Abstract
Insulin serves as a link between the metabolic and reproductive systems, communicating energy availability to the hypothalamus and enabling reproductive mechanisms. Adult Suffolk ewes prenatally exposed to testosterone (T) display an array of reproductive and metabolic dysfunctions similar to those seen in women with polycystic ovarian syndrome (PCOS), including insulin resistance. Moreover, prenatal T treatment alters neuropeptide expression in KNDy (co-expressing kisspeptin, neurokinin B/dynorphin) and agouti-related peptide (AgRP) neurons in the arcuate nucleus, two populations that play key roles in the control of reproduction and metabolism, respectively. In this study, we determined whether prenatal T treatment also altered insulin receptors in KNDy and AgRP neurons, as well as in preoptic area (POA) kisspeptin, pro-opiomelanocortin (POMC), and gonadotropin-releasing hormone (GnRH) neurons of the adult sheep brain. Immunoflourescent detection of the beta subunit of insulin receptor (IRβ) revealed that KNDy, AgRP and POMC neurons, but not GnRH or POA kisspeptin neurons, colocalize IRβ in control females. Moreover, prenatal T treatment decreased the percentage of KNDy and AgRP neurons that colocalized IRβ, consistent with reduced insulin sensitivity. Administration of the anti-androgen drug, Flutamide, during prenatal T treatment, prevented the reduction in IRβ colocalization in AgRP, but not in KNDy neurons, suggesting that these effects are programmed by androgenic and estrogenic actions, respectively. These findings provide novel insight into the effects of prenatal T treatment on hypothalamic insulin sensitivity and raise the possibility that decreased insulin receptors, specifically within KNDy and AgRP neurons, may contribute to the PCOS-like phenotype of this animal model.
Keywords: kisspeptin, agouti-related peptide, polycystic ovarian syndrome, insulin receptor, sheep
Graphical abstract
INTRODUCTION
Fertility is dependent on sufficient energy supply. Insulin is a key communicator of energy availability to the hypothalamus and specifically, gonadotropin-releasing hormone (GnRH) neurons, enabling reproductive function in times of sufficient energy supply (Bruning, 2000). In women with polycystic ovary syndrome (PCOS), infertility and insulin resistance are a major concern and are exacerbated by excess weight (Franks, 1995; Holte, 1996; Dunaif, 1997; Steckler, 2009). Indeed, insulin resistance affects 50–70% of women with PCOS leading to a number of co-morbidities including metabolic syndrome, hypertension, dyslipidemia, glucose intolerance, and diabetes; while 40% of PCOS women are infertile (Sirmans & Pate, 2014). The sheep model of PCOS, in which ewes are exposed to excess testosterone (T) during fetal life (from day 30–90 of gestation), is a well-characterized model that has been extensively utilized to understand and assess the molecular mediators involved in the reproductive and metabolic dysfunctions associated with this disorder (Dumesic et al., 2007). The prenatally androgenized sheep model mimics the reproductive and metabolic dysfunctions seen in women with PCOS (Padmanabhan et al., 2006; Dumesic et al., 2007; Abbott, 2008). The reproductive disruptions include polycystic ovaries (Padmanabhan, 2011; Padmanabhan & Veiga-Lopez, 2013), intermittent to absent ovulation (Birch et al., 2003; Manikkam et al., 2006; Steckler et al., 2007; Veiga-Lopez, 2008), early reproductive failure (Clarke et al., 1977; Birch et al., 2003; Manikkam et al., 2006; Steckler et al., 2007; Veiga-Lopez, 2008), neuroendocrine feedback defects (Wood & Foster, 1998; Robinson et al., 1999; Sharma et al., 2002; Sarma et al., 2005; Unsworth et al., 2005; Veiga-Lopez et al., 2009), and functional hyperandrogenism (Padmanabhan et al., 2010a). In addition, prenatal T-treated sheep are metabolically compromised and manifest reduced insulin sensitivity and compensatory hyperinsulinemia (Recabarren et al., 2005; Padmanabhan et al., 2010b). Although the reproductive and metabolic outcomes of prenatal T exposure are well described in the ewe, the mechanisms responsible for these detrimental effects, and the contribution of neural-level changes, are not well understood.
In sheep and other mammals, the arcuate nucleus (ARC) of the hypothalamus is a major center of convergence for reproductive and metabolic signals. This region contains two key populations of appetite regulating neurons, the orexigenic agouti-related peptide (AgRP) and the anorexigenic proopiomelanocortin (POMC) neurons, both of which have been shown to express insulin receptors (Morton & Schwartz, 2001; Benoit et al., 2002). Previous work from our lab has shown that prenatal T-treated ewes show a more than two-fold increase in the number of AgRP, but not POMC, neurons in the ARC, as well as an increase in the density of AgRP fiber projections (Sheppard et al., 2011). In addition to the metabolic control neurons, the ARC also contains a key neuronal group involved in the control of GnRH secretion, the KNDy (co-expressing the neuropeptides kisspeptin, neurokinin B (NKB), dynorphin) neuronal population. Kisspeptin (located in both KNDy and preoptic neurons), NKB and dynorphin have each been proposed to serve distinct roles in modulating GnRH release depending on the steroid hormone milieu, mediating pulsatile and/or surge secretion of GnRH (de Roux et al., 2003; Seminara et al., 2003; Caraty et al., 2007; Dungan, 2007; Lehman et al., 2010; Smith et al., 2011). Previous work has found that prenatal T-treated females show a reduction in dynorphin and neurokinin B immunoreactivity in KNDy neurons, but no change in kisspeptin (Cheng et al., 2010). This peptide imbalance within the KNDy population has been postulated to contribute to the steroid feedback dysregulation and reproductive deficits seen in prenatal T-treated females. In addition to these findings, it has been shown that insulin sensitizer treatment improves reproductive function in prenatal T-treated females (Veiga-Lopez et al., 2010) and this is also seen in women with PCOS (Baillargeon, 2004; Legro, 2007), suggesting that insulin signaling is an important component of the PCOS phenotype, and may have impact on both metabolic and reproductive functions.
There is evidence from rodent studies that insulin can have significant effects on reproduction, acting at a neural level. For example, diabetic female rats show decreased luteinizing hormone (LH) surges and fewer ovulatory cycles (Steckler, 2009). Moreover, deletion of neuronal insulin receptors in mice affects metabolic and reproductive function. For example, Bruning et al. showed that mice with CNS-specific disruption of the IR gene displayed increased food intake, diet-sensitive obesity, mild insulin resistance, and were sub-fertile (Bruning, 2000). Moreover, Burks et al. showed that mice lacking insulin receptor substrate-2 (IRS-2), a component of the insulin/insulin-like growth factor-1 signalling cascade, had small, anovulatory ovaries with reduced numbers of follicles and were infertile (Burks et al., 2000). Importantly, restoration of insulin receptor expression in the brain is required to maintain fertility in these females (Okamoto et al., 2004). These actions may be mediated by direct insulin action on GnRH neurons or by afferent signals from other insulin sensitive neurons, including kisspeptin, AgRP or POMC neurons. There is evidence in rodents for insulin receptor expression in each of these populations (Morton & Schwartz, 2001; Benoit et al., 2002), although selective deletion of the receptor in each population alone does not seem to compromise adult fertility at least in a major way (Könner, 2007; DiVall et al., 2010; Qiu et al., 2013). However, very little is known about the localization of insulin receptors in the sheep brain, and the possibility that prenatal T exposure alters its expression in specific neuronal subpopulations involved in reproduction and/or metabolic function has not been investigated.
Therefore, the goals of the present study were 1) to determine whether insulin receptors are present in kisspeptin and GnRH neurons in the adult sheep brain, and 2) to examine the effect of prenatal T exposure on insulin receptor colocalization in these neuronal populations, as well as in AgRP and POMC neurons. Because insulin appears to play an important role in relaying energy availability to the reproductive axis, we predicted that prenatal T-treated ewes would show a decreased colocalization of insulin receptors in metabolic and reproductive neurons of the ewe, rendering these neurons less sensitive to insulin and thereby affecting processes that require specific information about energy availability. Finally, we asked whether such effects are due to androgenic or estrogenic actions of prenatal T by examining colocalization of insulin receptors in animals in which the anti-androgen, Flutamide, was co-administered during prenatal T treatment.
