Abstract
The use of zebrafish for high throughput screening (HTS) for chemical bioactivity assessments is becoming routine in the fields of drug discovery and toxicology. Here we report current recommendations from our experiences in zebrafish HTS. We compared the effects of different high throughput chemical delivery methods on nominal water concentration, chemical sorption to multi-well polystyrene plates, transcription responses, and resulting whole animal responses. We demonstrate that digital dispensing consistently yields higher data quality and reproducibility compared to standard plastic tip-based liquid handling. Additionally, we illustrate the challenges in using this sensitive model for chemical assessment when test chemicals have trace impurities. Adaptation of these better practices for zebrafish HTS should increase reproducibility across laboratories.
Keywords: zebrafish, high throughput screening, developmental toxicity
1. Introduction
Modern toxicology is faced with the challenge of performing hazard and risk assessment for tens of thousands of chemicals, of which only a small subset has been evaluated for safety. These chemicals represent diverse structural classes that were developed for specific applications (e.g., flame retardants, plasticizers, insecticides, machine lubricants, drugs, etc.), while others are byproducts or breakdown products. With numerous classes spanning different physico-chemical properties (i.e., polarity, hydrophobicity) it is difficult to create “one-size-fits-all” testing models to establish quantitative structure-activity relationships (QSAR). Since QSAR models do not exist for most chemical classes, the focus is on toxicity testing of individual chemicals prior to use in commercial products, as mandated by regulatory agencies. This focus generates critical information, but is incapable of globally guiding safer chemical use decisions by the thousands of industrial interests that seek to incorporate new chemistries into commercial products. Thus, the huge backlog of chemicals that lack safety information is cause enough for a paradigm shift from high cost, low throughput mammalian models to alternative systems.
There is less uncertainty in accurately identifying hazardous chemical if whole animal models are used as these integrated systems are able to detect different modes of bioactivity and are well suited to uncover underlying molecular response pathways. Developmental life stage whole animal models are particularly useful as they possess nearly all potential cell types and express the full complement of gene products that must interact in concert throughout embryogenesis to accomplish normal developmental outcomes. This unique life stage offers an ideal time to determine if a test chemical has the inherent structure to interact with and perturb targets or signaling events that are necessary for normal biological processes. If sufficient perturbations occur, the normal developmental plan is disrupted, resulting in a chemical-induced phenotype.
The developmental zebrafish toxicity bioassay is one such model that has met these needs, and has dramatically accelerated high throughput chemical hazard detection and, in so doing, rapidly prioritize many chemicals for further testing [1–5].The purpose of screening in zebrafish is to more rapidly identify chemically-induced phenotypes. The zebrafish also has ~80% genetic homology to humans, which allows for immediate translation to humans [6]. Embryos in turn develop rapidly, and their small size consumes minute test chemical quantities [7]. The externally developing embryos are transparent during much of the process allowing for noninvasive visual tracking of chemical-induced defects in the brain, notochord, heart, jaw, snout, eyes, trunk and fins [8].
EPA-NCCT developed the ToxCast program to assess a large number of chemicals (>1000) in a diverse set of in vitro assays [9]. There are over 600 assays, and 800 assay endpoints that are evaluated to provide enough information to prioritize chemicals for further testing. As an effort to help with this initiative, the zebrafish model was used to screen the ToxCast Phase 1 and 2 library [2, 3, 10]. From these studies, there was a good concordance with historic animal data and amongst the different zebrafish assays, demonstrating this model is feasible for HTS screening. As the embryonic zebrafish becomes increasingly used in large toxicity screening programs, there is a growing need for more standardized HTS practices to reduce potential for incongruent results and improve zebrafish screening. From the results of the initial large scale screening endeavors, studies much has been learned and opportunities for improved experimental practices are evident.
Our efforts to adapt new and emerging technology to zebrafish HTS are driven by a better, faster, cheaper philosophy for detecting chemical-induced phenotypes. Here we report several case studies that demonstrate how the instrumentation used to deliver test chemicals to zebrafish assay plates can dramatically affect observed chemical bioactivities. Specifically, we compared robotic liquid handling using traditional disposable pipette tips to ink-jet technology-based digital dispensing. We also suggest protocol improvements that maximize chemical solubility and mixing in the plate well to reduce false negatives, and improved plate sealing. Lastly, we illustrate the potential pitfalls of reliance on the chemical manufacturer’s declared purity. We emphasize that, prior to large chemical screens, it is important to verify the chemical certificates of analyses before proceeding. Incorporation of these improvements, we believe, offers a better practice for high throughput chemical screening in the developmental zebrafish.
2. Materials and Methods
2.1.Chemicals
The chemicals used in this manuscript are: 17α-Ethinylestradiol (EE2), and 17β-Estradiol (E2), thiram, polycyclic aromatic hydrocarbons (PAHs: fluoranthene, pyrene, chrysene, benzo[a]pyrene), polybrominated diphenyl ether (BDE) congeners (BDE-77 and BDE-77 FF (furan-free)). EE2, E2 and thiram were purchased from Sigma Aldrich (www.sigmaaldrich.com). Both BDE-77 and BDE-77FF were acquired from AccuStandard (www.accustandard.com) as 100% purity, though BDE-77FF received additional purification by the vendor to remove potential dioxin/furan contaminants. Stock solutions were prepared using 100% dimethyl sulfoxide (DMSO) and stored in 4°C and room temperature, respectively. PAHs were purchased as neat standards from AccuStandard (New Haven, CT) and suspended in DMSO and stored in −20C until 1 hour prior to exposure.
2.2. Zebrafish husbandry
Tropical 5D wild type zebrafish were housed at the Oregon State University Sinnhuber Aquatic Research Laboratory. The fish were kept in 100 gallon tanks at a density of 1000. Each tank was kept at standard laboratory conditions of 28°C on a 14-h light/10-h dark photoperiod in fish water consisting of reverse osmosis water supplemented with a commercially available salt (Instant Ocean™). Spawning funnels were placed into the tanks the night prior, and embryos were collected and staged [11]. To increase bioavailability, the chorion was enzymatically removed using pronase (90 µL of 25.3 U/µl; Roche, Indianapolis, In, USA) at 4 hpf using a custom automated dechorionator [12].
2.3.Chemical delivery
For the ToxCast chemical library delivery, we used a Caliper Zephyr (Perkin Elmer; denoted in the manuscript as “Liquid Handler”) that contacts the test chemical via traditional format polypropylene micro-pipette tips. The chemical dilutions via Liquid Handler were carried out as described in Truong et al. [2]. Briefly, a 10× solution of each chemical was made in embryo media (EM), and 10µL was added to each well which already contained 90 µL EM with and embryo. For the NTP collaboration, the Hewlett-Packard D300 Digital Dispenser [13] (referred to in the manuscript as “BioPrinter”), was used as the chemical delivery system. The BioPrinter uses inkjet technology to digitally dispense chemicals in 11 pL droplets. Thus, delivery from a 20 mM stock (in 100% DMSO) was directly into the experimental chamber, eliminating the need for serial dilution.
