Abstract
The past several years have been marked by extraordinary advances in clinical applications of immunotherapy. In particular, adoptive cellular therapy utilizing chimeric antigen receptor (CAR) modified T cells targeted to CD19 has demonstrated substantial clinical efficacy in children and adults with relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL), and durable clinical benefit in a smaller subset of patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or B cell non-Hodgkin lymphoma (B-NHL). Early phase clinical trials are presently assessing CAR T cell safety and efficacy in additional malignancies. Herein, we discuss clinical results from the largest series to date investigating CD19-targeted CAR T cells in B-ALL, CLL, and B-NHL, including discussion of differences in CAR T cell design and production and treatment approach, as well as clinical efficacy, nature of severe cytokine release syndrome and neurologic toxicities, and CAR T cell expansion and persistence. We additionally review the current and forthcoming use of CAR T cells in multiple myeloma and several solid tumors, and highlight challenges and opportunities afforded by the current state of CAR T cell therapies, including strategies to overcome inhibitory aspects of the tumor microenvironment and enhance antitumor efficacy.
Keywords: CAR T cells, chimeric antigen receptors, adoptive cellular therapy, acute lymphoblastic leukemia, cytokine release syndrome
Chimeric antigen receptor modified T cells: a general introduction
The limitations of presently available therapies to induce remissions reliably and durably in patients with advanced, relapsed or refractory malignancies has brought renewed interest to the field of immunotherapy, including antibody-based and adoptive cellular therapies. The adoptive transfer of genetically modified autologous T cells aims to rapidly establish specific antitumor activity. This strategy requires targeting of autologous T cells by means of a transgene-encoded antigen receptor, consisting of a chimeric antigen receptor (CAR), as will be discussed herein, or T cell receptor (TCR) chains. In the past several years, we and others have presented early data demonstrating that CAR-modified T cells targeting CD19 can induce meaningful responses in patients with relapsed or chemotherapy-refractory B cell malignancies. Herein, we review clinical results from the largest series reported to date reflecting treatment of hematologic malignancies with CAR-modified T cells, and the challenges and opportunities ahead in improving antitumor efficacy, understanding and managing toxicity, and extending this technology to further clinical settings.
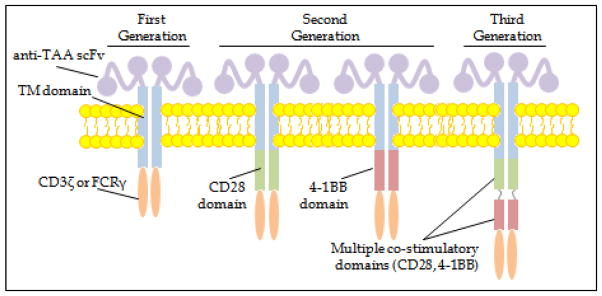
A CAR is a recombinant receptor construct composed of an extracellular single-chain variable fragment (scFv) derived from an antibody, linked to intracellular T cell signaling domains of the T cell receptor, thereby redirecting T cell specificity to the tumor in an HLA-independent manner.1 Multiple signals are required for the expansion and differentiation of naïve T cells. Antigen-specific interaction of the peptide:MHC complex with the T cell receptor (signal 1) is necessary but insufficient for full T cell activation. Costimulatory signaling, mediated through the interaction of costimulatory receptors on the T cell surface (e.g. CD28, 4-1BB, OX-40) with cognate ligands (e.g. CD80/86 [B7-1/B7-2], 4-1BB, OX-40L) on target cells or professional antigen-presenting cells (signal 2), is required for induction of IL-2/IL-2R expression and further T cell proliferation and differentiation to mature effector T cells. Initial first-generation CARs consisted of a target-specific scFv, fused to an inert CD8 transmembrane domain linked to a signaling domain of the T cell receptor (e.g. CD3ζ). As first-generation CAR T cells exhibited limited proliferative capacity and antitumor efficacy, second-generation CARs were designed with further genetic modifications to add cytoplasmic signaling domains of T cell costimulatory receptors and thereby provide “signal 2” on engagement with their target. Second-generation CD19-targeted CARs in present clinical use as described herein have incorporated a single costimulatory domain, most commonly CD28 or 4-1BBL (Figure 1). A third-generation CAR places multiple costimulatory domains in tandem; published clinical experience using third generation CARs remains limited.2 CAR T cells exert antitumor activity due to immune synapse formation with target cells (with release of perforin and granzyme, and expression of pro-apoptotic ligands) and secretion of pro-inflammatory cytokines (e.g. IFN-γ, IL-2), additionally resulting in recruitment of endogenous immune responses. Additionally, CAR T cells appear to be capable of sequential target cell destruction (i.e. “serial killing”).3
Figure 1.
Schematic depicting structure of chimeric antigen receptors in largest published series to date. scFv = single-chain variable region fragment; TAA = tumor associated antigen; TM = transmembrane.
Treatment of B cell ALL with CAR-modified T cells
Adult ALL
Adults with relapsed or refractory B-ALL have a poor prognosis when treated with standard salvage chemotherapy.4,5 The largest series of patients with B-ALL treated with CD19-targeted CAR T cells are summarized in Table 1. We (Memorial Sloan Kettering Cancer Center, MSKCC) were the first to report the efficacy of CD19-targeted CAR T cells incorporating a CD28 costimulatory domain (19-28z) in generating durable molecular remissions in 5 adults with relapsed ALL.6 Updated results were presented by the MSKCC group at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting.6–8 In brief, we reported on 51 treated patients, of whom 50 were eligible for response assessment. High-risk features of enrolled patients included Philadelphia chromosome-positive (Ph+) B-ALL (n=15), prior allogeneic hematopoietic stem cell transplantation (AlloHSCT, n=18), and ≥3 prior lines of treatment (n=31). Immediately prior to CAR T cell infusion, 31 patients had morphologic disease (≥5% blasts in the bone marrow [BM], or measurable extramedullary disease) and 20 patients had minimal disease (<5% blasts in marrow). Patients received either cyclophosphamide (Cy) alone or Cy in combination with fludarabine (Flu) as lymphodepleting chemotherapy two days prior to 19-28z CAR T cell infusion. During the initial phase of the trial, all patients received 3 × 106 19-28z CAR T cells/kg regardless of the pre-treatment disease burden. However, after observing a higher incidence of treatment-related toxicities at this dose in patients with morphologic disease, the CAR T cell dose was adjusted based on the disease burden, such that patients with morphologic disease received 1 × 106 19-28z CAR T cells/kg and patients with minimal disease continued to receive 3 × 106 19-28z CAR T cells/kg. In the entire cohort, inclusive of patients receiving all T cell dose levels, 41 patients (82%) achieved or remained in complete response (CR) following 19-28z CAR T cell infusion. 33 of 39 patients in CR following 19-28z CAR T cell infusion achieved minimal residual disease (MRD) negativity by multiparameter flow cytometry or deep sequencing. Similar rates of CR were observed regardless of age, disease burden prior to CAR T cell infusion, number of prior therapies, and prior AlloHSCT status. Following CAR T cell infusion, 16 of 41 patients who achieved CR underwent allo-HSCT.8 We previously reported 6-month overall survival (OS) did not differ significantly between who underwent post-CAR T cell AlloHSCT (79%) and those who did not (80%).9 15 of 33 patients achieving MRD-negative CR relapsed during follow-up, of whom 4 experienced morphologic relapse of B-ALL with undetectable CD19 expression.6–8,10
Table 1.
