Abstract
Prostate cancer (PCa), a hormonally-driven cancer, ranks first in incidence and second in cancer related mortality in men in most Western industrialized countries. Androgen and androgen receptor (AR) are the dominant modulators of PCa growth. Over the last two decades multiple advancements in screening, treatment, surveillance and palliative care of PCa have significantly increased quality of life and survival following diagnosis. However, over 20% of patients initially diagnosed with PCa still develop an aggressive and treatment-refractory disease. Prevention or treatment for hormone-refractory PCa using bioactive compounds from marine sponges, mushrooms, and edible plants either as single agents or as adjuvants to existing therapy, has not been clinically successful. Major advancements have been made in the identification, testing and modification of the existing molecular structures of natural products. Additionally, conjugation of these compounds to novel matrices has enhanced their bio-availability; a big step towards bringing natural products to clinical trials. Natural products derived from edible plants (nutraceuticals), and common folk-medicines might offer advantages over synthetic compounds due to their broader range of targets, as compared to mostly single target synthetic anticancer compounds; e.g. kinase inhibitors. The use of synthetic inhibitors or antibodies that target a single aberrant molecule in cancer cells might be in part responsible for emergence of treatment refractory cancers. Nutraceuticals that target AR signaling (epigallocatechin gallate [EGCG], curcumin, and 5α-reductase inhibitors), AR synthesis (ericifolin, capsaicin and others) or AR degradation (betulinic acid, di-indolyl diamine, sulphoraphane, silibinin and others) are prime candidates for use as adjuvant or mono-therapies. Nutraceuticals target multiple pathophysiological mechanisms involved during cancer development and progression and thus have potential to simultaneously inhibit both prostate cancer growth and metastatic progression (e.g., inhibition of angiogenesis, epithelial-mesenchymal transition (EMT) and proliferation). Given their multi-targeting properties along with relatively lower systemic toxicity, these compounds offer significant therapeutic advantages for prevention and treatment of PCa. This review emphasizes the potential application of some of the well-researched natural compounds that target AR for prevention and therapy of PCa.
Keywords: Androgen receptor inhibitors, regulation, protein stability, anticancer natural products, cancer prevention
1. Introduction
Prostate cancer (PCa) ranks first in incidence and second in mortality among men in Western industrialized countries. In the United States of America, over 220,000 cases of PCa were diagnosed in 2015, accounting for approximately 13% of all cancers combined or 26% of cancers diagnosed in men [1]. Circulating prostate specific antigen (PSA) has become a universally accepted marker for detection and surveillance of PCa, improving early detection of the disease.
Despite the significant effort made in the fight against PCa, it still remains the most common male malignancy and the second-leading cause of cancer related deaths for men in the United States [2, 3]. Despite significant improvement in disease-free survival following initial surgical or radiation therapy, the disease recurs in >30% of patients. Furthermore, since the recurring disease is non-localized, the treatment options available are limited to systemic therapies.
2. Current treatments for prostate cancer
When PCa is localized to the prostate and surrounding tissues, surgery or radiotherapy can be used effectively in most patients [4]. The standard therapy for advanced/metastatic disease has been androgen deprivation therapy (ADT). However, this approach often results in resistance and has recently become a less common therapeutic option [5]. Bilateral orchiectomy is one classical strategy for androgen suppression; however, this method is associated with numerous side effects and risk factors [4] and results in castration-resistant prostate cancer (CRPC). This method is rarely used in developed countries. Treatment with chemical compounds targeting gonadotropin-releasing hormone offers an alternative to surgical castration. These prevent the secretion of luteinizing hormone and follicle-stimulating hormone, thus inhibiting downstream testosterone synthesis. Unfortunately, these options lose efficacy over time as cancer cells develop resistance to ADT and proliferate despite the absence of androgen, progressing into CRPC. Before 2010, the only treatment shown to inhibit disease progression and improve overall survival for patients with CRPC was the drug docetaxel; however, this compound could only lengthen the life span of CRPC patients for a few months [4, 6]. Since 2010, five new therapeutics have been approved in the United States which extend median survival by only 3–5 months [7]. The autologous cellular immunotherapy, sipuleucel-T is used for minimally symptomatic disease. Cabazitaxel (microtubule inhibitor), enzalutamide (AR inhibitor), and abiraterone acetate (CYP17/androgen biosynthesis inhibitor) are used for pre- and post-docetaxel treatment; whereas, the radiopharmaceutical radium-223 is used for the treatment of post-docetaxel and docetaxel-ineligible symptomatic bone metastases in the absence of visceral metastases [6, 7].
A decrease in the mortality rate among PCa patients may be attributed to early diagnosis by PSA testing and other measures. However, a high mortality rate is still observed in CRPC patients [4]. Current therapies often fail to cure this malignancy and despite the development of novel therapeutic strategies, survival can only be extended for 4–6 months. Current approaches for palliative and curative treatment of PCa are associated with significant cost and morbidity [8] indicating that chemoprevention of PCa should be the main strategy to reduce PCa mortality [3].
3. Role of androgens and AR in PCa progression
PCa is an endocrine related malignancy driven by circulating androgens. First line systemic treatment for PCa is total androgen-blockade by anti-androgens and inhibitors of AR activity. Unfortunately, the regression of the disease following withdrawal of androgens or inhibiting AR signaling is often transient, lasting no more than a few years [9]. Most patients with advanced disease develop resistance to ADT and progress with CRPC [3]. PCa cells can overcome castration-induced growth arrest through an up-regulation of enzymes that drive the synthesis of androgens in the tumor tissue [9]. Intratumoral synthesis of androgens and conversion of adrenal androgens to testosterone and dihydrotestosterone (DHT) are often observed in CRPC [7, 10, 11]. Moreover, a gain-of-stability mutation in 3β-hydroxysteroid dehydrogenase type 1 (3βHSD1), an enzyme that mediates conversion of the weak androgen steroid dehydroepiandrosterone (DHEA) to the potent DHT, has been observed in CRPC. This mutation prevents 3βHSD1 from being ubiquitinated, thus protecting it from degradation by the proteasome. The accumulation of 3βHSD1 results in increased levels of DHT and constitutive AR activation [12].
Interestingly, recent studies suggest that CRPC cells, although resistant to ADT, remain addicted to AR-driven signaling [2]. AR becomes hyperactive in CRPC through increased AR expression. This hyperactivity may result from AR gene amplification, increased rate of AR gene transcription and/or increased AR transcript stability [13, 14], expression of constitutively active ligand-independent AR splice variants, or AR mutations that facilitate activation by non-androgens [15]. Moreover, the previously mentioned intratumoral steroidogenesis and overexpression of AR co-activators in tumor cells may also contribute to the hyperactivity of AR in CRPC [15]. In some PCa cell lines, androgen independence and elevated AR signaling can be attributed to AR trans-repressors and trans-activators. A recent publication identified 20 human genes that regulate expression of AR responsive genes in the absence of androgens. Among them, IGSF8 and RTN1 are shown to negatively regulate the progression from androgen dependence (castration sensitive) to an androgen-independent (castration resistant) state; their down-regulation enabled castration resistant cell proliferation of androgen-dependent PCa cells [15]. While strategies that inhibit androgen synthesis or interfere with androgen-AR interaction ultimately fail in the treatment of CRPC, methods which target the synthesis or activity of AR remain powerful therapeutic options [2].