MATERIALS AND METHODS
Animals and experimental groups
Suffolk ewes were cared for at the University of Michigan Sheep Research Facility (Ann Arbor, MI). Details of housing, nutrition, breeding, lambing and prenatal treatment have previously been described (Manikkam et al., 2004; Manikkam et al., 2006; Veiga-Lopez, 2008). Prenatal treatment involved administration of hormones to the pregnant mother between days 30 and 90 of gestation of a 147 day gestation period. The three experimental groups used in this study consist of adult female offspring treated prenatally with T (T group; n=5), with T and Flutamide (TF; n=5), or Flutamide alone (F; n=5). Control females (n=5) received an equal volume of vehicle (2 ml cottonseed oil). If twin births occurred, only one offspring from the pair was included. All procedures conducted were approved by the Institutional Animal Care and Use Committee of the University of Michigan and were in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
T (T-propionate; Sigma-Alderich) was injected i.m. twice weekly (100 mg/2ml dissolved in cottonseed oil; Sigma-Aldrich, St. Louis, MO). Previous analysis of maternal and fetal blood circulation has demonstrated that this dosage of T produces levels similar to adult males and fetal males, respectively (Veiga-Lopez et al., 2011). Females in the F group received daily injections of Flutamide (15mg/kg, Sigma-Alderich) dissolved in dimethylsulfoxide (400mg/ml, Sigma-Aldrich) administered subcutaneously. Females in the TF group received T-propionate as described above in addition to daily injections of Flutamide.
Tissue Collection
Brains were collected during the breeding season when females were 2 years of age. As in our previous studies (Sheppard et al., 2011), to normalize differences between prenatal T- treated and control ewes in endogenous steroid hormone levels present as a result of prenatal treatments, and any effect of these difference on insulin receptor or neuropeptide immunoreactivity, ewes were ovariectomized and implants used to normalize the endogenous hormonal milieu. Specifically, 3–4 weeks prior to tissue collection, animals were ovariectomized and were treated sequentially for 11–12 d with two controlled internal drug release P4 implants (CIDR) (InterAG, Hamilton, Waikato, New Zealand) and then for 1day with four 3-cm-long E2 implants to simulate ovarian steroid levels during the late follicular phase of the cycle. Eighteen hours after the E2 implants, animals were sacrificed. At time of tissue collection, all ewes received two intravenous injections (at 10 min intervals) of 25,000 U heparin (catalog # 402588B; Abraxiz Pharmaceuticals, Schumberry, IL). Ewes were anaesthetized intravenously with sodium pentobarbital (390 mg/ml/kg; Fatal Plus, Vortech, Dearborn, MI) and rapidly decapitated. The heads were immediately perfused through the internal carotid artery with 6L of 4% paraformaldehyde (Sigma-Aldrich) dissolved in 0.1M phosphate buffer (PB; Sigma- Aldrich) containing 0.1% sodium nitrate (Sigma- Aldrich), and 10 U/ml heparin (Abraxiz Pharmaceuticals). Brains were submerged in the same fixative at 4°C for 18hr. Blocks of tissue containing hypothalami were transferred in 30% sucrose (Caledon, Georgetown, Ont., Canada) at 4°C and sectioned coronally (45 µm) using a freezing microtome (Microm, Walldorf, Germany) into 12 parallel series for each animal. Sectioned tissues were stored in cryoprotectant (30% ethylene glycol, 0.1% sodium azide, 30% sucrose in PB) at −20°C until further processing.
Immunohistochemistry: general
One series of every sixth section through the POA and hypothalamus from each animal were processed for immunohistochemistry. All incubations occurred at room temperature with gentle agitation. Free-floating sections were rinsed thoroughly with 0.1 M phosphate-buffered saline (PBS) between incubations. Antibodies were dissolved in incubation solution consisting of 0.1M PBS, 0.4% Triton X-100 (catalog item BP151- 500; Sigma-Aldrich) containing 4% normal goat serum (NGS) (catalog item 005-000-121; Jackson Immuno Research Laboratories, West Grove, PA). Unless otherwise specified, tissue sections were washed extensively with 0.1 M PBS (pH 7.35) between steps. Prior to incubation with first primary antibody sections were incubated with 1% hydrogen peroxide (10 min, H2O2; catalog item H325; Fisher Scientific, Pittsburgh, PA) and incubation solution (1hr) to prevent non-specific background labeling. See Table 1 (Supplemental Material) for detailed information on all primary antibodies used.
Immunohistochemistry: triple-label immunofluorescence for AgRP/IRβ/POMC
Sections were incubated overnight (17 hours) with antibody specifically recognizing AgRP (raised in Guinea Pig, 1:800 dilution in incubation solution with 4% NGS; Antibodies Australia, catalogue # GPAAGRP.1, Lot # AS506), and with goat anti-guinea pig Alexa 488 (1:100 dilution, 30 minutes, Molecular Probes, Inc., Carlsbad, CA). Next, sections were incubated overnight with antibody recognizing the beta sub-unit of the insulin receptor (IRβ, raised in Rabbit, 1:300 dilution, Santa Cruz, C-19, SC-711), with biotinylated goat anti-rabbit (1:500 dilution, 1 hour, Vector Laboratories, Burlingame, CA, USA), ABC-elite (1:500 dilution in PBS, 1 hour, Vector Laboratories), biotinylated tyramide (BT; 1:250 dilution in PBS with 1 µl of 3% H2O2/ml, 10 minutes, Perkin Elmer Life Sciences, Woodbridge, Ont., Canada), and Alexa 555 conjugated streptavidin (1:100 dilution in PBS, 30 mins., Molecular Probes). Finally, sections were incubated with antibody specifically recognizing POMC (raised in rabbit, 1:4000 in incubation solution with 4% normal donkey serum, Phoenix Pharmaceuticals, Burlingame, CA, USA, catalogue # H-029-30) and donkey anti-rabbit Cy5 (1:100 dilution in PBS, 30 minutes, Molecular Probes). Sections were mounted on plus charged slides and cover slipped with gelvatol (Lennette, 1978).
Specificity of primary antibodies for POMC and AGRP has previously been demonstrated in sheep hypothalamic sections (Lennette, 1978). Pre-absorption of IRβ antibody with its corresponding peptide antigen (Santa Cruz) at concentration of 10 µg/ml for 24 hours at 4°C eliminated all immunoreactive staining (Supplemental Material, Fig. 1, A, B). Finally, elimination of any single primary antibody while performing all other steps of the protocol eliminated all staining for the corresponding antigen and no others, demonstrating lack of cross-reactivity (Supplemental Fig. 1, C–H). The reason for lack of cross-reactivity for this protocol rests on the fact that the concentration of reagents used in detection of the first antigen are optimized such that they completely saturate available binding sites and there is no cross-reactivity with reagents used to detect the second antigen (Supplemental Fig. 1F).
Immunohistochemistry: dual-label immunofluorescence for kisspeptin/GnRH with IRβ
Sections were incubated overnight with antibody against IRβ, as described above. Next, sections were incubated overnight with antibody specifically recognizing kisspeptin (raised in rabbit, 1:200,000 dilution, 17 h; kp10, lot 564; gift from Dr. Alain Caraty, Nouzilly, France) or antibody specifically recognizing GnRH (raised in Mouse, 1:400 dilution, 17 h, Sternberger Monoclonals, Inc., catalogue #SMI-41R, Lot #3). The next day, sections were washed and incubated with either goat anti-rabbit or goat anti-mouse Alexa 488 (1:100 dilution, 30 min., Molecular Probes). Sections were mounted on plus charged slides and cover slipped with gelvatol (Lennette, 1978).
Analysis
We performed two independent analyses on these tissue sections. In the first analysis, we performed cell counts of the total number of AgRP, POMC, kisspeptin, and GnRH neurons in sections through the POA at the level of the organum vasculosum of the lamina terminalis (GnRH and kisspeptin, 3 sections/animal), the middle ARC at the level of the tuberoinfundibular sulcus (AgRP, POMC, and kisspeptin, 5 sections/animal), and the MBH at levels of the rostral to middle arcuate nucleus (GnRH, 3 sections/animal). Sections were examined at 20× magnification using Neurolucida software (MicroBrightfield Bioscience, Williston, VT, USA) and a digital camera (Microfire A/R, Optronics, CA, USA) attached to a fluorescent microscope (DM500B, Leica Microsystems, Wetzlar, Germany). We calculated the mean number ± SEM for each region as appropriate (e.g., GnRH cells in the POA and MBH, kisspeptin cells in the POA and ARC).