CAR T cell design and production and clinical aspects of largest clinical trials to date investigating CD19-targeted CAR T cells in the treatment of B-ALL.
| Institution/ Reference |
# of Pts Reported |
Gene Transfer Method |
scFv | Co- stimulatory Domain |
Median age (range) |
Lymphodepleting Chemotherapy |
CAR T cell Doses |
Disease-Related Outcomes |
|---|---|---|---|---|---|---|---|---|
| Children’s Hospital of Philadelphia18 | 30 (25 children, 5 adults) | Lentivirus | FMC63 | 4-1BB | Pediatric ALL: 11 (5–22) Adult ALL: 57 (36–60) |
Cy 300–500 mg/m2/d × 2–3d + Flu 30 mg/m2/d × 3–4d (n=15) or Cy 440 mg/m2/d × 2d + VP 100 mg/m2/d × 2d (n=5) or other (n=10) | 0.76–17.36 ×106 CAR+ T cells/kg | CR: 90% (MRD-negative in 88% of those who achieved CR) 6-month EFS: 67% 6-month OS: 78% |
| National Cancer Institute19 | 20 | Gamma-retrovirus | FMC63 | CD28 | Pediatric ALL: 15 (5–27) | Cy 900 mg/m 2 and Flu 25 mg/m2/day × 3 days | 1×106 vs. 3×106 CAR+ T cells/kg | CR: 70% (MRD-negative in 60% of those who achieved CR) OS: 52% at 7.8 mon LFS: 79% at 4.8 mon 10 of 12 in MRD-negative CR underwent AlloHSCT |
| National Cancer Institute52 | 5 | Gamma-retrovirus | FMC63 | CD28 | Adult ALL | None (administered following AlloHSCT) | 4.2–7.1×106 CAR+ T cells/kg | CR: 80% (4/5, all MRD-negative) |
| Memorial Sloan Kettering Cancer Center6–8 | 51 | Gamma-retrovirus | SJ25C1 | CD28 | Adult ALL: (22–74) | Cy (n=42) or Cy/Flu (n=9) | 1×106 vs. 3×106 CAR+ T cells/kg | CR: 82% (MRD-negative in 85% of those who achieved CR) 16 of 41 in CR underwent AlloHSCT Median OS: 9 months (in pts with morphologic disease at CAR T cell infusion), not reached (in pts with minimal disease at CAR T cell infusion) |
| Fred Hutchinson Cancer Research Center11 | 30 | Lentivirus | FMC63 | 4-1BB | Adult ALL: 40 (20–73) | Cy 2–4 g/m2 ± VP 100 mg/m2/d × 3d or Cy 30–60 mg/kg + Flu 25/mg/m2/d × 3–5d | 2×105, 2×106, and 2×107 CAR+ T cells/kg; 1:1 CD4+:CD8+ | MRD-negative CR: 10/12 among pts receiving Cy monotherapy; 16/17 among pts receiving Flu/Cy |
| University of Pennsylvania13 | 27 | Lentivirus | FMC63 | 4-1BB | Adult ALL: 44 (21–72) | Investigator’s choice | 5×107 – 5×108 CAR+ T cells | CR: 15/27 (across all cohorts) |
scFv=Single chain fragment variable fragment; Cy=cyclophosphamide, Flu=fludarabine, VP=etoposide, OS=overall survival, EFS=event free survival, MRD=minimal residual disease, CR=complete response, pts=patients, AlloHSCT=allogeneic hematopoietic stem cell transplantation
Investigators from the Fred Hutchinson Cancer Research Center (FHCRC) recently reported mature results of their phase I trial of CD19-targeted CAR T cells in 30 adults with relapsed/refractory B-ALL.11 In contrast to the MSKCC approach as above, the FHCRC’s treatment protocol uses lentiviral transduction and a CD19-targeted CAR bearing a 4-1BB costimulatory domain (rather than the CD28 costimulatory domain). In addition, CD4+ and CD8+ T cells are expanded separately in vitro prior to infusion at a defined ratio of 1:1 CD4+:CD8+ CAR T cells, at total infused doses of 2 × 105, 2 × 106, and 2 × 107 CAR T cells/kg; the rationale for this approach is detailed in a subsequent section. FHCRC reported on 30 adults, of whom 11 had experienced relapse following AlloHSCT. Most patients had morphologic B-ALL at the time of CAR T cell infusion (median BM blasts 21%, range, 0.014–97%). 13 patients received lymphodepleting chemotherapy consisting of Cy without Flu; 10 of 12 evaluable patients achieved CR by flow cytometry (83%), though seven of 10 experienced relapse at a median of 66 days following CAR T cell infusion. While five of these patients were retreated, no response was observed; an endogenous CD8+ T cell response directed against the murine scFv component of the transgene was observed and hypothesized to contribute to CAR T cell rejection and in vivo expansion failure. As the investigators postulated that more intensive lymphodepletion would abrogate the CAR T cell rejection observed among patients receiving Cy alone, they administered Flu 25 mg/m2/day for 3–5 days along with Cy prior to CAR T cell infusion, and observed BM CR by flow cytometry and cytogenetic studies in 16 of 17 treated patients.11 Higher CAR T cell levels were observed 28 days following infusion of 2 × 106 CAR T cells (55.8 vs. 0.10 CD8+ CAR T cells/mL and 2.1 vs. 0.02 CD4+ CAR T cells/mL following Cy/Flu vs. Cy alone).12 Significantly improved disease-free survival (DFS) was observed among those receiving Cy/Flu as well.11
Investigators from the University of Pennsylvania (UPenn) and Children’s Hospital of Philadelphia (CHOP) have used a CD19-targeted CAR containing a 4-1BB costimulatory domain (similar to FHCRC as above) to generate their CAR T cell product, CTL019. Though the preponderance of UPenn’s reported experience treating relapsed and refractory ALL using CTL019 has been in pediatric patients, at the 2016 ASCO Annual Meeting they additionally reported results in 27 adults with relapsed or refractory B-ALL treated with 5 × 107 – 5 × 108 total CTL019 infused over 1–3 days following investigator’s choice of lymphodepleting chemotherapy. Across all CTL019 dose levels, 15 of 27 patients attained CR, including 9 of 112 treated with fractionated infusion of 5 × 108 CTL019. Of note, 3 of 6 patients treated with a single dose of 5 × 108 CTL019 died with CRS refractory to corticosteroids and tocilizumab, and all had concurrent culture-positive sepsis.13
Pediatric ALL
While outcomes have dramatically improved among children and adolescents diagnosed with B-ALL, those with chemotherapy-refractory or multiply relapsed B-ALL continue to have a poor prognosis.14–16 Therapeutic use of CD19-targeted CAR T cells remains under active investigation in this setting as well. Investigators from UPenn and CHOP have used a CD19-targeted CAR containing a 4-1BB costimulatory domain (similar to FHCRC as above) to generate their CAR T cell product, CTL019.17,18 UPenn has previously reported on 25 pediatric patients and 5 adults with refractory B-ALL; 25 of 30 total patients had primary refractory disease or were in second relapse or beyond, and 18 had undergone prior AlloHSCT. In addition to standard criteria of adequate organ function, successful test expansion of an aliquot of peripheral blood mononuclear cells in response to CD3/CD28 stimulation was required for eligibility. 24 patients had morphologic evidence of ALL, one had MRD, and 5 patients (all pediatric) had MRD-negative CR at the time of CTL019 therapy. 27 of 30 patients received one of several lymphodepleting regimens prior to infusion of CTL019 (median 3.6 × 106 CTL019/kg, range: 0.76–17.36 × 106 CTL019/kg). At first assessment following CTL019 infusion, 27 of 30 patients were in morphologic CR, and 22 of 25 evaluable patients in morphologic CR had achieved MRD-negative CR by multiparameter flow cytometry; one patient achieved MRD-negative CR after first assessment had revealed MRD. Higher peak proportions of CTL019 among total CD3+ T cells were detectable by flow cytometry among the 27 patients who achieved CR (median 39.8%, range 4.4–69.3%) than among the 3 who did not (0.2, 0.6 and 8.2%). Probability of CTL019 persistence by flow cytometry at 6 months was 68%; maximal CTL019 T cell persistence detected by flow cytometry was 11 months; CTL019 sequences were detectable by quantitative PCR as far as 2 years following CTL019 T cell infusion. Two patients with active CNS leukemia at the time of infusion ultimately cleared leukemic blasts from the CSF. 19 of 27 patients remained in CR during reported follow-up, 4 of whom left the study to receive further therapy. 7 patients experienced relapse between 6 weeks and 8.5 months post-infusion; relapsed disease was CD19-positive in 4 patients (3 of whom had experienced early loss of detectable CTL019) and CD19-negative in 3 patients. One patient developed MDS during CR. 6-month EFS was 67% (95% CI, 51–88%) and 6 month OS was 78% (95% CI, 65–95%).18