4. Chemodietary prevention of prostate cancer
Early chemoprevention strategies included long-term administration of 5α-reductase inhibitors such as finasteride, a drug approved by the Food and Drug Administration (FDA) of the United States. Finasteride is a selective inhibitor of type II and type III 5α-reductase with limited activity against the type I enzyme [16, 17]. 5α-reductase converts testosterone to DHT, the main hormone driving PCa cell proliferation in untreated patients. The finasteride clinical trial, also known as Prostate Cancer Prevention Trial (PCPT) was carried out on 18,882 men aged ≥55 years and lasted seven years [18, 19]. The main hypothesis was that reducing the level of DHT in older men will reduce the incidence of PCa and likely its progression. The trial was overall a success in which the incidence in the prevention group was ~ 30% lower than in the control (placebo) group [20]. However, an 18 year follow-up of this trial revealed that high-grade PCa (Gleason 8–10) was more common, although marginally, in the finasteride treated group than in the placebo group. Furthermore, there was no significant difference between treated and control groups in either overall survival or survival after the diagnosis of PCa [21]. A recent study reported by Chau et al. [22] analyzed the concentration of serum finasteride levels reported from the PCPT trial. They determined there was no correlation between the finasteride serum concentration and the risk for developing PCa. The authors investigated whether polymorphisms within the enzymes that are involved in finasteride metabolism have an effect on its serum concentration. They found that two single nucleotide polymorphisms (SNPs) within the enzyme CYP3A4 were associated with reduced plasma levels of finasteride. Other SNPs in CYP3A4 as well as CYP3A5 resulted in higher finasteride plasma levels [22].
In contrast to the PCPT trial, the results of another long-term trial called the SELECT trial using oral Vitamin E (α-tocopherol, 400 IU/day) and L-selenomethionine (0.2 mg/day) in similar population cohorts resulted in a negative outcome [23]. The SELECT trial found no decrease in PCa risk in the selenium supplement treated group and a statistically insignificant increase in PCa risk with vitamin E [23]. A silver lining in this study was the finding that PCa incidence decreased with respect to a linear increase in 25-hydroxy vitamin D in circulation of the patients enrolled in the study [24].
Few other defined drugs have been tested for prevention of PCa either among the general population or specifically the high risk population, such as men with a family history of prostate or breast cancers among their siblings, parents or first-degree cousins. A recent recommendation by the committee on prostate cancer treatment and prevention [25] suggests that patients with PSA<4 ng/ml should be given a choice for active surveillance as an alternative to treatment; thus heightening the need to develop strategies for prevention of PCa progression. Since about 20–30% of patients with PCa in the active surveillance group progress to more aggressive disease, there are palpable benefits in developing drugs or strategies that prolong the surveillance period and prevent rapid progression. Extensive studies on the activities of nature-derived compounds must be considered in the search for chemopreventative options for PCa.
5. Potential target population for natural product-based therapy in PCa
The present review is focused on the potential use of natural products that have proven inhibitory effects on the androgen-AR signaling pathway. A select set of natural compounds have been shown to be efficacious in a variety of animal models of PCa. Some of these compounds, as described in this review, have undergone limited clinical trials for some length of time mostly aimed to identify efficacy against all cancers in general. The populations of men that could benefit from the use of natural products include those with familial risk of developing PCa, those diagnosed with serum PSA between 2 and 4 ng/ml or those ineligible for both radiation and surgery on the grounds of pre-existing medical conditions. In addition, patients may also be considered for such treatments following localized therapy, surgery, brachytherapy or external beam radiation therapy.
6. Natural products against PCa
Most of the chemotherapy drugs used for treating cancers for the last 40 years have their root in natural products; furthermore, compounds from nature that are currently being characterized may provide various lead structures that can be used as templates for the synthesis of new, pharmacologically more effective agents [26]. Natural anticancer products are found in vegetables, fruits, herbs, and fermented plant products and extracts. The anticancer activity of these products might be related to their action on cells as antioxidants, free-radical scavangers, and inhibitors of DNA modifying enzymes. Together, these properties are likely to be protective against somatic mutations and unfavorable epigenetic DNA modifications [27]. These are potentially appropriate candidates for chemoprevention because associated adverse side effects, if any at all, are reported to be minimal. Epidemiological studies demonstrate that natural products protect against a wide array of malignancies such as lung, colorectal, stomach and esophageal cancers and to a lesser extent PCa. PCa has a long latency before clinical symptoms develop and a very high incidence rate in most economically developed countries which make this cancer type a favorable target for chemoprevention [28, 29]. Compounds that can prevent PCa initiation or delay its progression to CRPC should be able to reduce PCa related mortality.
Researchers have demonstrated anti-PCa activities of several natural compounds, including soy isoflavones (mostly genestein), elegiac acid and ellagitannins from pomegranate extract, green tea polyphenols, curcumin, lycopene, vitamins D and L-selenomethionine, both in vitro and in vivo. These agents target one or more signaling pathways that are deregulated in PCa, such as AR, nuclear factor κB (NF-κB), Wnt, Akt, Notch and Hedgehog (Hh) signaling [3]. This current review will focus on natural compounds that target AR-mediated cell signaling that leads to PCa growth and progression.
6.1. Products that target androgen synthesis
Several strategies involved in PCa therapy are focused on the direct inhibition of androgen-stimulated AR signaling. However, as PCa can escape this repression through up-regulation of AR or via mutations making AR more sensitive to a residual low concentration of androgens [30], approaches are necessary to target and eliminate androgen synthesis. To combat this mechanism of resistance, several natural compounds were analyzed for their efficacy in eliminating AR activating androgens [31]. One conventional method of anti-androgen therapy has been to inhibit the activity of the type I and type II 5-α-reductases which catalyze the conversion of testosterone to the much more potent AR activator, DHT. Some investigators tested the extracts from certain edible mushrooms to inhibit 5-α-reductase activity [32]. They identified several species of compounds with potent inhibitory activity against enzymes involved in androgen synthesis with the greatest effect derived from Ganoderma lucidum (Red Reishi) [31, 32]. Two triterpenoid compounds in the G. lucidum extract which yield the greatest suppression of 5-α-reductase activity were identified to be ganoderic acids DM and TR, both of which inhibited the enzyme activity with an IC50 of 10 µM [33]. Green tea extract has also been the subject of study regarding inhibition of 5-α-reductase activity. The major polyphenols present in green tea, epigallocatechin-3 gallate (EGCG) and epicatechin-3-gallate (ECG) have shown potent anti-PCa potential, due in part to their inhibition of 5-α-reductase [34, 35].
