Abstract
Background
The extent and severity of traumatic brain injuries (TBIs) can be difficult to determine with current diagnostic methods. To address this, there has been increased interest in developing biomarkers to assist in the diagnosis, determination of injury severity, evaluation of recovery and therapeutic efficacy, and prediction of outcomes. Several promising serum TBI biomarkers have been identified using hypothesis-driven approaches, largely examining proteins that are abundant in neurons and non-neural cells in the CNS.
New Method
An unbiased approach, phage display, was used to identify serum TBI biomarkers. In this proof-of-concept study, mice received a TBI using the controlled cortical impact model of TBI (1 mm injury depth, 3.5 m/s velocity) and phage display was utilized to identify putative serum biomarkers at 6h postinjury.
Results
An engineered phage which preferentially bound to injured serum was sequenced to identify the 12-mer ‘recognizer’ peptide expressed on the coat protein. Following synthesis of the recognizer peptide, pull down, and mass spectrometry analysis, the target protein was identified as glial fibrillary acidic protein (GFAP).
Comparison with Existing Methods and Conclusions
GFAP has previously been identified as a promising TBI biomarker. The results provide proof of concept regarding the ability of phage display to identify TBI serum biomarkers. This methodology is currently being applied to serum biomarkers of mild TBI.
Keywords: traumatic brain injury, biomarkers, rodents, animal models, concussion, blood
Introduction
Mild traumatic brain injury (mTBI), often referred to as a concussion for sports-related injuries, represents a major health concern (Jeter et al., 2013). mTBIs account for up to 90% of the brain injuries in the United States, affecting between 1.6 and 3.8 million people yearly, and representing a “silent epidemic” (Jordan, 2013; Langlois et al., 2006). While many cases fully resolve spontaneously, others result in long term consequences including chronic cognitive difficulties and postconcussive syndrome. Repetitive insults can result in chronic traumatic encephalopathy, a neurodegenerative dementing disorder (Butler, 2013; Carroll et al., 2004; Maroon et al., 2012; Topolovec-Vranic et al., 2011).
There is not a precise definition of mTBI, but it is generally considered to be a brief loss of consciousness (less than 30 minutes) or loss/alteration of neurologic function such as memory, caused by an interaction between biomechanical forces and the head, with negative radiology findings (Eakin and Miller, 2012; Rosenbaum and Lipton, 2012; Shultz et al., 2011). Within the mTBI category, there are a range of injury severities with 90% not resulting in a loss of consciousness (Ropper and Gorson, 2007; Rosenbaum and Lipton, 2012). Symptoms can include disorientation, confusion, amnesia, impaired concentration, sleep disturbance, irritability, anxiety, fatigue, headache, dizziness/vertigo, nausea, vacant stare, unsteady gait, impaired coordination, diplopia/ blurred vision, photophobia, hyperacusis, and concussive convulsion/ impact seizure (Jordan, 2013). These symptoms, however, can be associated with other conditions and thus accurate diagnosis and assessment as to when and whether mTBI has occurred is critical for proper therapeutics.
Accurate diagnosis of mTBI is particularly important in the acute stages when treatments could be most effective (Ponsford et al., 2001; Ponsford et al., 2002; Wade et al., 1998). To aid in the objective diagnosis and evaluation of mTBI, there is an urgent need for biomarkers as highlighted in NIH workshops and reviews (Jeter et al., 2013; Manley et al., 2010; Saatman et al., 2008; Zetterberg et al., 2013). The power of biomarkers is evident in cardiac injury, where cardiac troponin proteins and brain natriuretic peptide are now routinely utilized to help diagnose myocardial infarction and congestive heart failure.
Several potential cerebrospinal fluid (CSF) and serum biomarkers for TBI have been investigated, including S100β, neuron specific enolase, glial fibrillary acidic protein (GFAP), ubiquitin C-terminal hydrolase-L1, and spectrin breakdown products (for a recent review see (Kulbe and Geddes, 2016)). These biomarkers have the greatest sensitivity and specificity with severe TBI, but are less sensitive for mTBI (Agoston and Elsayed, 2012; Brophy et al., 2011; Czeiter et al., 2012; Mondello et al., 2012; Mondello et al., 2010; Topolovec-Vranic et al., 2011). mTBI biomarkers would also be useful to assist with prediction of outcomes, evaluation of recovery and therapeutic efficacy, and provide insight into the mechanisms involved for evidence-based therapeutic interventions.
