Abstract
Sickle cell disease (SCD) is a monogenic red cell disorder affecting over 300,000 annual births worldwide and leading to significant organ toxicity and premature mortality. While chronic therapies such as hydroxyurea have improved outcomes, more durable therapeutic and curative options are still being investigated. Newer understanding of the disease has implicated invariant NKT (iNKT) cells as a critical immune profile that potentiates SCD. Hence, targeting this cell population may offer a new approach to disease management. Hematopoietic stem cell transplant (HSCT) is a curative option for patients with SCD, but the under-representation of minorities on the unrelated donor registry means that this is not a feasible option for >75% of patients. Work in this area has therefore focused on increasing the donor pool and decreasing transplant-related toxicities to make this a treatment option for the majority of patients with SCD. This review focuses on the currently available cell and gene therapies for patients with SCD, and acknowledges that newer gene editing approaches to improve gene therapy efficiency and safety are the next wave of potentially curative approaches.
Keywords: Gene therapy, Hematopoietic stem cell transplant, Sickle Cell Anemia, Sickle Cell Disease
INTRODUCTION
Sickle cell disease (SCD) is a disorder with significant morbidity and shortened lifespan that results from the simplest of mutations – a single nucleotide change in the beta globin coding gene where adenine is replaced by thymine. The result is hemoglobin S (HbS), a molecule that has two normal alpha globin and two mutated beta globin chains that, in the deoxygenated state, tends to polymerize instead of remaining soluble as normal hemoglobin would. The polymerization changes the structure of the normally malleable, donut-shaped red cell into the characteristic “sickle”. These red cells are more likely to occlude blood vessels and lead to so-called “vaso-occlusive crises” and infarction. Additionally, the red cells have a shorter lifespan than typical red cells (10–20 vs. 120 days), and the resultant hemolysis leads to nitric oxide scavenging by free hemoglobin, which also contributes to a pro-inflammatory state [1, 2]. The overall effect is significant morbidity for patients from childhood, and the disease manifests with widespread organ dysfunction such as cerebrovascular stroke, pulmonary disease (acute and chronic), pulmonary artery hypertension, nephropathy and debilitating pain crises. The foreshortened average life expectancy in the U.S. is in the 30–40 year range, up to 50 years in very controlled cohorts [3, 4]. In parts of the developing world such in Africa, the mortality rate in children is 50–90% by age 5 with limited access to care being a prominent factor in childhood mortality [5]. This is most troubling since over 300000 patients with SCD are born worldwide each year, with the majority being in developing countries [6].
The survival of children with SCD has improved dramatically in the last 3 decades. Improved supportive care, routine penicillin prophylaxis and vaccination have decreased childhood mortality from approximately 50% to less than 5% in first world nations [7, 8]. However, higher mortality rates in young adults continues to be a problem as patients are still affected by appreciable organ dysfunction and dependence on the medical system. Red cell transfusions and the medication hydroxyurea can ameliorate disease but are limited by patient compliance and may not completely prevent organ injury. Hence, much work is focused on the development of more durable and curative treatments that avoid the requirement of daily patient adherence. This review will focus on the development of immune/ cell-based therapies as a novel treatment approach being explored for patients with SCD.
INVARIANT NATURAL KILLER T CELL (iNKT) TARGETED THERAPIES
The pathobiology of sickle cell disease is now recognized to not only be mediated through red blood cells containing sickle hemoglobin, but also through white blood cells that have been exposed to the hyper-inflammatory milieu of ongoing hemolysis. Invariant natural killer T (iNKT) cells are increased in number and activation in patients with sickle cell disease compared to healthy controls [15]. iNKT cells have recently been shown in SCD mouse models to be a major contributor to the inflammatory response through IFN-γ and production of chemotactic CXCR3 chemokines, leading to vaso-occlusion. Blockade of iNKT inflammatory mechanisms decreases pulmonary inflammation and injury [15]. Given these preclinical results, attempts have now focused on blocking the negative effects of iNKT cells in human disease. Adenosine A2A receptors (A2AR), when engaged, reduce inflammation in a variety of white blood cells and are a potential target to reduce the activation of iNKT cells. One such agonist, regadenson, has now entered clinical trials based on promising results in the SCD mouse model [15, 16]. In Phase I clinical trials, regadenson was administered intravenously for 12 or 24 hours to 27 adults. Twenty-one of the study participants were in a steady disease state and 6 were in painful vaso-occlusive crisis. The iNKT activation of study participants as measured by phospho-NF-κB p65 was compared to healthy controls. In patients with pain crisis, a 24 hour infusion resulted in a median decrease of 48% iNKT cell activation to levels similar to that of both controls and steady-state participants. A phase II clinical trial (NCT01788631) is ongoing.
A humanized iNKT cell depleting antibody, NKTT120, targets the invariant T cell receptor and has recently been developed for application in iNKT-mediated inflammatory conditions such as asthma and sickle cell disease [17]. This antibody has been tested in a Phase I clinical trial (NCT01783691), and early reports show that at the highest tested dose (0.3mg/kg), no dose limiting toxicities are seen and iNKT cells are depleted for a minimum of 28 days before recovery [18]. Further plans for this study have not been announced.