Other natural products have been discovered to act as anti-androgens by reducing the activity of enzymes involved in the pathway leading to the formation of testosterone [31]. Studies have shown that consumption of the extract from licorice root (Glycyrrhizia glabra) is associated with reduced circulating testosterone in humans [31]. A study into this effect has revealed that glycyrrhetinic acid (GA), a bioactive metabolite of glycyrrhizic acid (GL) found in licorice, is responsible for this activity [36]. This compound targets two enzymes involved in androgen synthesis. GA inhibits the enzyme, 17,20-lyase which is required for the conversion of 17-hydroxyprogesterone to androstenedione, the precursor molecule to testosterone. Furthermore, it prevents 17β-hydroxysteroid dehydrogenase from completing the conversion of androstenedione to testosterone [31, 36].
By targeting the mechanisms involved in the synthesis of androgens, these compounds have great potential to prevent at least some mechanisms of resistance to ADT for PCa. Since a low level of androgen may remain in a patient undergoing ADT, methods to further reduce androgen synthesis are important avenues of research. By inhibiting the pathways involved in testosterone synthesis, and preventing catalysis to the more potent ligand, DHT [37], these nature-derived compounds could be utilized in combination with standard ADT to improve efficacy of treatment and potentially prevent the onset of CRPC.
6.2. Products that target AR-initiated growth signaling
Therapies that target the synthesis of androgens or that interfere with the interaction of androgens with AR often fail since recurrent PCa during androgen deprivation is often characterized by an up-regulation of AR expression which compensates for the lower level of androgens [38]. In some instances, PCa can acquire genetic or epigenetic changes that alter the AR structure and confer an androgen-independent growth phenotype. For instance, mutations in the androgen binding domains may result in increased responsiveness of the receptor towards androgens or develop ligand-independent, constitutive AR activation [37]. Additionally, genetic changes might also turn receptor antagonists into agonists [2]. In vitro studies of T878A and H875Y AR ligand binding domain (LBD) mutations showed that both mutants are paradoxically activated, rather than inhibited, by the anti-androgens nilutamide and flutamide [37, 39, 40]. Thus, directly targeting the activity of AR may provide a more powerful tool for PCa therapy.
One study demonstrated anti-proliferative and proapoptotic effects of 3, 3’-diindolylmethane (DIM) in androgen-responsive LNCaP and androgen-insensitive C4-2B PCa cells which was attributed to its effects on AR, Akt, and NF-κB signaling [41]. DIM is a dimeric product of indole-3-carbitol (I3C) produced from plant derived glucossinolates from the Cruciferae family [3]. The authors claim that B-DIM (a formulated DIM with higher bioavailability) significantly reduced Akt activation, decreased NF-κB DNA binding activity, and inhibited AR phosphorylation. They also observed reduced expression of PSA. They conclude that cell proliferation inhibition and apoptosis induction are mediated in part through down-regulation of AR, Akt, and NF-κB signaling or by B-DIM mediated interruption of the crosstalk between these pathways [41]. A recent report on a human clinical trial of BR-DIM (formulated DIM manufactured by BioResponse) shows intraprostatic accumulation of BR-DIM and inhibition of multiple targets in AR signaling, including microRNAs that enhance AR transcription, stem cell characteristics and epithelial to mesenchymal transition of CRPC [42].
The bioactive compound curcumin, derived from turmeric (Curcuma longa) has received considerable attention due to its anti-carcinogenic activities as demonstrated in numerous studies in animal models [43–47]. Importantly, several curcumin analogs have been shown to function as AR antagonists in the presence of AR and the AR co-activator, ARA70 [48]. Unfortunately, the oral bioavailability of curcumin is low and making improvements to this is currently an active area of research [3]. Several investigators demonstrated that curcumin analogs inhibit testosterone- or DHT-induced AR activity and inhibit growth of CWR-22Rv1 and LNCaP cells [43, 49].
6.3. Drugs targeting AR transactivation activity (N-terminal domain)
Interestingly, the majority of PCa, including the androgen-independent forms, are addicted to AR activity and signaling, making AR an important target for this malignancy [2]. The AR’s N-terminal domain (NTD) is responsible for its transactivation potential; thus, treatment with agents that target this site may result in a sustained and more efficient inhibition of AR signaling. Several tyrosine and serine/threonine kinases can phosphorylate the NTD of the AR or its co-activators which may promote its nuclear translocation or transactivation in an androgen-independent manner [10, 50–55]. Increased kinase activity that drives ligand-independent AR signaling, together with over-expression of AR, may have a significant role in the development of CRPC [2]. There are several agents that have been investigated that interfere with the interaction of essential co-activators with the NTD of AR [2].
Compounds that target the amino-terminus of the AR have recently been isolated from marine organisms. Andersen et al. [56] demonstrated that the marine sponge derived small molecule EPI-001 is capable of inhibiting both the androgen-dependent and androgen-independent activation of AR. EPI-001 covalently binds to the AR-activation function-1 (AF1) region of the NTD [57] and was shown to block transcriptional activity of AR as well as transcriptional activity of a truncated form of the AR that lacks the LBD. EPI-001 did not reduce ligand binding; instead, it inhibited the androgen-dependent N/C interaction which is required for ligand mediated activation of AR. The authors also suggest that EPI-001 blocks interactions between AR and trans-activating proteins, thus inhibiting androgen induced proliferation as well as androgen-dependent growth in vivo without causing toxicity [56].
Meimetis et al. [58] showed that the glycerol ether lipids, niphatenones A and B, which are derived from the marine sponge Niphates digitalis, act as AR antagonists and interfere with AR transcriptional activity [58]. Niphatenone B alkynyl ether was also shown to bind covalently to the AR-AF1 region of the NTD and inhibited androgen-induced proliferation in androgen-dependent PCa cells (LNCaP), whereas it had no effect in PCa cells lacking functional AR (PC3) [58].
Furthermore, sintokamides A to E are small peptides with varying degrees of chlorination derived from the marine sponge Dysidea sp. [59]. They were determined to suppress AR activity in LNCaP cells. The compound sintokamide A was demonstrated to inhibit N-terminus transactivation of the AR and decrease proliferation in PCa cells [59].
Since it is becoming clear that treatment directed to the ligand binding site of AR only temporarily controls PCa growth, the above mentioned compounds may open new therapeutic avenues in the treatment of CRPC. However, it is possible that PCa cells develop mechanisms which allow them to acquire resistance against compounds that target transcriptional activity of the AR. For instance, mutations in the AR NTD may weaken binding of NTD targeted compounds [2] or even result in increased binding and interaction of activating kinases targeting the NTD, regardless of the presence of these therapeutic compounds.