There are two major approaches to identify biomarkers—hypothesis driven and unbiased. Hypothesis-driven approaches have largely focused on proteins abundant in the cells impacted by TBI, including neurons (neuron specific enolase, ubiquitin C-terminal hydrolase-L1, spectrin breakdown products, spectrin N-terminal fragment, tau, neurofilament proteins), astrocytes (S100B, glial fibrillary acidic protein), oligodendrocytes and myelin (myelin basic protein), as well as inflammatory markers and oxidized lipids (Dash et al., 2010; Giacoppo et al., 2012; Kochanek et al., 2008; Kulbe and Geddes, 2016; Pineda et al., 2004; Sandler et al., 2010; Siman et al., 2009; Yokobori et al., 2013).
Unbiased methods to identify TBI biomarkers have included 2D proteomics. This method has been successful in identifying proteins released from degenerating cultured neurons (Guingab-Cagmat et al., 2012; Loov et al., 2013; Siman et al., 2004; Siman et al., 2009) and proteins differentially expressed in lysates from injured vs. uninjured rat brain (Wang et al., 2005). More recently, 2D proteomics has been applied to biofluids obtained from animal models of mTBI (Ding et al., 2015). While proteomics is powerful, it can be problematic for serum biomarker identification (Diamandis, 2004). Moreover, 2D proteomics is most applicable to medium-large proteins (Zurbig and Jahn, 2012). Other separation technologies such as liquid chromatography-mass spectrometry (LC-MS), SELDI-MS, and capillary electrophoresis-MS also have limitations including restricted mass range and low sensitivity (Zurbig and Jahn, 2012). The difficulty in detecting low molecular weight peptides and proteins is relevant to biomarker discovery, as this group includes cytokines, chemokines, peptides, and proteolytic fragments of larger proteins. This is not to suggest that 2D proteomics or related methods are not valuable. However, additional methods may identify novel and complimentary proteins/peptides as biomarkers.
To identify novel biomarkers for TBI, phage display represents a powerful, unbiased approach (Azzazy and Highsmith, 2002; Bradbury, 2010) Phage display is a method to select peptides, proteins or antibodies with specific binding properties (Bratkovic, 2010). It is most widely used to investigate protein-protein interactions, receptor- and antibody-binding sites, and for selecting antibodies against a range of antigens (Bradbury, 2010; Bratkovic, 2010; Sidhu et al., 2000). Phage display uses bacteriophages in which DNA encoding peptides or proteins are inserted into the gene encoding a coat protein of a filamentous phage such as M13 phage. M13 is a filamentous bacteriophage in which a circular single stranded DNA, 6407 nucleotides long, encodes a major coat protein (P8) and several minor coat proteins (P3, P6, P9) on the ends. A DNA encoding a peptide of interest is inserted into the P3 phage coat protein. Five copies of the protein are expressed for P3. Following infection with bacteria, new protein is synthesized and expressed on the viral particle. These foreign proteins can then bind to proteins of interest and the binding partners can be identified by sequencing.
To determine the suitability of phage display for identification of TBI serum biomarkers, we utilized the mouse controlled cortical impact (CCI) model (1 mm depth, 3.5 m/s velocity) which results in moderate neuron degeneration and cortical tissue damage (Smith et al., 1995) (Saatman et al., 2006). Glial fibrillary acidic protein (GFAP) was identified as a putative serum TBI biomarker, providing proof-of-concept regarding the ability of phage display to identify serum biomarkers. The phage display methodology is currently being applied to serum obtained from rats following midline fluid percussion injury, a model of mTBI resulting in diffuse injury, to identify novel serum biomarkers of mTBI (Cao et al., 2012; McIntosh et al., 1987).