CURRENT STATE OF HEMATOPOIETIC STEM CELL TRANSPLANTATION FOR PATIENTS WITH SCD
The first report of a hematopoietic stem cell transplant (HSCT) curing SCD was in 1984 in a patient with SCD undergoing bone marrow transplant for acute myeloid leukemia using an HLA matched sibling donor. The patient was cured of both diseases, providing proof-of-principle that replacement of the host hematopoietic stem cells (HSCs) harboring the homozygous sickle mutation with healthy cells could lead to disease resolution [19]. Since this report, over 1200 HSCTs have been performed for SCD, with matched sibling marrow being the predominant donor source [20]. Transplant outcomes with matched sibling donors have improved over time, as demonstrated in a pivotal French study of 87 consecutive matched sibling transplants, the event-free survival improved from 76.7% to 95.3% when comparing those transplanted before and after January 2000 [21]. Overall and disease-free survival rates of approximately 95% and 92%, respectively, have subsequently been achieved by multiple groups in recent years. A noticeable change in the last decade is the increased use of less intense preparative regimens that may partially account for the improved outcomes [23]. In the past, HSCT had been reserved for pediatric patients due to concerns that adults with SCD already had developed too much organ injury and would have unacceptable transplant-related morbidity and mortality rates. However, with the use of intense preparative regimens, HSCT trials in adults with SCD have been successfully conducted and have shown excellent results, with overall survival and disease-free survival rates of over 95% and 87–95% respectively [24–27]. An interesting finding from these trials has been that many patients are found to have stable mixes of donor and recipient peripheral myeloid cells (stable mixed chimerism) in their peripheral blood (likely reflecting similar patterns in the bone marrow compartment), and yet still have a predominance of donor red cells in the peripheral blood with resolution of the SCD phenotype. The fact that healthy red blood cells survive longer than those from patients with SCD likely explains the shift to donor red cell predominance despite persistence of residual host cells in the white cell compartment. This data further supports the use of less intense preparative regimens, where the goal can be to achieve some, but not complete, donor hematopoiesis. The result can still be resolution of the SCD phenotype as long as the mixed donor chimerism in the marrow remains stable.
While results have been encouraging for those who have an HLA-matched sibling, there is unfortunately less than a 25% chance of having an acceptable HLA-matched sibling donor based upon inheritance and the potential for matched siblings to also be affected by SCD [28]. This has led to the investigation of unrelated and mismatched donors as a donor source (Table I). Based on case report data and promising experience in other diseases, using a fully HLA-matched unrelated donor is believed by many to be the next best HSCT option if a matched sibling is not available [29, 30]. A multicenter Clinical Trials Network (CTN) study evaluating matched unrelated donor bone marrow transplants for pediatric patients with SCD has recently completed accrual. The study enrolled 29 patients and preliminary reports show a one-year overall and disease-free survival of 86% and 76%, respectively [31]. However, the cumulative incidence of chronic graft-versus-host disease (GVHD) was high at 62%, including 6 patients who died from the complication, making these results less encouraging. While investigators will undoubtedly work toward decreasing this GVHD risk a major obstacle that remains is the probability of finding an HLA-matched unrelated donor who actually proceed with donation for SCD transplant is as low as 9% [32]. This likely reflects a larger issue of insufficient donors of African descent represented on donor registries given that the vast majority of patients with SCD are of African descent. Therefore efforts from groups such as the National Marrow Donor Program and United States government funding initiatives to recruit minority donors are extremely important [33]. Unrelated cord blood (UCB) transplantation is also being investigated as an alternative donor source; however, success has been limited by rejection, as evidenced one study that was prematurely closed after 4 of 8 enrolled patients rejected their grafts [34]. The reduced intensity regimen used in that study included alemtuzumab, melphalan and fludarabine. This platform has since been intensified by the addition of the alkylating agent thiotepa and there is an active UCB transplant study (NCT00920972) using this conditioning regimen for pediatric patients with SCD.
Table I.
Hematopoietic stem cell transplant trials for patients with sickle cell disease without an HLA-matched related donor.
| Ref | Donor | Conditioning Regimen GVHD prophylaxis |
Patient Numbers (Age range in yrs.) |
EFS/OS % at 1yr |
Acute GVHD |
Chronic GVHD |
|---|---|---|---|---|---|---|
| [31] CTN |
8/8 MUD | Alemtuz + Flu + Mel CSA/Tacro + MTX + Methylpred |
29 (5.9–19.3) |
76/86 | 31% | 62% 38% extensive |
| [34] CTN |
5-6/6 UCB | Alemtuz + Flu + Mel CSA/Tacro + MMF |
8 (7.4–16.2) |
38/100 | 66%* | 33%* |
| [35] Johns Hopkins |
Haplo BM | rATG + Flu + CY + TBI 2Gy CY 50mg/kg Day3&4 + MMF + tacro/siro |
14 (15–42) |
50/100 | 0% | 0% |
| [37] St. Jude |
Haplo PBSC, Ex-vivo T cell depleted |
1) rATG+Flu+Thio+Bu+OKT3 or 2)HU+Aza+Bu+Thio+CY+OKT3 MMF |
8 (4–17) |
50/88 38/75 at 3 years |
40%* (Grade II only) |
60%* |
| [39] NYMC multi- center |
Haplo PBSC, Ex-vivo T cell depleted |
HU+Aza+Flu+Bu+Thio+CY+TLI + rATG Tacro |
13 Study still accruing |
92/ N/A | N/A | N/A |
Studies shown have event-free survival (events = graft rejection, death) data of at least 1 year
Values calculated as a percentage of engrafted patients at risk for GVHD
Alemtuz = alemtuzumab, BM = bone marrow, Bu = busulfan, CSA = cyclosporine, CTN = Clinical Trials Network, CY = cyclophosphamide, EFS = event-free survival, Flu = fludarabine, Haplo = haploidentical donor, HU = hydroxyurea, Mel = melphalan, Methylpred = methylprednisolone, MMF = mycophenolate mofetil, MTX = methotrexate, MUD = matched unrelated donor, N/A = not available, NYMC =New York Medical College, OS = overall survival, PBSC = peripheral blood stem cells, rATG = rabbit anti-thymocyte globulin, Siro = sirolimus, Tacro = tacrolimus, TBI = total body irradiation, TLI = total lymphoid irradiation, UCB =unrelated cord blood.