6.4. Natural products that suppress AR synthesis
Another therapeutic strategy would be to use drugs that target synthesis of AR. There are several pathways that regulate AR synthesis, including the NF-κB signaling pathway [2]. NF-κB response elements are located in the AR promoter region and it has been demonstrated that activation of NF-κB signaling in PCa cells results in increased expression of AR [60–64]. Thus, inhibition of NF-κB may suppress AR expression and downstream signaling.
The green tea derived catechin EGCG may target AR expression indirectly through inhibition of NF-κB activity [2]. EGCG has been suggested to suppress the acetylation of the NF-κB subunit RelA, resulting in decreased NF-κB activity [65]. These observations are consistent with the finding that administration of EGCG down-regulates expression of AR as well as nuclear translocation of AR in PCa xenografts [34].
There are several other nutraceuticals such as the Cruciferae-derived compound diindolylmethane, or polyphenols derived from green tea, grape seed and pomegranate, that target NF-κB activation and have been shown to control growth in PCa cells in vitro and in vivo [66, 67]. Moreover, selenium, which is found in fish, meat, eggs and grains was shown to modulate genes involved in androgen regulation and could also reduce AR expression [68, 69]. Since selenium also inhibits binding of NF-κB to its target DNA sequences [70], reduced expression of AR following selenium intake could be attributed to inhibition of NF-κB-mediated AR gene expression. However, this needs to be further elucidated, since the clinical utility of selenium to prevent PCa progression and mortality was contraindicated (see section 3).
Another report shows that isoflavone inhibited phosphorylation of Akt and FOXO3a and increased expression of glycogen synthase kinase-3 beta (GSK-3β), resulting in a down-regulation of AR [71]. The authors argue that isoflavone alters AR expression through inhibition of FOXO1a binding to the AR promoter in PCa cells and suggest that isoflavone induces apoptosis and inhibits PCa cell proliferation though regulation of Akt/FOXO3a/GSK-3β/AR signaling [71]. Interestingly, the effect of soy isoflavones on PCa cells seems to be controversial and relates to the AR status of the respective cells. Various laboratories demonstrated that while the isoflavone, genistein, inhibited AR expression and cell proliferation in AR wild-type expressing LAPC-4 cells, it induced AR expression and growth in LNCaP cells when used at lower doses [72–76]. LNCaP cells have a threonine to alanine (T877A) mutation in the LBD of the AR which render them more promiscuous towards steroidal structures and potentially also towards the steroidlike structure of genistein [76].
It was recently shown that ericifolin, a compound purified form the Jamaican pepper, Pimenta dioica (Allspice) transcriptionally suppresses AR. Ericifolin was purified from an aqueous extract prepared from Allspice berries (AAE) that also inhibited PCa cell proliferation, colony formation and induced apoptosis and/or autophagy in PCa cells [77]. Importantly AAE inhibited AR promoter activity and mRNA stability leading to depletion of AR in AR-expressing cells [77].
The homovanillic acid derivative capsaicin is an active compound derived from the red pepper (Genus Capsicum) with potential therapeutic value. Mori et al. [78] showed that capsaicin (100 and 200 µM) inhibits proliferation and induces apoptosis in AR-negative (PC3) and AR-positive (LNCaP) cell lines. These effects were accompanied with an increase in p53, p21, and Bax, as well as a down-regulation of AR and PSA. The authors performed promoter assays to demonstrate that capsaicin interferes with DHT-mediated PSA promoter/enhancer activation. Since these results were also observed in the presence of exogenous AR, the authors concluded that capsaicin directly inhibited PSA transcription in addition to the observed down-regulation of AR expression [78]. Interestingly, Malagarie-Cazenave et al. [79] claim that capsaicin at lower doses (20 µM) induces expression of AR via PI3K and MAPK pathways in LNCaP cells. In addition, the authors show that capsaicin (20 µM) induces proliferation of LNCaP in the absence as well as in the presence of DHT [79]. In conclusion, capsaicin was shown to exert a biphasic effect in androgen-sensitive PCa cells, inducing AR expression and proliferation at lower doses and inhibiting AR expression and inducing apoptosis at doses over 200 µM [78–80]. Since this high dose of 200 µM is unlikely to be physiologically or pharmacologically relevant, capsaicin at present is more of AR stimulant than an inhibitor.
6.5. Induction of AR turnover
Agents that induce proteasomal degradation of the AR may also be beneficial in the treatment of androgen-independent PCa [2]. For instance, long-chain omega-3 fats have been shown to promote proteasomal degradation of AR in LNCaP PCa cells [81] as well as in the tumor cells in mice bearing PTEN-null CRPC [82].
Since heat shock protein 90 (Hsp90) and co-chaperones interact with AR and protect it from proteasomal degradation [83] they also provide potential therapeutic targets. For instance, the phytochemical berberine impedes the interaction of Hsp90 with AR, thus promoting the proteasomal degradation of AR [84].
HDAC6 regulates AR hypersensitivity and nuclear translocation in PCa cells [85]. Moreover, HDAC6 has been shown to reverse acetylation of Hsp90 [86]. Since acetylation of Hsp90 impedes its activity, HDAC6 positively regulates Hsp90 activity [86]. The natural product genistein modulates the HDAC6–Hsp90 chaperone function, which has been shown to cause a down-regulation/degradation of AR [2, 85, 87, 88]. Similarly, the isothiocyanate sulforaphane has been shown to inhibit HDAC6, resulting in the destabilization of AR in PCa cells [89].
Another compound with anti-tumor potential that specifically targets tumor cells but not normal cells is betulinic acid, a pentacyclic plant derived triterpenoid [90]. Betulinic acid is isolated from the bark of white-bark birch and other trees [90]. Reiner et al. recently showed that betulinic acid increases degradation of pro-survival proteins such as AR in PCa cell lines LNCaP and PC3, but not in normal cells [91]. The authors claim that these effects are possibly attributed to inhibition of deubiquitinase (DUB) activity [91], which is critical for the regulation of the ubiquitin-proteasome system-mediated protein degradation [92–94]. Importantly, betulinic acid (10 mg/kg) also inhibited DUB activity, reduced proliferation in primary tumors, and decreased the level of AR in the TRAMP transgenic mouse model of PCa. However, it had no effect on the ubiquitination or the AR protein expression level in normal mouse tissues [91].
The phytochemical 1,2,8-trihydroxy-6-methylanthraquinone (emodin), derived from Rheum palmatum, has been shown to be more potent against PCa than curcumin or genistein [95]. The investigators showed that emodin induces dissociation of AR from Hsp90 and increases its interaction with the E3 ligase MDM2, thus promoting the proteasome-mediated degradation of AR in LNCaP cells [95]. Importantly, emodin also promoted AR degradation in tumor tissues and suppressed tumor growth in C3(1)/SV40 transgenic mice with no effect on body weight and physical activity [96].