2.4.Developmental toxicity screen
To remove the potential confounding effects from the presences of the chorion for all developmental toxicity studies, embryos were dechorionated at 4 hours post fertilization (hpf) followed by robotic placement into individual wells prefilled with 100 µL of EM [12]. It is essential to place the fragile dechorionated embryos directly into aqueous solutions because direct contact with dry plates leads to immediate embryo damage. Using this experimental design, chemical delivery must follow embryo placement to avoid cross contamination between exposure wells using a common robotic capillary. A chemical stock concentration of 20 mM, was dispensed to the test plate with the BioPrinter using T8 cassettes to achieve the desired test concentration in a single step. All wells were normalized to 0.64% DMSO in a single step using D4 cassettes. During the previous screening of the ToxCast chemical library the Liquid Handler was at the time the most accurate automated technology available, but because of limitations in precision pipetting at lower volumes it was necessary to perform a series of dilutions of the 20 mM stocks to achieve the final concentration test concentration (in the low nM to µM ranges). The first dilution plate is made by serial diluting from the highest concentration down (without pipette tip replacement), and thorough mixing at each concentration. Changing tips at each dilution step would be cost-prohibitive if a routine practice. In total, 3 chemical transfer steps were needed to deliver a test chemical from the stock plate to the to the assay plate using the Liquid Handler. Each well was prefilled with 90 uL of EM to which 10 uL of the chemical dilution was added to achieve the assay concentration. For each delivery method, 32 embryos were exposed per concentration. All plates were sealed using parafilm to prevent evaporation and wrapped in aluminum foil to prevent photodegradation. Embryos were stored in a 28°C incubator and statically exposed until 120 hpf.
At 24 and 120 hpf, a total of 22 morphological endpoints were assessed according to Truong et al [8]. Briefly, at 24 hpf, mortality, developmental progression, normal spontaneous movement, and notochord were evaluated for presence of an aberrant phenotype. At 120 hpf, embryos were assessed for 17 morphological endpoints and collected in a laboratory information management system called the Zebrafish Acquisition and Analysis Program (ZAAP). Concentration response modeling was completed with the drm function in the drc package in R. Statistical significance was computed as described in Truong et al [2].
2.5. Assay plate mixing after chemical delivery
The BioPrinter’s default auto-shaking provides suitable mixing for most in vitro work, but is much too harsh for dechorionated zebrafish embryos at only 6 hpf. Due to the risk of chemical contamination if delivered prior to placement of embryos, we had to identify a suitable mixing protocol post chemical dispensing. Thus, we initially mixed the plates post-delivery on a stand-alone plate mixer (Variomag Teleshake) for 10 seconds (referred to as “offboard mixing”). This was adequate for moderate to highly water soluble chemicals as indicated by rapid and uniform dispersion of a concentrated bromophenol blue aliquot. But it was empirically inadequate for compounds such as aryl flame retardants, PAHs, and E2 and EE2. In collaboration with HP, a lower energy mixing protocol was scripted for the BioPrinter and implemented to automatically mix for 1 sec between all deliveries of at least 5 nL, again for 15 sec after each dispensehead on the cassette was exhausted (on average after every 16 wells, dependent on the protocol), then again for 15 sec after the BioPrinter completed the plate. This “onboard mixing” was an improvement for dispersion of flame retardant and PAH aliquots, but to achieve fully adequate mixing, an overnight (16 h) incubation at 235 rpm on an orbital shaker (VWR model 3500) was also implemented. The shaker platform was modified by attaching a 3-bay stainless steel freezer rack which, mounted on its back, securely held 48 microtiter plates. We noted that the round well bottoms of the 96-well plates served to restrict the eccentric motion of the embryo to a small, 1 mm radius thus, the motion was gentle enough to yield no detectable effects on development.
2.6.Nominal exposure concentration
Nominal exposure concentrations of 17β-estradiol and 17α-ethinylestradiol were measured in wells without embryos immediately following two methods of dispensing (BioPrinter and Liquid Handler). Nominal concentrations tested were 0, 0.01, 0.1, 1, and 10 µM in 0.64% DMSO. Two commercially available colorimetric based ELISA kits were used to quantify E2 (Enzo Life Sciences, PN: ADI-900-008) and EE2 (Abraxis, PN: 590051) following the manufacturer protocol with the following minor modifications: 30 wells (n=6 per concentration) were filled with 100 µL of EM and chemical was dispensed using the BioPrinter from a 20 mM stock. For the Liquid Handler, 90 µL of assay buffer was dispensed into 30 wells (n=6 per concentration) and 10 µL of 10× chemical was added afterwards (from 2nd transfer step). A Welch T-Test was applied to the nominal exposure concentration and the measured concentration for each dispensing method. Statistical significance was set at p < 0.05.
2.7.Analysis of mRNA expression
At the end of the developmental toxicity screen, animals exposed to 0, 0.01, 0.1, 1 and 10 µM of E2 and EE2 using either the Liquid Handler or BioPrinter were collected and pooled to create 3 biological replicates for quantitative RT-PCR analysis. Each biological replicate comprised 8 animals. RNA expression was quantified using a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, California). RNA expression was quantified using methods adapted from [14]. Briefly, total RNA was isolated from groups of 120 hpf larval zebrafish using RNAzol® RT (Molecular Research Center, Inc., Cincinnati, OH). One microgram of total RNA was converted to cDNA using the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using Power SYBR® Green PCR Master Mix (12.5 ng of cDNA in a 12.5 uL reaction) with a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, California) using the following thermal profile: 95 °C for 10 min; 40 cycles: 95 °C for 10 sec, 60 °C for 60 sec. β-actin was used as a housekeeping gene for normalization, and relative gene expression was quantified using the ΔΔCt method[15].
Primers for B-actin2 (ENSDART00000141737) were F: 5′-AAG CAG GAG TAC GAT GAG TC-3′ and 5′-TGG AGT CCT CAG ATG CAT TG -3′. Cytochrome P450 1A (cyp1a, ENSDART00000038200) primers were F: 5′-TGC CGA TTT CAT CCC TTT CC-3′ and R: 5′-AGA GCC GTG CTG ATA GTG TC-3′. Cytochrome P450 1B (cyp1b, ENSDART00000131147) primers were F: 5′- CTG CAT TGA TTT CCG AGA CGT G -3′ and R: 5′- CAC ACT CCG TGT TGA CAG C -3′. Cytochrome P450 1C1 (cyp1c1, ENSDART00000169530) primers were F: 5′- AGT GGC ACA GTC TAC TTT GAG AG -3′ and R: 5′- TCG TCC ATC AGC ACT CAG -3′. Cytochrome P450 1C2 (cyp1c2, ENSDART00000158830) primers were F: 5′- GTG GTG GAG CAC AGA CTA AG -3′ and R: 5′- TTC AGT ATG AGC CTC AGT CAA AC -3′. Vitellogenin 1 (vtg1, ENSDART00000050238) primers were F: 5′-GCC AAA AAG CTG GGT AAA CA-3′ and R: 5′-AGT TCC GTC TGG ATT GAT GG-3′. Cytochrome P450 1A (cyp19a1b, ENSDART00000130307) primers were F: 5′-ATG AAA TGG ACT TCG CAA CA-3′ and R: 5′-TCT CCT GTA CGA TTT GCT CT-3′. Luteinizing hormone beta (lhb, ENSDART00000051787) primers were F: 5′-CAC GCT GTG AGC TAG TAA AT-3′ and R: 5′-CCG GGT ATG TGA TCT GCG-3′.