Unrelated donor transplant for SCD is still in early stages, but even when including fully matched unrelated donors and suitable single cord blood units as graft sources, over half of the patients may still not have a suitable donor option [32]. Haploidentical, or HLA half-matched, donors have also been considered as a graft source since the majority of patients should have a first degree relative who could be a potential donor. To date, two general approaches have been reported to achieve the adequate T cell depletion required to remove alloreactive cells and prevent severe GVHD. The first approach reported was from the group at Johns Hopkins University, who transplanted haploidentical marrow into 14 patients (2 adolescents, 12 adults) with severe SCD after a non-myeloablative preparative regimen. High doses of cyclophosphamide were administered on days 3 and 4 after transplant as a T cell depletion strategy. All patients survived without evidence of severe GVHD but 6 of the 14 patients ultimately rejected the grafts and one patient remained on immunosuppression to maintain a very low donor chimerism of only 5–6% [35]. Preliminary reports from a different group in London who intensified the Hopkins non-myeloablative regimen in 16 patients (13 patients with sickle cell disease and 3 with beta thalassemia) showed a much higher rate of engraftment and disease resolution (15 out of 16 patients). However, one patient died after developing secondary graft failure and macrophage activation syndrome (MAS). The rate of acute GVHD (grade II or higher) was 12.5% and chronic GVHD was 6.3% [36]. The other haploidentical donor HSCT approach reported involves ex-vivo T cell depletion. In one report using this approach, 8 patients with SCD underwent haploidentical transplant using a reduced intensity conditioning regimen. In this study, there were two graft infusions: (i) a CD34 positively selected graft and (ii) a CD3 depleted graft with a goal of infusing a maximum of 1 × 105 CD3+ T cells/kg recipient weight. Three patients had sustained engraftment with resolution of their SCD, 3 patients rejected the graft, and 2 patients died of chronic GVHD [37]. In the two patients who died of chronic GVHD, one received a CD3/kilogram dose >1×105 and the other developed GVHD after receiving a donor lymphocyte infusion to prevent rejection. It is important to note that results of this T cell depletion strategy were more encouraging for children with thalassemia, with 71% of patients cured, 2 deaths (from viral infections) and no GVHD [38]. The caveat is that the pre-transplant preparative regimen was more intense for these thalassemia patients. Preliminary results from one trial (NCT01461837) of ex-vivo T cell depletion that added radiation to the conditioning regimen used for the thalassemia patients has an encouraging 92% 1-year event free survival in 13 reported patients with SCD [39]. Therefore, trials using both ex-vivo (NCT01461837, NCT02165007, NCT00968864) and in-vivo (NCT02678143, NCT02013375, NCT02105766) T cell depletion approaches are ongoing in an attempt to prevent rejection and improve outcomes for patients with SCD undergoing haploidentical transplant.
USE OF MESENCHYMAL STROMAL CELLS TO ENHANCE ENGRAFTMENT
Mesenchymal stromal cells (MSCs) are non-hematopoietic anti-inflammatory cells defined by their ability to differentiate into adipocytic, osteocytic and chondrocytic tissues. These cells have shown some efficacy for patients with GVHD and have been a promising strategy to enhance HSC engraftment after transplant outside the context of SCD [40–48]. Based on these results, a small multicenter center study was initiated to evaluate whether infusing bone marrow-derived MSCs from third-party sources on the day of and two days after transplant would prevent rejection in patients with SCD and thalassemia [49]. Six patients (four with SCD and two with thalassemia) underwent transplant with either a 7/8 HLA matched unrelated donor marrow (n=2) or with one or two 4–5/6 umbilical cord unit grafts (n=4). Patients were conditioned with a reduced intensity regimen of alemtuzumab, fludarabine and melphalan with cyclosporine and mycophenolate mofetil (MMF) for GVHD prophylaxis. Two SCD patients who received UCB grafts rejected, with autologous recovery within one-month post-transplant. The remaining 4 patients died at 24, 43, 59 and 141 days post-transplant from intracranial bleed, CMV pneumonitis, disseminated toxoplasmosis and steroid refractory GVHD respectively. The study was closed prematurely, and while no adverse effects related to the MSC infusions were noted, there was no evidence of efficacy and it remains unclear if there was any contribution to the infection risk.
CORD BLOOD EXPANSION STRATEGIES
While transplantation with cord blood increases the donor pool for patients with SCD, some units cannot be used due to insufficient size. The size of a unit recommended for transplant in SCD is higher than that used in malignant disease (5 vs. 2×107 total nucleated cells/kilogram), making finding a suitable cord unit relatively challenging in SCD transplants [50]. The ex-vivo expansion of cord blood-derived stem cells prior to transplant being investigated may be able to overcome this limitation. Culturing human cord blood HSCs in media with nicotinamide, a form of Vitamin B3, and the cytokines thrombopoietin, interleukin (IL)-6, fms-like tyrosine kinase–3 ligand, and stem cell factor, has been shown to expand CD34+ cells in-vitro [51]. Using this methodology, a Phase I trial was initiated for patients with hematologic malignancies. In this trial, the HSC expanded unit was co-transplanted with an unexpanded unit. Of the 11 patients treated, 8 patients showed stable complete or partial neutrophil and T cell engraftment from the expanded unit with a median follow-up of 21 months [52]. This data has prompted investigation using expanded cord blood units as graft sources for patients with SCD in both the double and single cord blood transplant settings (NCT01590628 and NCT02504619).