6.6. Inhibition of AR nuclear translocation (AR acetylation)
Acetylation of AR can promote its nuclear translocation and thus AR mediated gene transcription [2]. Several enzymes such as p300, PCAF, TIP60, and acetyl-transferase arrest-defect 1 protein can promote acetylation of lysine groups on AR within its hinge region [97–99]. Thus, agents or natural products that target those enzymes may also interfere with nuclear translocation of AR and indirectly inhibit AR mediated gene expression. For instance, the green tea derived EGCG, has been demonstrated to inhibit acetyltransferase activity up to 80% when used at a concentration of 25 µM [100]. Treatment with EGCG resulted in reduced AR acetylation and impeded androgen-mediated nuclear translocation of AR in PCa cell lines [100]. These effects were confirmed in vivo using a PCa xenograft model [34].
As discussed previously, B-DIM has been implicated in antiproliferative and proapoptotic effects in PCa cells, in part through an interruption of AR signaling [41]. In addition, the authors show in confocal studies that B-DIM also inhibits AR translocation to the nucleus, thereby preventing AR-mediated gene transcription [41]. Additionally, B-DIM significantly inhibited C4-2B cell growth in a severe combined immunodeficiency-human model of experimental PCa bone metastasis [41].
Natural products with a promising therapeutic effect were also isolated from the African prune (plum) tree Pygeum africanum [101, 102]. P. africanum is used for treatment of benign prostatic hypertrophy in Europe and to reduce PCa. An ethanolic extract of P. africanum plum extract inhibited the growth of two PCa cell lines LNCaP and PC-3 in vitro and the mouse TRAMP tumor in vivo [103]. Bioassay-guided fractionation resulted in the isolation of two antiandrogenic compounds from P. africanum, namely atraric acid (AA) and N-butylbenzene-sulfonamide (NBBS) [104, 105]. Conventional AR-antagonists competitively bind to AR and promote its translocation to the nucleus, where the AR-antagonist complex recruits corepressors resulting in repressed AR mediated transcriptional activity [101]. In contrast, the P. africanum derived compounds AA and NBBS were shown to inhibit AR-mediated transcription by blocking nuclear translocation of AR [102, 106].
7. Challenges to overcome: clinical efficacy
The major challenge facing the use of natural products is their low bioavailability in the absence of extensive structural modification. Most natural products have an efficacy, defined as 50% inhibitory concentration (IC50), of several micromoles (µM) or > 10 µg/ml, when tested in vitro. Although the compounds are well tolerated in experimental models, such as rodent models (Table 2), the plasma concentration of such compounds seldom reaches the IC50 level, even when administered at a very high dose (> 200 mg/kg). For example, the plasma concentration of curcumin is seldom seen > 1 µM, thus falling short of the micromolar concentration range necessary for the inhibitory activity against its targets [107]. Even when dosed at levels of 2 g/kg in rats, the maximum level achieved in plasma was less than 5 µg/ml [107]. It has been reported that the major reasons contributing to the low plasma and tissue levels of curcumin, even at a dose of 12 g/day in humans, is its poor absorption through the gastrointestinal tract, rapid metabolism in the liver, and rapid systemic elimination [108]. Recent advancements in drug delivery in the form of nanoparticles coated with curcumin or other compounds has enhanced the bioavailability by more than nine-fold [109]. Chemical modification of orally delivered natural products has shown improved efficacy in curcumin and other bioactive natural products [108, 110]. Similar studies in DIM have also shown improved bioavailability in an absorption enhanced form of DIM (BR-DIMNG) which has the potential for oral administration [111]. While numerous synthetic compounds have been tested in clinical trials for treatment of PCa in general, only a few natural products have been investigated in the clinic [42, 107, 108, 111].
Table 2.
Systemic toxicity of anticancer natural products*
| Natural product (Source) |
Mouse LD50 (mg/kg) Route of administration |
Common toxicity |
|---|---|---|
| Curcumin (Curcuma longa, Turmeric) |
1,500 (i.p.) >2,000 (p.o.) |
None |
| EGCG (Green tea extract) |
2,170 (p.o.) 4,200 g/m2 (Humans, p.o.) |
Minimal/none detected; Green tea extract-caffeine related insomnia in humans |
| Glycyrrhetinic acid (Licorice) |
308 (i.p.) 56 (i.v.) |
Mild skin irritant |
| Capsaicin (Chili peppers, Capscicum) |
6.5 (i.p.) 42.7 (p.o.) |
Muscle contraction, spasticity and convulsion. |
| Genistein (Soy isoflavone) |
>0.5 (i.p.) | None |
| Stilbene (Red wine, redgrapes) |
1.150 (i.p.) 0.034 (i.v.) |
None |
| Emodin (Rhamnus frangula) |
35 (i.p.) | Gastrointestinal (GI) and related toxicity; laxative |
| Berberine (Hydrastis canadensis, L.) |
329 (p.o.) | Parenteral toxicity |
| Butylbenzene Sulfonamide (bark of Pygeum africanum) |
2.5 (p.o.) | Not available. |
From ToxNet data network (http://chem.sis.nlm.nih.gov/chemidplus/name)
Abbreviations: LD50: Lethal dose, 50%; i.p.: intraperitoneal; p.o.: Per os; i.v.: intravenous
8. Conclusion and future perspectives
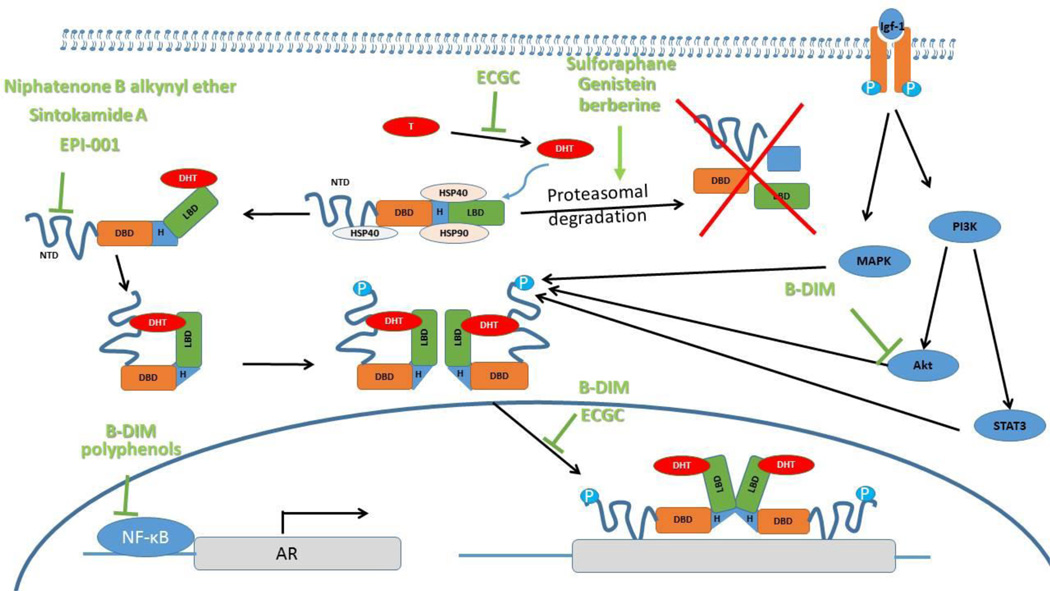
Because of its high incidence and long latency, PCa is an ideal candidate for chemoprevention at many stages. There is ample evidence that several natural products target AR signaling (Figure 1, Table 2), providing novel means to prevent or treat PCa, including advanced metastatic disease. AR antagonists only offer temporary efficacy in the treatment of PCa since PCa cells proceed to an androgen-independent survival and growth state; they become unresponsive to androgen ablation or agents that block the interaction between AR and its ligand. PCa cells depend on AR activity even in advanced CRPC disease. Therefore, the development of drugs that directly target AR and inhibit its transcriptional activity are of special interest. Several natural compounds were demonstrated to target synthesis and turnover, as well as transcriptional activity of AR in PCa cells, indicating a chemopreventive potential. Unfortunately, the nutritional interventions tested so far in clinical studies failed to reduce the incidence of PCa and at present there is no evidence for any nutritional supplement which can prevent PCa. However, several new natural products have been discovered that have the potential to be used in combination with existing strategies for targeting the “AR addiction” of PCa. Some of these compounds, such as BR-DIM, nanoparticle encapsulated curcumin, the marine sponge derived sintokamide A, niphatenones A and B, EPI-001 E and the allspice derived ericifolin open new avenues for treatment since, in contrast to conventional drugs, they are not based on androgen ablation and do not target the AR LBD, but rather block AR transcriptional activity.