2.8.Gas chromatography analysis for BDE
The analysis of standards and chemicals for purity was performed using an Agilent 7890 GC coupled to an Agilent 5975 MS in electron capture negative ionization (ECNI) mode using methods adapted from [16]. Full scans were carried out for both standards with a ranging of 50 to 650 m/z. For analysis, 1 µL of each standard was injected using an Agilent 7683 injector in a pulsed splitless mode (pulse at 20 psi until 0.74 min). A DB-5 ms capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness) was used for chromatographic separation with a helium carrier gas (99.999%) at a flow rate of 1 mL/min. The injection port was set to 300 °C. The oven temperature program was as follows: 120 °C held for 2 min, ramped at 4 °C/min to 275 °C, ramped again at 6 °C/min to 320 °C and held for 5 min for a total run time of 52.25 min. The ECNI's ion source was set to 150 °C. Methane (99.999%) was used as the chemical ionization moderating gas. The quadrupole mass analyzer temperature was 150 °C.
2.9.Plate sorption measurements
The final concentration of PAHs in the plates was ~0.32 µM for individual compounds. Fourteen plates were dosed with the liquid handler using protocols described above and embryos were placed into 8 of these plates (n=8), and 6 had none (n=6). Six additional plates were made using the BioPrinter to directly dispense chemicals into the wells, mixed onboard and then overnight as described above. The Bioprinter dispensed a total of 6 plates where 3 had embryos, and 3 did not. Plate extractions were performed at 5 days post fertilization. For each plate, the exposure solutions (and embryos, where appropriate) were removed, and combined into amber glass vials. Analytes were extracted from the wells by three sequential 20 minute extractions with hexane. The rinses were kept separate and analyzed independently by GC/MS as described in Chlebowski et al [17].
3. Results and Discussions
3.1. Rationale and approach
In collaboration with the National Toxicology Program (NTP), a subset of chemicals that were previously assessed as part of the ToxCast chemical set were procured and re-evaluated in the zebrafish model [2]. The chemicals selected were also part of the Tox21 10,000 chemical library, which were evaluated with a robotic HTS system. While re-testing these chemicals in the embryonic zebrafish model [8, 18, 19], a percentage of results were discordant between the studies. Upon further review, the discord centered on chemicals that were classified as prone to adsorbing (sticking) to metal transfer/delivery pins (unpublished data, National Center for Advancing Translational Science). The concentration response of two such sticky chemicals, 17α-Ethinylestradiol (EE2), and 17β-Estradiol (E2), known teratogens in the developmental zebrafish, were investigated to determine if an alternative approach could overcome adsorption challenges.
3.1.1. Chemical delivery method impacts concentration-response profile of bioactive compounds
We identified significant concentration-response differences resulting from direct, digital delivery (BioPrinter) and the multi-transfer process of the Liquid Handler (Figure 1). Delivery of 64 µM of EE2 using the BioPrinter caused 100% mortality at 120 hpf, but delivery via the Liquid Handler was associated with only 31% mortality at 64 µM and morphological abnormality in most of the remaining 69% of the animals. The discrepancy was greater with E2 where the LC100 at 120 hpf was 64 µM when dispensed by the BioPrinter, but no mortality or malformations were associated with delivery via the Liquid Handler. The discord between delivery technologies may be due to error propagation through serial dilution, by chemical adsorption onto the large surface area of the Liquid Handler’s plastic pipette tips, or both.
Figure 1. Heatmap comparison of developmental effects of 17α-Ethinylestradiol and 17β-Estradiol delivered with 2 different chemical delivery systems.
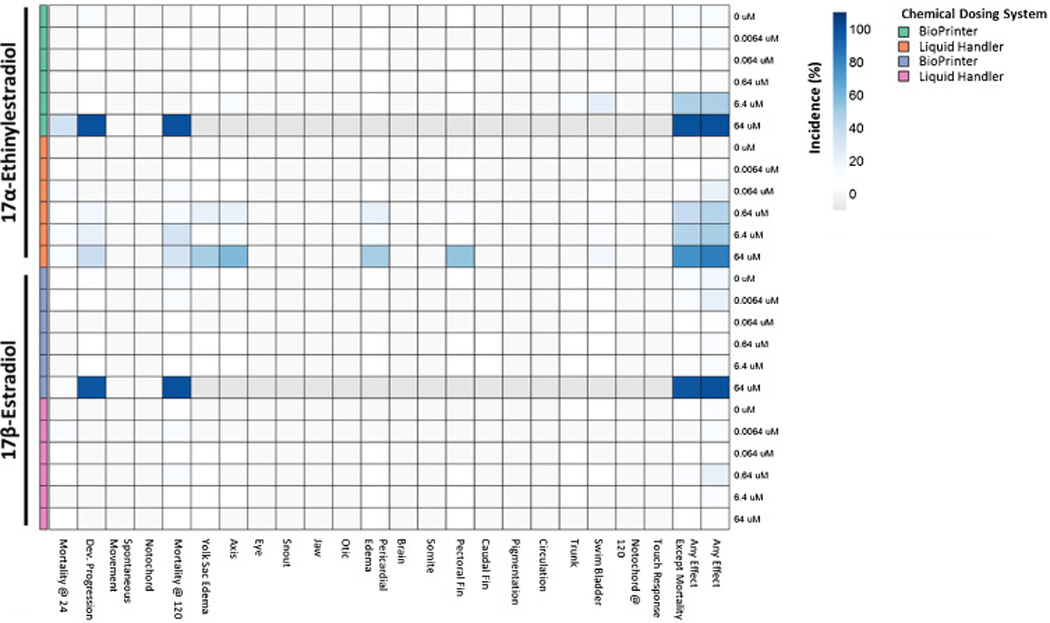
For each endpoint: chemical:delivery system:concentration and percent occurrence are denoted based on degree of shading. When 100% mortality was observed, all subsequent developmental endpoints were unavailable for evaluation, and are shaded in gray. In addition to 22 individual endpoints, two comprehensive endpoints are available (any effect, and any effect excluding mortality).