Materials and Methods
In a proof-of-concept study, we utilized a commercial phage display library (Ph.D. 12 phage display library kit, England Biolabs, Ipswich, MA) to identify phages that preferentially bind to serum following contusive TBI. CCI was used to model contusive TBI as described previously (Madathil et al., 2013; Saatman et al., 2006). Male C57BL/6 mice, 8–10 wks old, were anesthetized with isoflurane (3% induction, 2.5% maintenance). The head was secured in a stereotaxic frame (David Kopf Instruments, CA), and following a midline scalp incision a 5 mm diameter craniotomy was conducted over the left parietal cortex. Cortical contusion was produced using a pneumatically driven impactor (Precision Systems and Instrumentation LLC, Fairfax Station, VA) with a 3 mm diameter rounded impactor tip, 1 mm impact depth, and a velocity of 3.5 m/s. Following injury, the craniotomy was sealed with dental cement and the scalp was sutured. Body temperature was maintained at 37°C. Control animals were not injured. Six hours following injury, animals were euthanized and blood was collected transcardially in a BD SST tube, retained 30 minutes for clotting, and centrifuged at 1300xg for 15 minutes. Aliquoted serum was stored at −80°C. One aliquot of serum (5μl) from each of four injured animals was pooled and diluted in 1:100 in TBS. Similarly, serum aliquots from two control animals were pooled and diluted in TBS.
To identify phages selective for serum from injured mice, subtractive panning was performed using a commercial random peptide library (Ph.D.™ -12 Phage Display Peptide Library Kit, New England Biolabs). The library contains greater than 2 billion independent clones that express random 12 amino acid peptides on the N-terminus of the minor coat protein P3. Pooled serum form control mice, diluted 1:100 in TBS (100 μl) was added in one well of a 96 well plate (Nunc Immuno Plate, Maxisorp surface) and 100 μl of diluted pooled injured serum was added to a second well. The serum samples were incubated at 37°C for one hour with mild rocking, then discarded. Blocking buffer (5% BSA in TBS-T (TBS+0.05%Tween 20); 300 μl) was added to both wells (injured and uninjured) for one hour, then discarded. In the uninjured sample well, 100 μl of the phage library, diluted 1:100 in TBS, was added; 100 μl TBS was added to the injured sample well, followed by incubation for 30 minutes. TBS was discarded from the injured sample well and the phage solution was transferred from the uninjured to the injured sample well, followed by a second incubation for thirty minutes. The injured sample well was rinsed with 300 μl TBS-T to remove unbound phages. For elution of bound phages, 100 μl of 0.2M Glycine-HCl, 1mg/ml BSA, pH 2.5 was added to the well, incubated for 10mins, and neutralized with 10 μl of 1M Tris-HCl, pH 9.5. The eluate was then titered and amplified according to the manufacturer’s instructions. Two additional rounds of panning and amplification were performed, with the Tween concentration in the final panning round increased to 0.5%. Six phage plaques from the final eluate were randomly selected for ELISA and sequencing.
For ELISA, anti M13-HRP antibody (GE Healthcare, Cat#27-9421-01) was used to detect the phages using 3,3′,5,5′-tetramethyl benzidine (TMB) substrate and the absorbance was read at 450nm. For sequencing of phage clones, the phage pellet was suspended in 100 μl iodide buffer and incubated with 250 μl ethanol to preferentially precipitate the single-stranded phage DNA. After another spin and wash in 70% ethanol, the pellet was re-suspended in 30 μl TE buffer, quantitated by agarose gel electrophoresis, and sent for sequencing using the −96 g111 sequencing primer (5′-CCC TCA TAG TTA GCG TAA CG-3′) from the Phage Display Library Kit.
Following identification of a phage clone which preferentially bound to serum from injured mice, the encoded ‘recognizer’ 12-mer peptide was synthesized from Anaspec (Freemont, CA) along with a C-terminal Cys for linkage. A scrambled peptide was synthesized as a control, and both peptides were linked to magnetic beads (Life Technologies Dyna Beads Protein A). The peptide-coated beads (5mg) were incubated with 40μg of serum protein for 3h at room temperature. Following incubation and washings in PBS containing 0.5% Tween 20, the beads were immobilized using the magnet and the supernatant, containing the purified target, obtained. The eluate was separated on a 4–12% SDS-PAGE gel, the gel washed and stained with GelCode Blue, and destained overnight. Regions of the gel with bands exhibiting greater staining density with the recognizer peptide pull down, as compared to the scrambled peptide, were identified and submitted to the University of Kentucky Proteomics Core Facility for sequencing. Following trypsin digestion, the peptides were analyzed by Orbitrap LC/MS. MS data were searched in Mascot against Mus musculus sequences in SwissProt and a peptide high confidence value filter was applied.