CD19-targeted CAR T cell therapy has similarly been assessed in pediatric patients with relapsed ALL by investigators at the National Cancer Institute (NCI). Similar to the MSKCC approach as above, the CAR construct utilized by the NCI contains a CD28 costimulatory domain, with a protocol of retroviral transduction, though utilizing a different scFv. The NCI reported a dose escalation study enrolling 21 children and young adults with relapsed or refractory B-ALL (n=20) or B-NHL (n=1), with high-risk features including primary refractory disease (n=6), prior AlloHSCT (n=8), and active CNS leukemia (n=2) at the time of treatment. Median BM blast content was 26% (range, 0.03–96%) and most (n=16) had ≥ 5% BM blasts at the time of therapy. Enrolled patients received Flu and Cy followed by 1 × 106 CAR T cells/kg or 3 × 106 CAR T cells/kg in the context of a 3+3 dose escalation protocol. The target CAR T cell dose was not able to be generated in two patients, and the entire manufactured product was infused. Dose limiting toxicity (DLT) was observed (grade 3–4 cytokine release syndrome [CRS], detailed below) at the 3 × 106 CAR T cells/kg dose level, and the maximum tolerated dose (MTD) was ultimately defined by the trial as 1 × 106 CAR T cells/kg. 14 of 21 patients achieved CR, including 14 of 20 with B-ALL. 12 of 20 with B-ALL achieved MRD-negative CR. OS was 51.6% at 9.7 months; median follow-up was 10 months, and leukemia-free survival was 78.8% at 4.8 months among the 12 patients who achieved MRD-negative CR. 10 of 12 patients who achieved an MRD-negative CR after CAR T cell therapy underwent AlloHSCT and remained in continued CR; 2 of the 12 who were determined to be ineligible for AlloHSCT experienced CD19-negative relapse at 3 and 5 months. Retreatment of 3 patients not responding to initial infusion yielded no further responses. 11 of 17 assessed patients had evidence of CAR T cells in the CSF, including both patients with CNS leukemia at the time of infusion. In 18 of 21 patients, CAR T cells were identified post-infusion by flow cytometery; peak CAR T cell expansion as assessed by flow cytometry and qPCR was observed around day 14, and the degree of expansion appeared to correlate both with response and severity of cytokine release syndrome (as below). Maximal observed CAR T cell persistence was 68 days.19
An MSKCC-led multi-institutional consortium of investigators has reported very preliminary data at the 2015 ASH Annual Meeting reflecting 9 pediatric patients with relapsed B-ALL treated with 19-28z CAR T cells. Patients received 1–3 × 106 19-28z CAR T cells/kg, and CR was noted in 5/9 patients. Lower disease burden at the time of 19-28z CAR T cell infusion and greater expansion following in vitro CD3/CD28 bead activation during CAR T cell production appeared correlated with response. Peak CAR T cell levels in the BM post-infusion were observed at 1–2 weeks, with loss of detectable CAR T cells over 1–2 months post-infusion.20
Toxicities of CAR T cells in Patients with ALL
The aforementioned reports of CAR T cells in ALL reflect different methods of CAR T cell design, production, and dosing, as well as important differences in patients’ baseline characteristics, leukemic burden, and lymphodepleting chemotherapy. However, in addition to sharing impressive observed rates of MRD-negative CR, compared with commonly used salvage chemotherapy regimens, the above trials share observed toxicities of CRS, neurologic toxicity, and B cell aplasia. While the definition and clinical grading of CRS following CAR T cell therapy remains in evolution, CRS reflects a systemic inflammatory response syndrome in the hours to days following CAR T cell infusion, characterized by elevations of pro-inflammatory cytokines and T cell activation and expansion, with clinical features including fevers, myalgias, malaise, and, in more severe cases, a capillary leak syndrome associated with hypotension, hypoxia, and occasionally renal dysfunction and coagulopathy. Investigators from CHOP/UPenn reported that all 30 patients (including 25 pediatric patients) treated in their initial report exhibited CRS, which was mild-to-moderate in 22 patients, and severe in 8 patients (requiring intensive care with vasopressors and supplemental oxygen, up to and including intubation and mechanical ventilation), with clinical bleeding in the presence of coagulopathy in 3 patients. While cytokine levels were variable, those patients with severe CRS exhibited higher levels of C-reactive protein (CRP), ferritin, interleukin (IL)-6, IFN-γ, and soluble IL-2 receptor. Investigators from MSKCC first noted a positive correlation between greater leukemic burden in patients with B-ALL prior to CAR T cell infusion and greater elevations in IL-6, IFN-γ, and soluble IL-2 receptor as well as greater clinical severity of CRS.6 Similarly, investigators from CHOP/UPenn found higher leukemic burden in the BM prior to CTL019 infusion was correlated with greater risk of CRS, and patients with CRS demonstrated higher levels of CTL019+ CD8+ T cells and overall CTL019+ CD3+ T cells. None of the 3 patients not achieving CR following CTL019 experienced severe CRS. Treatment for CRS in this series included the anti-IL-6 receptor monoclonal antibody tocilizumab (n=9) and corticosteroids (n=6), and all patients demonstrated improvement.18 An updated report presented by investigators from UPenn/CHOP at the 2014 ASH Annual Meeting similarly noted severe CRS in 11 of 12 adults treated with CTL019, with 3 patients experiencing refractory, ultimately fatal shock despite administration of tocilizumab and steroids. Marked in vivo expansion of CTL019 was observed in these 3 patients. Each of the 3 patients additionally was experiencing a documented infection at the time of clinical decompensation, which may have served as an additional potentiating factor for CRS and complicated administration of immune-suppressive therapy.21
As discussed in the previous section, CRS constituted the dose-limiting toxicity in the NCI’s trial of CD19-targeted CAR T cells for children and adolescents. The NCI additionally created a novel grading system for CRS, as the widely-used NCI Common Terminology Criteria for Adverse Events v4.0 describes CRS as an infusion-related toxicity, which does not reflect its clinical characteristics following CAR T cell therapy. The NCI’s system for CRS defines grade 3 CRS as consisting of ≥20% drop of blood pressure from baseline, unresponsive to fluid administration, and/or grade 3 respiratory dysfunction, creatinine elevation, or neurologic dysfunction, and defines grade 4 CRS as CRS requiring ventilator and/or vasopressor support.19 Of the 21 pediatric patients in their report, 16 developed CRS of any grade, beginning a median of 4 days (range, 1–7) following CAR T cell infusion, and lasting a mean of 4.8 days (range, 1–9). Grade ≥3 CRS was observed in 6 of 21 total patients (grade 4 in 3 patients), including 2 of 4 patients treated with 3 × 106 CAR T cells/kg. One patient experienced cardiac arrest in the context of CRS and survived, with drop in left ventricular ejection fraction from 65 to <25%. CRS was reversible in all affected patients, with supportive care alone in most (n=12), tocilizumab and supportive care alone (n=2), or tocilizumab and corticosteroids (n=2).19
CRS has been observed in adults with relapsed or refractory ALL treated with CD19-targeted CAR T cells as well. 13 of 31 adults with morphologic B-ALL treated at MSKCC with 19-28z CAR T cells developed severe CRS (requiring vasopressors or mechanical ventilation), compared with one of 20 with MRD only at CAR T cell infusion. BM leukemic burden prior to 19-28z CAR T cell infusion and 19-28z CAR T cell dose appear to correlate with severity of CRS. We observed grade 5 toxicity (sepsis, multi-organ failure) in 3 patients treated with 3 × 106 19-28z CAR T cells/kg. Of note, while pre-existing infection may have contributed to these deaths, the dose of 19-28z CAR T cells was nonetheless subsequently lowered to 1 × 106 19-28z CAR T cells/kg for patients with morphologic disease (as previously defined) prior to CAR T cell therapy, and no grade 5 toxicities have been observed in patients treated subsequently.8 We have previously reported that the magnitude of CRP elevation, which is readily monitored in real-time in the clinical setting, appears to predict severe CRS.7 Similarly, investigators from the FHCRC observed a correlation between increased BM leukemic burden, higher CAR T cell dose, and development of severe CRS, with severe CRS (fever and hypotension requiring intensive care) reported in 7 of 30 patients; severe CRS was fatal in 2 patients. As in the aforementioned reports in pediatric patients, higher peak levels of CRP, ferritin, IFN-γ, and IL-6 were observed in patients with severe CRS. Similar to MSKCC, FHCRC has adopted a risk-adapted system to guide CAR T cell dosing depending on baseline leukemic burden.11