To further characterize the discord in delivery technologies, we assessed delivery method-dependent transcriptional responses in the absence of a phenotype. Embryos were exposed to 0.01, 0.1, 1.0, and 10.0 µM of E2 and EE2 (delivered with the same number of transfer steps by the Liquid Handler, and direct dispensing using the BioPrinter) to evaluate induction levels of vitellogenin (vtg1) and aromatase (cyp19a1b) expression [20]. These concentrations were selected based on the low incidence of mortality and adverse effects to reduce additional confounders. The expression of vtg1 and cyp19a1b was consistently higher in animals exposed to E2 via the BioPrinter than via the Liquid Handler. BioPrinter delivery of 0.01–0.1 µM E2 resulted in a ~300–700-fold greater induction of vtg1 and significantly greater induction of cyp19a1b compared to Liquid Handler delivery (Figure 2a and b). Similar but less dramatic discord was also observed for the EE2 transcriptional responses. As anticipated, the delivery method discord seen at the phenotype level was also readily detectable at the transcriptional level.
Figure 2. Concentration-responses for (a) cyp19a1b and (b) vtg1 expression for 17β-Estradiol and 17α-Ethinylestradiol.
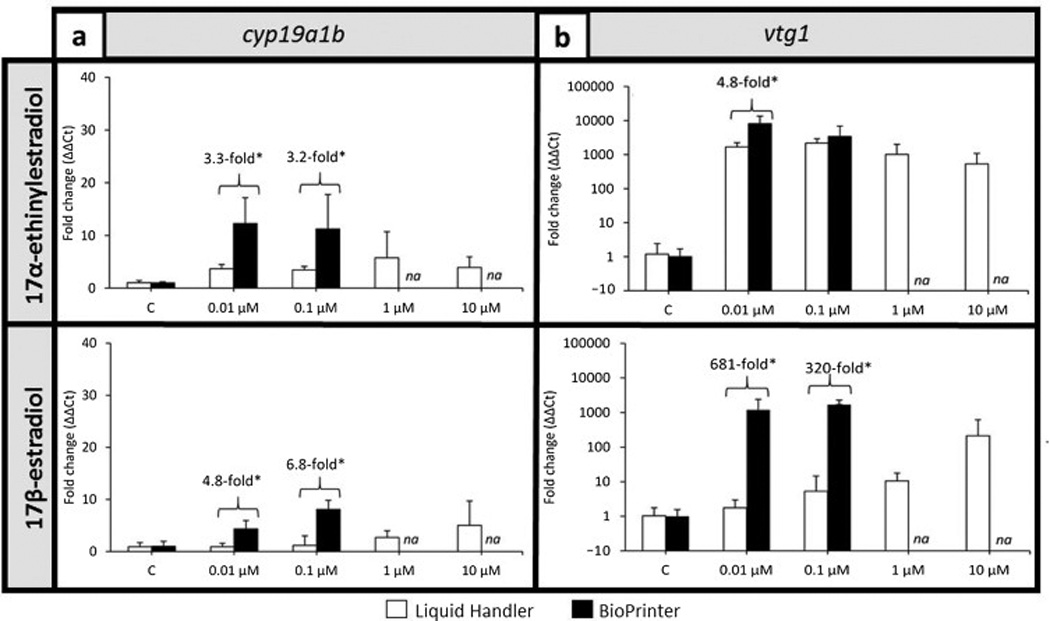
Data are reported as mean ± standard deviation. Fold changes are relative to the respective control group (0.64% DMSO). Asterisks indicate significant differences between chemical delivery methods within the treatment group (p≤0.05). Expression data for treatments with high mortality/morbidity are not available for 1 and 10 µM delivered with the BioPrinter are denoted with “NA”. N = 4 biological replicates per treatment group (10–12 larvae each).
We also evaluated whether a highly water soluble compound might show delivery method-dependent discord. Thiram, over a broad concentration range (0 – 64 µM), is a highly water soluble pesticide that reproducibly elicits a distorted notochord phenotype early in zebrafish development [21, 22]. Comparing the BioPrinter with Liquid Handler delivery, the distorted notochord phenotype was always readily evident, but the concentration response profiles associated with each method were dissimilar (Figure 3). The LC50 for Thiram dispensed with the BioPrinter is ~15 µM, and ~50 µM for the LiquidHandler. The BioPrinter delivery was associated with a higher incidence of adverse effects (across all endpoints), and increasing mortality with increasing Thiram concentration; 100% at the highest concentration. The Liquid Handler was associated with a more attenuated concentration-response where mortality at the highest Thiram concentration was well below 100% and incidences of craniofacial, somite and pectoral fin malformations, observed with the BioPrinter, were absent entirely in animals exposed via the Liquid Handler. Similar phenotype and analyte discord between the delivery technologies with hydrophilic Thiram and the more hydrophobic E2 and EE2 would suggest chemical retention on the plastic tips of the Liquid Handler may not be the issue.
Figure 3. Heatmap of developmental effects for non-sticky chemical: Thiram.
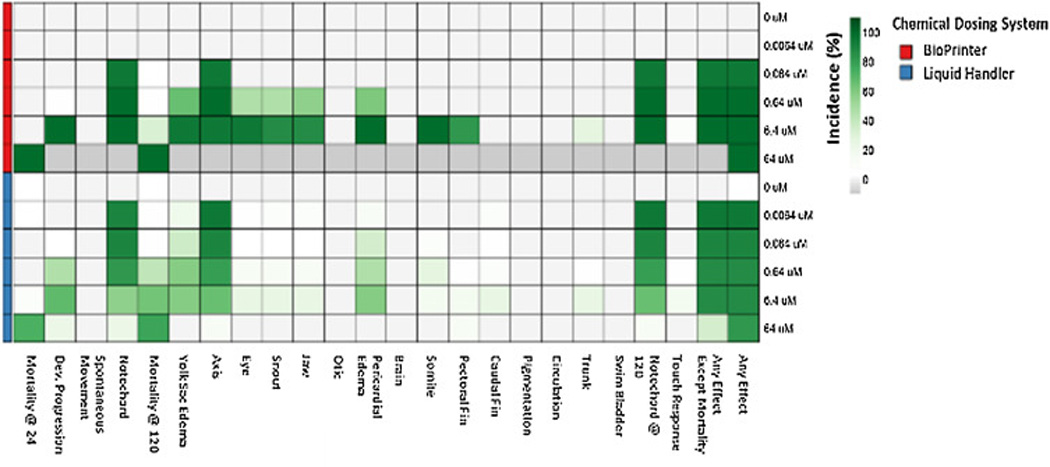
Embryos were exposed to 6 concentrations of Thiram using the BioPrinter or Liquid Handler. The exposed embryos were evaluated for 22 endpoints. The percent incidence was calculated for each endpoint and concentration and displayed in the heatmap. When 100% mortality occurred, the morphological endpoints were unavailable for evaluation and are shaded gray.
