1371/currents.RRN1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Stock J, De Winter F-L, Ahmad R, Sunaert S, Van Laere K, Vandenberghe W, Vandenbulcke M. Functional brain changes underlying irritability in premanifest Huntington’s disease. Human Brain Mapping. 2015;36:2681–2690. doi: 10.1002/hbm.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn E, Selis MA, Giltay EJ, Zitman FG, Roos RAC, van Pelt H, van der Mast RC. Hypothalamic-pituitary-adrenal axis functioning in Huntington’s disease mutation carriers compared with mutation-negative first-degree controls. Brain Res Bull. 2010;83:232–237. doi: 10.1016/j.brainresbull.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Vassos E, Panas M, Kladi A, Vassilopoulos D. Higher levels of extroverted hostility detected in gene carriers at risk for Huntington’s disease. Biol Psychi. 2007;62:1347–1352. doi: 10.1016/j.biopsych.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Victorson D, Carlozzi NE, Frank S, Beaumont JL, Cheng W, Gorin B, Duh MS, Samuelson D, Tulsky D, Gutierrez S, Nowinski CJ, Mueller A, Shen V, Sung V. Identifying motor, emotional-behaivoral, and cognitive deficits that comprise the triad of HD symptoms from patient, caregiver, and provider perspectives. Tremor Other Hyperkinet Mov. 2014;4:e1–e11. doi: 10.7916/D8JW8BWS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ross CA, Cai H, Cong W-N, Daimon CM, Carlson OD, et al. Metabolic and hormonal signatures in pre-manifest and manifest Huntington’s disease patients. Front Physiol. 2014;5:1–10. doi: 10.3389/fphys.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:1–10. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild EJ, Tabrizi SJ. Targets for future clinical trials in Huntington’s disease: What’s in the pipeline? Movement Disord. 2014;29:1434–1445. doi: 10.1002/mds.26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, et al. Mouse ENCODE Consortium: A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin AV, Cornish AS, Maudhoo MD, Gibbs RM, Zhang X, Pandey S, Meehan DT, Wipfler K, Bosinger SE, Johnson ZP, Tharp GK, Marçais G, Roberts M, Ferguson B, Fox HS, Treangen T, Salzberg SL, Yorke JA, Norgren RB., Jr A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol Direct. 2014;9:1–20. doi: 10.1186/1745-6150-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.