Comparisons between binding of different phages to the biofluids from injured vs. uninjured mice were performed using one-way ANOVA with posthoc analysis using Tukey’s HSD test. The null hypothesis was rejected at the P < 0.05 level.
Results
Following three rounds of subtractive panning, six individual phage clones were selected for analysis by ELISA and sequencing. ELISA analysis against the pooled serum samples revealed that four of the six clones exhibited increased binding to serum obtained from mice 6h following contusive TBI, as compared to serum from control (uninjured) mice (Fig. 1).
Figure 1. ELISA with individual phage clones.
Each phage clone was evaluated using ELISA against serum samples from uninjured (white bars) and injured (black bars) mice. Anti M13-HRP antibody (GE Healthcare, Cat#27-9421-01) was used to detect the phage, the signal was detected using 3,3′,5,5′-tetramethyl benzidine (TMB) substrate and the absorbance was read at 450nm. Individual phages 1, 2, 3,and 4 showed increased binding to serum from injured (black) as compared to uninjured (blue)mice. Data are the mean ± SEM, n=3.
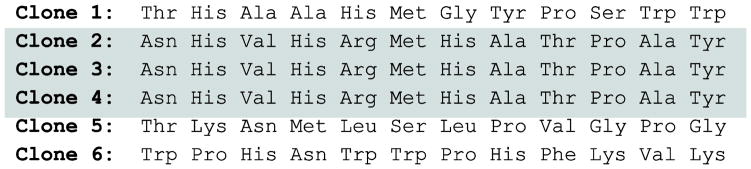
The DNA insert encoding the 12-mer peptide for the six phages was sequenced using primer sequences provided by the manufacturer (New England Biolabs) and translated using a standard amino acid codon table (Fig 2). The sequences for clones 2, 3 and 4 were identical indicating that they were amplified from the same original sequence.
Figure 2. Peptide sequence of individual phage clones.
The DNA insert encoding the 12-mer peptide expressed on the P8 coat protein for the six individual phages was sequenced using primer sequences provided by the manufacturer (New England Biolabs) and translated using a standard amino acid codon table. Note that the sequence for clones 2, 3 and 4 are identical (highlighted), indicating that they were amplified from the same original sequence.
To further examine the suitability of the identified phages, we screened phages 1 and 2 against novel serum samples collected from injured (1 mm CCI, 6h post-injury, n=4) and uninjured (n=3) mice using ELISA. The results demonstrate that phage 1, but not phage 2, consistently exhibited greater binding to serum obtained from mice following TBI as compared to serum from uninjured mice (Fig 3).
Figure 3. ELISA for Phages 1 and 2 vs. novel serum samples.
Phages 1 and 2 were evaluated using novel serum samples collected from injured (1 mm CCI, 6h post-injury, n=8) and uninjured (n=3) mice. Phage 1, but not Phage 2, exhibited preferential binding to the injured serum. (* denotes P<0.05)
Based on its enriched binding to TBI serum, the peptide expressed on the P3 coat protein of phage 1 was synthesized along with a C-terminal cysteine (THAAHMGYPSWW-C) and linked to magnetic beads. A scrambled peptide (WHAAHMPYGSWT-C) was used as a control. Following pull-down and subsequent proteomic analysis, three proteins were identified with high confidence: glial fibrillary acidic protein (GFAP, accession number P03995), oxidized glutathione peroxidase 3 (GPX3, accession number P46412), and serum paraxonase/arylesterase 1 (PON1, P52430). In contrast to other isozymes of glutathione peroxidase, GPX3 is extracellular and is abundant in plasma (Takahashi et al., 1987) and is therefore likely an artifact. PON1 is secreted by the liver and other tissues and is present in serum (Mackness et al., 1998). Other proteins identified included bovine serum albumin (P02769), an artifact of the blocking buffer; and several keratins from homo sapiens, contaminants from either the preparation or common use of the proteomic instrumentation.
