Highlights
-
•
We place affect-biased attention within a developmental neuroscience framework.
-
•
We suggest that affect-biased attention serves as a domain general mechanism.
-
•
Affect-biased attention broadly shapes socioemotional trajectories across development.
-
•
We review the developmental neurocognitive mechanisms of affect-biased attention.
-
•
A proposed model helps place affect-biased attention in a developmental context.
Keywords: Affect-biased attention, Attention bias, Temperament, Emotion, Brain development, Individual differences
Abstract
There is growing interest regarding the impact of affect-biased attention on psychopathology. However, most of the research to date lacks a developmental approach. In the present review, we examine the role affect-biased attention plays in shaping socioemotional trajectories within a developmental neuroscience framework. We propose that affect-biased attention, particularly if stable and entrenched, acts as a developmental tether that helps sustain early socioemotional and behavioral profiles over time, placing some individuals on maladaptive developmental trajectories. Although most of the evidence is found in the anxiety literature, we suggest that these relations may operate across multiple domains of interest, including positive affect, externalizing behaviors, drug use, and eating behaviors. We also review the general mechanisms and neural correlates of affect-biased attention, as well as the current evidence for the co-development of attention and affect. Based on the reviewed literature, we propose a model that may help us better understand the nuances of affect-biased attention across development. The model may serve as a strong foundation for ongoing attempts to identify neurocognitive mechanisms and intervene with individuals at risk. Finally, we discuss open issues for future research that may help bridge existing gaps in the literature.
1. Introduction
Attention mechanisms play an early and pervasive role in shaping behavior. Historically, much of the literature has focused on cognitive or “cool” components of attention development and functioning. Thus we have a strong literature base examining, for example, the impact of attention on learning and memory (e.g., Amso and Scerif, 2015). Recently, there has been more direct examination of the role attention may play in eliciting and supporting broad profiles of socioemotional functioning. As will be noted below, a rapidly growing literature suggests that attention bias to threat may play a causal role in the emergence of anxiety and non-clinical social withdrawal. Indeed, laboratory manipulations using attention bias modification (ABM; explained in Fig. 1) appear to impact even entrenched patterns of anxious thought and behavior (Eldar et al., 2008, Hakamata et al., 2010). While this literature has garnered a great deal of recent interest, it represents only a small portion of the complex relations across time and levels of analysis between attention and socioemotional behavior. Given the pervasiveness of attention as a cognitive mechanism, the distributed neural networks supporting attention, and the early emergence of individual differences in attention in infancy and childhood, we suggest that attention plays a broad and sustained role in socioemotional development.
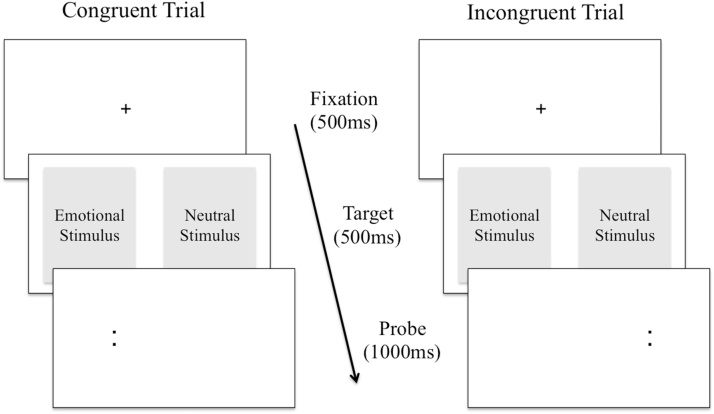
Fig. 1.
An illustration of the dot-probe task. In the dot-probe task, participants see a pair of stimuli simultaneously, one emotionally salient (e.g., threatening) and one neutral (e.g., non-threatening), most often for 500 ms. A probe replaces one of the two stimuli. The individual is required to respond as accurately and as quickly as possible to the probe. An attentional bias towards emotional stimuli is inferred when participants preferentially attend to emotional cues, resulting in decreased reaction times to probes replacing the emotional stimuli compared to the neutral stimuli. A direct extension of the dot-probe task has been attention bias modification (ABM), which is used to reduce affect-biased attention. The training procedure only uses incongruent trials. The logic is that by having the probe replace the emotional or neutral stimuli in all the trials, the individual implicitly learns to attend towards the neutral stimuli/away from the emotional stimuli.
Affect-biased attention, as used by Todd et al. (2012), refers to “attentional biases that cause preferential perception of [any] particular category of stimulus based on its relative affective salience” (p. 365). In this review, we propose a developmental model of affect-biased attention, in which individual traits and characteristics help shape the specific components of the environment that are deemed salient. At the extreme, salience may track constructs highlighted by condition-specific psychiatric concerns (e.g., food in eating disorders or spiders in arachnophobia). Salience may also track developmental concerns, as seen in normative data indicating an attention bias to negative facial stimuli in infancy. In addition, environmental experience can help define salience. For example, children exposed to violence or maltreatment are especially sensitive to anger cues. As a rough analogy, one can point to language mechanisms that are both experience expectant and experience dependent (Greenough et al., 1987, Werker and Tees, 1992). In our model, affect-biased attention acts as a general mechanism that highlights cues that reflect past history and are relevant to concurrent motivational states, guiding the individual to meet his/her goals. In this way, a single processing mechanism may be responsible for both positive and negative attentional biases.
In this model, we also suggest that affect-biased attention influences cognitive and emotional development from infancy. For example, preferential attention allocation toward emotionally salient objects emerges early in development, likely due to specific perceptual markers (e.g., the curvilinear body of a snake; LoBue et al., 2010). In the competition for limited attentional resources, infants prioritize objects that provide information about danger and reward (Peltola et al., 2008). No other object is as closely tied to survival, punishment, and reward as the human face (Hoehl and Striano, 2010). Due to the coupling of perceptual cues, rewarding daily events (e.g., feeding), and long hours of exposure, infants quickly begin to show preferential looking to human faces (Leppänen and Nelson, 2009). This preference is magnified when the face also conveys an emotional threat signal. Thus, this particular example of affect-biased attention is early appearing, likely rooted in evolutionary concerns, and has the potential to influence broad patterns of socioemotional behavior throughout life.
Expanding from this early foundation, our proposed model places the concept of affect-biased attention into a developmental framework. More specifically, it suggests that affect-biased attention, particularly if stable and entrenched, helps sustain early socioemotional and behavioral profiles over time, even in the face of internal and external forces that typically act to ameliorate early extreme tendencies. In order to account for normative developmental variations as well as for individual differences in attentional patterns, our model argues that affect-biased attention builds on the development of different attentional components proposed by the cognitive literature (Posner, 2012) and draws in the specific traits and characteristics of the individual. We use this model to make the following predictions: (I) Affect-biased attention is not a single construct; rather it emerges from the interaction of multiple attentional systems; (II) Affect-biased attention develops and its role in socioemotional functioning changes due to maturation and experience; (III) Affect-biased attention acts as a domain-general mechanism. This prediction suggests that affect-biased attention is not limited to attention bias towards threat and internalizing disorders, but that these relations may hold across multiple domains of interest (e.g., positive affect, externalizing behaviors, drug use, and eating behaviors); (IV) The relation between affect-biased attention and socioemotional functioning is reciprocal rather than unidirectional. In the following sections of the review, we examine the existing data that support each of these predictions. Finally, we discuss issues for future research that may help bridge existing gaps in the literature.
2. What are the basic attention mechanisms behind attentional bias?
Attention is not a single construct, but rather constitutes a series of mechanisms and processes that are both distinct and interrelated (Raz and Buhle, 2006). That is, while sub-components of attention are thought to play specialized roles in supporting cognition and behavior, multiple components of attention work together to support real-world behavior (Fan et al., 2009, Petersen and Posner, 2012). Perhaps one of the most prominent is Posner’s model of attention, which proposes three core systems: alerting, orienting, and executive attention. The alerting system serves to achieve and maintain a state of heightened sensitivity to incoming stimuli. It is associated with brain activity from the locus coeruleus, cingulate areas, as well as frontal and parietal areas of the cortex (Posner, 2012) and is modulated mainly by norepinephrine.
The orienting system prioritizes information from sensory inputs for further processing. This system is believed to be modulated by cholinergic activity and related to activity in the superior parietal lobe, temporal parietal junction, frontal eye fields, superior colliculus, and pulvinar (Petersen and Posner, 2012). These structures are heavily interconnected with emotion-related regions such as the amygdala. For example, the superior colliculus has projections to the amygdala via the pulvinar (Tamietto and de Gelder, 2010), supporting the claims that the amygdala mediates orienting responses (e.g., Carlson et al., 2009, Öhman and Mineka, 2001).
Finally, the executive attention system is thought to monitor and resolve conflicts between thoughts, emotions, and behavioral responses (Posner, 2012). This system is modulated by dopaminergic functioning and is mostly, but not exclusively, marked by activity in anterior regions of the brain such as the anterior cingulate cortex (ACC), anterior insula, prefrontal cortex, and basal ganglia. The executive attention system underlies the ability to suppress brain activity that is in conflict with the individual’s goals, hence its close relation to self-regulation and effortful control processes (Posner, 2012). Indeed, Posner and Rothbart have proposed that effortful control characterizes self-regulatory abilities linked to the functioning of the executive attention system (Posner and Rothbart, 2007 Rothbart et al., 2014). As such, this system allows the individual to override a prepotent response such as attending to specific stimuli (e.g., threat) and perform a subdominant response, such as disengaging attention and reorienting to other stimuli.
Research focused on attention mechanisms benefits from the fact that there is a fairly close coupling between the attention components captured by laboratory tasks and observations, and the underlying neural networks that support attention processes. However, layered onto this brain-behavior coupling are broad developmental and individual differences reflecting how, when, and where attention is deployed by an individual as he or she navigates the surrounding environment. The basic components of attention – alerting, orienting, and executive – may work together to support or implement more global patterns of affect-biased attention.
Traditionally, the alerting and orienting systems are thought to reflect reactive or exogenously-driven profiles of attention. This is largely due to the fact that the systems are early appearing developmentally and generally rooted in subcortical systems. Moreover, few studies have differentiated between individual differences in alerting versus orienting (reviewed in subsection “How Do We Capture Affect-Biased Attention?”). Given that both of these systems are exogenously driven, have a similar developmental trajectory, and the lack of studies evaluating their unique impact in socioemotional development, we will largely discuss them together, making distinctions between them only when data are available.
In contrast, much of the literature has focused on the executive system as a top-down regulator. Again, this is partially rooted in development (later appearing) and anatomy (prefrontal localization). In this model (Lonigan et al., 2004, Susa et al., 2014), effortful control works to dampen initial reactive, or ‘biased’, responses. For example, Lonigan and Vasey (2009) found that an attention bias towards threat was only present in children (9–18 year olds) with fearful temperament and low effortful control. Fearful children high in effortful control did not display an attention bias towards threat (Lonigan and Vasey, 2009). However, recent work (Henderson et al., 2015) suggests an alternative dual process model – such that for fearful children, the executive attention system may actually potentiate the child’s fear and wariness, rather than down-regulate them, creating a positive feedback loop. This parallels the model from Todd et al. (2012) suggesting that initial reactive tendencies may become practiced and canalized, coming to act in a preemptory, regulatory manner with the support of higher-order systems.
3. The emergence of affect-biased attention
It is unlikely that newborns display stable patterns of affect-biased attention. Given infants’ limited behavioral repertoires, attempts to capture early patterns of attention rely heavily on observations of gaze behavior, supplemented by non-invasive psychophysiological measures, such as event-related potentials (ERPs; Leppänen and Nelson, 2009). Infants are able to discriminate among emotional stimuli such as emotional facial expressions at 5–7 months of age (Nelson, 1987). The preference for specific basic emotions seems evident soon thereafter. For example, 8- to 14-month-olds orient faster towards angry faces over happy faces when presented side-by-side (LoBue and DeLoache, 2010). Similarly, 7-month-olds look longer at fearful facial expressions than happy ones (Nelson and Dolgin, 1985). Additionally, 7-month-olds are less likely to disengage from a fearful face than a happy or neutral facial expression when presented with a distractor (Peltola et al., 2008). This normative bias seems to predict positive outcomes such as a secure attachment – where a higher bias towards fearful faces at 7 months predicted being characterized as securely attached at 14 months (Peltola et al., 2015). The bias towards threatening information does not seem limited to faces as 8- to 14-month-old infants are also quicker to orient towards snakes over frogs, implying the presence of an early attentional bias towards non-social threats as well (LoBue and DeLoache, 2010).