DISEASE MODIFYING THERAPIES VERSUS HEMATOPOIETIC STEM CELL TRANSPLANT
Non-curative therapies that modify disease progression such as hydroxyurea and chronic red cell transfusions have not been formally compared to HSCT in a multicenter randomized format making it difficult to draw conclusions about which is the best therapy. A randomized multi-center Phase II study of matched donor HSCT compared to best standard therapy for adults with SCD (BMT CTN 1503, STRIDE, NCT02766465) is planned by the CTN to start in 2016 and may address this gap in knowledge. In the interim the comparative information is restricted to small cohorts and observational studies. One such French study evaluated SCD patients at risk of stroke identified by abnormal transcranial Doppler (TCD) velocities and placed initially on chronic transfusions [53]. In this study, all 24 patients that were transplanted (matched sibling donor BMT) were able to safely discontinue transfusions with 23 of them normalizing their TCD including four who did not normalize on transfusions. Of the 46 patients treated with hydroxyurea, 13 had reversion to abnormal TCD and had to be restarted on chronic transfusions. In another report from Belgium of 469 patients with SCD, the mortality rate was highest in the patients undergoing transplant at 5.6% compared to 1.6% for hydroxyurea, and 0% for those on chronic transfusions [13]. However it should be noted that the mortality rate was also quite low in patients who were not on hydroxyurea or transfusions in this cohort at 2.9% and may reflect other beneficial genetic factors and aspects of the medical care in Belgium. Thus in the absence of clear-cut guidelines providers are left to weigh the risks and benefits of each treatment approach. Red cell transfusions significantly reduce the risk of recurrent stroke, progression to stroke from abnormal TCD, acute chest syndrome and pain crises[54, 55]. Monthly red blood cell transfusion therapy has the significant side effect of iron accumulation, which if not managed properly can lead to cardiac, liver and endocrine dysfunction. Additionally, identifying red cell units that are sufficiently antigen-matched to prevent immunization and an antibody response is a challenge and can prevent some patients from obtaining regular transfusions. Hydroxyurea being an oral medication is convenient and additionally improves survival in adults, reduces symptoms (acute chest syndrome and pain crises) and is equivalent to transfusions in preventing progression from abnormal TCD to stroke in certain patients [10, 11]. However, hydroxyurea has not been able to completely prevent all disease injury as evidenced by its inability to prevent recurrent stroke as well as chronic transfusion does [14]. Additionally, other issues such as the need for daily adherence to a medication schedule and concern about long-term side effects have limited the widespread use of this agent for patients with SCD. Transplant is the only curative approach currently available and is shown to normalize TCD velocities, halt brain disease progression and resolve acute SCD symptoms [21, 37, 53, 56]. However the risk of mortality within the first 2 years of transplant is something significant to consider especially if transplant is done early in childhood and the otherwise increased mortality from SCD would be expected to start in early adulthood. Additionally, GVHD from HSCT can negatively impact the health-quality of life and the risk of this will likely increase when using donors other than HLA-matched related donors.
GENE THERAPY
Since SCD is a genetic disorder resulting from a single point mutation, another potentially curative strategy is to use gene therapy to change the thymine back to an adenine at the mutated 6th codon of the beta globin gene. For the last two decades, significant work has been done to address concerns of possible malignant transformation of cell modified by gene therapy vectors. An additional challenge for SCD has been the development of an efficient gene transfer strategy for HSCs that results in sufficient sustained production of gene modified cells that would lead to disease resolution. In 2015, two patients with SCD were enrolled in gene therapy protocols for the treatment of their disease, following some early success in thalassemia [57]. These two SCD patients received busulfan chemotherapy conditioning followed by autologous bone marrow cells that were genetically modified using a lentiviral vector that transferred an engineered beta globin gene (βA(T87Q)) [58, 59]. βA(T87Q) has anti-sickling properties similar to γ-globin, but has less oxygen affinity than γ-globin similar to normal β-globin. One 13-year-old male patient in Europe with SCD and a history of multiple vaso-occlusive crises, silent infarct, and acute chest syndrome on prophylactic transfusions received the βA(T87Q) lentivirally transduced autologous bone marrow after receiving busulfan at myeloablative doses (NCT02151526). This patient demonstrated polyclonal reconstitution without clonal dominance, was able to discontinue blood transfusions 3 months after gene therapy, and at 9 months has been free of severe symptoms related to his SCD. His total hemoglobin maintained off transfusions is 11.4gm/dL with anti-sickling hemoglobin A(T87Q) at 5.5gm/dL and sickle hemoglobin at 5.5gm/dL. The counterpart study in the United States (NCT02140554) is underway and at least one patient has been treated[59]. Two other gene therapy studies for patients with SCD involving lentiviral transfer of γ-globin (NCT02186418) and another anti-sickling globin, βAS3-globin, (NCT02247843) were opened in 2014 and results have yet to be presented.