Discussion
The goal of this study was to evaluate the suitability of phage display for the identification of serum biomarkers of TBI. Following subtractive panning phage display and subsequent pull-down with the recognizer peptide and subsequent mass spectrometry analysis, three proteins were identified with high confidence: GPX3, PON1, and GFAP. GPX3 and PON1 are blood/serum proteins and likely represent artifacts resulting from the high sensitivity of mass spectrometry. In contrast, elevated serum GFAP has previously been identified as a TBI biomarker in both animal studies and human cases (Papa et al., 2012)(Honda et al., 2010) (Yang and Wang, 2015)(Kulbe and Geddes, 2016; Wang et al., 2015). Thus, the identification of GFAP in the present study provides proof-of-concept for the utilization of phage display for identification of serum TBI biomarkers.
Of the six individual phages evaluated, three had the same DNA insert. This is due to the phage amplification steps between subtractive pannings. Fewer rounds of panning and amplification would reduce replication of individual phages, but may also reduce the pool of phages that bind to the target. Further panning/amplification rounds may result in a phage pool with greater specificity towards the target, but there is also the risk of some phages being enriched due to ease of amplification vs. target specificity. Thus, the three rounds of panning are considered optimal.
The present study utilized a single, commercial phage library consisting of randomized 12-mer peptides. Other libraries are available including randomized 7-mer peptides (Ph.D. 7, New England Biolabs) and a structurally constrained library (Ph.D. C7C, New England Biolabs). The 12-mer peptides allow for folding into short structural elements, while the 7-mer peptides require binding targets concentrated in a short stretch of amino acids. It is not possible to predict in advance which library will yield the greatest number of productive targets (Bonnycastle et al., 1996).
Additional methods of phage display are also available, with antibody phage display (Benhar and Reiter, 2002; McCafferty et al., 1990)(Hof et al., 2006) being of particular interest. In this protocol, large repertoires of single-chain Fv antibody fragment (scFv) or Fab antibody genes are cloned into phage or phagemid vectors, similar to the random peptides of the New England Biolabs library. This method has been used to develop antibodies for therapeutics and vaccines (Schirrmann et al., 2011).
Phage display has been successfully utilized to identify serum biomarkers in non-CNS disorders including ankylosing spondylitis (Wang et al., 2011), rheumatoid and osteoarthritis (Weng et al., 2012) (Araujo et al., 2015), heart disease (Park et al., 2010), hepatocellular carcinoma (Zhang et al., 2011), and lieshmaniasis (Coelho et al., 2015), and to identify a novel calpain inhibitor (Guttmann et al., 2005) but has not previously been applied to CNS disorders including TBI. Our results extend the utility of phage display to the discovery of serum TBI biomarkers. We are currently utilizing this methodology to search for novel biomarkers of mTBI.
Highlights.
Phage display is an unbiased approach to identify serum biomarkers
Applicable to acute CNS injuries as well as neurological disorders
Identification of GFAP as serum biomarker of TBI provides proof-of-concept
Acknowledgments
This research was supported by NIH grants R21 NS084088 and P30 NS051220 and by the Kentucky Spinal Cord and Head Injury Research Trust. We thank Kathleen Schoch, Ph.D. for the TBI surgeries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoston DV, Elsayed M. Serum-Based Protein Biomarkers in Blast-Induced Traumatic Brain Injury Spectrum Disorder. Front Neurol. 2012;3:107. doi: 10.3389/fneur.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo GR, Vaz ER, Fujimura PT, Fonseca JE, de Lima LM, Canhao H, Venturini G, Cardozo KH, Carvalho VM, Napimoga MH, Goulart LR, Goncalves J, Ueira-Vieira C. Improved serological detection of rheumatoid arthritis: a highly antigenic mimotope of carbonic anhydrase III selected in a murine model by phage display. Arthritis Res Ther. 2015;17:168. doi: 10.1186/s13075-015-0685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzazy HM, Highsmith WE., Jr Phage display technology: clinical applications and recent innovations. Clinical biochemistry. 2002;35:425–45. doi: 10.1016/s0009-9120(02)00343-0. [DOI] [PubMed] [Google Scholar]