This pattern of affect-biased attention appears to be conserved across the lifespan and across species. For example, work by LoBue and DeLoache (2008) demonstrated that 3- to 5-year-old children display the same pattern of biased attention as adults to a variety of putatively threatening stimuli. Most of these studies measure affect-biased attention by using visual search tasks performed with touch screens, taking advantage of the motor skills of older children. In one variant (LoBue and DeLoache, 2008), the participant is required to find a specific stimulus among an array of distractors (e.g., finding a snake among eight frogs). Attentional bias is measured as the difference in reaction time in finding the target between categories of stimuli (e.g., snakes among frogs vs. frogs among snakes). Using this paradigm, adults and children as young as three can detect snakes more quickly than other similar stimuli like frogs and caterpillars (LoBue and DeLoache, 2008). Moreover, 3-year-olds, just like adults, are quicker to detect spiders than cockroaches and mushrooms (LoBue, 2010a). Similarly, 5-year-old children and adults are faster to detect angry faces compared to other facial expressions (LoBue, 2009). Studies with 8–12 year olds have found analogous findings of quicker detection of social (Waters and Lipp, 2008b) and non-social (Waters et al., 2008a, Waters and Lipp, 2008a) threats in a similar visual search task. This effect has even been reported in non-human primates (Japanese monkeys), who in the same paradigm also detect evolutionary threats faster than non-threatening stimuli (Shibasaki and Kawai, 2009).
Together, although newborns do not reliably display affect-biased attention, attention biases emerge shortly after infants are able to perceptually distinguish among stimuli. Compelling evidence supports the proposition that infants show attentional bias towards threatening stimuli even when they have little experience or knowledge of the specific stimuli. This data can be interpreted as humans possessing an evolutionary-based, specialized neural system that is automatically activated in the presence of threatening stimuli to evoke fear (Öhman and Mineka, 2001). However, it is worth noting that across studies the expression of threat bias was independent of overt fear expressions in infants, children, and adults. Hence, the observed biases may reflect early perceptual mechanisms, perhaps enhanced by cognitive mechanisms that privilege the processing of certain stimuli (e.g., threats), rather than directly elicited emotional responses (LoBue, 2013). Instead, early appearing perceptual biases may act as a catalyst for fear learning, by drawing attention to threatening stimuli, making it easier to develop fear towards this type stimuli (e.g., snakes; LoBue, 2013, LoBue et al., 2010).
The fear learning mechanism can be potentiated if paired with inborn tendencies, an extreme experience, or combinations of the two. For instance, there is evidence of individual differences in affect-bias attention towards threat that stem from individual variations in allelic status (e.g., the serotonin-transporter gene; 5-HTTLRP; Pergamin-Hight et al., 2012). Emotional experiences may also shape patterns of affect-biased attention. For example, most 3-year-olds have had aversive experiences with syringes, causing fear and anxiety. However, most of them lack experience with knives. As such, 3-year-olds are quicker to detect syringes among pens, but not knives among spoons (LoBue, 2010b). On the other hand, adults, with considerable experience with both stimuli, are faster to detect both syringes and knives (e.g., Blanchette, 2006). Similarly, 7- to 9-year-old children display an attentional bias towards novel animals after receiving negative information about them (Field, 2006b), and this effect is more pronounced in anxious children (Field, 2006a).
Together, these findings suggest that there are both innate and learned influences in the development of attentional biases. This can lead some biases to be present in all individuals. At the same time, these biases might be more pronounced or more resistant to counter-evidence for some individuals, either because of inborn temperament-based fear tendencies, previous experiences, or a combination of both. For example, in a sample of 4- to 7-year-olds (LoBue and Pérez-Edgar, 2014), we found that all children displayed heightened attention towards social and non-social threatening stimuli. However, the bias towards social threat cues was significantly larger for temperamentally fearful children, who are constitutionally and/or environmentally more sensitive towards social threat cues.
3.1. Neural bases for the emergence of affect-biased attention
3.1.1. Networks supporting the development of reactive attention
As noted above, the data suggest that attention biases emerge quite rapidly after infants are able to perceptually distinguish among stimuli. This suggests that neural networks supporting these early appearing biases are also functional early in development. Given the limited neuroscientific literature during early human development, we first briefly review the neural networks implicated in affect-biased attention during adulthood.
Affect-biased patterns of attention in adults, primarily the aspects of behavior supported by alerting and orienting mechanisms, are believed to be mediated by emotion-related brain networks (including the amygdala and the orbitofrontal cortex; OFC). For example, amygdala activation increases perceptual processing of emotionally salient stimuli by increasing activity in visual-representation areas (e.g., Lim et al., 2009, Vuilleumier et al., 2004 Vuilleumier et al., 2004) via projections from the amygdala to the visual cortex (Amaral et al., 2003). The amygdala underlies the processing of salient and biologically-relevant stimuli, which can be threatening, rewarding, or unpredictable (Adolphs, 2008). This is in line with our proposal that affect-biased attention is a general mechanism in socioemotional functioning rather than limited to a specific class of salient stimuli.
Amygdala function is complemented by the OFC’s role in assigning emotional valence to stimuli (Anderson et al., 2003) and recognizing stimuli based on previous experience and contexts. This recognition and assessment process can, in turn, modulate perceptual processing via top-down regulatory mechanisms (Bar, 2003). Both the amygdala and the OFC receive low spatial frequency visual information via the magnocellular pathway, allowing their early involvement in visual processing (Bar et al., 2006). In this way, as proposed by Todd and Anderson (2013), the amygdala focuses attention on emotionally salient stimuli. The OFC, by helping determine emotional valence and stimulus recognition, influences amygdala and visual cortex processing (Bar et al., 2006). This reciprocal and pervasive network suggests that these affective influences are an integral part of visual processing (Barrett and Bar, 2009). These core neural systems are also associated with broader biological profiles, which are reflected in secondary markers of risk for psychopathology, such as right frontal encephalogram (EEG) asymmetry (Pérez-Edgar et al., 2013, Schutter et al., 2001) and allelic status (Fani et al., 2013, Jenness et al., 2015 Pérez-Edgar et al., 2010b; Pergamin-Hight et al., 2012), also associated with affect-biased attention.
If the structures and mechanisms of affect-biased attention evident in adults are also in place during infancy, the neural structures proposed should be at least nominally functional by the time affect-biased attention is observed behaviorally in infants (i.e., 7 months of age). There is limited information regarding the form and function of these neural structures in human infants. However, data from non-human primates can be highly informative. Although, to our knowledge, there is no evidence directly linking affect-biased attention in non-human primates to its related neural structures, there is data regarding the development of the neural structures involved in animal emotion (e.g., fear). This evidence can be particularly useful as the emergence of affect-biased attention in humans occurs during the same developmental period as related emotions occur. For instance, attention bias towards threat-related stimuli emerges during the developmental period when fear appears − a period in which across several mammal species, infants begin to actively explore the environment while spending less time with their caregiver (Leppänen and Nelson, 2012). This developmental period, in which both attention bias to threat and fear behaviors appear, is associated with changes in the amygdala in rats (Sullivan and Holman, 2010) and rhesus monkeys (Bauman et al., 2004, Suomi, 1999). Moreover ablation studies suggest the amygdala and OFC mediate fear- and anxiety-related behaviors (Kalin et al., 2004, Kalin et al., 2007). Developmental work from non-human primates suggests that the amygdala undergoes rapid development during the first months of life and stabilizes before total brain volume and other specific structures, such as the hippocampus (Payne et al., 2010). Similarly, the OFC appears relatively well developed at birth, although it still shows important postnatal changes (e.g., connections with temporal visual areas and myelination of the anterior region; Machado and Bachevalier, 2003). This converging evidence implies that the adult-like discrimination of aversive and appetitive cues observed in the second half of the first year of life is related to the development of a neural structure centered on frontolimbic networks (Leppänen and Nelson, 2012).
Although neuroimaging, especially of subcortical structures, is challenging in infants and young children, we do have evidence of distinct cortical activation to emotional stimuli early in life. Most of this evidence comes from event-related potential (ERP) studies, in which electrocortical activity measured from the scalp (ERP components) is time-locked to stimulus presentation (i.e., an event; Luck, 2014). Although ERPs do not directly reflect activity from subcortical structures such as the amygdala, it is possible that ERP components arise from circuits that involve the amygdala (e.g., activity from the visual cortex while viewing emotional stimuli as described above).
For example, Leppänen et al. (2007) demonstrated that 7-month-old infants display a larger P400 (a component that has been related to visual processing of faces) and larger Nc (a component related to orienting towards salient stimuli) when exposed to fearful faces compared to neutral and happy faces. This effect is akin to the effect observed in adults (Leppänen et al., 2007) and is not observed in 5-month olds (Peltola et al., 2009). 7-month-old infants, but not 5-month olds, display a larger Nc in response to only eye whites expressing fear, even in the absence of conscious perception (Jessen and Grossmann, 2014, Jessen and Grossmann, 2016). This suggests that infants, by the second half of their first year, possess detection mechanisms that operate outside conscious awareness (Jessen and Grossmann, 2015). Collectively (for review, see Leppänen and Nelson, 2009), this evidence suggests that the neural systems that underlie more automatic components of affect-biased attention are functional at the time these biases are observed behaviorally, during the first year of life (Leppänen et al., 2007).
3.1.2. Networks supporting the development of executive attention
The early emergence and stabilization of the orienting and alerting systems suggests that they play a central role in evoking and supporting early patterns of affect biased attention. In contrast, the executive system is believed to reflect more effortful mechanisms that are mediated by areas anterior areas such as the medial prefrontal cortex (mPFC), ventrolateral PFC, dorsolateral PFC, and ACC. These areas show a slower developmental course with important structural and functional changes during preadolescence that last until the end of adolescence (Giedd et al., 1999, Lenroot and Giedd, 2006) and emerging adulthood (Taber-Thomas and Pérez-Edgar, 2015). The anterior association areas, for example the prefrontal cortex, are one of the last to reach peak grey matter density, whereas the primary sensory motor areas are one of the first (Gogtay et al., 2004).
As in the previous discussion, evidence is derived from data showing a coupling between behavioral and neural markers. For example, structural characteristics, such as the size of the ACC, predict effortful attention behaviors across development, with the biggest impact during early childhood (4–8 years; Fjell et al., 2012). Studies using fMRI connectivity analyses suggest that the connectivity between these anterior areas (i.e., mPFC) and the amygdala do not resemble an adult-like pattern until age 10 (Gee et al., 2013). Moreover, resting state connectivity in these areas steadily increases from infancy to childhood (Gao et al., 2009) and from childhood to adolescence (Fair et al., 2009). Similarly, studies evaluating the development of behavioral functions related to these areas, such as effortful control, show clear patterns of improvement and maturation across childhood (Kochanska et al., 2001, Rueda et al., 2004) and do not reach adult levels of functioning until later in adolescence (Williams et al., 1999). Hence, it is expected that this system does not play as large a role as the orienting and alerting systems in attentional bias patterns observed during infancy. As a result, it has been proposed that during the first years of life, emotion regulation strategies associated with affect-biased attention are mostly driven by the orienting system.
The earliest forms of self-regulation and effortful control are rooted in the ability to disengage, shift gaze, and re-orient on a new focus of attention (Rothbart et al., 1994). Thus, while orienting in infancy can be reactive, it has regulatory consequences (Harman et al., 1997, Rothbart et al., 2011). For example, three- to six-month-old infants who orient away from provoking stimuli show decreases in distress (Harman et al., 1997). Infants with low levels of selective attention show steady increases in fearful temperament through out childhood and increased social discomfort as adolescents (Pérez-Edgar et al., 2010c). Although there have been recent concerns regarding published data outlining early functional connections in infancy and childhood (Power et al., 2012, Van Dijk et al., 2012), there is clear consensus that the neural regions subserving orienting are in place in the first year of life (Elison et al., 2013, Posner et al., 2012) and functionally mature between 3 and 6 months of age (Colombo, 2001, Courage et al., 2006).
Orienting, often measured as looking duration, in infancy predicts cognitive development (Colombo et al., 2004), including executive function during childhood (Cuevas and Bell, 2014) and adolescence (Rose et al., 2012). For instance, eye tracking measures of fixation duration at 7 months are related to effortful control at 42 months (Papageorgiou et al., 2014). This supports data suggesting that there is a functional overlap during infancy in the networks involved in orienting and executive attention (Gao et al., 2009), that later differentiate into two systems (Cuevas and Bell, 2014, Posner et al., 2012).