A spectrum of largely reversible neurologic toxicity has additionally been observed following CAR T cell infusion, including delirium, seizure-like activity, confusion, word-finding difficulty, aphasia, and frank obtundation.7 Such toxicities may occur independently of CRS. Investigators from CHOP/UPenn reported neurologic adverse effects in 13 of 30 patients in their study, ranging from delirium during the period of fevers to encephalopathy with delirium, hallucinations, confusion, and/or aphasia. Delayed encephalopathy following CRS-associated fevers was observed in 6 patients, which was self-limited, lasted 2–3 days and resolved over 2–3 days; one of these 6 patients exhibited seizures.18 The NCI similarly reported reversible neurotoxicity in a subset of pediatric patients treated with CAR T cells, including grade 1 visual hallucinations (n=5) and transient dysphasia (n=1). All patients with neurotoxicity had identifiable CAR T cells in the CSF, and their concentration appeared higher in affected vs. unaffected patients. Of note, this finding contrasts with data reported from MSKCC’s ongoing study in adults with ALL, which did not identify a correlation between the presence of CD19-targeted CAR T cells in the CSF and clinically evident neurotoxicity.7 Additionally, while CT/MRI findings were reportedly normal in assessed patients in the UPenn/CHOP cohort, reversible splenial lesion syndrome was observed by MRI in one patient in the NCI trial who exhibited mild encephalopathy.19 Within the MSKCC trial enrolling adults with B-ALL, 15 of 51 patients treated with 19-28z CAR T cells exhibited grade 3–4 neurologic toxicity. While severe CRS appeared largely restricted to patients with morphologic disease at the time of CAR T cell infusion in the MSKCC study, 4 of 20 patients with only minimal disease at the time of treatment developed grade 3–4 neurologic toxicity.8 A slightly higher proportion of adults in the FHCRC trial developed grade 3–4 neurologic toxicity (15 of 30 patients), which, like CRS, appeared correlated with elevations in pro-inflammatory cytokines (IL-6, IFN-γ, TNF-α) and appeared correlated with higher baseline BM leukemic burden and greater CAR T cell dose.11
Treatment of Chronic Lymphocytic Leukemia with CAR-modified T cells
The natural history of chronic lymphocytic leukemia (CLL) is considerably heterogeneous. While an initial course of observation is appropriate for many newly diagnosed patients, the majority of patients will ultimately require therapy. Patients with unfavorable cytogenetic or molecular features, or with persistent or recurrent disease following initial purine analog-based therapy, continue to have a guarded prognosis when treated with standard chemoimmunotherapy.22,23 The introduction and increasingly widespread use of oral molecularly targeted therapies such as ibrutinib has afforded a highly efficacious new line of treatment for patients with relapsed or refractory CLL.24,25 However, such therapies require an indefinite duration of treatment, and a significant subset of patients remain intolerant of such therapy due to toxicity. The largest series of patients with CLL and B cell NHL treated with CD19-targeted CAR T cells are summarized in Table 2. In the initial cohort of patients with CLL treated with 19-28z CAR-modified T cells at MSKCC, we described 8 patients with purine analog-refractory CLL and bulky lymphadenopathy, 7 of whom had received ≥2 lines of prior therapy, and 7 of whom had additional adverse cytogenetic or molecular features (del17p, del11q, and/or unmutated IgHV). Three patients received 1.2–3.0 × 107 19-28z CAR T cells/kg without lymphodepleting chemotherapy; no objectives responses were observed in these patients. A fourth patient developed fevers, hypotension, and renal failure following 19-28z CAR T cell infusion and died within 48 hours of a suspected sepsis syndrome; his case is detailed in a prior report.10 The subsequent four patients received Cy 1.5–3.0 g/m2 followed by 0.4–1.0 × 107 CAR T cells/kg, with T cell infusion split over 2 days. Of these patients, one demonstrated marked reduction of peripheral adenopathy after an initial period of stable to progressed disease, 2 others demonstrated stable disease, and another demonstrated progression. All patients developed fevers following 19-28z CAR T cell infusion, and 2 developed hypotension, including the patient above who died within 48 hours of infusion.26 In a more recent phase I trial at MSKCC, we enrolled 8 patients with untreated CLL, many of whom had unfavorable cytogenetic or molecular features (unmutated IgHV, n=7; del11q, n=1). These patients received pentostatin, Cy, and rituximab (PCR), and all attained partial response (PR). Patients subsequently received Cy followed two days later by escalating doses of 19-28z CAR T cells, administered outpatient. 4 of 5 patients receiving ≥1 × 107 19-28z CAR T cells/kg were readmitted with fevers and mild CRS. At median follow-up of 32.4 months, two patients achieved clinical CR as best response, 2 achieved PR, one had stable disease, and 3 had progression of disease, one of whom had rising ALC by the time of 19-28z CAR T cell infusion, and 2 of whom achieved BM response (MRD+ CR in one, PR in the other) with progression noted in lymph nodes.27
Table 2.
CAR T cell design and production and clinical aspects of largest clinical trials to date investigating CD19-targeted CAR T cells in the treatment of CLL and B-NHL.
| Institution/ Reference |
Population/ # Reported |
Gene Transfer Method |
scFv | Co- Stimulatory Domain |
Lymphodepleting Chemotherapy (LDC) |
CAR T cell Doses | Disease-Related Outcomes |
|---|---|---|---|---|---|---|---|
| Memorial Sloan Kettering Cancer Center26 | Adult CLL (n=8) | Gamma-retrovirus | SJ25C1 | CD28 | None or Cy 1.5–3 g/m2 | Cohort receiving no LDC: 1.2–3.0×107 CAR+ T cells/kg Cy cohort: 0.4–1.0×107 CAR+ T cells/kg |
ORR: 1/8 (PR); two others with ≥2 months of SD, all in Cy cohort |
| Memorial Sloan Kettering Cancer Center27 | Adult CLL (n=8) | Gamma-retrovirus | SJ25C1 | CD28 | Cy 600 mg/m2 | 3×106, 1×107, or 3×107 CAR+ T cells/kg |
ORR: 4/8 (CCR, n=2; PR, n=2) |
| National Cancer Institute34 | Adult CLL and B-NHL (n=8) | Gamma-retrovirus | FMC63 | CD28 | Cy 60 mg/kg × 2d + Flu 25 mg/m2 × 5d | 0.3–3.0×107 CAR+ T cells/kg | ORR: 6/8 (CLL, ¾; FL, 2/3); CR, n=1 (CLL) and PR, n=5 |
| National Cancer Institute35 | Adult B-NHL (n=15) | Gamma-retrovirus | FMC63 | CD28 | Cy 60 mg/kg × 1–2d + Flu 25 mg/m2 × 5d | 1 – 5×106 CAR+ T cells/kg | CR: 4/7 (refractory DLBCL), 4/6 (indolent B-NHL) |
| National Cancer Institute52 | Adult CLL and B-NHL (n=15) | Gamma-retrovirus | FMC63 | CD28 | None (administered following AlloHSCT) | 0.4–8.2×106 CAR+ T cells/kg | For pts with CLL, ORR: 2/5 (CR, n=1; PR, n=1; SD, n=1) For pts with other B-NHL, ORR: 2/10 (CR, n=1; PR, n=1; SD, n=7) |
| Fred Hutchinson Cancer Research Center37 | Adult CLL and B-NHL (n=28) | Lentivirus | FMC63 | 4-1BB | Cy 60 mg/kg ×1 ± etoposide or Cy 60 mg/kg ×1 + Flu 25 mg/m2 × 3d | 2×105, 2×106, and 2×107 CAR+ T cells/kg;1:1 CD4+:CD8+ |
ORR: 6/12 (CR, n=1, PR, n=5 in Cy cohort) and 8/12 (CR, n=5, PR, n=3 in Cy + Flu cohort) |
| University of Pennsylvania30 | Adult CLL (n=14) | Lentivirus | FMC63 | 4-1BB | Investigator’s choice | 0.14–11×108 CAR+ T cells | ORR: 8/14 (MRD negative CR, n=4; PR, n=4) Median PFS: 7 months Median OS: 29 months |
| University of Pennsylvania31 | Adult CLL (n=35 total; n=21 at stage 2 dose) | Lentivirus | FMC63 | 4-1BB | Investigator’s choice | Stage 1: 5×107 vs. 5×108 CAR+ T cells Stage 2: 5×108 CAR+ T cells |
ORR in stage 1: 10/23 (CR, n=5; PR, n=5) ORR in pts treated with stage 2 CAR T cell dose: 9/17 (CR, n=6; PR, n=3) |
| University of Pennsylvania36 | Adult B-NHL (n=24)† | Lentivirus | FMC63 | 4-1BB | Investigator’s choice | 3.08–8.87 ×106 CAR+ T cells/kg | ORR: 15/22 (DLBCL, 7/13; FL, 7/7, MCL, 1/2) PFS: 62% at 11.7 months |
scFv=Single chain fragment variable fragment, Cy=cyclophosphamide, Flu=fludarabine, VP=etoposide, OS=overall survival, EFS=event free survival, MRD=minimal residual disease, CR=complete response, CCR=clinical complete response, PR=partial response, pts=patients, DLBCL=diffuse large B cell lymphoma, FL=follicular lymphoma, MCL=mantle cell lymphoma, AlloHSCT=allogeneic hematopoietic stem cell transplantation.