Others have reported more precise chemical delivery using the BioPrinter. Jones et al. (2013) [23] observed differences in EC50 using the BioPrinter vs. delivery via 1536 pin tool. They found that 7 of the 9 compounds tested in their study had comparable EC50s, while the other two were less active (Cortisol and Tamoxifen) when delivered via pin tool. They attributed this to physical differences between the formats of the dispensing wells (384-well plates vs. 1536-well plates) and the need for multiple rounds of pin dips vs. single step BioPrinter delivery, direct to the assay well. Here we eliminated a potential format effect by standardizing on one plate format. Additionally, there is Organisation of Economic Co-operation and Development (OECD) guidelines for screening using zebrafish, which is substantially technically different and cost prohibitive or any lab to use with digital dispensing in a 20 well plate format since each well is about 2 mL, thus 200× the cost.
3.1.2. Nominal exposure concentration
To assess the actual concentrations delivered to the experimental chambers that resulted in detectable differences at the transcriptional level, we determined the nominal concentrations of 17β-Estradiol (E2) and 17α-Ethinylestradiol (EE2) in the exposure medium, prior to the addition of embryos. Each chemical was delivered to a final target concentration of 0, 0.01, 0.1, 1, and 10 µM using the number of transfer steps for the Liquid Handler, and BioPrinter described in the methods. Using the BioPrinter, the observed concentrations for E2 and EE2 were not statistically different than the target concentrations (Table 1, Welch T-Test, p<0.05). With the Liquid Handler, the measured nominal concentrations of E2 and EE2 were statistically different than the target concentrations. For E2, the measured concentration in the 10 µM wells was ~32% below the target concentration while the smaller target concentrations contained nominally higher concentrations of E2. Similar discord between target and nominal concentration was observed for EE2. The inaccuracy of the Liquid Handler here appeared more generally to be a function of error propagation through serial dilution and negative displacement pipetting, both inherent in use of the Liquid Handler platform. The data in Table 1, demonstrating both over- and undershooting of target concentrations in the same experiment, supports this conclusion. When comparing the concentrations measured at the initiation of an experiment to the embryonic malformation and transcriptional responses observed at 120 hpf, there is a discrepancy at the lower concentrations when dispensed with the Liquid Handler. A likely explanation for the overshooting of lower concentrations is the method used to prepare the chemical dilutions. The same pipette tips on the Liquid Handler were used to serial dilute the chemical. Using this method, the Liquid Handler would be expected to have an inherently greater potential for carryover of hydrophobic analytes that are loosely retained on the plastic pipette tips. Additionally, the adsorption would have occurred during the time after chemical dispensing and before embryo addition to the well. This was a 10 – 30 minute period depending on how long it took to dispense/mix the plates with the liquid handler before a person started manually adding embryos to the plate wells. The plates were sampled for target concentration during this period. We believe that the liquid handler artifact would have been avoided had the embryo been present during chemical dispensing, as was the case for the BioPrinter. The 6 hpf embryo is primarily a large yolk sac into which fat soluble compounds such as E2 and EE2 easily and preferentially partition. Its presence during chemical dispensing with the Bioprinter ensured that the embryo intercepted much of the E2 and EE2 dosing. Most of the liquid handler dosing was probably lost to the plate wells by the time the embryo was added to those plates.
Table 1.
Nominal exposure media concentration for 17α-Ethinylestradiol and 17β-estradiol delivered by the Bioprinter or Liquid Handler.
| Nominal Exposure Concentration (µM) |
Mean Measured Concentration ± SD (µM) | |||
|---|---|---|---|---|
| 17α-Ethinylestradiol | 17β-estradiol | |||
| Bioprinter | Liquid Handler | Bioprinter | Liquid Handler | |
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00* |
| 0.01 | 0.01 ± 0.01 | 0.07± 0.01* | 0.01 ± 0.00* | 0.19± 0.01* |
| 0.1 | 0.09 ± 0.06 | 0.49 ± 0.08* | 0.11 ± 0.00* | 1.18 ± 0.15* |
| 1 | 1.09 ± 0.29 | 1.76 ± 0.67* | 1.12 ± 0.09* | 1.77 ± 0.08* |
| 10 | 7.86 ± 3.38 | 8.24 ± 2.00 | 9.27 ± 0.33* | 6.71 ± 0.27* |
Asterisk indicates significance compared to nominal exposure concentration.
The accuracy of both chemical delivery systems was further evaluated using 4 different PAHs (fluoranthene, pyrene, chrysene, benzo[a]pyrene) as hydrophobic analytes already known to weakly adsorb to plastic surfaces. The performance metric was adsorption of the PAH species to the microtiter plate wall after dispensation via the BioPrinter or by the multi-step method described for the Liquid Handler. The expectation, based on the likelihood of tip carryover and the performances of the two platforms already described, was that Liquid Handler dispensation of the 4 PAHs would overshoot the target concentration resulting in excessive sorption of the lipophilic analytes to the plastic plate walls [17]. This was indeed the case (Table 2, Figure 4). Less than 10% of the BioPrinter’s target PAH dispensation was recovered from the plate wall, regardless of embryo inclusion in the assay. But adsorption ranged from 4.75–51.8% of the target with the Liquid Handler (Table 2; Figure 4). Inaccuracy of both platforms, in the form of target overshoot, was most pronounced for CHR and BaP, but the overshoot was ≥ 5 times worse with the Liquid handler, which can be explained by two operational factors: 1) plastic tip carryover that lead to concentration overshoot relative to the BioPrinter, in the first place and 2) the presence of the embryo with its large lipophilic yolk sac sequestered 25–40% of the overshoot PAH before it could be adsorbed to the plastic wall. Therefore, hydrophobic analyte carryover on the Liquid Handler’s plastic tips may be an additional source of error.
Table 2.
PAH Chemical names, abbreviations used, and measured percent sorption (%) for the Liquid Handler and BioPrinter protocols, in the presence and absence of zebrafish embryos, ±SE
| PAH Chemical Name |
Abbreviation | Liquid handler with embryos |
BioPrinter with embryos |
Liquid handler without embryos |
BioPrinter without embryos |
|---|---|---|---|---|---|
| Fluoranthene | FLA | 5.01 ±0.46 | 1.27 ±0.21 | 5.14 ±0.18 | 1.25 ±0.03 |
| Pyrene | PYR | 4.75 ±0.42 | 1.0 ±0.2 | 5.20 ±0.20 | 0.95 ±0.04 |
| Chrysene | CHR | 27.0 ±1.4 | 5.61 ±1.4 | 51.4 ±1.2 | 6.26 ±0.28 |
| Benzo[a]pyrene | BaP | 38.5 ±3.9 | 7.30 ±1.6 | 51.8 ±0.7 | 7.21 ±0.16 |
Figure 4. Difference in sorption of 4 PAHs to experimental chambers depending on chemical delivery system with or without embryos.