FUTURE DIRECTIONS AND CHALLENGES
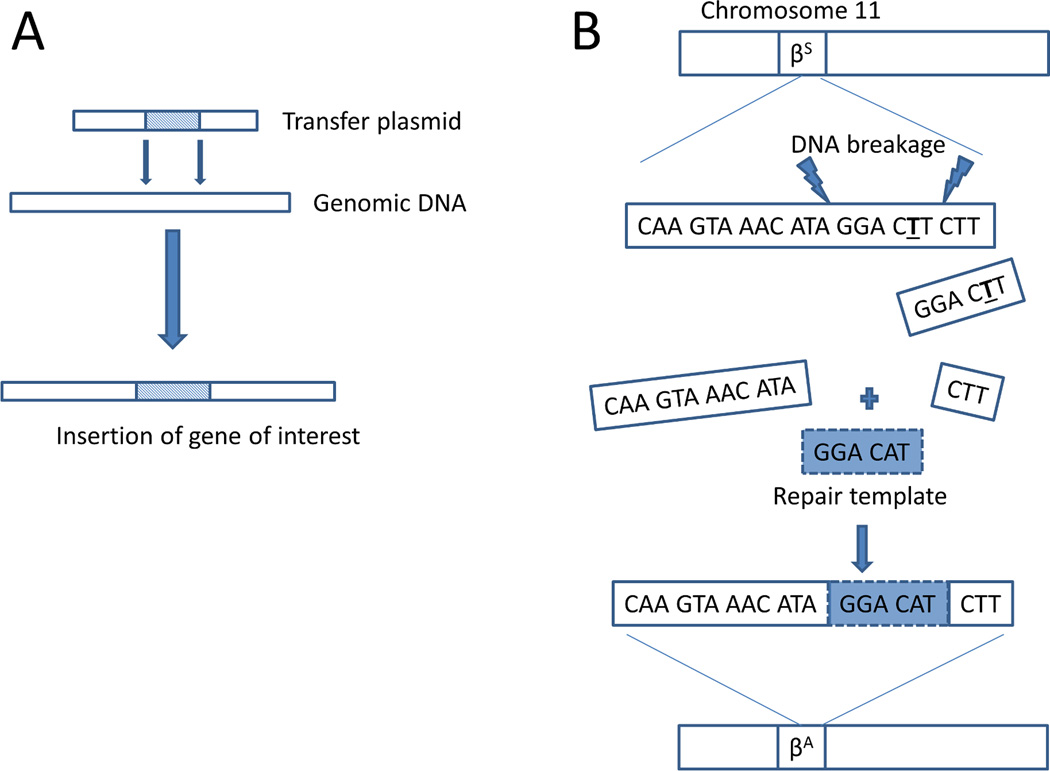
Gene therapy will most likely become the definitive treatment for SCD with the approach of gene transfer into autologous hematopoietic cell progenitors predominating until gene editing is ready for widespread clinical use (Figure 1). However, gene transfer approaches require fine-tuning to consistently offer high efficiency transduction and expression of the transgene without disturbing normal gene function or leading to a clonal expansion event [57, 60, 61]. Advancements in gene transfer technology have been seen, with newer more effective generations of lentivectors currently being clinically evaluated for patients with SCD. It remains to be seen if lentiviral vectors can be further optimized, if other transfer approaches such as sleeping beauty transposons are better, and if a γ-globin, engineered β-globin, or other type of globin is the best to transfer. The penultimate goal is, gene editing with replacement of the defective globin gene. Proof-of-principle in-vitro data using zinc finger nucleases to correct the sickle mutation in human hematopoietic cells with reasonable efficiency has already been described [62]. Gene editing, as opposed to a gene transfer approach, is likely to be the next wave of clinical trials particularly with development of more advanced gene editing tools such as TALEN and CRISPR/CAS9. Eventually, gene editing could allow for the modification of stored autologous umbilical cord blood obtained from patients with SCD, enabling them to receive a potentially curative transplant shortly after birth. However, there will have to be significantly more work done before this approach can be applied at the embryonic level. In utero transplant is currently being explored in animal models with some success[63]. However, gene editing of the endogenous beta globin gene in human embryonic stem cells led to significant off-target mutations, illustrating demonstrating how catastrophic such an approach could be if not rigorously tested in preclinical models [64].
Figure 1. Curative gene therapy strategies for Sickle Cell Disease.
(A) Gene transfer or gene addition approaches involve a transfer vector (lentiviral, retroviral, transposon) that introduces a globin gene (gamma globin, beta globin, anti-sickling beta globin mutant) into random sites in genomic DNA in hematopoietic stem cells. The transferred globin competes with sickle beta globin for alpha globin to form normal hemoglobin molecules leading to disease resolution (B) Gene editing or correction strategies involve inducing DNA breaks (Zinc finger nuclease, CRISPR/Cas9. TALEN) in the mutated sickle beta globin gene and providing a repair template with the normal DNA sequence to be used in the cell’s DNA repair mechanism.
In the meantime, until gene therapy is widely available, the current clinical curative approach for SCD is hematopoietic stem cell transplantation. However, this approach requires further optimization to offer it safely to the majority of patients. As most patients do not have a suitable HLA-matched sibling donor, investigators are exploring HLA-mismatched donor options, but success has been limited by graft rejection. More intense preparative regimens may be employed to non-specifically target the immune cells responsible for rejection. There will undoubtedly be an increase in short-term and long-term adverse effects, including regimen-related toxicity, infection, GVHD and infertility when more intense preparative regimens are utilized. Research is needed to elucidate the exact mechanisms at play in graft rejection so that more targeted, lower toxicity approaches can be developed for patients with SCD. In the interim, being able to treat post-transplant toxicities with novel cells therapies such as antiviral T-cells for infectious complications and MSC and/or Tregs for GVHD will be important to reduce transplant-related morbidity and mortality [40, 41, 47, 48, 65–69]. The best donor source, the infusion dose, and the treatment schedule of these cellular therapies to achieve efficacy are all important aspects that need to be explored.
Acknowledgments
This work was supported by a mentored K12 award (5K12HD001399-15) from the Eunice Kennedy Shriver National Institute of Child Health and Human development
Abbreviations
- A2AR
Adenosine A2A receptors
- CRISPR/CAS9
Clustered regularly-interspaced short palindromic repeats/CRISPR associated protein 9
- CTN
Clinical Trials Network
- CXCR3
chemokine (C-X-C motif) receptor 3
- GVHD
graft-versus-host-disease
- HbF
hemoglobin F
- HbS
hemoglobin S
- HLA
human leukcoyte antigen
- HSCT
hematopoietic stem cell transplant
- IFN-γ
interferon gamma
- IL-6
interleukin 6
- iNKT
invariant natural killer T cell
- MAS
macrophage activating syndrome
- MMF
mycophenolate mofetil
- MSC
mesenchymal stromal cell
- SCD
sickle cell disease
- TALEN
Transcription activator–like effector nuclease
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Almeida CB, Souza LE, Leonardo FC, Costa FT, Werneck CC, Covas DT, et al. Acute hemolytic vascular inflammatory processes are prevented by nitric oxide replacement or a single dose of hydroxyurea. Blood. 2015;126(6):711–720. doi: 10.1182/blood-2014-12-616250. Epub 2015/05/29. PubMed PMID: 26019278. [DOI] [PubMed] [Google Scholar]