- Benhar I, Reiter Y. Phage display of single-chain antibody constructs. Curr Protoc Immunol. 2002;Chapter 10(Unit 10):9B. doi: 10.1002/0471142735.im1019bs48. [DOI] [PubMed] [Google Scholar]
- Bonnycastle LL, Mehroke JS, Rashed M, Gong X, Scott JK. Probing the basis of antibody reactivity with a panel of constrained peptide libraries displayed by filamentous phage. J Mol Biol. 1996;258:747–62. doi: 10.1006/jmbi.1996.0284. [DOI] [PubMed] [Google Scholar]
- Bradbury AR. The use of phage display in neurobiology. Curr Protoc Neurosci. 2010;Chapter 5(Unit 5):12. doi: 10.1002/0471142301.ns0512s51. [DOI] [PubMed] [Google Scholar]
- Bratkovic T. Progress in phage display: evolution of the technique and its application. Cellular and molecular life sciences : CMLS. 2010;67:749–67. doi: 10.1007/s00018-009-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy GM, Mondello S, Papa L, Robicsek SA, Gabrielli A, Tepas J, 3rd, Buki A, Robertson C, Tortella FC, Hayes RL, Wang KK. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. Journal of neurotrauma. 2011;28:861–70. doi: 10.1089/neu.2010.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler IJ. Postconcussion Syndrome After Mild Traumatic Brain Injury in Children and Adolescents Requires Further Detailed Study. JAMA neurology. 2013:1–2. doi: 10.1001/jamaneurol.2013.2801. [DOI] [PubMed] [Google Scholar]
- Cao T, Thomas TC, Ziebell JM, Pauly JR, Lifshitz J. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience. 2012;225:65–75. doi: 10.1016/j.neuroscience.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pepin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Coelho EA, Chavez-Fumagalli MA, Costa LE, Tavares CA, Soto M, Goulart LR. Theranostic applications of phage display to control leishmaniasis: selection of biomarkers for serodiagnostics, vaccination, and immunotherapy. Rev Soc Bras Med Trop. 2015;48:370–9. doi: 10.1590/0037-8682-0096-2015. [DOI] [PubMed] [Google Scholar]
- Czeiter E, Mondello S, Kovacs N, Sandor J, Gabrielli A, Schmid K, Tortella F, Wang KK, Hayes RL, Barzo P, Ezer E, Doczi T, Buki A. Brain Injury Biomarkers May Improve the Predictive Power of the IMPACT Outcome Calculator. Journal of neurotrauma. 2012;29:1770–8. doi: 10.1089/neu.2011.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Zhao J, Hergenroeder G, Moore AN. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:100–14. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamandis EP. Analysis of serum proteomic patterns for early cancer diagnosis: drawing attention to potential problems. J Natl Cancer Inst. 2004;96:353–6. doi: 10.1093/jnci/djh056. [DOI] [PubMed] [Google Scholar]
- Ding J, Ding Z, Yuan F, Guo J, Chen H, Gao W, Wang R, Gu Y, Chen J, Guo Y, Tian H. Proteomics analysis after traumatic brain injury in rats: the search for potential biomarkers. Arq Neuropsiquiatr. 2015;73:342–9. doi: 10.1590/0004-282X20150006. [DOI] [PubMed] [Google Scholar]
- Eakin K, Miller JP. Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. Journal of neurotrauma. 2012;29:1180–7. doi: 10.1089/neu.2011.2192. [DOI] [PubMed] [Google Scholar]
- Giacoppo S, Bramanti P, Barresi M, Celi D, Foti Cuzzola V, Palella E, Marino S. Predictive biomarkers of recovery in traumatic brain injury. Neurocritical care. 2012;16:470–7. doi: 10.1007/s12028-012-9707-z. [DOI] [PubMed] [Google Scholar]
- Guingab-Cagmat JD, Newsom K, Vakulenko A, Cagmat EB, Kobeissy FH, Zoltewicz S, Wang KK, Anagli J. In vitro MS-based proteomic analysis and absolute quantification of neuronal-glial injury biomarkers in cell culture system. Electrophoresis. 2012;33:3786–97. doi: 10.1002/elps.201200326. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Day GA, 3rd, Wang X, Bottiggi KA. Identification of a novel calpain inhibitor using phage display. Biochemical and biophysical research communications. 2005;333:1087–92. doi: 10.1016/j.bbrc.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Hof D, Cheung K, Roossien HE, Pruijn GJ, Raats JM. A novel subtractive antibody phage display method to discover disease markers. Molecular & cellular proteomics : MCP. 2006;5:245–55. doi: 10.1074/mcp.M500239-MCP200. [DOI] [PubMed] [Google Scholar]