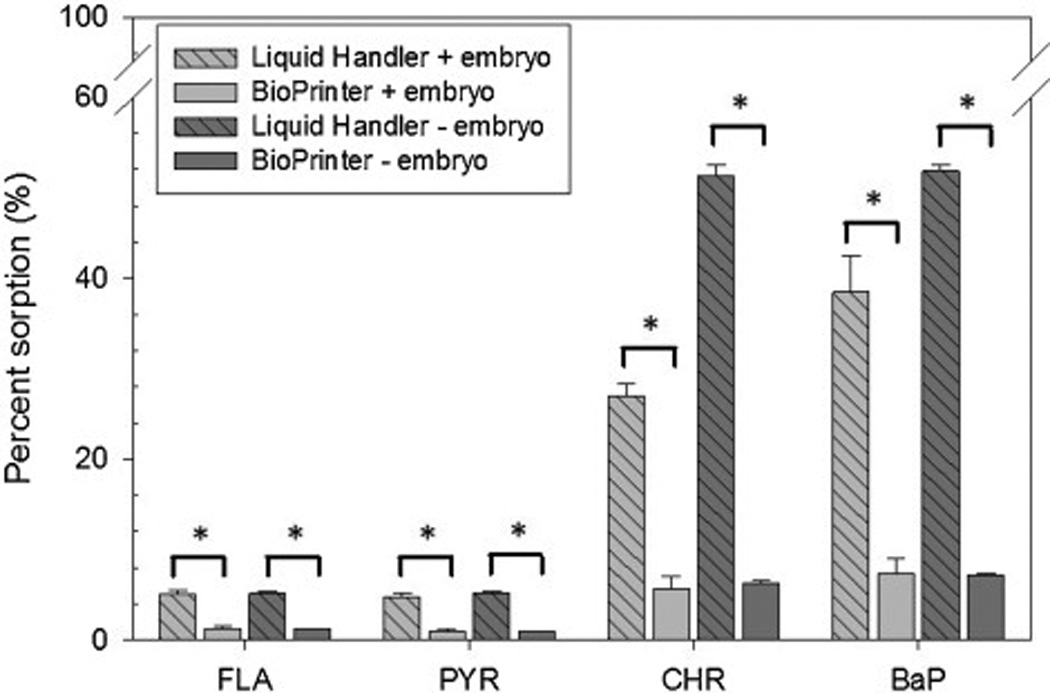
For each chemical delivery system (BioPrinter and Liquid Handler), PAHs were dispensed into plates with embryos and without (n=8 with embryos, n=6 without). For all 4 PAHs, there was a statistically significant (indicated by asterisk, p<0.05) difference in sorption for each chemical delivery system (with and without embryos).
We expected that the chemical delivery system would impact the biological readout primarily due to liquid handling technology typically requiring multiple transfer steps and serial dilutions to achieve lower concentrations {Jones, 2013 #14653}. Interestingly, the Tox21 in vitro testing used metal instead of plastic tips for their liquid handling, yet certain chemicals stuck to the tips, while others did not. This uncertainty can be minimized by moving towards direct digital dispensing, albeit with a higher consumable cost. The initial investment for the BioPrinter can range from $25 – $35,000 with individual experiment consumable costs ranging $6 to $45 per sample dispensed, dependent on stock concentration, number of replicates, and the range of test concentrations. Although more expensive than pipetting, the cost is offset by reduced likelihood of error, reduced labor costs, and an overall reduction in plastic consumable waste. Therefore, whenever possible, negative displacement, pipette-based liquid handling in plastic tips should be minimized/avoided.
3.2. Zebrafish HTS considerations post-digital dispensing
We have demonstrated that digital, inkjet-based delivery of chemicals, directly to the experimental chamber, sans serial dilutions, is a more accurate and thus, reproducible method of conducting chemical exposures for bioactivity detection. For HTS in the developmental zebrafish, chemical delivery typically occurs after placement of the embryos into the 96-well plate. But this order necessitates gentler mixing of the plate wells than would ordinarily be desirable to ensure solution uniformity. We collaborated with the BioPrinter development team at HP to script a gentler onboard mixing protocol that ensured solution uniformity in the plate, but not affect normal zebrafish development. We observed a substantial difference relative to offboard mixing (Figure 5). The lowest effect level (LEL), after exposure to a mixture of PAHs, for any morphological malformation (ie: any effect) occurred at the 1:50 dilution for the multifaceted onboarding mixing while the LEL after a single 10 sec mix using an offboard Variomag Teleshake was 1:100 dilution of the stock. Moreover, developmental abnormalities associated with these exposures manifested by 24 hpf following onboard mixing, with > 50% mortality in the highest concentration group by 120 hpf. But the exposures mixed offboard did not manifest abnormalities by 24 hpf, or significant mortality by 120 hpf.
Figure 5. An example of how additional mixing protocols influences the bioactivity of embryonic zebrafish.
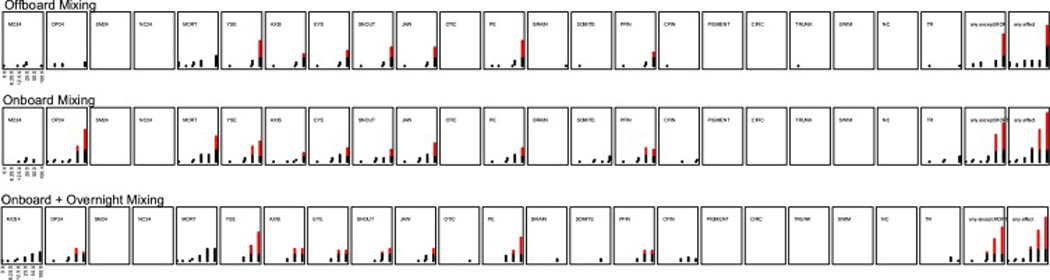
BioPrinter chemical delivery occurs in a specific order for HTS using developing zebrafish. When chemical is added by the BioPrinter, there are 3 mixing options: offboard mixing (not on the BioPrinter), onboard mixing (onboard the BioPrinter using a custom protocol), or onboarding and overnight mixing (onboard mixing with an additional 16hr orbital mixing at 235 rpm). There are 22 endpoint evaluated at 6 concentrations (n=32), and two comprehensive endpoints are available (any effect, and any effect excluding mortality). Once a significance threshold, the datapoints turn red.
We sought to determine if an overnight mixing of exposures to particularly low solubility compounds such as PAHs might alter apparent concentration bioactivity curves. We implemented a 16h orbital mixing at 235 rpm starting immediately after exposure. Since the experimental chambers have round bottoms, the embryo’s eccentric movement was minimized to a 1 mm radius at 235 rpm on a portable platform shaker. The addition of the overnight mixing shifted the LEL for any effect (Figure 5) and increased the prevalence of morphological defects. By implementing the addition of an overnight mixing, it encouraged uniform exposure solutions, and increased bioavailability. Embryos that underwent any mixing developed and behaved normally at 120hpf (data not shown). For a chemical non-renewal HTS approach when chemical is dispensed after embryo placement, the onboard and overnight mixing protocol should be a standard practice toward minimizing false negatives.