- 2.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. Epub 2013/11/28. PubMed PMID: 24277079; PubMed Central PMCID: PMCPMC3894494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–535. doi: 10.1002/ajh.23683. Epub 2014/01/31. PubMed PMID: 24478166; PubMed Central PMCID: PMCPMC3988218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. Epub 1994/06/09. PubMed PMID: 7993409. [DOI] [PubMed] [Google Scholar]
- 5.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 Suppl 4):S398–S405. doi: 10.1016/j.amepre.2011.09.013. Epub 2011/12/07. PubMed PMID: 22099364; PubMed Central PMCID: PMCPMC3708126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X. Epub 2012/10/30. PubMed PMID: 23103089; PubMed Central PMCID: PMCPMC3547249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. Epub 2010/03/03. PubMed PMID: 20194891; PubMed Central PMCID: PMCPMC2867259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr Blood Cancer. 2013;60(9):1482–1486. doi: 10.1002/pbc.24557. Epub 2013/05/03. PubMed PMID: 23637037. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Chin K, Aerbajinai W, Kumkhaek C, Li H, Rodgers GP. Hydroxyurea-inducible SAR1 gene acts through the Gialpha/JNK/Jun pathway to regulate gamma-globin expression. Blood. 2014;124(7):1146–1156. doi: 10.1182/blood-2013-10-534842. Epub 2014/06/11. PubMed PMID: 24914133; PubMed Central PMCID: PMCPMC4133487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. doi: 10.1016/S0140-6736(15)01041-7. Epub 2015/12/17. PubMed PMID: 26670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010;85(6):403–408. doi: 10.1002/ajh.21699. Epub 2010/06/01. PubMed PMID: 20513116; PubMed Central PMCID: PMCPMC2879711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. Epub 2011/05/17. PubMed PMID: 21571150; PubMed Central PMCID: PMCPMC3133619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le PQ, Gulbis B, Dedeken L, Dupont S, Vanderfaeillie A, Heijmans C, et al. Survival among children and adults with sickle cell disease in Belgium: Benefit from hydroxyurea treatment. Pediatr Blood Cancer. 2015;62(11):1956–1961. doi: 10.1002/pbc.25608. Epub 2015/07/16. PubMed PMID: 26173735. [DOI] [PubMed] [Google Scholar]
- 14.Ware RE, Helms RW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) Blood. 2012;119(17):3925–3932. doi: 10.1182/blood-2011-11-392340. Epub 2012/02/10. PubMed PMID: 22318199; PubMed Central PMCID: PMCPMC3350359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, et al. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114(3):667–676. doi: 10.1182/blood-2009-02-205492. Epub 2009/05/13. PubMed PMID: 19433855; PubMed Central PMCID: PMCPMC2713467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field JJ, Lin G, Okam MM, Majerus E, Keefer J, Onyekwere O, et al. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 2013;121(17):3329–3334. doi: 10.1182/blood-2012-11-465963. Epub 2013/02/05. PubMed PMID: 23377438; PubMed Central PMCID: PMCPMC3637009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuplein F, Thariath A, Macdonald S, Truneh A, Mashal R, Schaub R. A humanized monoclonal antibody specific for invariant Natural Killer T (iNKT) cells for in vivo depletion. PLoS One. 2013;8(9):e76692. doi: 10.1371/journal.pone.0076692. Epub 2013/10/03. PubMed PMID: 24086759; PubMed Central PMCID: PMCPMC3785425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majerus EM, Ataga KI, Vichinsky E, Eaton CA, Mazanet R, Nathan DG, et al. NKTT120 Reduces iNKT Cells without Dose Limiting Toxicity in Stable Adult Sickle Cell Patients in a Phase 1 Trial. Blood. 2014;124(21):2718. [Google Scholar]
- 19.Johnson F, Look A, Gockerman J, Ruggiero M, Dalla-Pozza L, Billings Fr. Bone-marrow transplantation in a patient with sickle-cell anemia. N Engl J Med. 1984;311(12):780–783. doi: 10.1056/NEJM198409203111207. PubMed PMID: 6382010. [DOI] [PubMed] [Google Scholar]
- 20.Gluckman E. Allogeneic transplantation strategies including haploidentical transplantation in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013;2013:370–376. doi: 10.1182/asheducation-2013.1.370. Epub 2013/12/10. PubMed PMID: 24319206. [DOI] [PubMed] [Google Scholar]
- 21.Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110(7):2749–2756. doi: 10.1182/blood-2007-03-079665. PubMed PMID: 17606762. [DOI] [PubMed] [Google Scholar]
- 22.Walters MC, De Castro LM, Sullivan KM, Krishnamurti L, Kamani N, Bredeson C, et al. Indications and Results of HLA-Identical Sibling Hematopoietic Cell Transplantation for Sickle Cell Disease. Biol Blood Marrow Transplant. 2016;22(2):207–211. doi: 10.1016/j.bbmt.2015.10.017. Epub 2015/10/27. PubMed PMID: 26500093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzhugh CD, Abraham AA, Tisdale JF, Hsieh MM. Hematopoietic stem cell transplantation for patients with sickle cell disease: progress and future directions. Hematol Oncol Clin North Am. 2014;28(6):1171–1185. doi: 10.1016/j.hoc.2014.08.014. Epub 2014/12/03. PubMed PMID: 25459186; PubMed Central PMCID: PMCPMC4254544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh MM, Fitzhugh CD, Weitzel RP, Link ME, Coles WA, Zhao X, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. Jama. 2014;312(1):48–56. doi: 10.1001/jama.2014.7192. Epub 2014/07/25. PubMed PMID: 25058217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh M, Kang E, Fitzhugh C, Link M, Bolan C, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361(24):2309–2317. doi: 10.1056/NEJMoa0904971. doi: 361/24/2309 [pii] 10.1056/NEJMoa0904971. PubMed PMID: 20007560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraf SL, Oh AL, Patel PR, Jalundhwala Y, Sweiss K, Koshy M, et al. Nonmyeloablative Stem Cell Transplantation with Alemtuzumab/Low-Dose Irradiation to Cure and Improve the Quality of Life of Adults with Sickle Cell Disease. Biol Blood Marrow Transplant. 2016;22(3):441–448. doi: 10.1016/j.bbmt.2015.08.036. Epub 2015/09/09. PubMed PMID: 26348889. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamurti L, Sullivan KM, Kamani NR, Waller EK, Abraham A, Campigotto F, et al. Results of a Multicenter Pilot Investigation of Bone Marrow Transplantation in Adults with Sickle Cell Disease (STRIDE) Blood. 2015;126(23):543. [Google Scholar]