In the second analysis, we used confocal microscopy to examine AGRP, POMC, kisspeptin, and GnRH neurons for colocalization with IRβ. Specifically, we used a Zeiss LSM-510 laser-scanning confocal microscope system (Zeiss, Heidelberg, Germany), to capture images of the ARC, POA and MBH in Z-stacks comprised of 1 µm optical sections. Alexa 555 fluorescence (IRβ) was imaged with a HeNe1 laser and a 543 nm emission filter. Alexa 488 fluorescence (kisspeptin, GnRH or AgRP) was imaged with an Argon laser and a 488 nm emission filter. Cy5 fluorescence (POMC) was imaged with a HeNe2 laser and a 633 nm emission filter. For each cell type in the ARC (AgRP, POMC, kisspeptin), between 39 to 44 neurons per animal were examined in serial 1 µm optical sections throughout their entire extent. For GnRH and POA kisspeptin neurons, every cell identified in the captured Z stacks was analyzed for colocalization with IRβ. Percentages of the kisspeptin (ARC and POA), GnRH, AgRP and POMC-ir neurons that colocalized IRβ-ir were calculated per animal, and the mean percentage ± SEM determined for each group. Differences between groups, for both total cell counts and percentage colocalization, were determined using Two Way Analysis of Variance, and Tukey's Multiple Comparison Test for posthoc comparisons. For pairwise comparisons between treatment groups, Dunn’s Method or Fisher LSD Method post hoc tests were performed. 95% confidence levels were applied to all tests. In addition, for comparison of the magnitude of treatment effects on total cell counts, we also performed effect size analysis (Cohen 1992, Nakagawa and Cuthill 2007, Padmanabhan, et al. 2015). This analysis allows comparison of the means between treatments with respect to the magnitude of difference between them. The computed statistic is Cohen's d value, and values above 0.2, 0.5, and 0.8 were considered as small, medium, and large effect sizes, respectively (Cohen 1992, Nakagawa and Cuthill 2007).
RESULTS
Colocalization of IRβ with AgRP, POMC and arcuate kisspeptin neurons in control ewes
In the ARC of control ewes, the vast majority of AgRP (85%) and POMC (92%) cell bodies colocalized IRβ (Fig. 1, top panel; also see Supplemental Figure 2). Similarly, 94% of ARC kisspeptin (KNDy) neurons colocalized IRβ (Fig. 2, top panel). By contrast, we observed no instances of colocalization among the 104 preoptic area (POA) kisspeptin neurons examined in 5 control animals (Fig. 3). Similarly, while IRβ-immunoreactive neurons were present in areas of the POA and MBH where GnRH neurons were located, of over 300 GnRH neurons examined in 5 control animals, none colocalized IRβ (Fig. 4). Despite the lack of colocalization of IRβ in POA kisspeptin or GnRH cell bodies, we observed numerous instances of IRβ-positive axonal boutons that were in direct contact with POA kisspeptin (Fig. 3) and GnRH (Fig. 4) somas. IRβ-positive boutons were also seen in direct contact with ARC kisspeptin neurons (e.g., Fig. 2, bottom panel). We observed no obvious regional differences in the distribution of colocalized cells, or in the location of IRβ-positive axon terminals, within the POA or ARC of control ewes.
Figure 1.
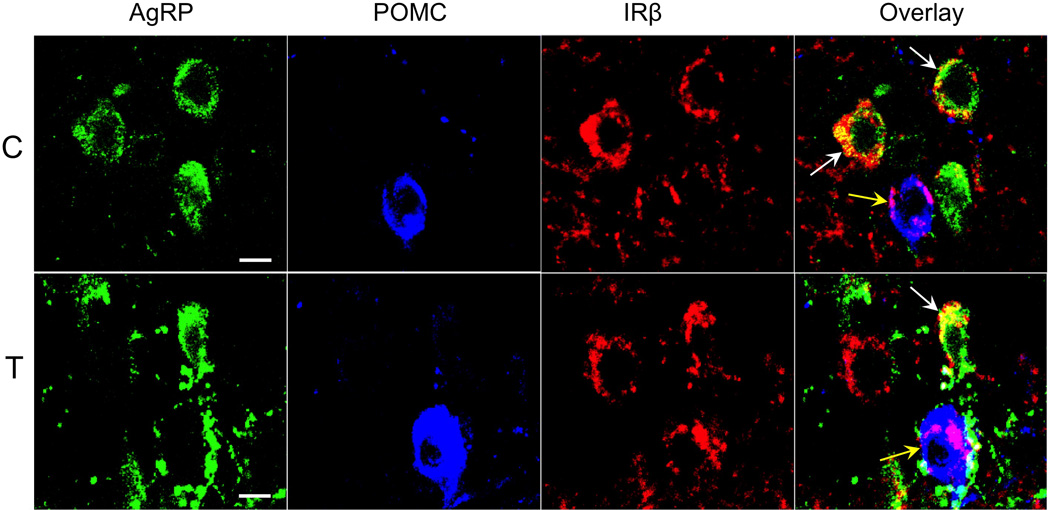
Confocal images of triple-labeled 1µm thick optical sections showing examples of AgRP- (green), POMC- (blue), and IRβ-immunolabeled neurons (red) in the arcuate nucleus of control (C, top panel) and prenatal T (T, bottom panel) ewes. Overlay images (far right panels) show colocalization of IRβ with AgRP (white arrows) and POMC (yellow arrows) neurons. Scale bars = 10µm.
Figure 2.
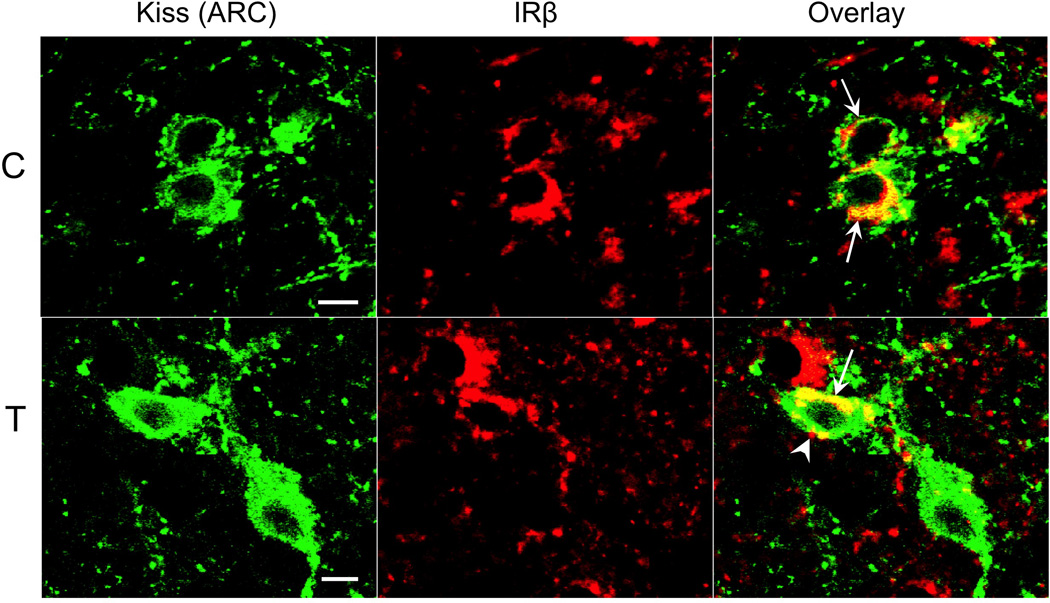
Confocal images of dual-labeled 1µm thick optical sections showing kisspeptin- (green) and IRβ-immunoreactive (red) neurons and fibers in the arcuate nucleus (ARC) of control (C, top panel) and prenatal T (T, bottom panel) ewes. Examples of dual-labeled kisspeptin/IRβ neurons (arrows) are indicated in the overlay images. In addition, an IRβ-positive terminal bouton (arrowhead) is seen in direct contact with a kisspeptin cell body in the lower panel. Scale bars = 10µm.
Figure 3.