Figure 1. A schematic of how natural products may affect AR.
Binding of DHT to AR results in release of Hsps and subsequent N/C dimerization and exposure of the nuclear localization signal. In the nucleus, AR binds DNA and recruits factors that initiate transcription of target genes. Many chemopreventive natural products mediate their activity though inhibition of AR expression or transcriptional activity. Other compounds interfere with the association of the chaperone Hsp90 with AR, resulting in the degradation of AR. Abbreviations: Akt, RAC serine/threonine protein kinase; AR, androgen receptor; Hsp90, heat shock protein 90; IGF-I, insulin-like growth factor 1; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; NTD, N-terminal domain; D, DNA binding domain (DBD); L, ligand binding domain (LBD); T, testosterone; DHT, dihydrotestosterone.
A better understanding of the three-dimensional structure of AR may contribute to the development of novel inhibitors of AR transcriptional activity [112]. While several compounds have been identified that target the AR NTD, few inhibitors of the AR DNA binding domain (DBD) have been developed to date [113]. High throughput screening of natural product libraries, including marine organism derived compounds and bioactivity directed fractionation may lead to the discovery of molecules that target the AR DBD. AR DBD inhibitors may have the potential to target the transcriptional activity of wild type AR as well as constitutively activated truncated variants. However, they might cross-react with other nuclear receptors due to high sequence similarity between DBDs of all steroid receptors [112]- the DBD is the most highly conserved domain in the nuclear receptor family [113].
Furthermore, the effects of dietary supplements and nutraceuticals on chemoprevention might differ within populations and could largely depend on the gastrointestinal microbiota composition, which influences the level of bioavailable nutrients and anti-cancer compounds [114]. Therefore, studies that evaluate differences in the microbiome between high risk and low risk populations, or between healthy individuals and patients with latent or metastatic and androgen-insensitive disease, may provide valuable insight into the role of the microbiome in the development and progression of PCa. As such, modulating the human microbiome might have beneficial effects in chemoprevention of PCa.
Table 1.
Natural products targeting AR signaling
| Natural product | Origin | Mechanism of action/target |
References |
|---|---|---|---|
| AAE/Ericifolin |
Pimenta dioica (Allspice) |
AR synthesis | [77] |
| Atraric Acid |
Pygeum africanum (African prune tree) |
AR nuclear translocation | [102] |
| B-DIM | Family Cruciferae (Cruciferous vegetables) |
AR activation/signaling, AR nuclear translocation |
[41] |
| Berberine | Several plants including Hydrastis Canadensis (Goldenseal), Coptis trifolia (Goldthread), Mahonia aquifolium (Oregon Grape) |
AR turnover/degradation | [84] |
| Betulinic acid |
Betula sp. (White-bark birch) |
AR turnover/degradation | [91] |
| Capsaicin |
Capsicum sp. (Red pepper) |
AR synthesis | [78–80] |
| Curcumin |
Curcuma longa (Turmeric) |
AR activation/signaling | [43, 48, 49] |
| ECG |
Camellia sinensis (Green tea) |
Androgen synthesis | [35] |
| EGCG |
Camellia sinensis (Green tea) |
Androgen synthesis, AR nuclear translocation |
[34] |
| Emodin |
Rheum palmatum (Rhubarb) |
AR turnover/degradation | [95, 96] |
| EPI-001 | Marine sponge Geodia lindgreni |
AR activation/signaling | [56, 57] |
| Ganoderic acids DM and TR |
Ganoderma sp. (Red Reishi) |
Androgen synthesis | [33] |
| Glycyrrhetinic acid |
Glycyrrhiza glabra (Licorice) |
Androgen synthesis | [36] |
| Isoflavones/Genistein | Glycine max (Soy) | AR synthesis , AR turnover/degradation |
[2, 72–76, 85, 87, 88] |
| N-butylbenzene- sulfonamide |
Pygeum africanum (African prune tree) |
AR nuclear translocation | [106] |
| Niphatenones A and B |
Marine sponge Niphates digitalis |
AR activation/signaling | [58] |
| Sintokamide A | Marine sponge Dysidea sp. |
AR activation/signaling | [59] |
| Sulforaphane | Family Cruciferae (Cruciferous vegetables, primarily broccoli sprouts) |
AR turnover/degradation | [89] |
Abbreviations: AAE, aqueous allspice extract; AR, androgen receptor; DIM, 3, 3’-diindolylmethane; B-DIM, a formulated DIM with higher bioavailability; ECG, epicatechin-3 gallate; EGCG, epigallocatechin-3 gallate; EPI-001, ESSA Pharma Inc.