- 28.Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16(1):27–29. Epub 1994/02/01. PubMed PMID: 8311169. [PubMed] [Google Scholar]
- 29.Mynarek M, Bettoni da Cunha Riehm C, Brinkmann F, Weissenborn K, Tell-Luersen M, Heuft HG, et al. Normalized transcranial Doppler velocities, stroke prevention and improved pulmonary function after stem cell transplantation in children with sickle cell anemia. Klin Padiatr. 2013;225(3):127–132. doi: 10.1055/s-0033-1333754. Epub 2013/04/30. PubMed PMID: 23625683. [DOI] [PubMed] [Google Scholar]
- 30.Pasquini M, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. 2014 Available from: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx#DownloadSummarySlides. [Google Scholar]
- 31.Shenoy S, Eapen M, Wu J, Walters MC, Levine JE, Logan B, et al. A Multicenter Phase II Trial of Unrelated Donor Reduced Intensity Bone Marrow Transplantation for Children with Severe Sickle Cell Disease (SCURT): Results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0601) Study. 2015 [Google Scholar]
- 32.Justus D, Perez-Albuerne E, Dioguardi J, Jacobsohn D, Abraham A. Allogeneic donor availability for hematopoietic stem cell transplantation in children with sickle cell disease. Pediatr Blood Cancer. 2015;62(7):1285–1287. doi: 10.1002/pbc.25439. Epub 2015/02/11. PubMed PMID: 25663074. [DOI] [PubMed] [Google Scholar]
- 33.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. Epub 2014/07/24. PubMed PMID: 25054717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamani NR, Walters MC, Carter S, Aquino V, Brochstein JA, Chaudhury S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18(8):1265–1272. doi: 10.1016/j.bbmt.2012.01.019. Epub 2012/02/22. PubMed PMID: 22343376; PubMed Central PMCID: PMCPmc3618440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285–4291. doi: 10.1182/blood-2012-07-438408. Epub 2012/09/08. PubMed PMID: 22955919; PubMed Central PMCID: PMCPmc3507140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Fuente J, O'Boyle F, Harrington Y, Bradshaw A, Hing S, Chakravorty S, et al. Haploidentical BMT with a Post-Infusion of Stem Cells Cyclophosphamide Approach Is Feasible and Leads to a High Rate of Donor Engraftment in Haemoglobinopathies Allowing Universal Application of Transplantation. Blood. 2015;126(23):4317. [Google Scholar]
- 37.Dallas MH, Triplett B, Shook DR, Hartford C, Srinivasan A, Laver J, et al. Long-term outcome and evaluation of organ function in pediatric patients undergoing haploidentical and matched related hematopoietic cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2013;19(5):820–830. doi: 10.1016/j.bbmt.2013.02.010. Epub 2013/02/19. PubMed PMID: 23416852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sodani P, Isgrò A, Gaziev J, Polchi P, Paciaroni K, Marziali M, et al. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood. 2010;115(6):1296–1302. doi: 10.1182/blood-2009-05-218982. doi: blood-2009-05-218982 [pii] 10.1182/blood-2009-05-218982. PubMed PMID: 19897573. [DOI] [PubMed] [Google Scholar]
- 39.Hamill D, Shenoy S, Morris E, Fabricatore S, Brand P, Ayello J, et al. Robust Donor Chimerism and Engraftment Following Familial Haploidentical (FHI) (CD34 Enriched and T-Cell Addback) Allogeneic Stem Cell Transplantation in Patients with High Risk Sickle Cell Disease (SCD) Biology of Blood and Marrow Transplant. 2016;22(3) [Google Scholar]
- 40.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. PubMed PMID: 18468541. [DOI] [PubMed] [Google Scholar]
- 41.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–2767. doi: 10.1182/blood-2007-04-087056. Epub 2007/07/20. PubMed PMID: 17638847. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Wang Z, Cao Y, Xu L, Li X, Liu P, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol. 2013 doi: 10.1007/s00277-013-1831-0. Epub 2013/07/12. PubMed PMID: 23842707. [DOI] [PubMed] [Google Scholar]
- 43.Wu KH, Sheu JN, Wu HP, Tsai C, Sieber M, Peng CT, et al. Cotransplantation of Umbilical Cord-Derived Mesenchymal Stem Cells Promote Hematopoietic Engraftment in Cord Blood Transplantation: A Pilot Study. Transplantation. 2012 doi: 10.1097/TP.0b013e31827a93dd. Epub 2013/01/01. PubMed PMID: 23274973. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Chen Y, Zeng Y, Xu L, Liu D, Chen H, et al. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev. 2011;20(10):1679–1685. doi: 10.1089/scd.2010.0447. Epub 2010/12/15. PubMed PMID: 21142788. [DOI] [PubMed] [Google Scholar]
- 45.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17(4):534–541. doi: 10.1016/j.bbmt.2010.04.014. Epub 2010/05/12. PubMed PMID: 20457269. [DOI] [PubMed] [Google Scholar]
- 46.Macmillan ML, Blazar BR, DeFor TE, Wagner JE. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant. 2009;43(6):447–454. doi: 10.1038/bmt.2008.348. PubMed PMID: 18955980. [DOI] [PubMed] [Google Scholar]
- 47.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. Epub 2006/05/30. PubMed PMID: 16732175. [DOI] [PubMed] [Google Scholar]
- 48.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. doi: 10.1016/j.bbmt.2005.02.001. PubMed PMID: 15846293. [DOI] [PubMed] [Google Scholar]