3.3. Sealing the assay plate
The 5 day (6 hpf – 120 hpf) assay at 28°C requires that the plate wells be sealed to prevent exposure solution loss due to evaporation. Heretofore, we routinely sealed our 96 well assay plates by sandwiching a piece of parafilm between the lid and plate. While this did not completely isolate individual wells from potential vapor exchange with each other, it did effectively seal the plate en masse. We note that removal of the parafilm occurred for the developmental evaluation at 24 hpf, but after that, the plate was sealed once again until 120 hpf. No additional input of oxygen is required for normal zebrafish development through day 5 [24]. We have encountered chemical classes for which well-to-well vapor exchanged has impacted zebrafish development over the 5 day assay (unpublished) thus, we sought to further isolate the assay wells as a routine HTS practice. Not all adhesive backed sealing membranes are biocompatible, but we have found that non-tacky, pressure activated silicone PCR sealing tapes (VWR, Catalog number: 89134-428) are completely biocompatible in this assay and create a tight and full seal around each well, even if removed and reapplied. This sealing tape is optically clear and has low-autofluorescence, which allows for imaging of embryos through the seal, avoiding the need for removal and replacement for the 24 hpf evaluations.
3.4.Chemical contamination
We routinely utilize genome-wide transcriptomics to better classify chemical bioactivity and to begin to define the mechanism of action. During a recent screen of brominated diphenyl ether (BDE) bioactivity in zebrafish, we observed several instances of severe developmental toxicity associated with certain commercially obtained BDEs (BDE-77 and 99), results that were discordant with other published studies. For instance, a concentration response study with BDE-77 at 99.9% purity and a furan-free (BDE-77FF) preparation that was further purified to remove dioxin/furan contamination, were compared for adverse effects over a 1 – 20 µM range. BDE-77 induced morphological malformations (Figure 6a), while BDE-77FF did not. BDE-77 at ≥1 µM was associated with mortality or a morphological defect in 100% of animals (24/24 for each concentration), whereas BDE-77FF was not associated mortality or aberrant morphology at any test concentration (0/24). Transcriptomic profiling of the apparent BDE anomalies indicated that they were also associated with strong activation of the aryl hydrocarbon receptor as high levels of induction were also observed for four well known AHR target cyp genes in response to BDE-77 exposure, whereas BDE-77FF elicited little or no induction (Figure 6b). We analytically characterized the BDE-77 preparations (Figure 6c) and found that Peak 7 matched an isotopically-labeled BDE-77 standard. Peak 5 was identified as BDE-66, and Peak 6 had a BDE-like spectrum, but could not be identified with available standards. Peak 11's spectrum showed a base peak at m/z 127, uncharacteristic of BDEs. Peaks 8–23 contain m/z 127, though not all exhibit it as the base peak of the spectra. Spectrum for Peak 17 showed a relatively small peak of m/z 127, normal m/z 79/81 Br patterns and an unusual halogen pattern around m/z 437. When comparing the gas chromatography-mass spectrometry spectrum for the two preparations, the m/z 127 was distinct and not found in BDE-77FF, which lead us to hypothesize that the congener was contaminated with a very low concentration of dioxin or furans. The presence of potent low level contaminants in test solutions will be a challenge for zebrafish screening. In this example, if a single contaminant is present as 0.1% of the sample, and assuming a similar MW, when the test concentration is 64 µM this contaminant could be present at 64 nM. Based on our previous studies, we know that many chemicals are identified as bioactive in the nanomolar range. In other words, assignment of toxicity to the parent compound would be completely incorrect and driven by the contaminant. It is important to emphasize that the zebrafish screening model is uniquely sensitive to the presence of contaminants during early development because at this life stage all the potential targets for chemicals are present and available for chemical interactions.
Figure 6. Unexpected contamination provides a signature developmental impact and transcriptional response for a brominated diphenyl ether.
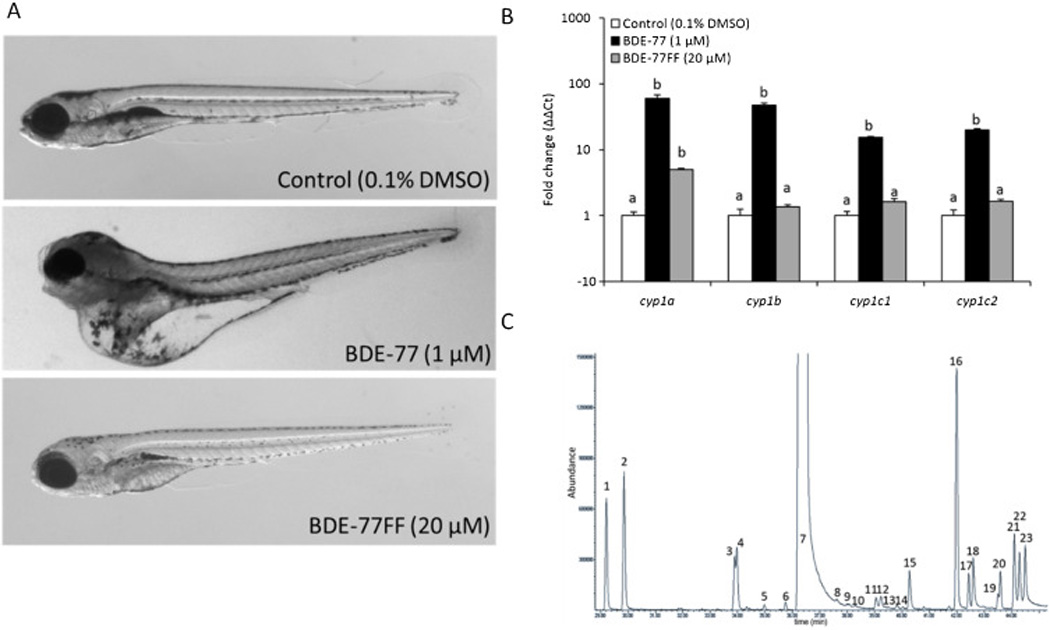
(A) Example photomicrographs of 120 hpf embryos exposed to Control (0.1% DMSO), BDE-77 (1 µM), and BDE-77FF (20 µM; BDE-77 preparation further purified to remove dioxin/furan contamination). (B) Whole animal expression of AHR-dependent cytochrome P450 genes (cyp1a, cyp1b, cyp1c1, cyp1c2) was analyzed in 120 hpf animals treated from 6–8 hpf to 120 hpf with either the EC100 for BDE-77 (1 µM), or highest concentration tested for BDE-77FF (20 µM). Bars not labelled with the same letter were significantly different (ANOVA on ranks, Student-Newman-Keuls, p ≤ 0.05, N = 4 replicates with 12 animals each). (C) Gas chromatography-mass spectrometry analysis for BDE-77 shows a complex mixture of over twenty low level contaminant compounds present in the BDE lot that elicited developmental toxicity.