38 enrolled, 24 received the protocol-specified dose and were included in the analysis
Investigators from UPenn have treated >40 patients with relapsed or refractory CLL with CTL019.21 They initially reported on a patient with refractory CLL with deletion of TP53 on chromosome 17p who achieved durable MRD-negative CR following pentostatin and Cy followed by 1.5 × 105 CTL019/kg.28,29 UPenn most recently reported mature data from their phase I study of CTL019 in 14 patients with refractory CLL. High-risk featured included unmutated IgHV (n=9), loss of TP53 or chromosome 17p (n=6), and a median of 5 prior therapies (range, 1–11). Patients received one of several lymphodepleting regimens followed by infusion of CTL019 over 1–3 days. 8 of 14 patients completed the full 3-days of CTL019, with the others receiving only one (n=3) or two fractions (n=3) due to fevers within 24 hours of infusion. At median 19 months of follow-up, 8 of 14 patients attained objective response, including 4 patients who achieved MRD-negative CR, 3 of whom have enjoyed durable responses (28–53 months) and one of who died of unrelated causes with no evidence of disease 21 months post-infusion. CTL019 expansion and persistence was assessed by flow cytometry and qPCR. Peak expansion was observed within the first month following CTL019 infusion, and greater CTL019 expansion was associated with better response; patients achieving CR exhibited peak expansion of median 73,237 copies/μg (range, 25,070–409,645) by qPCR and CTL019 comprised median 34.3% of CD3+ cells. Persistence of CTL019 was evident by flow cytometry and/or qPCR to most recent follow-up in the patients who achieved CR, and all 4 additionally exhibited ongoing B cell aplasia. An additional 4 patients achieved PR within the first month of infusion, lasting 5–13 months. 6 patients had no objective response and all progressed within 9 months of CTL019 infusion. Median OS for all patients was 29 months, 18-month OS was 71% and 18-month PFS was 28.6%. Though analysis is limited by the small number of patients, number of previous therapies, abnormalities of chromosome 17p, IgHV mutation status, and CTL019 dose did not appear correlated with response.30 9 of 14 patients developed CRS a median of 7 days (range, 1–14) following CTL019 infusion. 5 patients received tocilizumab and/or corticosteroids, and 4 required ICU care. Elevations in ferritin, CRP, and soluble IL-2 receptor were observed in patients experiencing CRS, consistent with observations in the context of ALL as previously discussed.30 This group subsequently conducted a phase II dose optimization study in which 28 patients with relapsed or refractory CLL were randomly assigned to receive 5 × 107 vs. 5 × 108 CTL019 following lymphodepleting chemotherapy. Among 24 patients who received the target CTL019 dose, they noted an overall response rate of 42% (CR, n=5, and PR, n=5); a clear dose-response relationship was not evident; patients were subsequently enrolled on a second stage of the study and treated with 5 × 108 CTL019.31
In summary, a subset of patients with refractory CLL appear to experience clinically significant benefits from second-generation CD19-targeted CAR T cells, though efficacy appears limited compared with the experience to date in ALL described in the previous section. In addition, the risks of severe CRS in patients with CLL may be decreased compared to patients with morphologic evidence of ALL. The challenges inherent in designing adoptive cellular therapeutics for CLL, and potential strategies to overcome these challenges, are discussed in the final section.
Treatment of B cell non-Hodgkin Lymphoma with CAR-modified T cells
Investigators from the NCI have described the investigational use of CD19-targeted T cells in management of relapsed or refractory CD19-expressing B cell non-Hodgkin lymphomas (NHL) and CLL in several reports. The NCI initially described a patient with refractory follicular lymphoma (FL) who achieved a partial response lasting 32 weeks and B cell aplasia following Cy/Flu and treatment with CD19-targeted autologous T cells bearing a CD28 costimulatory domain.32,33 They subsequently demonstrated that administration of CAR T cells with this design, followed by administration of IL-2, appeared to have clinical activity in patients with refractory indolent B cell malignancies, including CLL (n=4), FL (n=3), and splenic marginal zone lymphoma (MZL, n=1). Patients received lymphodepleting chemotherapy consisting of Cy and Flu followed by a single infusion of 0.3–3.0 × 107 CAR T cells/kg and administration of IL-2 every 8 hours until toxicity precluded further cytokine administration (median 3, range 1–10 doses). 6 of 8 enrolled patients achieved objective response (3 of 4 with CLL; 2 of 3 with FL) lasting 7–18+ months, with one patient with CLL attaining durable CR (15+ months) associated with B cell aplasia.34 CAR+ cells constituted >0.01% of PBMCs for <20 days in 6 of 8 patients. However, in 2 of 8 patients, CAR T cell persistence was demonstrated by qPCR and flow cytometry for up to 181 days in the peripheral blood, with CAR+ cells detectable the BM at 8 and 14 weeks post-infusion in these patients. Peak levels were noted prior to day 10 post-infusion and were widely variable.34 Notable toxicities described in this report included hypotension (n=4), capillary leak syndrome (n=4), acute renal failure (n=3), obtundation (n=2), and hypoxemia (n=2). An association between development of capillary leak syndrome and elevated levels of IFN-γ and TNF in the 10 days following CAR T cell administration was observed. While patients received IL-2 following CAR T cell administration, which may additionally predispose to fever and TH1 immunoregulatory cytokine induction, the final dose of IL-2 was administered ≥4 days prior to development of elevations in IFN-γ and TNF in 3 of 4 patients, suggesting this toxicity was at least in part associated with CAR T cell administration.32,34 The NCI group subsequently reported on 15 patients with chemotherapy-refractory DLBCL or indolent B cell malignancies who received Cy and Flu as lymphodepleting chemotherapy, followed by 1–5 × 106 CD19-targeted CAR T cells/kg, without post-infusion IL-2. Peak CAR T cell levels post-infusion varied considerably, with highest levels detected 7–17 days following infusion and ranging between 9–777 CAR+ cells/μL, with rapid decline in CAR T cell levels observed thereafter; 4 of 7 evaluable patients with refractory DLBCL achieved CR, which appeared durable in 3 of 4 cases (9–22+ months). Additionally, 4 of 6 patients with indolent B cell malignancies (CLL or indolent lymphoma) in the cohort achieved CR. 3 patients with CLL achieved durable CR confirmed by BM flow cytometry (14–23+ months). A clear correlation between peak expansion and response was not evident, and maximal persistence was not specified. 13 of 15 patients developed ≥grade 3 toxicities, largely within the 2 weeks following CAR T cell infusion, including fevers (n=12), hypotension (n=4), and neurologic sequelae including confusion, obtundation, aphasia and encephalopathy (n=6, in aggregate). Elevations in IL-6 and/or IFN-γ were observed at the time of peak toxicity.35
Investigators from UPenn and FHCRC have also presented preliminary data on adults with relapsed or refractory B-NHL treated with CD19-targered CAR T cells.36,37 In this trial as in their B-ALL described above, the FHCRC utilizes a procedure in which CD4+ and CD8+ T cells are expanded separately and infused in a 1:1 ratio. 16 of 28 patients with B-NHL in FHCRC’s report had undergone prior HSCT (autologous, n=13; alloHSCT, n=3). As observed in their BALL trial, some patients receiving lymphodepleting chemotherapy consisting of Cy alone developed an endogenous CD8+ T cell-mediated response to the murine component of the CAR transgene. Flu was added to the lymphodepleting chemotherapy regimen, and preliminary results suggest improved responses in the 16 patients treated subsequently.37
Investigators from MSKCC have additionally reported preliminary results of a study utilizing 19-28z CAR T cells in patients with relapsed/refractory B cell NHL with PET positivity and/or BM involvement following salvage therapy. In this study, 5 × 106 – 1 × 107 19-28z CAR T cells/kg were administered following high-dose chemotherapy and autologous stem cell rescue; dose limiting toxicity (CRS) was noted at the 1 × 107 19-28z CAR T cells/kg dose level. As of the 2015 ASCO Annual Meeting, 6 of 11 enrolled patients had achieved CR, which were durable and ongoing in 4 of 6 patients (13–21+ months). In total, 6 of 11 patients developed severe CRS, which was treated effectively with tocilizumab and corticosteroids.38 Of note, in addition to utilizing a different CD19 scFv from the NCI, MSKCC CAR T cell production procedure includes isolation and activation of the thawed leukapheresis product with Dynabeads Human T-Activator CD3/CD28 magnetic beads whereas the NCI’s procedure for adults with B-NHL includes suspension of collected PBMCs in IL-2-containing medium and subsequent stimulation of T cell proliferation using the anti-CD3 monoclonal antibody OKT3.6,34 A higher proportion of CD8+ T cells in the T cell products infused, and at the time of peak CAR+ T cell levels, a markedly increased ratio of CD3+CD8+CAR+ T cells to CD3+CD4+ CAR T cells, have been observed by the NCI group.6,34,35
Treatment of Other Malignancies with CAR Modified T Cells