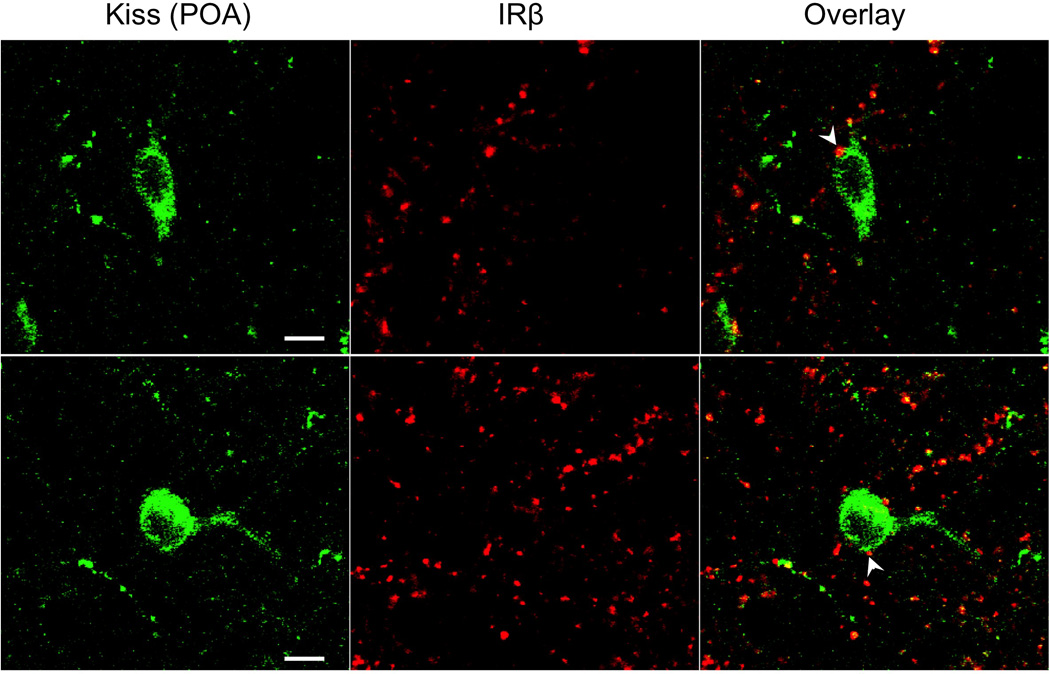
Confocal images showing the absence of IRβ (red) colocalization in kisspeptin neurons (green) of the preoptic area (POA) in control ewes. Despite the lack of cellular colocalization, IRβ-positive boutons were frequently seen in association with kisspeptin cell bodies (e.g., arrowheads). Scale bars = 10µm.
Figure 4.
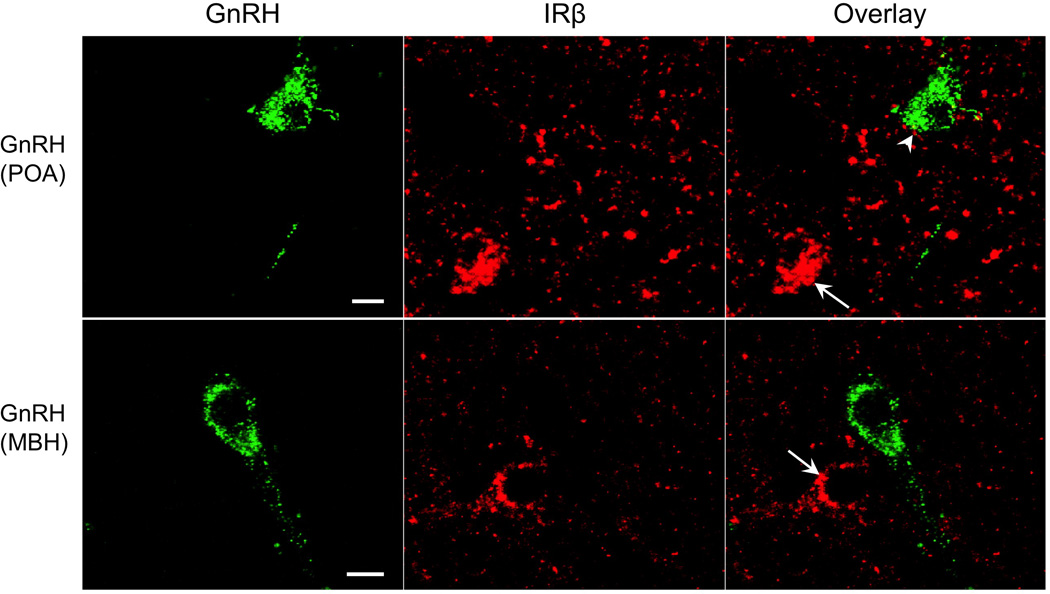
Confocal images showing examples of GnRH neurons in the POA (top panel) and the mediobasal hypothalamus (MBH, bottom panel) of control ewes, and the absence of IRβ (red) colocalization with these neurons despite the presence of adjacent IRβ-positive neurons (arrows). Arrowhead indicates an example of an IRβ-positive bouton in contact with a POA GnRH neuron. Scale bar = 10µm.
Effects of prenatal T treatment on total number of AgRP, POMC and arcuate kisspeptin neurons, and their colocalization with IRβ
Prenatal T treatment had no effect on either the number of POMC (F(3,18):3.16, p=0.833) or arcuate kisspeptin (KNDy) neurons (Fig. 5; F(3,16):3.24, p=0.141). Although there was a trend toward increased numbers of AgRP neurons in prenatal T animals compared to control ewes, this difference did not reach significance (F(3,19):2.65, p=0.077). Analysis of effect size showed a large effect of prenatal T on the total numbers of AgRP neurons (d=1.373) but not on POMC (d=0.062) or KNDy (d=0.289) cells. Co-treatment with the anti-androgen, Flutamide, did not significantly alter the number of AgRP, POMC or KNDy neurons compared to those numbers seen in control ewes (Fig. 5; p=0.077, p=0.833, and p=0.141, respectively). Although AgRP and KNDy neuron numbers tended to be higher and lower, respectively, in ewes receiving anti-androgen treatment alone, these differences did not reach statistical significance (F(3,19):2.65, p=0.077; F(3,16):2.11, p=0.141).
Figure 5.
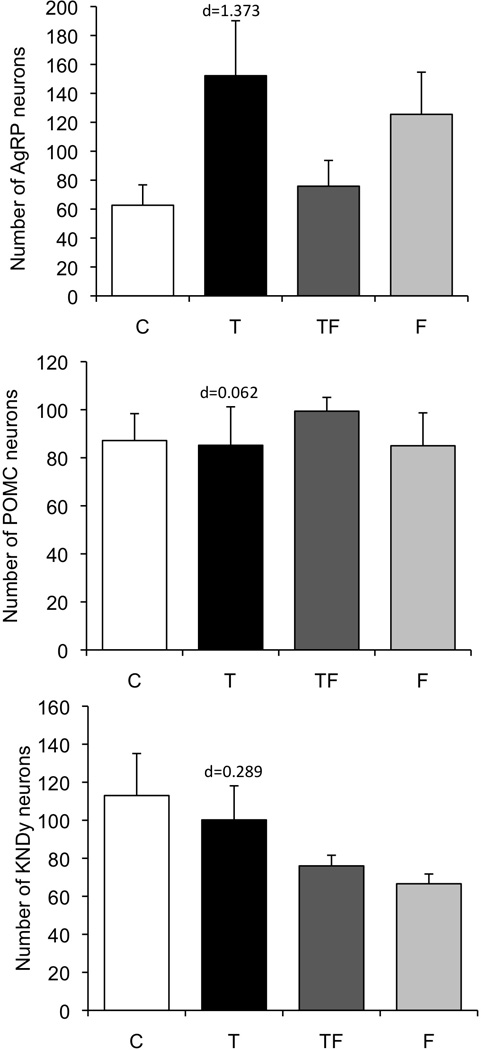
Mean ± SEM total number of AgRP (top), POMC (middle), and kisspeptin (KNDy, bottom) neurons in the arcuate nucleus of control (n=5), prenatal T (n=5), prenatal TF (n=5), and prenatal F (n=5) ewes. Although the total numbers of cells did not differ significantly across groups, size effect analysis (Cohen’s d value) indicates a large difference between control and T groups in number of AgRP but not POMC or KNDy cells.
Our analysis of a similar-sized subsets of AgRP, POMC, and KNDy neurons revealed that prenatal T treatment significantly decreased the percentage of IRβ colocalization within AgRP neurons (Fig. 6, top; F(3,22):4.35, p=0.003), but not within POMC neurons (Fig. 6, middle). Co-treatment with the anti-androgen, Flutamide, blocked this effect of prenatal T, with prenatal TF-treated females showing a similar percentage of IRβ colocalization with AgRP neurons as controls (85% vs 83%) and a significantly greater percentage of colocalization than prenatal T females (83% vs 73%, p<0.05). Anti-androgen treatment alone had no effect on the percentage of IRβ colocalization in either AgRP or POMC neurons, relative to controls (Fig. 6, top and middle panels; 85% vs 83% and 92% vs 94%, respectively, ns).