- Honda M, Tsuruta R, Kaneko T, Kasaoka S, Yagi T, Todani M, Fujita M, Izumi T, Maekawa T. Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J Trauma. 2010;69:104–9. doi: 10.1097/TA.0b013e3181bbd485. [DOI] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, Dash PK. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. Journal of neurotrauma. 2013;30:657–70. doi: 10.1089/neu.2012.2439. [DOI] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nature reviews Neurology. 2013;9:222–30. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RS. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Current opinion in critical care. 2008;14:135–41. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- Kulbe JR, Geddes JW. Current status of fluid biomarkers in mild traumatic brain injury. Exp Neurol. 2016;275(Pt 3):334–52. doi: 10.1016/j.expneurol.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Loov C, Shevchenko G, Geeyarpuram Nadadhur A, Clausen F, Hillered L, Wetterhall M, Erlandsson A. Identification of injury specific proteins in a cell culture model of traumatic brain injury. PLoS One. 2013;8:e55983. doi: 10.1371/journal.pone.0055983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen Pharmacol. 1998;31:329–36. doi: 10.1016/s0306-3623(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Madathil SK, Carlson SW, Brelsfoard JM, Ye P, D’Ercole AJ, Saatman KE. Astrocyte-Specific Overexpression of Insulin-Like Growth Factor-1 Protects Hippocampal Neurons and Reduces Behavioral Deficits following Traumatic Brain Injury in Mice. PLoS One. 2013;8:e67204. doi: 10.1371/journal.pone.0067204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Diaz-Arrastia R, Brophy M, Engel D, Goodman C, Gwinn K, Veenstra TD, Ling G, Ottens AK, Tortella F, Hayes RL. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil. 2010;91:1667–72. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Maroon JC, Lepere DB, Blaylock RL, Bost JW. Postconcussion syndrome: a review of pathophysiology and potential nonpharmacological approaches to treatment. The Physician and sportsmedicine. 2012;40:73–87. doi: 10.3810/psm.2012.11.1990. [DOI] [PubMed] [Google Scholar]
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–4. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Noble L, Andrews B, Faden AI. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119–34. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- Mondello S, Linnet A, Buki A, Robicsek S, Gabrielli A, Tepas J, Papa L, Brophy GM, Tortella F, Hayes RL, Wang KK. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery. 2012;70:666–75. doi: 10.1227/NEU.0b013e318236a809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Robicsek SA, Gabrielli A, Brophy GM, Papa L, Tepas J, Robertson C, Buki A, Scharf D, Jixiang M, Akinyi L, Muller U, Wang KK, Hayes RL. alphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. Journal of neurotrauma. 2010;27:1203–13. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Lewis LM, Falk JL, Zhang Z, Silvestri S, Giordano P, Brophy GM, Demery JA, Dixit NK, Ferguson I, Liu MC, Mo J, Akinyi L, Schmid K, Mondello S, Robertson CS, Tortella FC, Hayes RL, Wang KK. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59:471–83. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JP, Cropek DM, Banta S. High affinity peptides for the recognition of the heart disease biomarker troponin I identified using phage display. Biotechnology and bioengineering. 2010;105:678–86. doi: 10.1002/bit.22597. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Wang KK, Hayes RL. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14:202–9. doi: 10.1111/j.1750-3639.2004.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng K. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108:1297–303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, Cameron P, Kelly AM, Nelms R, Curran C. Impact of early intervention on outcome following mild head injury in adults. Journal of neurology, neurosurgery, and psychiatry. 2002;73:330–2. doi: 10.1136/jnnp.73.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropper AH, Gorson KC. Clinical practice. Concussion The New England journal of medicine. 2007;356:166–72. doi: 10.1056/NEJMcp064645. [DOI] [PubMed] [Google Scholar]