It is in the transition from early childhood (∼3–4 years) to emerging adulthood that the executive attention system increasingly mediates emotion regulation behaviors (Posner et al., 2012, Posner et al., 2014). However, there is a dearth of longitudinal evidence observing the shift between orienting and executive attention in self-regulation. Sheese et al. (2008) measured anticipatory looking in 6- to 7-month-olds during a sequential looking task, thought to reflect voluntary control as opposed to reactive orienting. Anticipatory looking was related to concurrent fear-related behaviors and self-regulation of fear, such as looking away from disturbing stimuli and self-soothing. This led the authors to conclude that there is “some degree of executive attention during the first year of life” (Sheese et al., 2008, p. 501). However, later studies did not find a relation between anticipatory looking in infancy and standard measures of executive attention at 3–4 years of age. Indeed, infant anticipatory looking showed stronger associations with the orienting system than the executive attention system (Posner et al., 2012, Rothbart et al., 2011).
In sum, the orienting system may emerge early in development and play a role throughout the life span in affect-biased attention. The development of the executive system seems to be slower; thus orienting may lay the groundwork for affect-biased attention during early development. As the executive system develops, it seems to interact with the other attentional systems and have an important function in the regulatory control of attention, potentially overriding biases in other attentional systems for some children. However, in other children, the executive system may potentiate early biases (Henderson et al., 2015).
3.2. Developmental models of affect-biased attention
Recently, Field and Lester (2010) proposed the following three potential models for the development of affect-biased attention: (i) The integral bias model (Field and Lester, 2010) suggests that individual factors (e.g., temperament, anxiety) determine the extent of any attention bias. This bias should be evident across the lifespan, assuming that the task is developmentally appropriate. As such, infants with early signs of negative affect would already show a more pronounced bias to threat relative to infants without this temperamental profile. This pattern would continue across time and across other markers of risk, such as fearful temperament and social withdrawal. Much of the current clinical literature makes this implicit assumption. (ii) The moderation model (Field and Lester, 2010) suggests that development moderates the expression of an existing bias to threat, such that under certain circumstances (e.g., in children at temperamental risk for anxiety) the initial normative bias may be linked to the later emergence of elevated fear and social withdrawal (LoBue, 2013, LoBue and DeLoache, 2008, Todd et al., 2012). This is in line with Kindt’s (Kindt et al., 1997, Kindt et al., 2000) findings that all children have an attention bias to spiders. However, this bias persists or increases with age (8–12 years) for spider phobic, but decreases for control children (however, see Morren et al., 2003). (iii) Finally, the acquisition model suggests that developmental experiences shape the acquisition of an attention bias gradually over time (Field and Lester, 2010), either in tandem or subsequent to the emergence of fear and anxiety. Here, affect-biased attention is a symptom of disorder.
Based on their review of the literature at the time, Field and Lester (2010) argued that there was more evidence for the moderation and the acquisition models than for the integral bias model. However, the developmental evidence regarding attention bias towards threat in infants reported in this review does not fit perfectly any of the three models. Thus, it is important to consider other models that fit the existing data.
The evidence appears to be most consistent with a hybrid model (Field and Lester, 2010), such that there are innate biases based on individual factors (e.g., fearful temperament) that are later moderated by factors intrinsic and/or extrinsic to the child. Our proposed model, illustrated in Fig. 2, is a hybrid model. More specifically, our model suggests that there are early emerging individual differences in attentional bias towards threat that are determined by constitutional and environmental factors, most likely guided by reactive attentional systems (i.e., alerting and orienting). With the development of the executive attention system in childhood, most children are able to modulate these early biases. Contextual factors, such as parenting, interpersonal relations (e.g., peer relations), and non-familial contexts (e.g., daycare and school), also work hand in hand to support self-regulation (Degnan et al., 2010, Degnan and Fox, 2007). Affect-biased attention acts to shape broad patterns of socioemotional functioning by creating a habitual filtering process that colors the information children use to categorize and act on their environments (Todd et al., 2012). For most children, early biases support healthy and necessary patterns of approach and avoidance. However, for a subgroup of children at risk for psychopathology, these early perceptual biases reinforce risk, calcifying into a pattern of maladaptation throughout childhood and into adulthood. These attention mechanisms act to bind children to specific trajectories that resist the normal amelioration of early risk through maturation and experience (Pérez-Edgar et al., 2010c, Pérez-Edgar et al., 2014b). From this perspective, these stable developmental trajectories grow out the child’s individual early traits or biases. These biases evoke an environmental response that is processed and interpreted by the child, framing subsequent behaviors. As illustrated in Fig. 2, this pattern of provocation and response can become cyclical, growing progressively more entrenched (and biased) with each successive iteration.
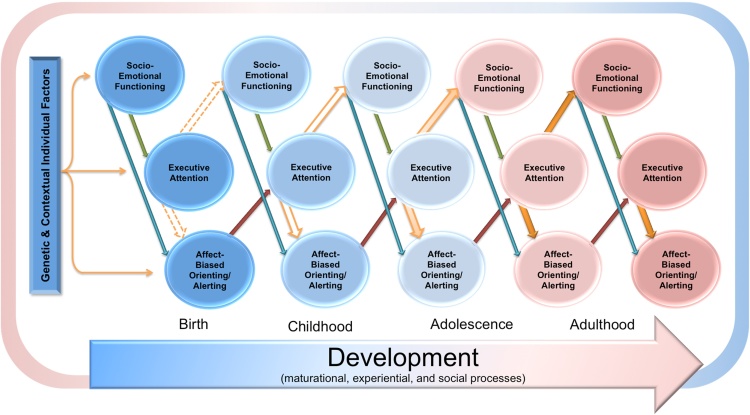
Fig. 2.
Conceptual cascade model depicting the normative relations of socioemotional functioning, affect-biased attention in orienting and alerting, and executive attention across development. Attention bias, as measured by tasks like the dot-probe, likely emerges from an interaction of the three attentional systems (alerting, orienting, and executive attention). There are early appearing biases based on individual factors (e.g., emotionality). These biases are later shaped by developmental processes (e.g., maturational, experiential, and social processes), represented in the “development” arrow. The influence of executive attention increases across development, illustrated by the change in color and shape of its paths.
It is worth noting that even though this illustration would suggest a statistical meditation processes, these factors may also act as moderators. For instance, as portrayed, early socioemotional functioning impacts later affect-biased attention in orienting and alerting via executive attention. In turn, the relation of these biases on later socioemotional functioning is mediated by executive attention. There is some support for this in the exuberance–externalizing literature (e.g., Morales et al., 2016a). However, in the anxiety literature, there is evidence to suggest that executive attention might moderate this link rather than mediate it (e.g., Susa et al., 2012).
4. The Breadth of affect-biased attention
The proposed model accounts for the fact that while a perceptual sensitivity to particular cues may be normative and adaptive, displaying a large and fixed attention bias may be associated with psychopathology. For instance, in the anxiety literature, a perceptual sensitivity is considered an evolution-based safety mechanism. However, there is growing evidence that a pronounced bias in this attention mechanism may lay the foundation for anxiety. Our work has focused on the temperamental trait of behavioral inhibition (BI). BI is chacterized in infancy by increased sensitivity towards novelty, social withdrawal and reticence in childhood (Kagan et al., 1984), and a four-fold increased risk for anxiety in adolescence (Chronis-Tuscano et al., 2009, Clauss and Blackford, 2012, Pérez-Edgar and Fox, 2005). Over the last three decades, multiple labs have documented strong similarities between BI and social anxiety across social (e.g., fewer friends and experience more social rejection) (Chen et al., 2006, Pedersen et al., 2007, Rubin et al., 2009), cognitive/behavioral (e.g., error-monitoring and decision-making) (Lahat et al., 2014, Lamm et al., 2014, McDermott et al., 2009), and neural (e.g., limbic, striatal, and PFC aberrations) (Fu et al., 2015, Guyer et al., 2006, Guyer et al., 2012, Jarcho et al., 2013, Jarcho et al., 2014, Pérez-Edgar et al., 2007, Pérez-Edgar et al., 2014a, Schwartz et al., 2011, Schwartz et al., 2003) levels of functioning, even in the absence of disorder among BI participants (Caouette and Guyer, 2014, Henderson et al., 2015). Within BI, most studies fail to find a zero-order relation between temperament and attention bias (except, Pérez-Edgar et al., 2010a). Rather, affect-biased attention seems to act as a moderator to social withdrawal and anxiety, such that early temperament is associated with patterns of socioemotional maladjustment only if also accompanied by attention bias (Cole et al., 2016, Morales et al., 2015, Pérez-Edgar et al., 2010a, Pérez-Edgar et al., 2011; White et al., in press).
Thus, the data suggest that individual differences in affect-biased attention associated with risks might be rooted in normative patterns of attention. That is, patterns of attention bias to threat act as risk factors only when coupled with secondary markers of risk. For example, LoBue and Pérez-Edgar (2014) found that their sample of young children (4–7 years) displayed heightened attention towards both social (angry faces) and non-social (snakes) threats in line with normative patterns. However, temperamentally fearful children manifested a potentiated bias only to social threat (angry faces), relative to non-fearful peers. Likewise, studies with older children (9–12 years) have found an increased (Waters et al., 2008b, Waters and Lipp, 2008a) and longer lasting (Kindt et al., 2000) bias towards phylogenetically threatening stimuli (i.e., snakes or spiders) for children who feared that specific stimuli. Moreover, this pattern of individual differences emerging from normative patterns of attention may be evident early in life (e.g., Peltola et al., 2015, Pérez-Edgar et al., 2010c), suggesting that early appearing differences in attention orienting and control may work to bias development by shaping interactions with, and interpretations of, emotionally-salient components of the environment.
These studies, while illustrative, focus on a very specifc sliver of functioning − fearful temperament, social withdrawal, and early anxiety. However, if as suggested by our model, attention mechanisms truly play a central domain-general role in socioemotional development, the relations analogous to those noted above should be evident across other areas of functioning.
A pivotal meta-analysis (Bar-Haim et al., 2007) found that attention bias towards threat was present in all the anxiety-related disorders considered (PTSD, GAD, obsessive compulsive disorder, panic disorder, social phobia, simple phobia, and anxiety with comorbid mood disorders) and that there was no difference in the magnitude of the effect for these disorders. This suggests that attention patterns (e.g., attention bias towards threat) are not specific to a type of anxiety. Instead, they act as a general anxiety risk mechanism. Although Bar-Haim et al. (2007) found that this relation was present across development, recent meta-analyses (Dudeney et al., 2015) and reviews (Roy et al., 2015) of attention bias to threat in anxious children (4–18 years) find that there is equivocal support for the presence and direction of the attention bias in this age group. Compared to the adult literature, the effect size for bias in children was smaller, needed longer stimulus presentation times to emerge, and increased with age. However, in both the adult and child literature, this evidence comes from just one domain of functioning (i.e., internalizing behaviors), in which attentional patterns are evaluated with threat-related cues that are thought to trigger socioemotional withdrawal when encountered. Stronger evidence for affect-biased attention as a domain-general mechanism would come from studies in which affect-biased attention plays a role in disorders or behavior patterns marked by bias towards appetitive cues that signal approach.
In surveying the literature, support for attention biases towards appetitive cues appears in the substance abuse and obesity literatures. For example, attention bias towards drug-related cues has been found among alcohol (Field et al., 2004), tobacco (Bradley et al., 2003, Mogg et al., 2005), caffeine (Yeomans et al., 2005), and opioid (Garland et al., 2013, Lubman et al., 2000) users. As an example, Garland et al. (2013) found that opioid-dependent chronic pain patients exhibited an attentional bias towards opioid cues (e.g., pictures of Oxycontin and Vicodin pills or bottles), whereas non-dependent opioid users did not. Moreover, among opioid-dependent individuals, the magnitude of the attentional bias was associated with the self-reported relief obtained from pain treatments (Garland et al., 2013). Similarly, heavy alcohol drinkers showed an attentional bias towards alcohol cues in comparison to light social drinkers, and self-reported alcohol craving was correlated with the magnitude of the attentional bias (Field et al., 2004). In addition, studies are beginning to explore the effects of training the attentional bias away from substance-relevant cues. For instance, in an attention bias modification paradigm similar to that used with anxiety disorders, training attentional bias away from alcohol cues significantly reduced the level of drinking compared to the control condition (McGeary et al., 2014). In line with our model, attention bias to alcohol-related cues is positively related to alcohol use during early adolescence (van Hemel-Ruiter et al., 2015) and alcohol bias predicts alcohol use in the following year (Janssen et al., 2015). Moreover, as predicted by our model, the relation between attention bias to alcohol cues and alcohol use seems to be moderated by effortful control – such that alcohol bias is related to alcohol use only for adolescents with low effortful control (van Hemel-Ruiter et al., 2015).