Acknowledgments
This work was supported in parts by the Veterans Health Administration Merit Award: BX 001517-01 (BLL); the United States’ Public Health Services awards: NIH 1R01CA156776-01 (BLL) and Augusta University Cancer Center funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.McCarty MF, Hejazi J, Rastmanesh R. Beyond androgen deprivation: ancillary integrative strategies for targeting the androgen receptor addiction of prostate cancer. Integr Cancer Ther. 2014;13(5):386–395. doi: 10.1177/1534735414534728. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar FH, et al. Novel targets for prostate cancer chemoprevention. Endocr Relat Cancer. 2010;17(3):R195–R212. doi: 10.1677/ERC-10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi G, et al. Growth factors mediated cell signalling in prostate cancer progression: Implications in discovery of anti-prostate cancer agents. Chem Biol Interact. 2015;240:120–133. doi: 10.1016/j.cbi.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Amato R, Stepankiw M, Gonzales P. A phase II trial of androgen deprivation therapy (ADT) plus chemotherapy as initial treatment for local failures or advanced prostate cancer. Cancer Chemother Pharmacol. 2013;71(6):1629–1634. doi: 10.1007/s00280-013-2163-4. [DOI] [PubMed] [Google Scholar]
- 6.Ezzell EE, Chang KS, George BJ. New agents in the arsenal to fight castrate-resistant prostate cancer. Curr Oncol Rep. 2013;15(3):239–248. doi: 10.1007/s11912-013-0305-9. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal N, et al. New agents for prostate cancer. Ann Oncol. 2014;25(9):1700–1709. doi: 10.1093/annonc/mdu038. [DOI] [PubMed] [Google Scholar]
- 8.Violette PD, Saad F. Chemoprevention of prostate cancer: myths and realities. J Am Board Fam Med. 2012;25(1):111–119. doi: 10.3122/jabfm.2012.01.110117. [DOI] [PubMed] [Google Scholar]
- 9.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93(22):1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 10.Titus MA, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery RB, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang KH, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Molecular and Cellular Endocrinology. 2008;295(1–2):115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards J, et al. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. British Journal of Cancer. 2003;89(3):552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levina E, et al. Identification of novel genes that regulate androgen receptor signaling and growth of androgen-deprived prostate cancer cells. Oncotarget. 2015;6(15):13088–13104. doi: 10.18632/oncotarget.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal S, et al. An overview on 5alpha-reductase inhibitors. Steroids. 2010;75(2):109–153. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Yamana K, Labrie F, Luu-The V. Human type 3 5alpha-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm Mol Biol Clin Investig. 2010;2(3):293–299. doi: 10.1515/HMBCI.2010.035. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Coltman CA, Jr, Crowley J. Chemoprevention of prostate cancer: the Prostate Cancer Prevention Trial. Prostate. 1997;33(3):217–221. doi: 10.1002/(sici)1097-0045(19971101)33:3<217::aid-pros11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, Tangen C, Goodman P. The Prostate Cancer Prevention Trial: design, status, and promise. World J Urol. 2003;21(1):28–30. doi: 10.1007/s00345-002-0315-y. [DOI] [PubMed] [Google Scholar]
- 20.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 21.Thompson IM, Jr, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369(7):603–610. doi: 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau CH, et al. Finasteride concentrations and prostate cancer risk: results from the Prostate Cancer Prevention Trial. PLoS One. 2015;10(5):e0126672. doi: 10.1371/journal.pone.0126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein EA, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GG. Vitamin D in blood and risk of prostate cancer: lessons from the Selenium and Vitamin E Cancer Prevention Trial and the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1447–1449. doi: 10.1158/1055-9965.EPI-14-0520. [DOI] [PubMed] [Google Scholar]
- 25.Wilt TJ, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2(2):143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 27.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99(1):1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 28.Horie S. Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean J Urol. 2012;53(10):665–672. doi: 10.4111/kju.2012.53.10.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimino S, et al. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid Med Cell Longev. 2012;2012:632959. doi: 10.1155/2012/632959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad F, Fizazi K. Androgen Deprivation Therapy and Secondary Hormone Therapy in the Management of Hormone-sensitive and Castration-resistant Prostate Cancer. Urology. 2015;86(5):852–861. doi: 10.1016/j.urology.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Grant P, Ramasamy S. An update on plant derived anti-androgens. Int J Endocrinol Metab. 2012;10(2):497–502. doi: 10.5812/ijem.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita R, et al. Anti-androgenic activities of Ganoderma lucidum. J Ethnopharmacol. 2005;102(1):107–112. doi: 10.1016/j.jep.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, et al. Quantitative determination of the representative triterpenoids in the extracts of Ganoderma lucidum with different growth stages using high-performance liquid chromatography for evaluation of their 5α-reductase inhibitory properties. Food Chemistry. 2012;133(3):1034–1038. [Google Scholar]
- 34.Siddiqui IA, et al. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25(4):1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao S, Hiipakka RA. Selective inhibition of steroid 5 alpha-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem Biophys Res Commun. 1995;214(3):833–838. doi: 10.1006/bbrc.1995.2362. [DOI] [PubMed] [Google Scholar]
- 36.Hawthorne S, Gallagher S. Effects of glycyrrhetinic acid and liquorice extract on cell proliferation and prostate-specific antigen secretion in LNCaP prostate cancer cells. J Pharm Pharmacol. 2008;60(5):661–666. doi: 10.1211/jpp.60.5.0013. [DOI] [PubMed] [Google Scholar]
- 37.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 39.Tan J, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11(4):450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 40.Veldscholte J, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 41.Bhuiyan MM, et al. Down-regulation of androgen receptor by 3,3’-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66(20):10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Sarkar FH. Role of BioResponse 3,3’-Diindolylmethane in the Treatment of Human Prostate Cancer: Clinical Experience. Med Princ Pract. 2015 doi: 10.1159/000439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou DY, et al. Curcumin analogues with high activity for inhibiting human prostate cancer cell growth and androgen receptor activation. Mol Med Rep. 2014;10(3):1315–1322. doi: 10.3892/mmr.2014.2380. [DOI] [PubMed] [Google Scholar]
- 44.Kuttan R, et al. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29(2):197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 45.Huang MT, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54(22):5841–5847. [PubMed] [Google Scholar]
- 46.Huang MT, et al. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–5946. [PubMed] [Google Scholar]
- 47.Huang MT, et al. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13(11):2183–2186. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsu H, et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45(23):5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 49.Wei X, et al. Synthesis and evaluation of curcumin-related compounds for anticancer activity. Eur J Med Chem. 2012;53:235–245. doi: 10.1016/j.ejmech.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Culig Z, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54(20):5474–5478. [PubMed] [Google Scholar]
- 51.Yeh S, et al. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96(10):5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Z, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10(4):309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Mellinghoff IK, et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 54.Gregory CW, et al. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005;11(5):1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 55.Mahajan NP, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104(20):8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen RJ, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Myung JK, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123(7):2948–2960. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meimetis LG, et al. Niphatenones, glycerol ethers from the sponge Niphates digitalis block androgen receptor transcriptional activity in prostate cancer cells: structure elucidation, synthesis, and biological activity. J Med Chem. 2012;55(1):503–514. doi: 10.1021/jm2014056. [DOI] [PubMed] [Google Scholar]