Figure 6.
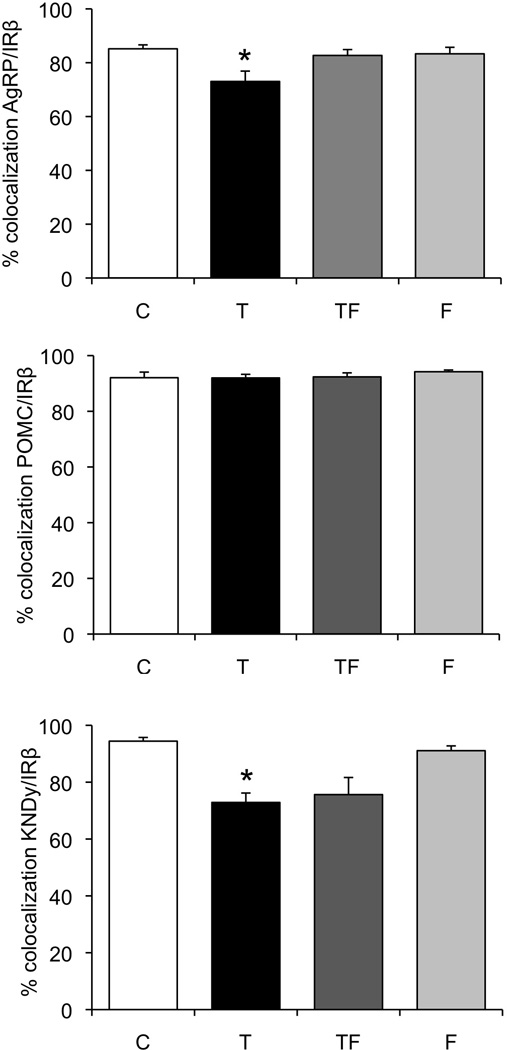
Mean ± SEM percentage colocalization of AgRP (top), POMC (middle), and kisspeptin (KNDy, bottom) neurons with IRβ in the arcuate nucleus of control (n=5), prenatal T (n=5), prenatal TF (n=5), and prenatal F (n=5) ewes. * indicates significant difference (p<0.05) from controls.
Similar to the effects seen in AgRP neurons, prenatal T treatment significantly decreased the percentage of IRβ colocalization in ARC kisspeptin neurons (F(3,19):9.07, p=0.001; Fig. 6, bottom). Unlike the effects of prenatal T on IRβ /AgRP colocalization, co-treatment with anti-androgen did not reverse the decrease in the percentage of IRβ colocalization within ARC kisspeptin neurons in prenatal T ewes (Fig. 6, bottom panel; 73% vs 76%; ns). Females treated prenatally with anti-androgen alone showed a similar percentage of IRβ/kisspeptin colocalization as control females (94% vs 91%, ns).
DISCUSSION
This study is the first to provide evidence of changes in brain insulin receptors in the prenatally androgenized sheep model of PCOS (Dumesic et al., 2007). Since one of the hallmarks of this model is insulin resistance (Padmanabhan et al., 2010b), changes in insulin receptor at the level of the hypothalamus, and within identified neuron groups important for metabolism (AgRP) and reproduction (KNDy), may play a key role in long-term changes in insulin sensitivity observed in these animals. In our previous study (Sheppard et al., 2011), we found that prenatal T treatment increased the number of AgRP neurons, but not POMC neurons, in the middle portion of the ARC. In the current study, the difference between control and prenatal T animals in number of AgRP cells failed to reach significance, although effect size analysis confirmed a large difference between control and prenatal T animals in the number of AgRP but not POMC neurons. In light of this, for the co-localization analysis, we examined a similar sample size of neurons across groups. We demonstrated that prenatal T treatment decreased the percentage of colocalization of IRβ in AgRP, but not in POMC neurons, suggesting that these changes are specific to the AgRP population. Since AgRP expression is inhibited by insulin (Qu, 2001; Breen et al., 2005), decreased colocalization of insulin receptors in AgRP but not POMC neurons could potentially provide an explanation for the increase in AgRP but not POMC peptide suggested by our previous work (Sheppard et al., 2011). Finally, we found that co-treatment with the androgen antagonist, Flutamide, blocked the effects of prenatal T on IRβ colocalization within AgRP, but not KNDy neurons, indicating that the changes in these two populations may be organized by different actions of prenatal T, androgenic and estrogenic, respectively.
Effects of prenatal T treatment on IRβ colocalization in AgRP and POMC neurons
Prenatal T treatment significantly decreased the percentage of AgRP, but not POMC, neurons colocalizing IRβ in the ARC compared with controls. The AgRP-specific decrease in IRβ is consistent with our previous results showing increased numbers of AgRP-ir neurons, but not POMC-ir neurons in prenatal T-treated females (Sheppard et al., 2011). As noted above, since insulin decreases AgRP mRNA levels (Qu, 2001) and peptide release (Breen et al., 2005) in normal females, the decreased IRβ colocalization within AgRP neurons may be partly responsible for the increased AgRP cell number seen in prenatal T females (Sheppard et al., 2011). In turn, both reduced IRβ colocalization in AgRP neurons, and increased AgRP peptide expression, may be contributing factors to the metabolic and reproductive deficits seen in these animals. Previous studies have found that prenatal T-treated females are insulin resistant and develop compensatory hyperinsulinemia as adults (Recabarren et al., 2005; Padmanabhan et al., 2010b). However, in the present study, we did not correlate metabolic defects with the observed neuroanatomical changes, and evidence for correlations between dysregulated insulin signaling and receptor colocalization in the same animals should be explored as a next step. In addition, neither POMC or AgRP-specific IR knockout mice show major defects in body weight or fertility, although control of hepatic glucose production is impaired in these mice (Könner, 2007). Nevertheless, the changes observed in our study represent only a portion of the disruptions caused by prenatal T exposure, and therefore, when combined with additional alterations may result in a more severe phenotype than previously reported. Although IR in AgRP neurons are not essential for adult fertility (Könner, 2007), this neuronal subpopulation may still play an important role relaying the influence of metabolic cues to the reproductive neuroendocrine system. AgRP neurons project directly to GnRH neurons in the rat (Li et al., 1999) and inhibit reproduction by suppressing pulsatile LH release in the rhesus monkey (Vulliémoz et al., 2005). Similarly, neuropeptide Y (NPY), an orexigenic neuropeptide, which is largely colocalized with AgRP (Hahn, 1998; Sheppard et al., 2011), inhibits reproduction by hindering GnRH neuronal firing in mice (Sullivan & Moenter, 2004; Klenke, 2010) and tonic secretion of LH in the sheep (McShane et al., 1993; Adam et al., 1997). We do not know whether NPY peptide, like AgRP, is increased in prenatal T sheep, but it is conceivable that increased AgRP and/or NPY peptide and its release may be a means by which this population could potentially inhibit GnRH release in prenatal T-exposed females, and contribute to some of the observed defects including a decrease in the magnitude of the LH surge (see below).
Prenatal TF treatment successfully rescued the effects of T on IRβ colocalization with AgRP neurons, suggesting that the decrease in IRβ in this subpopulation is prenatally organized by the androgenic actions of T. These effects of T parallel organizational effects of prenatal T on AgRP expression (Sheppard et al., 2011), albeit more completely. We previously showed that co-treatment of T with androgen antagonist (TF) was able to largely, but not completely, rescue the effects of prenatal T on AgRP neuron numbers (Sheppard et al., 2011). Moreover, the effects of prenatal T on insulin resistance and AgRP neuron numbers are mimicked by prenatal dihydrotestosterone (DHT, a non-aromatizable androgen) treatment (Padmanabhan et al., 2010b; Sheppard et al., 2011), further implicating an androgenic programming role of T in this population. However, prenatal T-induced hyperinsulinemia and insulin resistance were not reversed by Flutamide co-treatment (TF) in a recent study (Table 1; (Cardoso et al., 2016), suggesting that the change in IR colocalization in AGRP neurons is not sufficient by itself to account for the metabolic alterations seen in this model. A caveat to this conclusion is that the metabolic changes in aforementioned study (Cardoso et al., 2016) were observed in juvenile animals; hence the decreased IRβ colocalization in AgRP neurons may still be a contributing factor for hyperinsulinemia and insulin resistance in adult prenatal T ewes.
Table 1.