- 49.Kharbanda S, Smith AR, Hutchinson SK, McKenna DH, Ball JB, Lamb LS, Jr, et al. Unrelated donor allogeneic hematopoietic stem cell transplantation for patients with hemoglobinopathies using a reduced-intensity conditioning regimen and third-party mesenchymal stromal cells. Biol Blood Marrow Transplant. 2014;20(4):581–586. doi: 10.1016/j.bbmt.2013.12.564. Epub 2013/12/29. PubMed PMID: 24370862; PubMed Central PMCID: PMCPMC3998675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggeri A, Eapen M, Scaravadou A, Cairo MS, Bhatia M, Kurtzberg J, et al. Umbilical cord blood transplantation for children with thalassemia and sickle. Biol Blood Marrow Transplant. 2011;17(9):1375–1382. doi: 10.1016/j.bbmt.2011.01.012. Epub 2011/02/01. PubMed PMID: 21277376; PubMed Central PMCID: PMCPmc3395002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40(4):342.e1–355.e1. doi: 10.1016/j.exphem.2011.12.005. Epub 2011/12/27. PubMed PMID: 22198152. [DOI] [PubMed] [Google Scholar]
- 52.Horwitz ME, Frassoni F. Improving the outcome of umbilical cord blood transplantation through ex vivo expansion or graft manipulation. Cytotherapy. 2015;17(6):730–738. doi: 10.1016/j.jcyt.2015.02.004. Epub 2015/03/18. PubMed PMID: 25778757. [DOI] [PubMed] [Google Scholar]
- 53.Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Hau I, Leveille E, et al. Long-term treatment follow-up of children with sickle cell disease monitored with abnormal transcranial Doppler velocities. Blood. 2016;127(14):1814–1822. doi: 10.1182/blood-2015-10-675231. Epub 2016/02/07. PubMed PMID: 26851292. [DOI] [PubMed] [Google Scholar]
- 54.Miller ST, Wright E, Abboud M, Berman B, Files B, Scher CD, et al. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139(6):785–789. doi: 10.1067/mpd.2001.119593. Epub 2001/12/18. PubMed PMID: 11743502. [DOI] [PubMed] [Google Scholar]
- 55.Adams R, McKie V, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. PubMed PMID: 9647873. [DOI] [PubMed] [Google Scholar]
- 56.Walters M, Storb R, Patience M, Leisenring W, Taylor T, Sanders J, et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood. 2000;95(6):1918–1924. PubMed PMID: 10706855. [PubMed] [Google Scholar]
- 57.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328. doi: nature09328 [pii] 10.1038/nature09328. PubMed PMID: 20844535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavazzana MR, J Payen E, Beuzard Suarez F, Touzot Y, Cavallesco F, Lefrere R, Chretien F, Bourget S, Monpoux P, Pondarre F, Neven C, Schmidt B, von Kalle M, Kuypers C, Sandler FA, Soni L, Hermine S, Blanche O, De Montalembert S, Hacien-Bey-Abina M, Leboulch P S. Outcomes of Gene Therapy for Severe Sickle Disease and Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex Vivo with a Lentiviral Beta AT87Q-Globin Vector. Blood. 2015;126(23):202. [Google Scholar]
- 59.Kanter JW, MC Hsieh M, Krishnamurti Thompson AA, Kwiatkowski L, Kamble J, von Kalle RT, Kuypers C, Cavazzana FA, Leboulch M, Sandler P, Soni L, Tisdale JF S. Initial Results from Study Hgb-206: A Phase 1 Study Evaluating Gene Therapy By Transplantation of Autologous CD34+ Stem Cells Transduced Ex Vivo with the Lentiglobin BB305 Lentiviral Vector in Subjects with Severe Sickle Cell Disease. Blood. 2015;126(23):3233. [Google Scholar]
- 60.Howe S, Mansour M, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798. PubMed PMID: 18688286; PubMed Central PMCID: PMCPMC2496964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu B, Evans-Galea M, Gray J, Bodine D, Persons D, Nienhuis A. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111(4):1866–1875. doi: 10.1182/blood-2007-04-085506. doi: blood-2007-04-085506 [pii] 10.1182/blood-2007-04-085506. PubMed PMID: 17991809; PubMed Central PMCID: PMCPMC2234041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoban MD, Cost GJ, Mendel MC, Romero Z, Kaufman ML, Joglekar AV, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125(17):2597–2604. doi: 10.1182/blood-2014-12-615948. Epub 2015/03/04. PubMed PMID: 25733580; PubMed Central PMCID: PMCPMC4408287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peranteau WH, Hayashi S, Abdulmalik O, Chen Q, Merchant A, Asakura T, et al. Correction of murine hemoglobinopathies by prenatal tolerance induction and postnatal nonmyeloablative allogeneic BM transplants. Blood. 2015;126(10):1245–1254. doi: 10.1182/blood-2015-03-636803. Epub 2015/07/01. PubMed PMID: 26124498; PubMed Central PMCID: PMCPMC4559936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6(5):363–372. doi: 10.1007/s13238-015-0153-5. Epub 2015/04/22. PubMed PMID: 25894090; PubMed Central PMCID: PMCPMC4417674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21(11):2113–2121. doi: 10.1038/mt.2013.151. Epub 2013/06/21. PubMed PMID: 23783429; PubMed Central PMCID: PMCPMC3831033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. Epub 2009/08/25. PubMed PMID: 19700662; PubMed Central PMCID: PMCPmc2774556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. Epub 2013/04/24. PubMed PMID: 23610374; PubMed Central PMCID: PMCPmc3695359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato K, Ozaki K, Mori M, Muroi K, Ozawa K. Mesenchymal stromal cells for graft-versus-host disease : basic aspects and clinical outcomes. J Clin Exp Hematop. 2010;50(2):79–89. doi: 10.3960/jslrt.50.79. Epub 2010/12/03. PubMed PMID: 21123965. [DOI] [PubMed] [Google Scholar]
- 69.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–1051. doi: 10.1182/blood-2015-06-653667. Epub 2015/11/14. PubMed PMID: 26563133; PubMed Central PMCID: PMCPMC4768428. [DOI] [PMC free article] [PubMed] [Google Scholar]