- 59.Sadar MD, et al. Sintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cells. Org Lett. 2008;10(21):4947–4950. doi: 10.1021/ol802021w. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamura K, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21(4):825–830. [PubMed] [Google Scholar]
- 62.Araki S, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67(14):6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 63.Hoshino K, et al. Regulation of androgen receptor expression through angiotensin II type 1 receptor in prostate cancer cells. Prostate. 2011;71(9):964–975. doi: 10.1002/pros.21312. [DOI] [PubMed] [Google Scholar]
- 64.Lee JW, Kim GY, Kim JH. Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression. Biochemical and Biophysical Research Communications. 2012;420(2):428–433. doi: 10.1016/j.bbrc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Choi KC, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69(2):583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 66.Sainz RM, et al. Critical role of glutathione in melatonin enhancement of tumor necrosis factor and ionizing radiation-induced apoptosis in prostate cancer cells in vitro. J Pineal Res. 2008;45(3):258–270. doi: 10.1111/j.1600-079X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 67.Rettig MB, et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol Cancer Ther. 2008;7(9):2662–2671. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong Y, et al. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63(1):52–59. [PubMed] [Google Scholar]
- 69.Zhao H, et al. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15(2):506–519. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen MJ, et al. High selenium reduces NF-kappaB-regulated gene expression in uninduced human prostate cancer cells. Nutr Cancer. 2007;58(2):197–204. doi: 10.1080/01635580701328701. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, et al. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283(41):27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahmoud AM, et al. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8(10):e78479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarevic B, Karlsen SJ, Saatcioglu F. Genistein differentially modulates androgen-responsive gene expression and activates JNK in LNCaP cells. Oncol Rep. 2008;19(5):1231–1235. [PubMed] [Google Scholar]
- 74.Gao S, Liu GZ, Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59(2):214–225. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- 75.Maggiolini M, et al. The mutant androgen receptor T877A mediates the proliferative but not the cytotoxic dose-dependent effects of genistein and quercetin on human LNCaP prostate cancer cells. Mol Pharmacol. 2002;62(5):1027–1035. doi: 10.1124/mol.62.5.1027. [DOI] [PubMed] [Google Scholar]
- 76.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shamaladevi N, et al. Ericifolin: a novel antitumor compound from allspice that silences androgen receptor in prostate cancer. Carcinogenesis. 2013;34(8):1822–1832. doi: 10.1093/carcin/bgt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mori A, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66(6):3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 79.Malagarie-Cazenave S, et al. Capsaicin, a component of red peppers, induces expression of androgen receptor via PI3K and MAPK pathways in prostate LNCaP cells. FEBS Lett. 2009;583(1):141–147. doi: 10.1016/j.febslet.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 80.Diaz-Laviada I. Effect of capsaicin on prostate cancer cells. Future Oncol. 2010;6(10):1545–1550. doi: 10.2217/fon.10.117. [DOI] [PubMed] [Google Scholar]
- 81.Friedrichs W, et al. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63(5):771–777. doi: 10.1080/01635581.2011.570892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S, et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis. 2012;33(2):404–412. doi: 10.1093/carcin/bgr290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30(5):709–716. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 84.Li J, et al. Berberine suppresses androgen receptor signaling in prostate cancer. Mol Cancer Ther. 2011;10(8):1346–1356. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ai J, et al. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009;23(12):1963–1972. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovacs JJ, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Davis JN, Kucuk O, Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34(2):91–101. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 88.Basak S, et al. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther. 2008;7(10):3195–3202. doi: 10.1158/1535-7163.MCT-08-0617. [DOI] [PubMed] [Google Scholar]
- 89.Gibbs A, et al. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A. 2009;106(39):16663–16668. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fulda S. Betulinic Acid for cancer treatment and prevention. Int J Mol Sci. 2008;9(6):1096–1107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reiner T, et al. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: possible role for inhibition of deubiquitinase activity. PLoS One. 2013;8(2):e56234. doi: 10.1371/journal.pone.0056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8(11):1688–1697. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 94.Sacco JJ, et al. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 2010;62(2):140–157. doi: 10.1002/iub.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cha TL, et al. Emodin modulates epigenetic modifications and suppresses bladder carcinoma cell growth. Mol Carcinog. 2015;54(3):167–177. doi: 10.1002/mc.22084. [DOI] [PubMed] [Google Scholar]
- 96.Cha TL, et al. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65(6):2287–2295. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 97.Fu MF, et al. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. Journal of Biological Chemistry. 2000;275(27):20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 98.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. Journal of Endocrinology. 2012;215(2):221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 99.Wang ZH, et al. Inactivation of androgen-induced regulator ARD1 inhibits androgen receptor acetylation and prostate tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):3053–3058. doi: 10.1073/pnas.1113356109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee YH, et al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 2012;30(1):69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- 101.Roell D, Baniahmad A. The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth. Mol Cell Endocrinol. 2011;332(1–2):1–8. doi: 10.1016/j.mce.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Papaioannou M, et al. The natural compound atraric acid is an antagonist of the human androgen receptor inhibiting cellular invasiveness and prostate cancer cell growth. J Cell Mol Med. 2009;13(8B):2210–2223. doi: 10.1111/j.1582-4934.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shenouda NS, et al. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007;31(1):72–81. doi: 10.1007/s12020-007-0014-y. [DOI] [PubMed] [Google Scholar]
- 104.Schleich S, et al. Activity-guided isolation of an antiandrogenic compound of Pygeum africanum. Planta Med. 2006;72(6):547–551. doi: 10.1055/s-2006-941472. [DOI] [PubMed] [Google Scholar]
- 105.Schleich S, et al. Extracts from Pygeum africanum and other ethnobotanical species with antiandrogenic activity. Planta Med. 2006;72(9):807–813. doi: 10.1055/s-2006-946638. [DOI] [PubMed] [Google Scholar]
- 106.Papaioannou M, et al. NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Invest New Drugs. 2010;28(6):729–743. doi: 10.1007/s10637-009-9304-y. [DOI] [PubMed] [Google Scholar]
- 107.Anand P, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 108.Meyskens FL, Jr, et al. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaikh J, et al. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3–4):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 110.Angelo Siviero EG, Maggini Valentina, Gori Luigi, Mugelli Alessandro, Firenzuoli Fabio, Vannacci Alfredo. Curcumin, a golden spice with a low bioavailability. Journal of Herbal Medicine. 2015;5(2):57–70. [Google Scholar]
- 111.Gee JR, et al. Phase Ib placebo-controlled, tissue biomarker trial of diindolylmethane (BR-DIMNG) in patients with prostate cancer who are undergoing prostatectomy. Eur J Cancer Prev. 2015 doi: 10.1097/CEJ.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 112.Lallous N, et al. Targeting alternative sites on the androgen receptor to treat castration-resistant prostate cancer. Int J Mol Sci. 2013;14(6):12496–12519. doi: 10.3390/ijms140612496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caboni L, Lloyd DG. Beyond the ligand-binding pocket: targeting alternate sites in nuclear receptors. Med Res Rev. 2013;33(5):1081–1118. doi: 10.1002/med.21275. [DOI] [PubMed] [Google Scholar]
- 114.Amirian ES, et al. Potential role of gastrointestinal microbiota composition in prostate cancer risk. Infect Agent Cancer. 2013;8(1):42. doi: 10.1186/1750-9378-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]