A similar pattern of findings is present in the obesity literature, in which higher attentional bias towards food cues is present in obese and overweight adults compared to individuals with a healthy weight (e.g., Castellanos et al., 2009, Werthmann et al., 2011). Indeed, higher attention bias towards food cues has been related to higher craving and hunger in healthy weight and obese individuals (Kemps and Tiggemann, 2009, Werthmann et al., 2011). When required to fast, individuals (healthy or obese) displayed a bias towards food (Castellanos et al., 2009). However, only obese individuals were more likely to orient and maintain attention to food cues after eating. Moreover, across the entire sample, self-reported hunger and stimuli depicting higher calorie foods were positively related to increased likelihood of orienting towards food cues. Recent work training participants to attend towards or away from food-related cues in an anti-saccade task increased and reduced chocolate intake, accordingly (Werthmann et al., 2014). In support for our developmental model, the relation between attention bias towards food cues and obesity appears to be present during childhood – such that overweight 8- to 10-year-old children displayed a larger attention bias to food cues compared to normal weight children (Folkvord et al., 2015). Moreover, attentional bias to food cues seems to have developmental implications as it predicted weight gain six months later in a group of obese children (Werthmann et al., 2015).
However, the role of executive attention in this domain is still unclear. One study found that effortful control moderated the relation between approach bias, another type of cognitive bias related to the behavioral tendency to move towards food, such that individuals with high approach bias and low effortful control consumed higher amounts of unhealthy food than individuals with high effortful control (Kakoschke et al., 2015). Although there was a similar pattern with attention bias to food cues, in which individuals with high attention bias to food and low effortful control consumed the most food, the interaction was not significant (Kakoschke et al., 2015). Overall, the data suggest that attentional bias, as a general mechanism, is related to the motivational state of the individual (e.g., hunger). However, for some individuals, these rewarding cues remain salient across states, leading them to unhealthy outcomes (Castellanos et al., 2009).
Studies examining attention bias towards happy faces are scarce. In the internalizing literature, increased attention towards happy faces is most commonly conceptualized as reflecting a bias towards rewards. Evidence suggesting that happy faces may be socially rewarding comes from fMRI studies in which happy faces activate the distributed “reward circuitry,” such as the ventral striatum and OFC (e.g., Monk et al., 2008, O’Doherty et al., 2003, Phillips et al., 1998, Shechner et al., 2012 for a review). This evidence has been used as the basis to use happy facial expressions to study social reward processing in adults and children (e.g., children with autism spectrum disorder, Sepeta et al., 2012). Studies find that anxious and depressed individual have a bias away from rewards such as positive/happy words, faces, and pictures (Frewen et al., 2008; Shechner et al., 2012). In addition, training anxious individuals to attend towards reward leads to a reduction in anxiety symptoms, anxious behavior, and anxiety-related physiology (Heeren et al., 2012). Similarly, studies training anxious children to attend towards reward (i.e., happy faces) find a significant reduction in anxiety (Britton et al., 2013, Waters et al., 2013). Moreover, positive affect is related to attention bias to reward (i.e., emotionally positive words; Tamir and Robinson, 2007) and training adults to attend towards reward increases positive affect (Grafton et al., 2012, Taylor et al., 2011). Finally, children abandoned in infancy to institutional care in Romania, but randomly placed in high-quality foster care displayed a bias towards reward at age 8 (Troller-Renfree et al., 2015) and 12 years (Troller-Renfree et al., 2016), compared to post-institutionalized children not in foster care. Moreover, individual differences in attention bias towards reward among post-institutionalized children were concurrently related to more social engagement, more prosocial behavior, fewer externalizing and internalizing problems, and less social withdrawal (Troller-Renfree et al., 2016, Troller-Renfree et al., 2015).
In normative samples, however, emerging evidence suggests a different pattern. A recent study (Morales et al., 2016a) indicates that attention bias towards rewards (i.e., happy faces) is related to externalizing problems and temperamental exuberance, a trait characterized by high approach and impulsive behavior and related to later emergence of externalizing disorders (Fox et al., 2001b; Polak-Toste and Gunnar, 2006). We found that attention bias towards reward in childhood (70 months) was related to children’s exuberance during toddlerhood (20 months) and externalizing behaviors during kindergarten. Moreover, the longitudinal relation between toddler exuberance and childhood attentional bias towards reward was mediated by effortful control, such that increased exuberance predicted increased bias toward reward via lower effortful control (Morales et al., 2016a). A recent study replicated the negative relation between attention bias towards reward and effortful control in childhood (Cole et al., 2016).
These findings suggest that attention bias towards reward may be an index of a high behavioral approach system coupled with reduced activity from the behavioral inhibition system, leading to exuberance, and at the extreme, externalizing problems. These recent findings may seem contradictory to the existing literature that reports attention bias towards reward is associated with positive outcomes (e.g., less anxiety, increased positive affect, prosocial behavior, and social engagement). However, most of these studies have been carried out with selected populations, such as clinically anxious individuals or post-institutionalized children. As suggested by our model, the meaning of affect-biased attention depends on individual differences in developmental experiences and context. Thus, it is possible that attention bias towards reward differs in its meaning and manifestation between a population exposed to early depravation, in which a bias towards reward is associated with positive outcomes (Troller-Renfree et al., 2016, Troller-Renfree et al., 2015), and a normative sample, in which a bias towards reward is associated with early temperamental exuberance, lower effortful control, and externalizing problems (Morales et al., 2016a).
Overall, the findings from multiple literatures (internalizing, externalizing, addiction, and obesity) closely parallel each other, supporting the proposed model. Across literatures there is evidence of: a) differences in attention bias between target individuals and controls to disorder-relevant cues, b) attention bias correlates with symptom levels, c) these relations are present at different stages of development, d) there is some evidence that training attentional biases changes feelings, thoughts, and behavior towards cues of interest, and e) the executive attention system seems to influence the relation between attention bias and behavioral manifestations of the disorder − in some domains executive attention serves as a moderator, wheareas in other domains, it serves as a mediator. Together, this evidence implies that attention bias plays a role in shaping broad patterns of emotion and behavior, most likely building on idiosyncratic patterns of susceptibility (e.g., anxiety vs. addiction vs. obesity).
5. What is the causal relation between affect-biased attention and socioemotional functioning?
Statements such as “attentional bias towards threat is implicated in the development, and maintenance of anxiety” are not uncommon in the literature. Indeed, we have made the same statement in the current review. While this statement suggests a clear directionality, with attention causing the disorder, the actual direct evidence is quite thin. In other words, we do not know if attentional bias causes the observed outcomes in socioemotional traits (e.g., anxiety) or if attentional bias is a symptom of the socioemotional outcome (e.g., anxiety). A recent in-depth review of the anxiety literature by Van Bockstaele et al. (2013) addresses this issue and concludes that the strongest evidence suggests a bidirectional relation. Most of the evidence reviewed thus far (although often correlational) has been interpreted as attention causing anxiety. Evidence on the causal relation between attention bias and anxiety has largely come from studies that employ 1) experimental paradigms, such as ABM studies (Bar-Haim, 2010, Eldar et al., 2008, Hakamata et al., 2010), which specifically manipulate affect-biased attention to examine the effects on socioemotional outcomes (Fig. 1); 2) prospective longitudinal studies in which the long-term impacts of affect-biased attention are assessed; 3) studies examining the impact on affect-biased attention from therapies that are aimed at curing the disorder without specifically targeting attention bias; and 4) studies in which experimental fear learning (i.e., fear conditioning) is followed by an attention bias to threat.
In ABM studies, experimental manipulations of the attentional bias (i.e., reducing or augmenting the bias) in children and adults are examined to see if they lead to the expected changes in anxious thought and behavior (i.e., reduction or augmentation of anxiety, respectively). Manipulating the contingency of threat cues is thought to implicitly train the individual to attend away from threat cues or towards safety cues. For example, Amir et al. (2009) randomized individuals diagnosed with generalized anxiety disorder (GAD) into either ABM or a control condition. After eight sessions, the ABM group showed significant reductions in attentional bias towards threat and anxiety, as evaluated by self-reports and clinical interview (Amir et al., 2009). On the other hand, as previously discussed, training anxious adults and children to attend towards reward leads to a reduction in anxiety symptoms, anxious behavior, and anxiety-related physiology (Britton et al., 2013, Heeren et al., 2012, Waters et al., 2013). Moreover, training adults to attend towards reward increases their levels of positive affect (Grafton et al., 2012, Taylor et al., 2011). While some meta-analyses of ABM studies support the argument that attentional bias play a role in the etiology and maintenance of anxiety (Bar-Haim, 2010, Hakamata et al., 2010), others questioned the reliability and breadth of the relation by finding important moderators (e.g., ABM delivered in the clinic vs. at home; Cristea et al., 2015, Heeren et al., 2015, Linetzky et al., 2015, Mogoaşe et al., 2014).
More direct evidence of the casual relation between attentional bias and anxiety in development would come from longitudinal studies. Although uncommon, several studies are beginning to emerge. For example the studies reviewed above indicate that attention bias at an earlier time point predicted weight gain (Werthmann et al., 2015) or alcohol use (Janssen et al., 2015) months later. Similarly, in the anxiety literature there are several studies demonstrating that attentional bias towards threat at an earlier time point predicts later psychophysiological reactivity to stress (Egloff et al., 2002 Fox et al., 2010). However, in the only longitudinal study to our knowledge, attention bias to threat at age 5 failed to predict later anxiety at age 7 (White et al., in press). Rather, concurrent affect-biased attention towards both threat and reward moderated the relation between early fearful temperament and anxiety − such that early fearful temperament only predicted anxiety for children who displayed a bias toward threat or those who did not display a bias toward reward. These results are in line with the findings previously discussed from concurrent measures of anxiety/social withdrawal and attention (Cole et al., 2016, Morales et al., 2015, Pérez-Edgar et al., 2010a, Pérez-Edgar et al., 2011).
At the same time, there is also evidence suggesting that changing profiles of socioemotional functioning without specifically targeting attention (e.g., via anxiety therapy) produce changes in affect-biased attention. For example, several studies find that successful anxiety treatments, which do not directly target affect-biased attention, cause changes in attentional bias towards threat (Baños et al., 2008, Mathews, 1995, Pishyar et al., 2008). Pishyar et al. (2008) randomly placed individuals diagnosed with social anxiety in either a cognitive-behavioral group therapy (CBGT) or a waitlist control group. As expected, before therapy, both groups displayed equally high levels of anxiety as well as a bias towards threat. After eight weeks of therapy, CBGT showed significant reductions in anxiety and attention bias towards threat, as well as an increase in bias towards reward, whereas the control group showed no changes. Moreover, the change in anxiety correlated with the change in attentional bias, suggesting that changes in anxiety levels can lead to changes in attentional biases.
Further evidence comes from experimental studies that use fear-conditioning to induce fear and anxiety and then evaluate the impact of fear learning on affect-biased attention towards the conditioned stimuli (Koster et al., 2005, Van Damme et al., 2004b, Van Damme et al., 2006). In these studies, during acquisition, a cue (the reinforced conditioned stimulus, CS+) is intermittently paired with an aversive experience (e.g., white noise burst or mildly painful electric shock; the unconditioned stimulus, UCS). The other cue (the unreinforced conditioned stimulus, CS−) is presented without aversive experience. Participants learn to associate the aversive experience with the CS+ cues and report increased fear towards that cue. Importantly, participants show an attentional bias towards this cue by displaying increased orienting (Koster et al., 2005, Van Damme et al., 2004b) and reduced disengagement from the CS+ cue (Koster et al., 2005; Van Damme et al., 2004a). During extinction phase, both cues (CS+ and CS−) are presented without the aversive experience. In this phase, attentional bias towards the CS+ cue is reduced (Koster et al., 2005) and in some studies disappears (Van Damme et al., 2006). Furthermore, a reinstatement phase in which the previously extinguished CS+ is paired again with the UCS, can cause fear and the attentional bias towards the CS+ cue to reappear (Van Damme et al., 2006). Finally, as discussed above, children display an attentional bias towards novel animals after learning negative information about them (Field, 2006a, Field, 2006b). This provides persuasive evidence that affect-biased attention may be a consequence of fear and anxiety.
Together, these findings suggest a reciprocal relation between affect-biased attention and socioemotional functioning (Van Bockstaele et al., 2013). As outlined in our model, affect-biased attention impacts socioemotional outcomes and these outcomes, in turn, impact affect-biased attention. Placing such intricate relations into a theoretical framework that may be empirically tested is a challenging task. For the most part, the literature has a tacit understanding of the affect-attention link based on concurrent correlational data and mechanistic laboratory-based manipulations of attention. As such, we are missing a truly developmental approach to this issue. Prospective longitudinal studies will be crucial to further our developmental understanding of affect-biased attention.