Summary of results from prior studies on the ability of prenatal co-treatment with anti-androgen (Flutamide) to reverse metabolic and reproductive defects produced by prenatal testosterone (T) treatment
| Defect/changes produced by prenatal T treatment |
Reversal by Flutamide co- treatment (TF)? |
Reference |
|---|---|---|
| Metabolic | ||
| Hyperinsulinemia (juvenile) | No | (Cardoso et al., 2016) |
| Insulin resistance (juvenile) | No | |
| Reproductive | ||
| Decreased LH surge amplitude | No/Yes | (Abi-Salloum et al., 2012); (Padmanabhan et al., 2015) |
| Delayed LH surge timing | No | |
| Advanced puberty | Yess | (Jackson et al., 2008); (Padmanabhan et al., 2015) |
Effects of prenatal T treatment of IRβ colocalization in ARC kisspeptin (KNDy) neurons
We found that approximately 94% of KNDy neurons colocalize IRβ and that prenatal T treatment results in a significant decrease in IRβ colocalization in these neurons. Reproductive deficits in prenatal T-treated females include increased LH pulsatility and a delayed and dampened LH surge culminating in ablation of ovulatory cycles (Robinson et al., 1999; Sharma et al., 2002; Sarma et al., 2005; Unsworth et al., 2005; Veiga-Lopez et al., 2009). As noted earlier, postnatal insulin sensitizer treatment improves reproductive function in prenatal T-treated females (Veiga-Lopez et al., 2010). Moreover, insulin has been shown to stimulate GnRH and LH secretion in vivo (Moret, 2009) and in vitro (Burcelin et al., 2003), an effect which is mediated at the level of the hypothalamus and not the pituitary. However, because GnRH neurons in sheep do not appear to contain IRβ, the influence of insulin upon the reproductive axis in this species is likely relayed to GnRH neurons via afferent neurons expressing this receptor.
KNDy neurons represent one such potential relay. KNDy neurons have been strongly implicated in the control of pulsatile GnRH secretion (Wakabayashi, 2010; Okamura, 2013) and, in sheep, also appear to be activated during the preovulatory GnRH surge (Merkley, 2012). A role for insulin in facilitation of the GnRH/LH surge has been widely described (Bruning, 2000; Steckler, 2009; Veiga-Lopez et al., 2010) and recent work in prenatal T-treated sheep showed that postnatal insulin sensitizer treatment increased the magnitude of the LH surge (Abi-Salloum et al., 2012). Furthermore, ovine KNDy neurons are activated by leptin treatment (Backholer et al., 2010) albeit indirectly (Louis et al., 2011), and colocalize IRβ (this study) rendering this subpopulation responsive to metabolic signals (Fu & van den Pol, 2010). Finally, there is an array of substantial evidence in rodents that KNDy neurons play an important role in mediating metabolic control of reproduction (Castellano et al., 2005; Castellano, 2006; Castellano et al., 2009; Castellano et al., 2010; Pinilla et al., 2012). However, recent studies of female mice with kisspeptin-specific deletion of phospholipid enzyme phosphoinositide 3-kinase (PI3K), a major signaling target for insulin, show that normal pubertal development, LH levels, and estrous cyclicity is unaffected in these animals ((Beymer, 2012) suggesting that insulin signaling via kisspeptin neurons (including KNDy neurons) may not be essential by itself for female adult reproductive function. In addition, mice with insulin receptors conditionally deleted from either GABA-, glutamate- or CamKII alpha-expressing neurons are completely fertile (Evans et al., 2014b). Thus the influence of insulin upon GnRH secretion is most probably not conveyed by any single neuronal population, and likely requires a larger aggregate of neurons and their projections that collectively funnel this input to the GnRH neurosecretory unit. In the sheep, KNDy neurons may participate in conveying the influence of insulin upon GnRH secretion in concert with other neurons, since they have direct contacts with AgRP and POMC neurons (Backholer et al., 2010), and, in turn, both AgRP/NPY and POMC neurons send projections that synapse on GnRH neurons (Norgren & Lehman, 1989; Jansen et al., 2003). Finally, it should be noted that insulin can act at either hypothalamic, pituitary, or ovarian levels to regulate follicular development (Adashi, 1981; Poretsky & Kalin, 1987). Thus, alterations in IRβ expression in KNDy neurons, together with changes in receptor expression in other neurons and/or levels of the reproductive axis, may ultimately contribute to the defects in reproductive function seen in this model.
In contrast to IRβ colocalization in AgRP neurons, co-treatment with androgen antagonist did not rescue the effects of prenatal T on IRβ colocalization in KNDy neurons, suggesting that the decrease in IRβ in this subpopulation is organized by the estrogenic actions of T. It is unlikely that the inability of anti-androgen (Flutamide) co-treatment to reverse this effect was due to an insufficient dose since the same co-treatment was able to completely reverse the decrease in IR/AgRP colocalization. Moreover, this dose has been previously shown to block the effect of prenatal T on AgRP neuron number (Sheppard et al., 2011) and reverse the external masculinization seen in this model (Jackson et al., 2008). An alternate possibility is that the changes in IRβ in KNDy neurons are programmed by androgenic effects of T via other intermediaries, for example those leading to an increase in gestational insulin levels (Abi-Salloum et al., 2012). However, recent findings that prenatal co-treatment with insulin sensitizer fails to rescue the effects of prenatal T treatment on GnRH/LH surge (Abi-Salloum et al., 2012) argue against this possibility. Nonetheless, increased insulin levels during the period of fetal exposure to excess T, when insulin levels in the mother are also increased (Abi-Salloum et al., 2012), might intensify the degree of disturbance in this model.
Previous studies of the ability of anti-androgen co-treatment to ameliorate reproductive defects produced by prenatal T treatment have suggested that some but not all of these defects are due to androgenic actions (Table 1). Specifically, previous results showed that Flutamide co-treatment reversed the advanced puberty seen in prenatal T ewes (Jackson et al., 2008; Padmanabhan et al., 2015) but not the delayed timing of the LH surge (Abi-Salloum et al., 2012; Padmanabhan et al., 2015). Data regarding the ability of androgen antagonist co-treatment to reverse decreased peak amplitude of the surge is equivocal with an earlier study testing positive feedback in ovary-intact animals showing increased magnitude of the LH surge in prenatal T ewes receiving Flutamide co-treatment (Abi-Salloum et al., 2012) but a more recent study characterizing hormonal dynamics during natural follicular phase finding no effect of prenatal androgen antagonist treatment on LH surge amplitude in prenatal T sheep (Padmanabhan et al., 2015). Preliminary data from the current cohort of animals studied during the artificial follicular phase is similar to the positive feedback results (Abi-Salloum et al., 2012), with prenatal T ewes showing a significant decrease in peak LH surge amplitude compared to controls that is reversed in the TF group (unpublished observations). Thus the decreased IRβ colocalization in KNDy neurons seen in prenatal T ewes is unlikely by itself to be responsible for the decreased LH surge amplitude, although they could still contribute to this defect.
Absence of IRβ localization in GnRH neurons and POA kisspeptin neurons in the ewe
In contrast to AgRP, POMC and KNDy neurons, we found no evidence of colocalization of IRβ in either GnRH neurons or POA kisspeptin neurons of the brains of control ewes. The absence of IRβ colocalization with GnRH neurons suggests that metabolic signals controlling reproductive status in sheep are relayed via an upstream population of neurons. Although insulin receptors have been previously detected in immortalized GnRH cell lines (Kim et al., 2005) and insulin receptor mRNA and protein have been recently shown in GnRH neurons in mice (Evans et al., 2014a), insulin-induced phospho-Akt or phospho-extracellular-signal-regulated kinase (pERK) ½ was not observed in these GnRH neurons (Evans et al., 2014a), suggesting that insulin may exert its effects via upstream neuronal populations. In addition, only 5% (Evans et al., 2014a) to 22% (Qiu, 2013) of murine kisspeptin neurons colocalize IRβ, compared to 94% of ARC kisspeptin neurons in the sheep, suggesting that the mechanism of insulin action in the sheep brain, and particularly its effects on GnRH, differs from that in the mouse. As noted above, KNDy neurons as well as AgRP neurons represent possible candidates for conveying insulin signals to GnRH neurons, since both populations of neurons colocalize IRβ, have direct projections to GnRH neurons (Li et al., 1999; Lehman et al., 2010; Roa & Herbison, 2012), and have been shown to regulate GnRH activity (Sullivan & Moenter, 2004; Vulliémoz et al., 2005; Caraty et al., 2007; Klenke, 2010; Lehman et al., 2010). While we found no colocalization of IRβ in POA kisspeptin neurons, we cannot exclude the possibility that this subset of kisspeptin neurons may participate in these actions via inputs from other insulin-sensitive neurons. Finally, although recent work suggests that insulin receptors in KNDy and POA kisspeptin neurons in mice may not be essential for adult reproduction (Qiu et al., 2013), these neurons may play redundant roles with other populations, including AgRP neurons, in this function.