- Rosenbaum SB, Lipton ML. Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain imaging and behavior. 2012;6:255–82. doi: 10.1007/s11682-012-9162-7. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. Journal of neurotrauma. 2008;25:719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. Journal of neurotrauma. 2006;23:1241–53. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- Sandler SJ, Figaji AA, Adelson PD. Clinical applications of biomarkers in pediatric traumatic brain injury. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2010;26:205–13. doi: 10.1007/s00381-009-1009-1. [DOI] [PubMed] [Google Scholar]
- Schirrmann T, Meyer T, Schutte M, Frenzel A, Hust M. Phage display for the generation of antibodies for proteome research, diagnostics and therapy. Molecules. 2011;16:412–26. doi: 10.3390/molecules16010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behavioural brain research. 2011;224:326–35. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Lowman HB, Cunningham BC, Wells JA. Phage display for selection of novel binding peptides. Methods in enzymology. 2000;328:333–63. doi: 10.1016/s0076-6879(00)28406-1. [DOI] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis. 2004;16:311–20. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Siman R, Toraskar N, Dang A, McNeil E, McGarvey M, Plaum J, Maloney E, Grady MS. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. Journal of neurotrauma. 2009;26:1867–77. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. Journal of neurotrauma. 1995;12:169–78. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Avissar N, Whitin J, Cohen H. Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme. Arch Biochem Biophys. 1987;256:677–86. doi: 10.1016/0003-9861(87)90624-2. [DOI] [PubMed] [Google Scholar]
- Topolovec-Vranic J, Pollmann-Mudryj MA, Ouchterlony D, Klein D, Spence J, Romaschin A, Rhind S, Tien HC, Baker AJ. The value of serum biomarkers in prediction models of outcome after mild traumatic brain injury. J Trauma. 2011;71:S478–86. doi: 10.1097/TA.0b013e318232fa70. [DOI] [PubMed] [Google Scholar]
- Phage Display Libraries: Instruction Manual. New England Biolabs; Ipswich, MA: Various Ph.D. [Google Scholar]
- Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow up after head injury: a second randomised controlled trial. Journal of neurology, neurosurgery, and psychiatry. 1998;65:177–83. doi: 10.1136/jnnp.65.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Ottens AK, Liu MC, Lewis SB, Meegan C, Oli MW, Tortella FC, Hayes RL. Proteomic identification of biomarkers of traumatic brain injury. Expert Rev Proteomics. 2005;2:603–14. doi: 10.1586/14789450.2.4.603. [DOI] [PubMed] [Google Scholar]
- Wang KK, Yang Z, Yue JK, Zhang Z, Winkler EA, Puccio A, Diaz-Arrastia R, Lingsma H, Yuh EL, Mukherjee P, Valadka A, Gordon WA, Okonkwo DO, Manley GTMDPD. Plasma Anti-Glial Fibrillary Acidic Protein (GFAP) Autoantibody Levels During the Acute and Chronic Phases of Traumatic Brain Injury - A TRACK-TBI Pilot Study. Journal of neurotrauma. 2015 doi: 10.1089/neu.2015.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li X, Chen J, Zhou Y, Cao H, Wu X, Jiang H. Screening and evaluating the mimic peptides as a useful serum biomarker of ankylosing spondylitis using a phage display technique. Rheumatology international. 2011;31:1009–16. doi: 10.1007/s00296-010-1403-8. [DOI] [PubMed] [Google Scholar]
- Weng X, Liao Q, Li K, Li Y, Mi M, Zhong D. Screening serum biomarker of knee osteoarthritis using a phage display technique. Clinical biochemistry. 2012;45:303–8. doi: 10.1016/j.clinbiochem.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364–74. doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S, Bullock R. Biomarkers for the clinical differential diagnosis in traumatic brain injury--a systematic review. CNS neuroscience & therapeutics. 2013;19:556–65. doi: 10.1111/cns.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nature reviews Neurology. 2013;9:201–10. doi: 10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu L, Wang Z. Screening serum biomarkers for early primary hepatocellular carcinoma using a phage display technique. Journal of clinical laboratory analysis. 2011;25:402–8. doi: 10.1002/jcla.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbig P, Jahn H. Use of proteomic methods in the analysis of human body fluids in Alzheimer research. Electrophoresis. 2012;33:3617–30. doi: 10.1002/elps.201200360. [DOI] [PubMed] [Google Scholar]