6. Future directions
Systematic, developmental work that examines attention bias patterns, as well as socioemotional (e.g., temperament) profiles repeatedly over time, would allow us to delineate the time course of any emerging interrelations between attention and socioemotional behavior, providing evidence for the proposed model. Importantly, a longitudinal study extending over time can incorporate additional biopsychosocial markers of risk. This may allow us to create differential predictions of trajectories and outcomes across levels of analysis. There is a large gap in the literature as infant attention studies have focused on capturing normative developmental milestones, while the infant temperament literature has examined individual differences in affect and the later moderating mechanisms (e.g., social relationships, self-regulation) that shape subsequent socioemotional trajectories. The same parallels can be seen in the literature with older children and in the adult clinical literature. As such, there is a clear need for studies that couple attention and affect over time and across levels of analysis. However, in order to do this, there are a number of open questions that need to be addressed. In the next sections, we outline two of such questions that we believe are fundamental to evaluate and refine the proposed model; thus, furthering our understanding of affect-biased attention and its impact on socioemotional development. We first discuss how to measure affect-biased attention such that we are able to capture its dynamic components as well as distinguish among the different attentional systems. We then discuss how to capture the real-life context in which attention bias occurs.
6.1. How do we capture affect-biased attention?
Of course, a call for more research then begs the question of how do we best go about capturing the dynamic components of affect-biased attention. Most evidence for the link between affect-biased attention and socioemotional functioning is derived from studies using a variant of the dot-probe task (Fig. 1; Roy et al., 2015, Todd et al., 2012). For instance, in the original dot-probe task, MacLeod et al. (1986) found that anxious individuals were faster to respond to probes following threatening words. On the other hand, control participants, were faster to respond to probes following neutral words, exhibiting a bias away from threat (MacLeod et al., 1986). Children diagnosed with an anxiety disorder also display an attentional bias towards threat compared to non-anxious controls (Roy et al., 2008). In addition, temperamentally at-risk, but healthy, adolescents displayed the same bias pattern (Pérez-Edgar et al., 2010a). Finally, the magnitude of attention bias has been found to predict levels of anxiety symptoms (Waters et al., 2008b), suggesting a relation across the anxiety dimension and in childhood as well as adulthood.
Despite the early enthusiasm for the dot-probe task as a simple and efficient task for capturing affect-biased attention, recent concerns suggest that the task may not be reliable (Rodebaugh et al., 2016, Schmukle, 2005, Staugaard, 2009). For example, there is a good deal of inconsistency in the literature as some data point to anxiety-linked attention avoidance (Brown et al., 2013; Chen et al., 2002, Mansell et al., 1999, Salum et al., 2013, Stirling et al., 2006, Waters et al., 2014) and other data show null findings when comparing anxious/at-risk participants against healthy controls (Gotlib et al., 2004, Morales et al., 2016b, Pérez-Edgar et al., 2011 Pineles and Mineka, 2005, Pishyar et al., 2004, Waters et al., 2004).
Part of the difficulties may derive from the fact that the dot-probe task is not designed to disambiguate potential subcomponents of attention that may shape bias − namely orienting and disengagement (Cisler and Koster, 2010). Second, the variability in the data may simply reflect the “noise” of any task that relies on a reaction-time based difference score. Attention bias difference scores are derived from a contrast of behavioral responses for across trial types, which assumes that RTs provide an accurate measure of attention allocation (Harrison and Gibb, 2014). Even if this assumption were correct, RTs measures cannot track the pattern of attention across a trial, reducing all contributing processes to a single number. That is, individual differences in cue processing, response speed, and competing approach/avoidance mechanisms may all be in play (Mogg et al., 2008). This blurring of multiple processes can obscure any true signal of bias. In response, multiple strategies have been used to attempt and better capture the “core” bias seen within and across individuals.
One approach is to analyze the dot-probe task in alternative way by comparing trials containing threat-neutral pairs to trials containing neutral–neutral pairs (for details see Koster et al., 2004). Several studies performing this analysis have found evidence for biases in disengagement, rather than orienting, among anxious individuals (e.g., Cooper and Langton, 2006, Koster et al., 2004, Salemink et al., 2007). Others have suggested that variability in bias throughout attention tasks best capture the fluctuating patterns of attention that mark individual differences and risk (Amir et al., 2016, Naim et al., 2015, Zvielli et al., 2014, Zvielli et al., 2015).
Another approach is to use different tasks that also aim to capture an attentional bias. Although there are several tasks that capture affect-biased attention (for a review, see Van Bockstaele et al., 2013 Yiend, 2010), few of them are able to evaluate individual differences in attentional patterns. One such task is the attentional cueing task developed by Posner (Posner, 1980). In the traditional version of the task, a single cue appears on either side of the screen (left or right) normally for 250–500 ms. Following the cue, a target probe appears either behind the cue or on the opposite side of the cue. The validity score is then computed by contrasting the RTs from probes that appear on the other side of the cue minus the probes that appear behind the cue. This difference, called validity effect, represents the effort required to disengage from the cue. In the emotional version of the task, the cues are presented as positive or negative stimuli such as punishment and reward cues, cues as facial expressions, or words with emotional valence. In such a task, individual differences in emotionality also relate to differences in attentional bias (as indexed by the validity effect), supporting the role of disengagement (Cisler and Olatunji, 2010, Derryberry and Reed, 2002, Fox et al., 2001a). For instance, Derryberry and Reed (2002) found that trait anxious participants displayed an early and late attentional bias towards threat with cues presented for 250 ms and 500 ms, respectively. Moreover, the late bias, 500 ms, was moderated by attentional control, such that only anxious individuals low in attentional control displayed a bias (Derryberry and Reed, 2002). This finding is in line with our model as it suggests that the ability to disengage may be able to supersede biases in other attentional systems. However, this process is slow and effortful such that it might not be effective under fast presentations of stimuli (Derryberry and Reed, 2002).
The use of multiple tasks may also help us examine more thoroughly the expected underlying attention sub-components. A promising approach is to compare or look for convergence across attention bias tasks. However, few studies have evaluated individuals on multiple attention bias tasks and compared their performance. Most of these studies found no relation between tasks (Broeren et al., 2011, Brown et al., 2014, Dalgleish et al., 2003). One exception is one of our studies that examined the relation between the dot-probe task and the affective Posner task in a sample of BI and non-BI children (Morales et al., 2016b). We found that there was no relation between the two tasks for the sample as a whole. However, there was a significant relation between tasks only for BI children, indicating that convergence across attention bias measures may be dependent on individuals’ predispositions (e.g., temperament). Moreover, children who displayed a consistent bias across tasks, regardless of direction (bias towards or away from threat), had higher levels of anxiety, suggesting that convergence across attention bias measures may serve as an index of information processing patterns that influences socioemotional outcomes (Morales et al., 2016b).
Thus, there is evidence for the role of both orienting and disengagement in the emergence of bias patterns of attention. However, there is limited evidence regarding the involvement of the alerting system. Most studies examining the alerting system do so by contrasting trials in which a warning is given before a target event and trials without the warning. This aims to capture the alertness (vigilance) or preparedness to respond of the individual (Posner, 2008). However, few studies have evaluated individual differences in alertness or vigilance, as defined above. In particular, there is a dearth of studies that consider individual differences in alertness with respect to individual differences in emotionality (e.g., ADHD, Swanson et al., 1991).
One measure of alertness comes from the Attention Network Test (ANT), which is aimed to test the altering, orienting, and executive attention systems (Fan et al., 2002, Rueda et al., 2004). In its original form, this task does not involve emotion. In an attempt to create an emotional variant, studies have included affective contexts by adding facial expressions before each trial, providing positive or negative feedback, or using emotional stimuli as the alerting cue (Cohen et al., 2011, Dennis and Chen, 2007, Dennis et al., 2008). Whereas most of these studies do not find differences in alertness, Dennis et al. (2008) found that state anxiety positively correlated with alertness and that fearful faces decreased alerting efficiency.
Future investigations should adapt or develop paradigms that might capture individual differences in alertness based on emotionality. For instance, it would be feasible to develop an eye-tracking paradigm that could evaluate if certain individuals are quicker to fixate on stimuli that they are biased towards – for example, based on the evidence reviewed above regarding alertness and its relation to amygdala activity, individuals characterized with a fearful temperament might be quicker to detect threatening and novel stimuli.
While the use of multiple tasks helps balance the over-reliance on the dot-probe task, it does not directly deal with the argument that we rely on a measure, RTs, which is too remote from the process of interest. Eye-tracking methods can measure where the individual is visually attending and may provide a level of analysis a step closer to underlying processes, relative to behavioral responses. The few studies employing this methodology have mostly found that attention bias is rooted in individual differences in orienting (Castellanos et al., 2009, Shechner et al., 2013, Werthmann et al., 2011). For example, Shechner et al. (2013) found that anxious 8- to 17-year-olds were faster to fixate to threatening versus neutral stimuli, and were more likely to fixate on the threatening stimuli first, indicating a bias in the early stages of attention. Similarly, overweight participants are more likely to direct their gaze towards appetitive cues (food pictures) than healthy controls and their self-reported craving was related to initial orientation towards these cues (Castellanos et al., 2009, Werthmann et al., 2011).
Eye-tracking technology has the advantage that it can be employed across the lifespan (Oakes, 2012). Thus, the same task can be used repeatedly from infancy onward, supporting the call for systematic, longitudinal study of affect-biased attention. However, even here, traditional eye-tracking protocols suffer from the same core limitation found with computer-based reaction time measures. That is, these studies all rely on computerized paradigms that present static and simple visual displays, which restrict the potential targets to be attended. Hence, these tasks tap into how children and adults orient to preselected stimuli, rather than how they select a focus of attention when under threat from among an array of choices (Birmingham and Kingstone, 2009, Todd et al., 2012). Furthermore, measuring attention functioning removed from social interactions does not directly test if and how selective attention and behavioral responses influence each other – the very premise of the current affect-biased attention literature. Indeed, it may be that threat-related attention and behavioral approach/avoidance tendencies in context are better predictors of socioemotional adjustment in children (Kiel and Buss, 2011) than “cool” computer-based measures of attention.
6.2. How do we best capture the context of affect-biased attention?
We know that threat-related attention plays a role in the transition from early fearful temperament to later social withdrawal and anxiety. In addition, we know that temperamentally fearful children recruit aberrant bottom-up automatic and top-down regulatory mechanisms when attending to affectively and/or motivationally salient stimuli. However, the existing research cannot speak to whether there are temperament-related individual differences in affect-biased attention towards novelty and potential threat in real-life environments, nor can they assess how attention and approach/avoidance behavior influence each other in real-time as children encounter novel environments and interact with unfamiliar people.
Emerging adult (Foulsham et al., 2011, Freeth et al., 2013, Isaacowitz et al., 2015, Laidlaw et al., 2011, Lange et al., 2004) and infant studies (Franchak and Adolph, 2010, Franchak et al., 2011, Kretch and Adolph, 2015 Franchak et al., 2011; Kretch and Adolph, 2015 Kretch et al., 2014) have assessed attention patterns using mobile (i.e., ambulatory) eye tracking in real-life settings. Mobile eye tracking systems extend the advantages of stationary eye tracking by offering an ecologically-valid, real-time measurement of how attention processes unfold as individuals navigate the environment. Isaacowitz et al. (2015) found that when participants were given the discretion to select objects to attend, there were no differences in the affective valence of the objects attended between young and older adults. This was inconsistent with results from computerized paradigms indicating that older adults had a greater tendency to fixate on positive versus negative stimuli (Isaacowitz, 2012). In Lange et al.’s study (2004), adults with spider phobia increased their fixations to both the threat (a live tarantula) and safety stimuli (the exit of the room), and increasingly scanned the room in the threat-present versus threat-absent condition. The emerging data indicate that patterns of attention and behavior may differ with the level of threat, proximity to threat, and the perceived ability to modulate engagement with threat.
Mobile eye tracking paradigms that provide the freedom to selectively attend to and behaviorally approach competing threat and benign stimuli could reveal different or more nuanced attention patterns than screen-based tasks. The differences between screen-based and real-life stimuli reside not solely in their physical attributes (e.g. simple vs. complex, or static vs. dynamic), but more fundamentally, real-life settings provide the potential for social interactions (Risko et al., 2012, Schilbach et al., 2013). Using mobile eye tracking measures, Laidlaw et al. (2011) found that adult participants initiated fewer fixations to the experimenter who were physically sitting in the room (the potential for social interactions as present) than participants who viewed the videotaped experimenter (no potential for social interactions). In a subsequent study (Freeth et al., 2013), participants fixated more to the live experimenter’s face during a conversation when the experimenter imitated eye contact than when the experimenter averted his gaze away. The effect of eye contact manipulation was not significant in adults who spoke with the videotaped experimenter. The reciprocity of social interactions requires the interactors to execute moment-by-moment socially adequate reactions (Klin et al., 2003). Hence, their practical know-how may influence looking behaviors. Devoid of the potential for social interactions, paradigms using screen-based stimuli may not be valid for delineating the functioning of attention in real-world social contexts.