IRβ localization in axon terminals: an additional site of insulin action on the reproductive neuroendocrine system?
In addition to IRβ colocalization in arcuate neurons (KNDy, AgRP, and POMC), we also observed frequent instances of IRβ-positive boutons that were in direct contact with kisspeptin neurons in both the ARC (Fig. 2) and POA (Fig. 3), as well as in contact with GnRH cell bodies (Fig. 4). Earlier studies in rodents have shown insulin receptors, detected by IRβ immunoreactivity, are present in synapses of cultured hippocampal neurons (Abbott et al., 1999) as well as in axon terminal regions of the hippocampal formation (Park et al., 2009), and that insulin can act presynaptically to induce phosphorylation of insulin receptor substrate-1 and Akt (Heras-Sandoval et al., 2012). More relevant to the current findings is older work showing that insulin binding sites in the rat are present within the external zone of the median eminence (van Houten et al., 1980) and that these binding sites are dependent on projections arising from the ARC (van Houten et al., 1983). While we did not specifically examine the sheep median eminence for IRβ immunoreactivity, KNDy neurons project to the median eminence where they may modulate GnRH secretion via synapses onto GnRH dendrons (Iremonger & Herbison, 2015) and axo-axonic contacts with GnRH terminals (Lehman et al., 2013). Therefore, we would speculate that contacts between IRβ-containing axon terminals and kisspeptin or GnRH neurons may represent an additional presynaptic site of action for the effects of insulin on control of GnRH secretion. While confirmation of the IRβ-positive close contacts we observed as bona fide synapses awaits co-localization with markers such as synaptophysin (Cernea et al., 2015), their appearance as strings of beaded varicosities (e.g., Fig. 3, middle panel) are consistent with that of peptidergic axons and suggest that at least some of the boutons are presynaptic in nature.
Conclusions
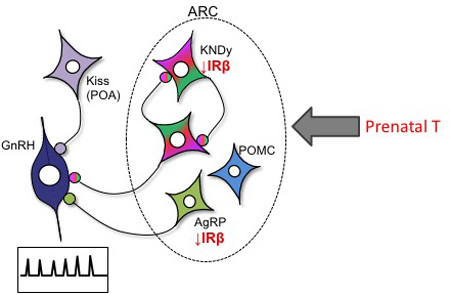
A summary of our current findings of changes in insulin receptors and metabolic peptides as a consequence of prenatal exposure to T in female sheep, and their relationship to the reproductive neuroendocrine system, is shown in Figure 7. KNDy neurons have been posited as a major intermediary, conveying metabolic signals to GnRH neurons (Castellano et al., 2005; Castellano, 2006; Castellano et al., 2009; Castellano et al., 2010; Pinilla et al., 2012); although direct projections of AgRP and POMC neurons to GnRH neurons are also present (Leranth, 1988; Li et al., 1999; Rondini, 2004; Roa & Herbison, 2012). KNDy neurons form a reciprocally interconnected neuronal population whose activity is hypothesized to play a key role in regulating GnRH pulsatile secretion, as well as participate in the control of the GnRH surge in sheep and primates (Lehman et al., 2010). Prenatal T exposure leads to decreased IRβ in KNDy neurons, and also decreased expression of two of the three KNDy peptides, neurokinin B and dynorphin (not shown). In addition, prenatal T exposure results in decreased in IRβ in AgRP but not POMC neurons, and our previous findings suggest a corresponding increase in AgRP but not POMC peptide in these populations. The precise functional impact of each of these changes on the control of GnRH secretion in the adult, specifically with respect to the defects in steroid feedback responsiveness seen in this animal model (Robinson et al., 1999; Sharma et al., 2002; Sarma, 2005; Unsworth et al., 2005; Veiga-Lopez et al., 2009), remains to be determined; however, a first step will be to determine whether pre- or postnatal treatments with insulin sensitizers, such as Rosiglitazone, can reverse any of these receptor/peptide changes. Ultimately, the challenge will be to understand the early developmental cascade of events initiated by exposure to excess T that result in adult reproductive and metabolic dysfunction, and to determine which of the long-term hypothalamic alterations seen in the adult offspring may contribute to functional deficits. Our results reveal the programming effects of prenatal T on insulin receptor levels in identified, subsets of neurons in the sheep brain, and raises the possibility that decreased insulin receptors, specifically within KNDy and AgRP neurons, may contribute in part to the metabolic disruptions seen in the PCOS-like phenotype of this animal model.
Figure 7.
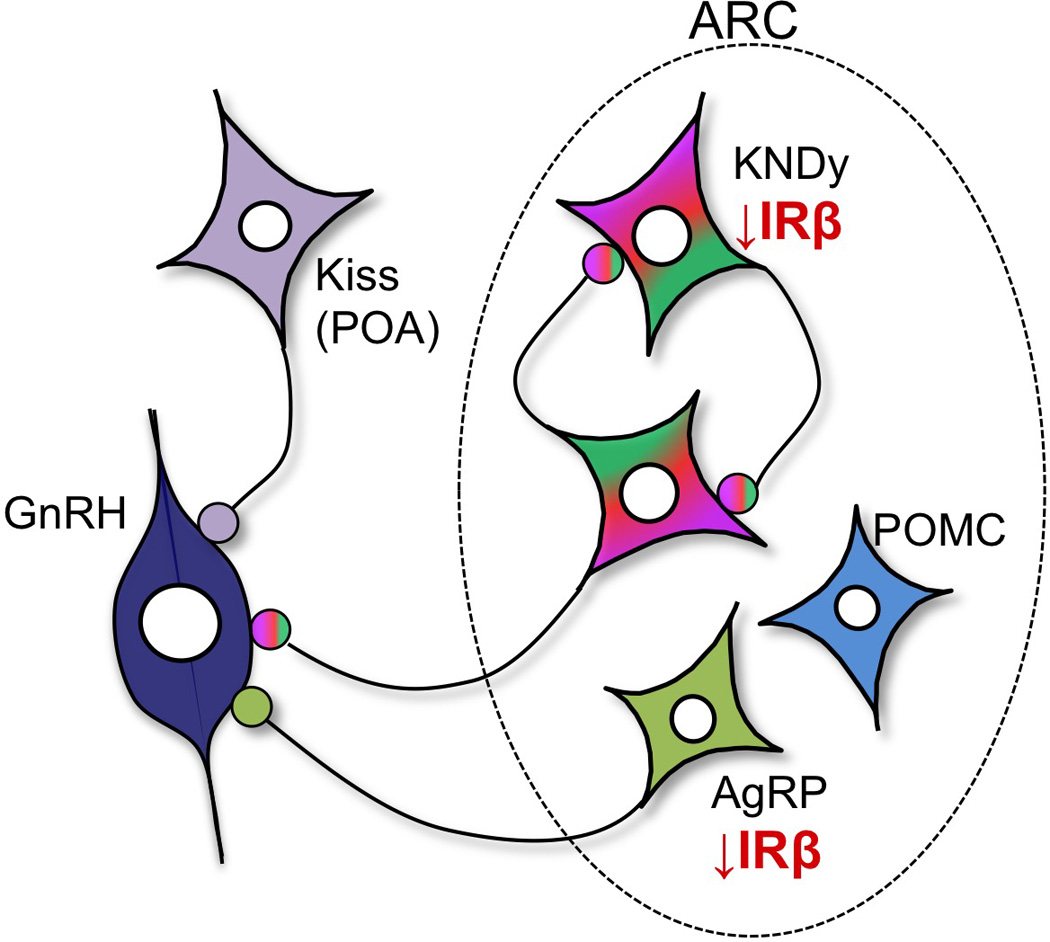
Schematic diagram illustrating the long-term consequences of prenatal T treatment on insulin receptor beta (IRβ) colocalization within AgRP, POMC and KNDy neurons in the female sheep hypothalamus. Decreased IRβ in AgRP neurons may, in part, underlie the increase in AgRP peptide previously observed in prenatal T ewes (Sheppard et al., 2013). Moreover, decreased IRβ colocalization within AgRP and KNDy neurons may result in decreased insulin sensitivity in these neurons and altered steroid feedback control of GnRH secretion, thereby contributing to the metabolic and reproductive disruptions seen in the PCOS-like phenotype of this animal model.