The above referenced studies have explored the use of CAR T cells in the treatment of CD19-expressing B cell malignancies. Investigators from UPenn recently reported treatment of 10 patients with multiple myeloma with CTL019, 4 of whom achieved objective response (stringent CR, n=1, VGPR, n=1, PR, n=2), and 6 of whom remained progression free. The rationale for targeting CD19 lies in reports that a disease-propagating, clinical behavior-defining component of the neoplastic plasma cell close has a CD19+ B cell phenotype. The authors highlighted a single patient with highly refractory IgA kappa multiple myeloma who received Cy 1200 mg/m2 during screening and CTL019 manufacturing, and then ultimately received melphalan 140 mg/m2, ASCR, and 5 × 107 CTL019 CAR T cells/kg on day 12 post-ASCR, followed by post-ASCR lenalidomide. She experienced no CRS and ultimately achieved stringent CR, which persisted for 12+ months, despite detectable expression of CD19 on only 0.05% of the dominant neoplastic plasma cell population and no evidence of CTL019 persistence by flow cytometry or qPCR at day 100 post-ASCR.39 Notwithstanding this single impressive response, the utility of CD19 as a therapeutic target in multiple myeloma remains unclear. Alternative targets for CAR T cell therapy for multiple myeloma have been proposed, including the plasma and NK cell surface antigen SLAMF7, presently targeted by the monoclonal antibody elotuzumab, which itself has demonstrated efficacy in relapsed/refractory multiple myeloma, and which may act via direct activation of NK cells in addition to antibody-dependent cell-mediated cytotoxicity.40,41 Most recently, the NCI reported on 11 patients with relapsed or refractory multiple myeloma enrolled on a phase I trial utilizing CAR T cells targeted to B cell maturation antigen (BCMA) and incorporating a CD28 costimulatory domain. Enrolled patients had received a median of 7 prior lines of therapy. Patients received Cy and Flu followed by escalating doses of 0.3–9 × 106 CAR T cells. While the best response observed on the lower two dose levels (n=6) was a transient PR, one of 3 patients on the third dose level achieved VGPR and both patients on the highest dose level attained objective responses, including one patient achieving stringent CR. Fevers developed in 5 of 11 patients treated, accompanied by tachycardia and hypotension in the three patients achieving best response.42 Of note, BCMA expression is largely restricted to plasma cells and preclinical studies have additionally suggested activity of BCMA-directed naked antibody or antibody-drug conjugates and BCMA/SLAMF7 bispecific antibody therapies.43,44
Several groups have shown interest in extending CAR T cell therapies to patients with solid tumors. The membrane-bound receptor tyrosine kinase human epidermal growth factor receptor 2 (HER2), which is overexpressed in multiple malignancies, has emerged as a logical target for adoptive cellular therapies. Investigators from the Baylor College of Medicine reported on 19 patients with refractory HER2+ tumors, 16 of whom had osteosarcoma, treated with autologous T cells expressing a HER2-specific CAR linked to CD28 costimulatory domain in escalating doses of 1 × 104 – 1 × 108 CAR+ T cells/m2. Efficacy in this very preliminary study appeared modest, with 4 patients achieving stable disease for 3 – 14 months, though this was sufficient for 3 patients to undergo clinically beneficial surgical intervention.45 HER2-specific CAR T cells are additionally being investigated in treatment of glioblastoma multiforme (NCT02442297). Of note, the NCI had reported previously on a single patient with colon cancer metastatic to liver and lung treated with Cy followed by 1010 autologous T cells transduced with a third generation HER2-targeted CAR incorporating CD28 and 4-1BB costimulatory domains. This patient rapidly developed dyspnea, hypoxia, pulmonary infiltrates, and marked increases in IL-6, IFN-γ, GM-CSF, TNF-α and IL-10 following CAR T cell infusion, required mechanical ventilator support, and died 5 days following CAR T cell infusion. Investigators suspected her death was related to CAR T cell recognition of HER2 on normal lung tissue upon first-pass clearance in the lung, leading to local release of inflammatory cytokines, pulmonary toxicity, and an ensuing cytokine storm leading to multi-organ failure. The anti-HER2 scFv in the CAR is derived from the HER2-directed monoclonal antibody trastuzumab, which is FDA-approved for the treatment of HER2-overexpressing breast and gastric cancer. While trastuzumab infusion has not been associated with toxicities of this severity, several patients previously treated with a bispecific antibody targeted to HER2/FcγRIII experienced fevers, dyspnea, hypoxia, and brisk elevations in IL-6, TNF-α, IL-8, GM-CSF, and IFN-γ.46,47 At MSKCC, we have opened a clinical trial of CAR T cells for patients with relapsed/refractory high-grade serous cancers (e.g. ovarian cancer) expressing MUC16ecto, the retained cytoplasmic and extracellular portion of CA 125, which is overexpressed in the majority of women with epithelial ovarian cancers (NCT02498912). The vector with which autologous T cells are transduced includes a MUC16ecto targeted CAR (4H1128z), a flexi human IL-12 domain (fIL-12) to overcome inhibitory aspects of the tumor microenvironment through constitutive tumor-targeted cytokine secretion, and a truncated epidermal growth factor receptor (EGFRt) elimination gene as a “safety switch” in the event of toxicity. 4H11-28z/fIL-12/EGFRt CAR T cells are administered IP and IV.48 We and others have additionally explored mesothelin as a potential target of CAR T cell therapy, as this is an immunogenic cell surface protein overexpressed in several human tumors, including mesothelioma and ovarian and pancreatic adenocarcinoma. To this end, MSKCC is actively investigating the use of mesothelin-targeted CAR T cells in the treatment of malignant pleural disease (NCT02414269), and investigators from UPenn are conducting a clinical trial of mesothelin-directed CAR T cell therapy in patients with metastatic pancreas cancer (NCT02465983). The monoclonal antibody from which the anti-mesothelin scFv in clinical use at MSKCC was derived has itself demonstrated antitumor activity in vitro, and prior mesothelin-targeted therapies, including the recombinant immunotoxin SS1P, have shown clinical benefit in a subset of patients with refractory mesothelin-expressing malignancies.49,50
Principles of Current Clinical Use of CAR T cells
Lymphodepletion
Multiple lines of preclinical evidence, as well as most current clinical experience with CAR T therapies to date, support the need for adequate lymphodepletion prior to CAR T cell infusion. While the precise mechanisms by which lymphodepletion enhances CAR T cell efficacy remain uncertain, lymphodepleting chemotherapy may eradicate homeostatic “cytokine sinks” and regulatory T cells and may enhance antigen presenting cell activation. We and others have observed poor CAR T cell expansion and persistence, limited efficacy, and no significant rise in pro-inflammatory cytokine levels following CD19-targeted CAR T cell infusion in patients who received no antecedent lymphodepleting chemotherapy.26,51 Additionally, investigators from the FHCRC observed improved CAR T cell expansion following addition of Flu to Cy as lymphodepleting chemotherapy in patients with B-ALL.11 Several patients with BALL and B-NHL receiving Cy alone additionally appeared to develop endogenous cytotoxic T lymphocyte responses directed at the transgene, which may have been abrogated by the addition of Flu. Improved DFS was observed among adults with B-ALL receiving Flu in addition to Cy (vs. Cy alone), and a suggestion of improved responses was also observed among patients with B-NHL receiving Flu.11,37 Interestingly, investigators from the NCI recently reported responses in several adults with progression of B cell malignancies following AlloHSCT following treatment with allogeneic-derived CD19-targeted CAR T cells, including MRD-negative CR in 4 of 5 patients with B-ALL, despite no antecedent lymphodepleting chemotherapy, and despite CAR T cell persistence of generally ≤4 weeks; whether lymphodepleting chemotherapy would further improve CAR T cell expansion and clinical responses post-AlloHSCT remains uncertain.52
Costimulation
As summarized in Tables 1–2, the second-generation CD19-targeted CAR T cells in clinical trials with reported results to date have incorporated either a 4-1BB or CD28 costimulatory domain. UPenn has reported CTL019 persistence (assessed by qPCR) of >2 years in patients with B-ALL and >4 years in patients with CLL achieving MRD-negative CR, in contrast to CAR T cell persistence of 1–6 months among adults with B-ALL treated with 19-28z CAR T cells at MSKCC and ≤68 days in pediatric patients with B-ALL treated by the NCI.8,18,19,30 Early observations to date suggest greater rates of MRD-negative CR in pediatric patients with B-ALL treated with CD19 CAR T cells incorporating a 4-1BB costimulatory domain rather than CD28.18,19,53 However, high rates of MRD-negative CR have been observed in adults with relapsed or refractory B-ALL treated with one of several CAR T cell products (Table 1), as well as among a subset of adults with refractory CLL or B-NHL treated with CD19-targeted CAR T cells bearing either a 4-1BB or CD28 costimulatory domain. Among patients with B-ALL treated with CD19-targeted CAR T cells, rates of relapse following achievement of MRD-negative appear roughly similar, though CD19-negative relapse may be more common in those treated with a 4-1BB-containing CAR.8,11,18,53 Moreover, whether greater CAR T cell persistence is associated with more durable response, or whether brisk expansion and initial antitumor potency is sufficient in the absence of extended CAR T cell persistence, remains uncertain.