7. Concluding remarks
This review suggests that we should consider several factors when examining affect-biased attention and its relation to socioemotional functioning: 1) Affect-biased attention is not a single construct, but arises from the interaction of multiple attentional systems. 2) Affect-biased attention and its role in socioemotional functioning might change over the course of development as subcomponents of attention emerge and strengthen, and are coupled with experience. 3) Affect-biased attention is not limited to attention bias towards threat and anxiety-related outcomes. Rather, it is a domain-general mechanism that shapes the type of information an individual has available when characterizing, acting on, and responding to the environment. 4) The relation between affect-biased attention and socioemotional functioning seems to be reciprocal rather than unidirectional. Based on these factors, we propose a model (Fig. 2) suggesting that attentional biases are present early in life and emerge from reactive attentional processes rooted in orienting mechanisms. These biases are modulated as the child matures (e.g., the development of executive attention) and gains experience (e.g., variations in peer relations), triggering potentially cyclical socioemotional trajectories leading some to positive outcomes and adaptive socioemotional functioning, while leading others to maladaptation and poor functioning.
Finally, this review advocates for a more fine-grained understanding of affect-biased attention and its nuanced relation to socioemotional functioning across development. This includes broadening targeted outcomes (e.g., both externalizing and internalizing patterns), examining multiple levels of analysis (e.g., behavioral and experiential), and expanding our experimental repertoire (e.g., capturing attention in context). This more complex understanding might help us better understand the nuances of affect-biased attention and serve as a stronger foundation for our already ongoing attempts to identify and intervene with individuals at risk.
Conflict of interest
The authors declare no conflict of interest in relation to the present manuscript.
Acknowledgement
Support for manuscript preparation was provided by grants from the National Institutes of Health (MH094633, MH103627) to Koraly Pérez-Edgar.
References
- Adolphs R. Fear, faces, and the human amygdala. Curr. Opin. Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D., Behniea H., Kelly J. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Amir N., Beard C., Burns M., Bomyea J. Attention modification program in individuals with generalized anxiety disorder. J. Abnorm. Psychol. 2009;118(1):28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir I., Zvielli A., Bernstein A. (De)Coupling of our eyes and our mind’s eye: a dynamic process perspective on attentional bias. Emotion. 2016 doi: 10.1037/emo0000172. [DOI] [PubMed] [Google Scholar]
- Amso D., Scerif G. The attentive brain: insights from developmental cognitive neuroscience. Nat. Rev. Neurosci. 2015;16(10):606–619. doi: 10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Stappen I., Panitz D., Ghahremani D.G., Glover G., Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Baños R.M., Quero S., Botella C. Detection and distraction effects for threatening information in social phobia and change after treatment. Depress. Anxiety. 2008;25(1):55–63. doi: 10.1002/da.20269. [DOI] [PubMed] [Google Scholar]
- Bar M., Kassam K.S., Ghuman A.S., Boshyan J., Schmid A.M., Dale A.M., Halgren E. Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. 2006;103(2):449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J. Cogn. Neurosci. 2003;15(4):600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y. Research Review: attention bias modification (ABM): a novel treatment for anxiety disorders. J. Child Psychol. Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Barrett L.F., Bar M. See it with feeling: affective predictions during object perception. Phil. Trans. R. Soc. B: Biol. Sci. 2009;364(1521):1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M.D., Lavenex P., Mason W.A., Capitanio J.P., Amaral D.G. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J. Cogn. Neurosci. 2004;16(8):1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Birmingham E., Kingstone A. Human social attention: a new look at past, present, and future investigations. Ann. N. Y. Acad. Sci. 2009;1156(1):118–140. doi: 10.1111/j.1749-6632.2009.04468.x. [DOI] [PubMed] [Google Scholar]
- Blanchette I. Snakes, spiders, guns, and syringes: how specific are evolutionary constraints on the detection of threatening stimuli? Q. J. Exp. Psychol. 2006;59(8):1484–1504. doi: 10.1080/02724980543000204. [DOI] [PubMed] [Google Scholar]
- Bradley B.P., Mogg K., Wright T., Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychol. Addict. Behav. 2003;17(1):66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Bar-Haim Y., Clementi M.A., Sankin L.S., Chen G., Shechner T., Pine D.S. Training-associated changes and stability of attention bias in youth: implications for attention bias modification treatment for pediatric anxiety. Dev. Cogn. Neurosci. 2013;4:52–64. doi: 10.1016/j.dcn.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S., Muris P., Bouwmeester S., Field A., Voerman J. Processing biases for emotional faces in 4- to 12-year-old non-clinical children: an exploratory study of developmental patterns and relationships with social anxiety and behavioral inhibition. J. Exp. Psychopathol. 2011 [Google Scholar]
- Brown H.M., Mcadams T.A., Lester K.J., Goodman R., Clark D.M., Eley T.C. Attentional threat avoidance and familial risk are independently associated with childhood anxiety disorders. J. Child Psychol. Psychiatry. 2013;54(6):678–685. doi: 10.1111/jcpp.12024. [DOI] [PubMed] [Google Scholar]
- Brown H.M., Eley T.C., Broeren S., MacLeod C., Rinck M., Hadwin J.A., Lester K.J. Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. J. Anxiety Disord. 2014;28(1):97–107. doi: 10.1016/j.janxdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Caouette J.D., Guyer A.E. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Reinke K.S., Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47(5):1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Castellanos E.H., Charboneau E., Dietrich M.S., Park S., Bradley B.P., Mogg K., Cowan R.L. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int. J. Obes. 2009;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Ehlers A., Clark D., Mansell W. Patients with generalized social phobia direct their attention away from faces. Behav. Res. Ther. 2002;40(6):677–687. doi: 10.1016/s0005-7967(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Chen X., DeSouza A.T., Chen H., Wang L. Reticent behavior and experiences in peer interactions in Chinese and Canadian children. Dev. Psychol. 2006;42(4):656–665. doi: 10.1037/0012-1649.42.4.656. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., Perez-Edgar K., Henderson H.A., Diaz Y., Fox N.A. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H.W. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin. Psychol. Rev. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Olatunji B.O. Components of attentional biases in contamination fear: evidence for difficulty in disengagement. Behav. Res. Ther. 2010;48(1):74–78. doi: 10.1016/j.brat.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(10):1066–1075.e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N., Henik A., Mor N. Can emotion modulate attention? Evidence for reciprocal links in the attentional network test. Exp. Psychol. (formerly Zeitschrift Für Experimentelle Psychologie) 2011;58(3):171–179. doi: 10.1027/1618-3169/a000083. [DOI] [PubMed] [Google Scholar]
- Cole C.E., Zapp D.J., Fettig N.B., Pérez-Edgar K.E. Impact of attention biases to threat and effortful control on individual variations in negative affect and social withdrawal in very young children. J. Exp. Child Psychol. 2016;141:210–221. doi: 10.1016/j.jecp.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Ann. Rev. Psychol. 2001;52(1):337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Cooper R., Langton S. Attentional bias to angry faces using the dot-probe task? It depends when you look for it. Behav. Res. Ther. 2006;44(9):1321–1329. doi: 10.1016/j.brat.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Courage M.L., Reynolds G.D., Richards J.E. Infant’ attention to patterned stimuli: developmental change from 3 to 12 months of age. Child Development. 2006;77(3):680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea I.A., Kok R.N., Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. Br. J. Psychiatry. 2015;206(1):7–16. doi: 10.1192/bjp.bp.114.146761. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Bell M.A. Infant attention and early childhood executive function. Child Dev. 2014;85(2):397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Taghavi R., Neshat-Doost H., Moradi A., Canterbury R., Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. J. Clin. Child Adolesc. Psychol. 2003;32(1):10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Degnan K.A., Fox N.A. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Dev. Psychopathol. 2007;19(03):729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Degnan K.A., Almas A.N., Fox N.A. Temperament and the environment in the etiology of childhood anxiety: temperament, anxiety, and environment. J. Child Psychol. Psychiatry. 2010;51(4):497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Chen C.-C. Neurophysiological mechanisms in the emotional modulation of attention: the interplay between threat sensitivity and attentional control. Biol. Psychol. 2007;76(1-2):1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Chen C.-C., McCandliss B.D. Threat-related attentional biases: an analysis of three attention systems. Depress. Anxiety. 2008;25(6):E1–10. doi: 10.1002/da.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D., Reed M.A. Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 2002;111(2):225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dudeney J., Sharpe L., Hunt C. Attentional bias towards threatening stimuli in children with anxiety: a meta-analysis. Clin. Psychol. Rev. 2015;40:66–75. doi: 10.1016/j.cpr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Egloff B., Wilhelm F.H., Neubauer D.H., Mauss I.B., Gross J.J. Implicit anxiety measure predicts cardiovascular reactivity to an evaluated speaking task. Emotion. 2002;2(1):3–11. doi: 10.1037/1528-3542.2.1.3. [DOI] [PubMed] [Google Scholar]
- Eldar S., Ricon T., Bar-Haim Y. Plasticity in attention: implications for stress response in children. Behav. Res. Ther. 2008;46(4):450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Elison J.T., Paterson S.J., Wolff J.J., Reznick J.S., Sasson N.J., Gu H., for the IBIS Network White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Petersen S.E. Functional brain networks develop from a local to distributed organization. PLoS Comput. Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J., Gu X., Guise K.G., Liu X., Fossella J., Wang H., Posner M.I. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70(2):209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Gutman D., Tone E., Almli L., Mercer K., Davis J., Ressler K. FKBP5 modulates attention bias for threat: associations with hippocampal function and morphology. Psychol. Faculty Publ. 2013 doi: 10.1001/2013.jamapsychiatry.210. (Retrieved from http://scholarworks.gsu.edu/psych_facpub/108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P., Lester K.J. Is there room for development in developmental models of information processing biases to threat in children and adolescents? Clin. Child Fam. Psychol. Rev. 2010;13(4):315–332. doi: 10.1007/s10567-010-0078-8. [DOI] [PubMed] [Google Scholar]
- Field M., Mogg K., Zetteler J., Bradley B.P. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl.) 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field A.P. The behavioral inhibition system and the verbal information pathway to children’s fears. J. Abnormal Psychol. 2006;115(4):742–752. doi: 10.1037/0021-843X.115.4.742. [DOI] [PubMed] [Google Scholar]
- Field A.P. Watch out for the beast: fear information and attentional bias in children. J. Clin. Child Adolesc. Psychol. 2006;35(3):431–439. doi: 10.1207/s15374424jccp3503_8. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Gruen J. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkvord F., Anschütz D.J., Buijzen M. Radboud University Nijmegen; 2015. Attentional Bias for Food Cues in Advertising Among Overweight and Hungry Children. (Retrieved from http://fransfolkvord.nl/wp-content/uploads/2014/12/ThesisFransFolkvord_Childrens-Reactivity-to-Embedded-Food-Cues-in-Advergames.pdf#page=141) [Google Scholar]
- Foulsham T., Walker E., Kingstone A. The where, what and when of gaze allocation in the lab and the natural environment. Vision Res. 2011;51(17):1920–1931. doi: 10.1016/j.visres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Fox E., Russo R., Bowles R., Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol. Gen. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Rubin K.H., Calkins S.D., Schmidt L.A. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox E., Cahill S., Zougkou K. Preconscious processing biases predict emotional reactivity to stress. Biol. Psychiatry. 2010;67(4):371–377. doi: 10.1016/j.biopsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak J.M., Adolph K.E. Visually guided navigation: head-mounted eye-tracking of natural locomotion in children and adults. Vision Res. 2010;50(24):2766–2774. doi: 10.1016/j.visres.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchak J.M., Kretch K.S., Soska K.C., Adolph K.E. Head-mounted eye tracking: a new method to describe infant looking: head-mounted eye tracking. Child Dev. 2011;82(6):1738–1750. doi: 10.1111/j.1467-8624.2011.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeth M., Foulsham T., Kingstone A. What affects social attention? Social presence, eye contact and autistic traits. PLoS One. 2013;8(1):e53286. doi: 10.1371/journal.pone.0053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P., Dozois D., Joanisse M., Neufeld R. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clin. Psychol. Rev. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fu X., Taber-Thomas B.C., Pérez-Edgar K. Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biol. Psychol. 2015 doi: 10.1016/j.biopsycho.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland E.L., Froeliger B.E., Passik S.D., Howard M.O. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J. Behav. Med. 2013;36(6):611–620. doi: 10.1007/s10865-012-9455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human Amygdala–Prefrontal circuitry. J. Neurosci. 2013:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Kasch K.L., Traill S., Joormann J., Arnow B.A., Johnson S.L. Coherence and specificity of information-processing biases in depression and social phobia. J. Abnorm. Psychol. 2004;113(3):386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Grafton B., Ang C., MacLeod C. Always look on the bright side of life: the attentional basis of positive affectivity: attentional basis of positive affectivity. Eur. J. Pers. 2012;26(2):133–144. [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. Experience and brain development. Child Dev. 1987:539–559. [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., Hardin M.G., Roberson-Nay R., Monk C.S., Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J. Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Detloff A., Benson B., Nelson E.E., Perez-Edgar K., Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am. J. Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y., Lissek S., Bar-Haim Y., Britton J.C., Fox N.A., Leibenluft E., Pine D.S. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol. Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A.J., Gibb B.E. Attentional biases in currently depressed children: an eye-tracking study of biases in sustained attention to emotional stimuli. J. Clin. Child Adolesc. Psychol. 2014:1–7. doi: 10.1080/15374416.2014.930688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman C., Rothbart M.K., Posner M.I. Distress and attention interactions in early infancy. Motiv. Emotion. 1997;21(1):27–44. [Google Scholar]