Supplementary Material
Acknowledgments
We thank Mr. Douglas Doop for assistance with breeding / lambing, his expert and conscientious animal care, and Sheep Facility management. This research was supported by NIH P01 HD044232 to V.P., M.N.L., and L.C.
Footnotes
The authors have no no conflicts of interest to declare.
REFERENCES
- Abbott DH, Bruns CM, Barnett DK, Tarantal AF, Hoffmann SM, Zhou R, Levine JE, Dumesic DA. Fetal origins of the polycystic ovarian syndrome. In: Dunaif A, Chang RJ, Franks S, Legro RS, editors. Polycystic ovarian syndrome. 1st. 2008. pp. 87–106. [Google Scholar]
- Abi-Salloum B, Herkimer C, Lee JS, Veiga-Lopez A, Padmanabhan V. Developmental Programming: Prenatal and Postnatal Contribution of Androgens and Insulin in the Reprogramming of Estradiol Positive Feedback Disruptions in Prenatal Testosterone-Treated Sheep. Endocrinology. 2012;153:2813–2822. doi: 10.1210/en.2011-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam CL, Findlay PA, Kyle CE, Young P, Mercer JG. Effect of chronic food restriction on pulsatile luteinizing hormone secretion and hypothalamic neuropeptide Y gene expression in castrate male sheep. Journal of Endocrinology. 1997;152:329–337. doi: 10.1677/joe.0.1520329. [DOI] [PubMed] [Google Scholar]
- Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin Cells in the Ewe Brain Respond to Leptin and Communicate with Neuropeptide Y and Proopiomelanocortin Cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82:893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beymer M, Aziz R, Mayer C, Fukuda M, Lin RZ, Boehm U, Acosta-Martinez M. The Effects of a Kisspeptin-Cell Specific Deletion of PI3K Catalytic Subunits p110α and p110β on the Hypothalamic-Pituitary-Gonadal Axis. Endocr Rev. 2012;33 SUN-701. [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Breen TL, Conwell IM, Wardlaw SL. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain research. 2005;1032:141–148. doi: 10.1016/j.brainres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-Releasing Hormone Secretion from Hypothalamic Neurons: Stimulation by Insulin and Potentiation by Leptin. Endocrinology. 2003;144:4484–4491. doi: 10.1210/en.2003-0457. [DOI] [PubMed] [Google Scholar]
- Burks DJ, de Mora JF, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- Cardoso RC, Veiga-Lopez A, Moeller J, Beckett E, Pease A, Keller E, Madrigal V, Chazenbalk G, Dumesic D, Padmanabhan V. Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Adiposity and Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep. Endocrinology. 2016;157:522–535. doi: 10.1210/en.2015-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: Bridging energy homeostasis and reproduction. Brain research. 2010;1364:129–138. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Roa J, Pineda R, Sanchez-Garrido MA, Garcia-Galiano D, Vigo E, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Alterations in hypothalamic KiSS-1 system in experimental diabetes: early changes and functional consequences. Endocrinology. 2009;150:784–794. doi: 10.1210/en.2008-0849. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernández-Fernández R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J Endocrinol. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Reviews in endocrine & metabolic disorders. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MC, Rizwan M, Mayer C, Boehm U, Anderson GM. Evidence that Insulin Signalling in Gonadotrophin-Releasing Hormone and Kisspeptin Neurones does not Play an Essential Role in Metabolic Regulation of Fertility in Mice. Journal of Neuroendocrinology. 2014a;26:468–479. doi: 10.1111/jne.12166. [DOI] [PubMed] [Google Scholar]
- Evans MC, Rizwan MZ, Anderson GM. Insulin Action on GABA Neurons Is a Critical Regulator of Energy Balance But Not Fertility in Mice. Endocrinology. 2014b;155:4368–4379. doi: 10.1210/en.2014-1412. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Holte J. Disturbances in insulin secretion and sensitivity in women with the polycystic ovary syndrome. Baillieres Clin Endocrinol Metab. 1996;10:221–247. doi: 10.1016/s0950-351x(96)80085-1. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual Differentiation of the External Genitalia and the Timing of Puberty in the Presence of an Antiandrogen in Sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, DiVall SA, Deneau RM, Wolfe A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Molecular and cellular endocrinology. 2005;242:42–49. doi: 10.1016/j.mce.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons viaY1receptors. Endocrinology. 2010;151:2736–2746. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, Dodson WC. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402. doi: 10.1016/j.ajog.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette DA. An improved mounting medium for immunofluorescence microscopy. Am J Clin Pathol. 1978;69:647–648. doi: 10.1093/ajcp/69.6.647. [DOI] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Morphological Evidence for Direct Interaction between Arcuate Nucleus Neuropeptide Y (NPY) Neurons and Gonadotropin-Releasing Hormone Neurons and the Possible Involvement of NPY Y1 Receptors. Endocrinology. 1999;140:5382–5390. doi: 10.1210/endo.140.11.7093. [DOI] [PubMed] [Google Scholar]
- Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG. Molecular Mapping of the Neural Pathways Linking Leptin to the Neuroendocrine Reproductive Axis. Endocrinology. 2011;152:2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- McShane TM, Petersen SL, McCrone S, Keisler DH. Influence of food restriction on neuropeptide-Y, proopiomelanocortin, and luteinizing hormone-releasing hormone gene expression in sheep hypothalami. Biology of reproduction. 1993;49:831–839. doi: 10.1095/biolreprod49.4.831. [DOI] [PubMed] [Google Scholar]
- Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (Kisspeptin/Neurokinin B/Dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5414. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret M, Stettler R, Rodieux F, Gaillard RC, Waeber G, Wirthner D, Giusti V, Tappy L, Pralong FP. Insulin Modulation of Luteinizing Hormone Secretion in Normal Female Volunteers and Lean Polycystic Ovary Syndrome Patients. Neuroendocrinology. 2009;89:131–139. doi: 10.1159/000160911. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25:S56–S62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nakae J, Kitamura T, Park B-C, Dragatsis I, Accili D. Transgenic rescue of insulin receptor–deficient mice. The Journal of Clinical Investigation. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297–323. doi: 10.1007/978-1-4614-6199-9_14. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Molecular and cellular endocrinology. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. International journal of andrology. 2010a;33:394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Molecular and cellular endocrinology. 2013;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental Programming: Impact of Prenatal Testosterone Excess and Postnatal Weight Gain on Insulin Sensitivity Index and Transfer of Traits to Offspring of Overweight Females. Endocrinology. 2010b;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Herkimer C, Salloum BA, Moeller J, Beckett E, Sreedharan R. Developmental Programming: Prenatal and Postnatal Androgen Antagonist and Insulin Sensitizer Interventions Prevent Advancement of Puberty and Improve LH Surge Dynamics in Prenatal Testosterone-Treated Sheep. Endocrinology. 2015;156:2678–2692. doi: 10.1210/en.2015-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental Origin of Reproductive and Metabolic Dysfunctions: Androgenic Versus Estrogenic Reprogramming. Semin Reprod Med. 2011;29:173–186. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiological reviews. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Brüning JC, Elias CF, Hill JW. Delayed Puberty but Normal Fertility in Mice With Selective Deletion of Insulin Receptors From Kiss1 Cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu SY, Yang YK, Li JY, Zeng Q, Gantz I. Agouti-related protein is a mediator of diabetic hyperphagia. Regul. Pept. 2001;98:69–75. doi: 10.1016/s0167-0115(00)00230-5. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. American Journal of Physiology - Endocrinology And Metabolism. 2005;289:E801–E806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Roa J, Herbison AE. Direct Regulation of GnRH Neuron Excitability by Arcuate Nucleus POMC and NPY Neuron Neuropeptides in Female Mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone, responsiveness to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. The New England journal of medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biology of reproduction. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol. 2011;23:401–411. doi: 10.1111/j.1365-2826.2011.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity -- implication to PCOS. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. y-Aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:1194–1202. doi: 10.1210/en.2003-1374. [DOI] [PubMed] [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biology of reproduction. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biology of reproduction. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental Programming: Insulin Sensitizer Treatment Improves Reproductive Function in Prenatal Testosterone-Treated Female Sheep. Endocrinology. 2010;151:4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biology of Reproduction. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- Vulliémoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M. Central Infusion of Agouti-Related Peptide Suppresses Pulsatile Luteinizing Hormone Release in the Ovariectomized Rhesus Monkey. Endocrinology. 2005;146:784–789. doi: 10.1210/en.2004-1093. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