The hypothesis that the BDE-77 was contaminated with dioxon/furans was supported by the upregulation of AhR-dependent cyp genes in embryos exposed to BDE-77, but not in those exposed to BDE-77FF. AhR binds dioxins and furans and induces morphological malformations that was similar to those observed after exposure of BDE-77. Others have demonstrated that nanomolar concentrations of dioxin activate the AhR [25–28]. These findings mirror those in Wahl et al (2008) who identified trace contamination of brominated furans in BDE-47 samples [29]. Even though BDEs are highly susceptible to contamination, many other chemicals may be prone to this effect and caution should be exercised. It may often be impractical for a typical HTS facility to perform independent purity assessments; an alternative and eminently practical exercise of caution would be to 1) secure the manufacturer’s purity data beforehand, and 2) evaluate the methods used to assess purity to determine validity.
4. Conclusions
Critical aspects of high throughput chemical bioactivity screening in the developmental zebrafish can be improved. Digital, ink-jet technology, applied to small molecule dispensation, we view is an improvement over the requisite use of serial dilution in negative displacement pipetting of concentration series. Adoption of digital dispensing technology, routine sealing of individual wells when screening, and more attention to chemical purity and should quickly improve the accuracy and reproducibility of bioactivity detection.
Highlights.
Choice of chemical delivery method affects the bioactivity profile
Digital dispensing results in more accurate chemical delivery
Extensive mixing increases chemical dispersion apparent bioactivity
Zebrafish model is sensitive to chemical impurities
Acknowledgments
We would like to acknowledge the NIEHS National Toxicology Program for providing the test chemicals, and their valuable advice. The authors would also like to thank members of the Tanguay laboratory, Sinnhuber Aquatic Research Laboratory for assistance with fish husbandry and especially Greg Gonnerman for chemical screening. This work was supported by NIEHS grants P42 ES016465 (Project 3 and 5), P30 ES000210, and Environmental Protection Agency (EPA) STAR Grants #835168.
Abbreviations
- hpf
hours post fertilization
- DMSO
Dimethyl sulfoxide
- HTS
high-throughput screening
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sipes NS, Padilla S, Knudsen TB. Zebrafish: as an integrative model for twenty-first century toxicity testing. Birth Defects Res., Part C. 2011;93(3):256–267. doi: 10.1002/bdrc.20214. [DOI] [PubMed] [Google Scholar]
- 2.Truong L, et al. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 2014;137(1):212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reif DM, et al. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2015 doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugel SM, Tanguay RL, Planchart A. Zebrafish: A marvel of high-throughput biology for 21 century toxicology. Curr Environ Health Rep. 2014;1(4):341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knecht AL, et al. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol. 2013;271(2):266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson RT, et al. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97(24):12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dix DJ, et al. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95(1):5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- 10.Padilla S, et al. Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod Toxicol. 2012;33(2):174–187. doi: 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Kimmel CB, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 12.Mandrell D, et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom. 2012;17(1):66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tecan. Rapid generation of dose-response curves for high throughput screening. Tecan Journal. 2015 [Google Scholar]
- 14.Bugel SM, White LA, Cooper KR. Inhibition of vitellogenin gene induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin is mediated by aryl hydrocarbon receptor 2 (AHR2) in zebrafish (Danio rerio) Aquat Toxicol. 2013;126:1–8. doi: 10.1016/j.aquatox.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trumble SJ, et al. Assessment of legacy and emerging persistent organic pollutants in Weddell seal tissue (Leptonychotes weddellii) near McMurdo Sound, Antarctica. Science of The Total Environment. 2012;439:275–283. doi: 10.1016/j.scitotenv.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski AC, Tanguay RL, Simonich SL. Quantitation and prediction of sorptive losses during toxicity testing of polycyclic aromatic hydrocarbon (PAH) and nitrated PAH (NPAH) using polystyrene 96-well plates. Neurotoxicol Teratol. doi: 10.1016/j.ntt.2016.05.001. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saili KS, et al. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291(1–3):83–92. doi: 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong L, et al. Zebrafish Assays as Developmental Toxicity Indicators in The Design of TAML Oxidation Catalysts. Green Chem. 2013;15(9):2339–2343. doi: 10.1039/C3GC40376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muncke J, Eggen RI. Vitellogenin 1 mRNA as an early molecular biomarker for endocrine disruption in developing zebrafish (Danio rerio) Environ Toxicol Chem. 2006;25(10):2734–2741. doi: 10.1897/05-683r.1. [DOI] [PubMed] [Google Scholar]
- 21.Tilton F, et al. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol Appl Pharmacol. 2006;216(1):55–68. doi: 10.1016/j.taap.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Tilton F, Tanguay RL. Exposure to sodium metam during zebrafish somitogenesis results in early transcriptional indicators of the ensuing neuronal and muscular dysfunction. Toxicol Sci. 2008;106(1):103–112. doi: 10.1093/toxsci/kfn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones RE, et al. An alternative direct compound dispensing method using the HP D300 digital dispenser. J Lab Autom. 2013;18(5):367–374. doi: 10.1177/2211068213491094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10(10):901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, et al. Generation of Tg(cyp1a:gfp) Transgenic Zebrafish for Development of a Convenient and Sensitive In Vivo Assay for Aryl Hydrocarbon Receptor Activity. Mar Biotechnol (NY) 2015;17(6):831–840. doi: 10.1007/s10126-015-9669-1. [DOI] [PubMed] [Google Scholar]
- 26.Tanguay RL, et al. Dioxin toxicity and aryl hydrocarbon receptor signaling in fish. In: Schecter A, editor. Dioxins and Health. New York: Plenum Press; 2003. pp. 603–628. [Google Scholar]
- 27.Andreasen EA, et al. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci. 2002;68(2):403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- 28.Abnet CC, et al. Transactivation activity of human, zebrafish, and rainbow trout aryl hydrocarbon receptors expressed in COS-7 cells: greater insight into species differences in toxic potency of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners. Toxicol Appl Pharmacol. 1999;159(1):41–51. doi: 10.1006/taap.1999.8719. [DOI] [PubMed] [Google Scholar]
- 29.Wahl M, et al. A technical mixture of 2,2',4,4'-tetrabromo diphenyl ether (BDE47) and brominated furans triggers aryl hydrocarbon receptor (AhR) mediated gene expression and toxicity. Chemosphere. 2008;73(2):209–215. doi: 10.1016/j.chemosphere.2008.05.025. [DOI] [PubMed] [Google Scholar]