- Heeren A., Reese H.E., McNally R.J., Philippot P. Attention training toward and away from threat in social phobia: effects on subjective, behavioral, and physiological measures of anxiety. Behav. Res. Ther. 2012;50(1):30–39. doi: 10.1016/j.brat.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Heeren A., Mogoaşe C., Philippot P., McNally R.J. Attention bias modification for social anxiety: a systematic review and meta-analysis. Clin. Psychol. Rev. 2015;40:76–90. doi: 10.1016/j.cpr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Henderson H.A., Pine D.S., Fox N.A. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz D.M., Livingstone K.M., Harris J.A., Marcotte S.L. Mobile eye tracking reveals little evidence for age differences in attentional selection for mood regulation. Emotion. 2015;15(2):151–161. doi: 10.1037/emo0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz D.M. Mood regulation in real time: age differences in the role of looking. Curr. Dir. Psychol. Sci. 2012;21(4):237–242. doi: 10.1177/0963721412448651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen T., Larsen H., Vollebergh W.A.M., Wiers R.W. Longitudinal relations between cognitive bias and adolescent alcohol use. Addict. Behav. 2015;44:51–57. doi: 10.1016/j.addbeh.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Etkin A., Leibenluft E., Shechner T., Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol. Psychol. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Leibenluft E., Shechner T., Degnan K.A., Ernst M. Enduring influence of early temperament on neural mechanisms mediating attention–emotion conflict in adults. Depress. Anxiety. 2014;31(1):53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness J.L., Hankin B.L., Young J.F., Smolen A. Stressful life events moderate the relationship between genes and biased attention to emotional faces in youth. Clin. Psychol. Sci. 2015 doi: 10.1177/2167702615601000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S., Grossmann T. Unconscious discrimination of social cues from eye whites in infants. Proc. Natl. Acad. Sci. 2014;111(45):16208–16213. doi: 10.1073/pnas.1411333111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S., Grossmann T. Neural signatures of conscious and unconscious emotional face processing in human infants. Cortex. 2015;64:260–270. doi: 10.1016/j.cortex.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Jessen S., Grossmann T. The developmental emergence of unconscious fear processing from eyes during infancy. J. Exp. Child Psychol. 2016;142:334–343. doi: 10.1016/j.jecp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Clarke C., Snidman N., Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Dev. 1984;55(6):2212–2225. [Google Scholar]
- Kakoschke N., Kemps E., Tiggemann M. Combined effects of cognitive bias for food cues and poor inhibitory control on unhealthy food intake. Appetite. 2015;87:358–364. doi: 10.1016/j.appet.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Kalin N.H., Shelton S.E., Davidson R.J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N.H., Shelton S.E., Davidson R.J. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol. Psychiatry. 2007;62(10):1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemps E., Tiggemann M. Attentional bias for craving-related (chocolate) food cues. Exp. Clin. Psychopharmacol. 2009;17(6):425–433. doi: 10.1037/a0017796. [DOI] [PubMed] [Google Scholar]
- Kiel E.J., Buss K.A. Toddlers’ duration of attention toward putative threat. Infancy. 2011;16(2):198–210. doi: 10.1111/j.1532-7078.2010.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M., Bierman D., Brosschot J.F. Cognitive bias in spider fear and control children: assessment of emotional interference by a card format and a single-trial format of the stroop task. J. Exp. Child Psychol. 1997;66(2):163–179. doi: 10.1006/jecp.1997.2376. [DOI] [PubMed] [Google Scholar]
- Kindt M., van den Hout M., Jong P., de Hoekzema B. Cognitive bias for pictorial and linguistic threat cues in children. J. Psychopathol. Behav. Assess. 2000;22(2):201–219. [Google Scholar]
- Klin A., Jones W., Schultz R., Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Phil. Trans. R. Soc. B: Biol. Sci. 2003;358(1430):345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Coy K.C., Murray K.T. The development of self-regulation in the first four years of life. Child Dev. 2001;72(4):1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Koster E.H.W., Crombez G., Verschuere B., De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav. Res. Ther. 2004;42(10):1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Koster E.H.W., Crombez G., Van Damme S., Verschuere B., De Houwer J. Signals for threat modulate attentional capture and holding: fear-conditioning and extinction during the exogenous cueing task. Cogn. Emotion. 2005;19(5):771–780. [Google Scholar]
- Kretch K.S., Adolph K.E. Active vision in passive locomotion: real-world free viewing in infants and adults. Dev. Sci. 2015;18(5):736–750. doi: 10.1111/desc.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch K.S., Franchak J.M., Adolph K.E. Crawling and walking infants see the world differently. Child Dev. 2014;85(4):1503–1518. doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A., Lamm C., Chronis-Tuscano A., Pine D.S., Henderson H.A., Fox N.A. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(4):447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw K.E.W., Foulsham T., Kuhn G., Kingstone A. Potential social interactions are important to social attention. Proc. Natl. Acad. Sci. 2011;108(14):5548–5553. doi: 10.1073/pnas.1017022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Walker O.L., Degnan K.A., Henderson H.A., Pine D.S., McDermott J.M., Fox N.A. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev. Sci. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W.G.T., Tierney K.J., Reinhardt-Rutland A.H., Vivekananda-Schmidt P. Viewing behaviour of spider phobics and non-phobics in the presence of threat and safety stimuli. Br. J. Clin. Psychol. 2004;43(3):235–243. doi: 10.1348/0144665031752989. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leppänen J.M., Nelson C.A. Tuning the developing brain to social signals of emotions. Nat. Rev. Neurosci. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen J.M., Nelson C.A. Early development of fear processing. Curr. Dir. Psychol. Sci. 2012;21(3):200–204. [Google Scholar]
- Leppänen J.M., Moulson M.C., Vogel-Farley V.K., Nelson C.A. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.-L., Padmala S., Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc. Natl. Acad. Sci. 2009;106(39):16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetzky M., Pergamin-Hight L., Pine D.S., Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification threatment for anxiety disorders. Depress. Anxiety. 2015;32(6):383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- LoBue V., DeLoache J.S. Detecting the snake in the grass attention to fear-relevant stimuli by adults and young children. Psychol. Sci. 2008;19(3):284–289. doi: 10.1111/j.1467-9280.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- LoBue V., DeLoache J.S. Superior detection of threat-relevant stimuli in infancy: threat detection in infancy. Dev. Sci. 2010;13(1):221–228. doi: 10.1111/j.1467-7687.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- LoBue V., Pérez-Edgar K.E. Sensitivity to social and non-social threats in temperamentally shy children at-risk for anxiety. Dev. Sci. 2014;17(2):239–247. doi: 10.1111/desc.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V., Rakison D.H., DeLoache J.S. Threat perception across the life span: evidence for multiple converging pathways. Curr. Dir. Psychol. Sci. 2010;19(6):375–379. [Google Scholar]
- LoBue V. More than just another face in the crowd: superior detection of threatening facial expressions in children and adults. Dev. Sci. 2009;12(2):305–313. doi: 10.1111/j.1467-7687.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- LoBue V. And along came a spider: an attentional bias for the detection of spiders in young children and adults. J. Exp. Child Psychol. 2010;107(1):59–66. doi: 10.1016/j.jecp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- LoBue V. What’s so scary about needles and knives? Examining the role of experience in threat detection. Cogn. Emotion. 2010;24(1):180–187. [Google Scholar]
- LoBue V. What are we so afraid of? How early attention shapes our most common fears. Child Dev. Perspect. 2013;7(1):38–42. [Google Scholar]
- Lonigan C.J., Vasey M.W. Negative affectivity, effortful control, and attention to threat-relevant stimuli. J. Abnorm. Child Psychol. 2009;37(3):387–399. doi: 10.1007/s10802-008-9284-y. [DOI] [PubMed] [Google Scholar]
- Lonigan C.J., Vasey M.W., Phillips B.M., Hazen R.A. Temperament, anxiety, and the processing of threat-relevant stimuli. J. Clin. Child Adolesc. Psychol. 2004;33(1):8–20. doi: 10.1207/S15374424JCCP3301_2. [DOI] [PubMed] [Google Scholar]
- Lubman D.I., Peters L.A., Mogg K., Bradley B.P., Deakin J.F.W. Attentional bias for drug cues in opiate dependence. Psychol. Med. 2000;30(01):169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; 2014. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- MacLeod C., Mathews A., Tata P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Machado C.J., Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J. Child Psychol. Psychiatry. 2003;44(1):64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Mansell W., Clark D.M., Ehlers A., Chen Y.-P. Social anxiety and attention away from emotional faces. Cogn. Emotion. 1999;13(6):673–690. [Google Scholar]
- Mathews A. Effect of psychological treatment on cognitive bias in generalized anxiety disorder. Behav. Res. Ther. 1995;33(3):293–303. doi: 10.1016/0005-7967(94)e0022-b. [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Perez-Edgar K., Henderson H.A., Chronis-Tuscano A., Pine D.S., Fox N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary J.E., Meadows S.P., Amir N., Gibb B.E. Computer-delivered, home-based, attentional retraining reduces drinking behavior in heavy drinkers. Psychol. Addict. Behav. 2014;28(2):559–562. doi: 10.1037/a0036086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K., Field M., Bradley B.P. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology (Berl) 2005;180(2):333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Mogg K., Holmes A., Garner M., Bradley B.P. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behav. Res. Ther. 2008;46(5):656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogoaşe C., David D., Koster E.H.W. Clinical efficacy of attentional bias modification procedures: an updated meta-analysis: clinical efficacy of attention retraining. J. Clinical Psychology. 2014 doi: 10.1002/jclp.22081. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Klein R.G., Telzer E.H., Schroth E.A., Mannuzza S., Moulton J.L., Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Morales S., Pérez-Edgar K.E., Buss K.A. Attention biases towards and away from threat mark the relation between early dysregulated fear and the later emergence of social withdrawal. J. Abnormal Child Psychol. 2015;43(6):1067–1078. doi: 10.1007/s10802-014-9963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Pérez-Edgar K.E., Buss K. Longitudinal relations among exuberance, externalizing behaviors, and attentional bias to reward: the mediating role of effortful control. Dev. Sci. 2016;19(5):853–862. doi: 10.1111/desc.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S., Taber-Thomas B.C., Pérez-Edgar K.E. Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Dev. Sci. 2016 doi: 10.1111/desc.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morren M., Kindt M., van den Hout M., van Kasteren H. Anxiety and the processing of threat in children: further examination of the cognitive inhibition hypothesis. Behav. Change. 2003;20(03):131–142. [Google Scholar]
- Naim R., Abend R., Wald I., Eldar S., Levi O., Fruchter E., Bar-Haim Y. Threat-related attention bias variability and posttraumatic stress. Am. J. Psychiatry. 2015;172(12):1242–1250. doi: 10.1176/appi.ajp.2015.14121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Dolgin K.G. The generalized discrimination of facial expressions by seven-month-old infants. Child Dev. 1985;56(1):58. [PubMed] [Google Scholar]
- Nelson C.A. The recognition of facial expressions in the first two years of life: mechanisms of development. Child Dev. 1987;58(4):889. [PubMed] [Google Scholar]
- O’Doherty J., Winston J., Critchley H., Perrett D., Burt D., Dolan R. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Oakes L.M. Advances in eye tracking in infancy research. Infancy. 2012;17(1):1–8. doi: 10.1111/j.1532-7078.2011.00101.x. [DOI] [PubMed] [Google Scholar]