Product Composition
Most of the trials to date of CD19-targeted CAR T cell therapeutics to date have expanded collected autologous or allogeneic T cells collectively and ultimately used products of random composition with demonstrable variability in CD4+/CD8+ subsets and memory phenotypes.6,19,26,30 Whether variation in product composition, beyond total CAR cell dose alone, influences toxicity and efficacy in B cell malignancies remains uncertain. However, in contrast to most studies, the FHCRC has reported on the use of CAR T cell products of defined composition in patients with B-ALL and B-NHL, as noted earlier in the manuscript.11,37 Autologous CD4+ and CD8+ T cells are expanded separately in vitro prior to infusion at a defined ratio of 1:1 CD4+:CD8+ CAR T cells. This group has previously reported preclinical data demonstrating that transduced CAR T cell subsets exhibit different effector functions. For example, CD8+ CAR T cells with a central memory phenotype appear to demonstrate greatest direct antitumor potency. CD4+ CAR T cells exhibit weaker lytic activity compared with CD8+ CAR T cells, but naïve CD4+ CAR T cells are capable of producing greatest IFN-γ, TNF-α, and IL-2 production following stimulation with CD19+ tumor cells. Additionally, treatment with patient-derived CAR T cells products with defined subset composition significantly enhanced survival in NOD/SCID/γc−/− mice engrafted with Raji/ffluc (CD19+) tumor cell lines.54 Prior receipt of cytotoxic chemotherapy may also skew T cell subsets at the time of collection, possibly resulting in a decrease of naïve CD4+ and CD8+ T cells and an increase in the proportion of effector memory T cells as observed in the above study.54 As such, this strategy may allow for a lower overall CAR T cell dose and more uniform product composition between patients. Further investigation will be required to assess CAR T cell expansion, persistence, and efficacy utilizing this approach, and to determine optimal CAR T cell subset composition, if any, for clinical use.
Challenges in Current Clinical Use of CAR T cells
Cytokine Release Syndrome and Neurologic Toxicity
Several challenges relevant to current clinical use of CAR T cells are summarized in Table 3. As discussed above in the context of B-ALL, CLL, and B-NHL, CRS and neurologic toxicity appear to be common following second generation CAR T cell therapy, with severe CRS particularly common in patients with B-ALL with higher disease burden prior to infusion. Risk-adapted CAR T cell dosing strategies have been employed the MSKCC, FHCRC, and UPenn groups as well in response to the greater incidence of severe CRS observed among patients with greater baseline disease burden treated with higher doses of CAR T cells.8,11,13 While these toxicities largely appear to be reversible, management of severe CRS nonetheless requires intensive supportive care, and in some instances may be life threatening. The use of the IL-6 receptor monoclonal antibody tocilizumab appears to be effective in many cases, and has been incorporated into the clinical management algorithm of CRS at our center and others.7 While corticosteroids may be effective as a broadly lymphotoxic therapy, such therapy carries the risk of abrogating anti-tumor responses mediated by CAR T cells, as well as destruction of endogenous T cells. We and others have investigated the use of “elimination genes” (also known as “suicide genes”) to permit targeted destruction of infused CAR T cells. Preclinical studies have explored the use of several elimination genes, including HSV thymidine kinase, E. coli nitroreductase, inducible caspase 9, CD20, OmomycER, and EGFRt.55 As additional genetic manipulations are introduced to enhance the expansion, persistence, and potential efficacy of CAR T cells as discussed below, incorporation of a specific “safety switch” may help to protect against additional toxicity of therapy if encountered. The pathogenesis of neurologic toxicities observed following CAR T cell therapy is incompletely understood. CAR T cells have commonly been observed in the CSF of recipients, though a correlation between the presence and levels of CAR T cells in the CSF and development of neurologic toxicity has not been consistently observed. Additionally, neuroimaging abnormalities characteristic of such neurologic changes following CAR T cell therapy have not yet been identified.7,19 Further investigation into the pathogenesis, monitoring, and management (beyond supportive care alone) of such neurologic toxicity is ongoing.
Table 3.
Challenges facing current clinical use of CAR-modified T cell therapeutics and potential strategies to address each.
| Challenge | Potential Strategies to Overcome Challenge |
|---|---|
| Poor expansion | Optimization of lymphodepleting chemotherapy prior to T cell infusion Optimization of costimulatory domain Further genetic modification of T cells, including expression of additional costimulatory ligands or cytokines (e.g. IL-12) |
| Suboptimal CAR T cell function | Co-administration of checkpoint blockade agents or ibrutinib Further genetic modification of T cells as above |
| Loss of expression of target antigen (e.g. CD19-negative relapse) | Targeting of multiple tumor-associated antigens at once |
| Cytokine release syndrome | Risk-adapted CAR T cell dosing Optimization of prediction models to permit earlier recognition/treatment in select patients Incorporation of “elimination genes” into the CAR construct |
| Neurologic toxicity | Risk-adapted CAR T cell dosing Improved understanding of pathogenesis Incorporation of “elimination genes” into the CAR construct |
| B cell aplasia (for CD19-targeted CAR T cells) | IVIG for hypogammaglobulinemia Antigen-specific inhibitory CAR to protect normal B cells73 |
Antigen Escape
Several reports to date have noted loss of detectable CD19 by flow cytometry in patients with previously CD19-positive B cell hematologic malignancies experiencing relapse following CD-19 targeted CAR T cell therapy, similar to reports of CD19-negative relapse following therapy with the bispecific T cell engager blinatumomab.19,21,56,57 Recently, investigators from CHOP/UPenn and others have reported that alternatively spliced CD19 mRNA bypassing exon 2 permits expression of an N-terminal truncated CD19 variant, compromising the target epitope, and in turn, CAR T cell-mediated killing, but preserving cytoplasmic domains critical for recruiting PI3K and Src family tyrosine kinases, and in turn, signaling required for leukemic maintenance. Accumulation of alternatively spliced CD19 mRNA may be driven in part by accumulation of frameshift mutations in exon 2 as well as downregulation of the splicing factor SRSF3.58 Several further strategies may be incorporated into future studies to address the problem of antigen escape in B cell malignancies, including modification of CAR T cells to target multiple tumor associated antigens, administration of checkpoint inhibitors, and as discussed in the next section, introduction of additional genetic manipulations to autologous T cells to overcome inhibitory features of the tumor microenvironment.59 Additionally, the use of CAR T cells targeting the immunoglobulin superfamily member CD22, expressed on mature and some immature B cells, is currently being investigated in relapsed CD22+ B-ALL and other CD22+ B cell malignancies, including but not restricted to patients with CD19-negative relapse following CD19-targeted CAR T cell therapy (NCT02650414, NCT02315612).
Enhancements
Despite the impressive results observed to date using CD19-targeted CAR T cells in children and adults with refractory B-ALL, clinical efficacy has appeared more modest in the setting of CLL and B-NHL. In part, the hostile tumor microenvironments characteristic of many human cancers may limit the efficacy of CAR T cells in present form. CLL cells, for example, exploit a variety of mechanisms to escape elimination from the endogenous immune system, such as secretion of inhibitory factors (e.g. soluble BAG6, leading to suppression of NK cell cytotoxicity), up-regulation of inhibitory ligands inducing impairment of T cell immunologic synapses (e.g. CD200, PD-L1), release of exosomes promoting a cancer-associated fibroblast phenotype in stromal cells and supporting leukemic maintenance, and induction of T cell dysfunction/exhaustion (as marked by decreased proliferative and cytotoxic capacity and increased expression of exhaustion markers PD-1, CD160, and CD244).60–65 Several strategies to overcome this inhibitory microenvironment have been described, including additional genetic modification of CD19-targeted CAR T cells with an additional costimulatory ligand, such as 4-1BB ligand (4-1BBL) or CD40 ligand (CD40L), or incorporation of the pro-inflammatory cytokine IL-12.66–69 Such modifications are being investigated in forthcoming clinical studies, including the aforementioned use of 4H11-28z/fIL-12/EGFRt CAR T cells in patients with refractory high grade serous cancers.48,70 An increase in the proportion of CAR T cells expressing markers of T cell exhaustion including PD-1 has been observed following CAR T cell infusion; administration of exogenous checkpoint blockade (e.g. anti PD-1 antibodies) may abrogate CAR T cell exhaustion and dysfunction and thereby enhance efficacy.52,71 Most recently, prolonged (≥5 months) therapy with the Bruton’s tyrosine kinase inhibitor ibrutinib has also been shown to improve CTL019 expansion and enhance CD8+ T cell function by decreasing immune suppressive PD-1 and CD200 expression, suggesting a potential rationale for combining these therapies in patients with CLL.72
Conclusions
CD19-targeted CAR-modified T cells have emerged as one of the most effective available therapies in treating children and adults with refractory B-ALL, with high rates of complete response observed over multiple trials using different T cell transduction methods, anti-CD19 scFvs, and costimulatory domains (e.g. CD28 vs. 4-1BB). More intensive lymphodepletion prior to CAR T cell administration additionally appears to be of benefit. Responses are rapid and often durable, even in the absence of subsequent AlloHSCT. In contrast, a smaller subset of patients with CLL or B-NHL treated with second-generation CD19-targeted CAR T cells have achieved clinically meaningful responses. Ongoing and planned studies will assess the safety and efficacy of present CAR T cells in patients with multiple myeloma and several solid malignancies. The optimal strategy, if any, for consolidating response following CAR T cell administration in patients with B-ALL remains unknown. In CLL and B-NHL, methods to enhance CAR T cell response and to overcome challenges associated with a hostile tumor microenvironment are being explored. CAR T cell therapy, particularly in patients with BALL, is frequently complicated by severe CRS and neurologic toxicity. While patterns of elevation in pro-inflammatory cytokines correlating with T cell activation and expansion are clearly observed in the setting of clinically severe CRS, the pathogenesis of neurologic toxicity remains less fully understood and warrants further investigation. As additional enhancements are made to augment CAR T cell expansion and persistence, development of strategies to modulate CAR T cell responses may become even more clinically relevant. Expansion of the repertoire of available tumor associated antigenic targets, as well as further strategies to overcome immune escape associated within the cancer microenvironment, holds promise in extending this therapy to a greater breath of malignancies.
Acknowledgments
Funding Disclosure
Renier J. Brentjens is supported by Leukemia and Lymphoma Society Career Development Grant (J.H.P.), National Comprehensive Cancer Center Young Investigator Award (J.H.P.), CA13873801, The Damon Runyon Clinical Investigator Award, The Translational and Integrative Medicine Fund Research Grant (MSKCC), The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York, Carson Family Charitable Trust, Kate’s Team, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC, The Geoffrey Beene Cancer Foundation, and The Bocina Cancer Research Fund.
Mark B. Geyer is supported by UL1TR0045, NIH/National Center for Advancing Translational Sciences (NCATS), administered by the Clinical and Translational Science Center at Weill Cornell Medical Center and MSKCC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park JH, Brentjens RJ. Adoptive immunotherapy for B-cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discovery medicine. 2010;9:277–288. [PMC free article] [PubMed] [Google Scholar]
- 2.Till BG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport AJ, et al. CAR-T Cells Inflict Sequential Killing of Multiple Tumor Target Cells. Cancer immunology research. 2015;3:483–494. doi: 10.1158/2326-6066.CIR-15-0048. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116:5568–5574. doi: 10.1002/cncr.25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gokbuget N, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 6.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, et al. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol. 2016;34(suppl) abstr 7003. [Google Scholar]
- 9.Park JH, et al. Implications of Minimal Residual Disease Negative Complete Remission (MRD-CR) and Allogeneic Stem Cell Transplant on Safety and Clinical Outcome of CD19-Targeted 19-28z CAR Modified T Cells in Adult Patients with Relapsed, Refractory B-Cell ALL. Blood. 2015;126:682–682. [Google Scholar]
- 10.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turtle CJ, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turtle CJ, et al. Addition of Fludarabine to Cyclophosphamide Lymphodepletion Improves In Vivo Expansion of CD19 Chimeric Antigen Receptor-Modified T Cells and Clinical Outcome in Adults with B Cell Acute Lymphoblastic Leukemia. Blood. 2015;126:3773–3773. [Google Scholar]
- 13.Frey NV, et al. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL) J Clin Oncol. 2016;34(suppl) abstr 7002. [Google Scholar]
- 14.Nguyen K, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. doi: 10.1111/j.1365-2141.2005.05773.x. [DOI] [PubMed] [Google Scholar]
- 16.Reismuller B, et al. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): a population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Munster) study group. J Pediatr Hematol Oncol. 2013;35:e200–204. doi: 10.1097/MPH.0b013e318290c3d6. [DOI] [PubMed] [Google Scholar]
- 17.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran KJ, et al. Multi-Center Clinical Trial of CAR T Cells in Pediatric/Young Adult Patients with Relapsed B-Cell ALL. Blood. 2015;126:2533. [Google Scholar]
- 21.Frey NV, et al. Refractory Cytokine Release Syndrome in Recipients of Chimeric Antigen Receptor (CAR) T Cells. Blood. 2014;124:2296–2296. [Google Scholar]
- 22.Tam CS, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay NE, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd JC, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrd JC, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geyer MB, et al. Updated results: phase I trial of autologous CD19-targeted CAR T cells in patients with residual CLL following initial purine analog-based therapy. J Clin Oncol. 2016;34(suppl) doi: 10.1016/j.ymthe.2018.05.018. abstr 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter DL, et al. Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL. J Clin Oncol. 2016;34(suppl) abstr 3009. [Google Scholar]
- 32.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochenderfer JN, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster SJ, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood. 2015;126:183. [Google Scholar]
- 37.Turtle CJ, et al. Anti-CD19 Chimeric Antigen Receptor-Modified T Cell Therapy for B Cell Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Fludarabine and Cyclophosphamide Lymphodepletion Improves In Vivo Expansion and Persistence of CAR-T Cells and Clinical Outcomes. Blood. 2015;126:184. [Google Scholar]
- 38.Sauter CS, et al. Phase I trial of 19-28z chimeric antigen receptor modified T cells (19-28z CART) post-high dose therapy and autologous stem cell transplant (HDT-ASCT) for relapsed and refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL) J Clin Oncol. 2015;33(suppl) abstr 8515. [Google Scholar]
- 39.Garfall AL, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danhof S, et al. CAR-Engineered T Cells Specific for the Elotuzumab Target SLAMF7 Eliminate Primary Myeloma Cells and Confer Selective Fratricide of SLAMF7+ Normal Lymphocyte Subsets. Blood. 2015;126:115. [Google Scholar]
- 41.Lonial S, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. The New England journal of medicine. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 42.Ali SA, et al. Remissions of Multiple Myeloma during a First-in-Humans Clinical Trial of T Cells Expressing an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor. in. ASH Annual Meeting: Late-Breaking Abstracts LBA-1; 2015. [Google Scholar]
- 43.Ryan MC, et al. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Molecular cancer therapeutics. 2007;6:3009–3018. doi: 10.1158/1535-7163.MCT-07-0464. [DOI] [PubMed] [Google Scholar]
- 44.Ramadoss NS, et al. An anti-B cell maturation antigen bispecific antibody for multiple myeloma. Journal of the American Chemical Society. 2015;137:5288–5291. doi: 10.1021/jacs.5b01876. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed N, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiner LM, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer research. 1995;55:4586–4593. [PubMed] [Google Scholar]
- 48.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassan R, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Molecular cancer therapeutics. 2009;8:1113–1118. doi: 10.1158/1535-7163.MCT-08-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz CR, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brudno JN, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grupp SA, et al. Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019) Blood. 2015;126:681. [Google Scholar]
- 54.Sommermeyer D, et al. Chimeric antigen receptor-modified T cells derived from defined CD8(+) and CD4(+) subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minagawa K, Zhou X, Mineishi S, Di Stasi A. Seatbelts in CAR therapy: How Safe Are CARS? Pharmaceuticals. 2015;8:230–249. doi: 10.3390/ph8020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topp MS, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 57.Park KH, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. Journal of Clinical Oncology. 2015;33(suppl) abstr 7010. [Google Scholar]
- 58.Sotillo E, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson HJ, Brentjens RJ. Overcoming Antigen Escape with CAR T-cell Therapy. Cancer Discov. 2015;5:1238–1240. doi: 10.1158/2159-8290.CD-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiners KS, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121:3658–3665. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. The Journal of clinical investigation. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClanahan F, et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood. 2015;126:203–211. doi: 10.1182/blood-2015-01-622936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riches JC, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paggetti J, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015 doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curran KJ, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–778. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pegram HJ, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pegram HJ, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29:415–422. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephan MT, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature medicine. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 70.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2:e26286. doi: 10.4161/onci.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraietta JA, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016 doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]