- Öhman A., Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol. Rev. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Fox N.A. Temperament and anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14(4):681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Roberson-Nay R., Hardin M.G., Poeth K., Guyer A.E., Nelson E.E., Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Bar-Haim Y., McDermott J.M., Gorodetsky E., Hodgkinson C.A., Goldman D., Fox N.A. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biol. Psychol. 2010;83(3):269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., McDermott J.N.M., Korelitz K., Degnan K.A., Curby T.W., Pine D.S., Fox N.A. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Dev. Psychol. 2010;46(6):1723–1730. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Reeb-Sutherland B.C., McDermott J.M., White L.K., Henderson H.A., Degnan K.A., Fox N.A. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J. Abnormal Child Psychol. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Kujawa A., Nelson S.K., Cole C., Zapp D.J. The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cogn. 2013;82(3):337–343. doi: 10.1016/j.bandc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Hardee J.E., Guyer A.E., Benson B.E., Nelson E.E., Gorodetsky E., Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Soc. Cogn. Affective Neurosci. 2014;9(4):445–453. doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Taber-Thomas B., Auday E., Morales S. Temperament and attention as core mechanisms in the early emergence of anxiety. In: Lagattuta K.H., editor. Vol. 26. S. KARGER AG; Basel: 2014. pp. 42–56. (Contributions to Human Development). Retrieved from http://www.karger.com/Article/Pdf/354350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou K.A., Smith T.J., Wu R., Johnson M.H., Kirkham N.Z., Ronald A. Individual differences in infant fixation duration relate to attention and behavioral control in childhood. Psychol. Sci. 2014;25(7):1371–1379. doi: 10.1177/0956797614531295. [DOI] [PubMed] [Google Scholar]
- Payne C., Machado C.J., Bliwise N.G., Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Vitaro F., Barker E.D., Borge A.I.H. The timing of middle-childhood peer rejection and friendship: linking early behavior to early-adolescent adjustment. Child Dev. 2007;78(4):1037–1051. doi: 10.1111/j.1467-8624.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Palokangas T., Hietanen J.K. Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Dev. Sci. 2008;11(1):60–68. doi: 10.1111/j.1467-7687.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Maki S., Hietanen J.K. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc. Cogn. Affect. Neurosci. 2009;4(2):134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J., Forssman L., Puura K., van IJzendoorn M.H., Leppänen J.M. Attention to faces expressing negative emotion at 7 months predicts attachment security at 14 months. Child Dev. 2015;86(5):1321–1332. doi: 10.1111/cdev.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L., Bakermans-Kranenburg M.J., van IJzendoorn M.H., Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol. Psychiatry. 2012;71(4):373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35(1):73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Bullmore E.T., Howard R., Woodruff P.W., Wright I.C., Williams S.C., David A.S. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. Neuroimaging. 1998;83(3):127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Pineles S.L., Mineka S. Attentional biases to internal and external sources of potential threat in social anxiety. J. Abnorm. Psychol. 2005;114(2):314–318. doi: 10.1037/0021-843X.114.2.314. [DOI] [PubMed] [Google Scholar]
- Pishyar R., Harris L.M., Menzies R.G. Attentional bias for words and faces in social anxiety. Anxiety Stress Coping. 2004;17(1):23–36. [Google Scholar]
- Pishyar R., Harris L.M., Menzies R.G. Responsiveness of measures of attentional bias to clinical change in social phobia. Cogn. Emotion. 2008;22(7):1209–1227. [Google Scholar]
- Polak-Toste C.P., Gunnar M.R. Temperamental exuberance: correlates and consequences. In: Marshall P.J., Fox N.A., editors. The Development of Social Engagement: Neurobiological Perspectives. Oxford University Press; New York, NY, US: 2006. pp. 19–45. [Google Scholar]
- Posner M.I., Rothbart M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007;58(1):1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. Control networks and neuromodulators of early development. Dev. Psychol. 2012;48(3):827–835. doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. Developing attention: behavioral and brain mechanisms. Adv. Neurosci. 2014;2014:e405094. doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Q. J. Exp. Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner M.I. Measuring alertness. Ann. N. Y. Acad. Sci. 2008;1129(1):193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- Posner M.I. University Press; Oxford: 2012. Attention in a Social World. [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Buhle J. Typologies of attentional networks. Nat. Rev. Neurosci. 2006;7(5):367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Risko E.F., Laidlaw K., Freeth M., Foulsham T., Kingstone A. Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh T.L., Scullin R.B., Langer J.K., Dixon D.J., Huppert J.D., Bernstein A., Lenze E.J. Unreliability as a threat to understanding psychopathology: the cautionary tale of attentional bias. J. Abnorm. Psychol. 2016;125(6):840–851. doi: 10.1037/abn0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.A., Feldman J.F., Jankowski J.J. Implications of infant cognition for executive functions at age 11. Psychol. Sci. 2012;23(11):1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Sheese B.E., Rueda M.R., Posner M.I. Developing mechanisms of self-regulation in early life. Emotion Rev. 2011;3(2):207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K., Posner M.I., Rosicky J. Orienting in normal and pathological development. Dev. Psychopathol. 1994;6(04):635. doi: 10.1017/S0954579400004715. [DOI] [Google Scholar]
- Roy A.K., Vasa R.A., Bruck M., Mogg K., Bradley B.P., Sweeney M., Pine D.S. Attention bias toward threat in pediatric anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Dennis T.A., Warner C.M. A critical review of attentional threat bias and its role in the treatment of pediatric anxiety disorders. J. Cogn. Psychother. 2015;29(3):171–184. doi: 10.1891/0889-8391.29.3.171. [DOI] [PubMed] [Google Scholar]
- Rubin K.H., Coplan R.J., Bowker J.C. Social withdrawal in childhood. Annu. Rev. Psychol. 2009;60(1):141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Salemink E., van den Hout M.A., Kindt M. Selective attention and threat: quick orienting versus slow disengagement and two versions of the dot probe task. Behav. Res. Ther. 2007;45(3):607–615. doi: 10.1016/j.brat.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Salum G.A., Mogg K., Bradley B.P., Gadelha A., Pan P., Tamanaha A.C., Pine D.S. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychol. Med. 2013;43(4):733–745. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., Costall A., Bente G., Schlicht T., Vogeley K. Toward a second-person neuroscience. Behav. Brain Sci. 2013;36(04):393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schmukle S.C. Unreliability of the dot probe task. Eur. J. Person. 2005;19(7):595–605. [Google Scholar]
- Schutter D.J.L.G., Putman P., Hermans E., van Honk J. Parietal electroencephalogram beta asymmetry and selective attention to angry facial expressions in healthy human subjects. Neurosci. Lett. 2001;314(1-2):13–16. doi: 10.1016/s0304-3940(01)02246-7. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Wright C.I., Shin L.M., Kagan J., Rauch S.L. Inhibited and uninhibited infants grown up: adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Kunwar P.S., Greve D.N., Kagan J., Snidman N.C., Bloch R.B. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol. Psychiatry. 2011;17(10):1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeta L., Tsuchiya N., Davies M.S., Sigman M., Bookheimer S.Y., Dapretto M. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. J. Neurodev. Disorders. 2012;4(1):17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Britton J.C., Pérez-Edgar K., Bar-Haim Y., Ernst M., Fox N.A., Pine D.S. Attention biases, anxiety, and development: toward or away from threats or rewards? Depress. Anxiety. 2012;29(4):282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Jarcho J.M., Britton J.C., Leibenluft E., Pine D.S., Nelson E.E. Attention bias of anxious youth during extended exposure of emotional face pairs: an eye-tracking study. Depress. Anxiety. 2013;30(1):14–21. doi: 10.1002/da.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese B.E., Rothbart M.K., Posner M.I., White L.K., Fraundorf S.H. Executive attention and self-regulation in infancy. Infant Behav. Dev. 2008;31(3):501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shibasaki M., Kawai N. Rapid detection of snakes by Japanese monkeys (Macaca fuscata): an evolutionarily predisposed visual system. J. Comp. Psychol. 2009;123(2):131–135. doi: 10.1037/a0015095. [DOI] [PubMed] [Google Scholar]
- Staugaard S.R. Reliability of two versions of the dot-probe task using photographic faces. Psychol. Sci. 2009;51(3):339. [Google Scholar]
- Stirling L.J., Eley T.C., Clark D.M. Preliminary evidence for an association between social anxiety symptoms and avoidance of negative faces in school-age children. J. Clin. Child Adolesc. Psychol. 2006;35(3):431–439. doi: 10.1207/s15374424jccp3503_9. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Holman P.J. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci. Biobehav. Revi. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi, S. J., 1999. Attachment in rhesus monkeys. Handbook of Attachment Theory, Research, and Clinical Applications, Ed. J. Cassidy., P. R. Shaver., 181–197.
- Susa G., Pitică I., Benga O., Miclea M. The self regulatory effect of attentional control in modulating the relationship between attentional biases toward threat and anxiety symptoms in children. Cogn. Emotion. 2012;26(6):1069–1083. doi: 10.1080/02699931.2011.638910. [DOI] [PubMed] [Google Scholar]
- Susa G., Benga O., Pitica I., Miclea M. Child temperamental reactivity and self-regulation effects on attentional biases. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.M., Posner M.I., Potkin S., Bonforte S., Youpa D., Fiore C., Crinella F. Activating tasks for the study of visual-spatial attention in ADHD children: a cognitive anatomic approach. J. Child Neurol. 1991;61(Suppl 1):S119–S127. doi: 10.1177/0883073891006001s12. [DOI] [PubMed] [Google Scholar]
- Taber-Thomas B., Pérez-Edgar K.E. Emerging adulthood brain development. In: Jensen J. Arnett., editor. The Oxford Handbook of Emerging Adulthood. 2015. pp. 126–141. [Google Scholar]
- Tamietto M., de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 2010;11(10):697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- Tamir M., Robinson M.D. The happy spotlight: positive mood and selective attention to rewarding information. Pers. Soc. Psychol. Bull. 2007;33(8):1124–1136. doi: 10.1177/0146167207301030. [DOI] [PubMed] [Google Scholar]
- Taylor C.T., Bomyea J., Amir N. Malleability of attentional bias for positive emotional information and anxiety vulnerability. Emotion. 2011;11(1):127–138. doi: 10.1037/a0021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.M., Anderson A.K. Salience, state, and expression: the influence of specific aspects of emotion on attention and perception. In: Ochsner K.N., Kosslyn S., editors. Vol 2. The Cutting Edges; 2013. pp. 11–31. (The Oxford Handbook of Cognitive Neuroscience). [Google Scholar]
- Todd R.M., Cunningham W.A., Anderson A.K., Thompson E. Affect-biased attention as emotion regulation. Trends Cogn. Sci. 2012;16(7):365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S., McDermott J.M., Nelson C.A., Zeanah C.H., Fox N.A. The effects of early foster care intervention on attention biases in previously institutionalized children in Romania. Dev. Sci. 2015;18(5):713–722. doi: 10.1111/desc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S., McLaughlin K.A., Sheridan M.A., Nelson C.A., Zeanah C.H., Fox N.A. The beneficial effects of a positive attention bias amongst children with a history of psychosocial deprivation. Biol. Psychol. 2016 doi: 10.1016/j.biopsycho.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B., Verschuere B., Tibboel H., De Houwer J., Crombez G., Koster E.H.W. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychol. Bull. 2013 doi: 10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- Van Damme S., Crombez G., Eccleston C., Goubert L. Impaired disengagement from threatening cues of impending pain in a crossmodal cueing paradigm. Eur. J. Pain. 2004;8(3):227–236. doi: 10.1016/j.ejpain.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Van Damme S., Lorenz J., Eccleston C., Koster E.H.W., De Clercq A., Crombez G. Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophys. Clin./Clin. Neurophysiol. 2004;34(1):33–39. doi: 10.1016/j.neucli.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Van Damme S., Crombez G., Hermans D., Koster E.H.W., Eccleston C. The role of extinction and reinstatement in attentional bias to threat: a conditioning approach. Behav. Res. Ther. 2006;44(11):1555–1563. doi: 10.1016/j.brat.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemel-Ruiter M.E., de Jong P.J., Ostafin B.D., Wiers R.W. Reward sensitivity, attentional bias, and executive control in early adolescent alcohol use. Addict. Behav. 2015;40:84–90. doi: 10.1016/j.addbeh.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Lipp O.V. The influence of animal fear on attentional capture by fear-relevant animal stimuli in children. Behav. Res. Ther. 2008;46(1):114–121. doi: 10.1016/j.brat.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Lipp O.V. Visual search for emotional faces in children. Cogn. Emotion. 2008;22(7):1306–1326. [Google Scholar]
- Waters A.M., Lipp O.V., Spence S.H. Attentional bias toward fear-related stimuli: an investigation with nonselected children and adults and children with anxiety disorders. J. Exp. Child Psychol. 2004;89(4):320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Lipp O., Spence S. Visual search for animal fear-relevant stimuli in children. Aust. J. Psychol. 2008;60(2):112–125. [Google Scholar]
- Waters A.M., Mogg K., Bradley B.P., Pine D.S. Attentional bias for emotional faces in children with generalized anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(4):435–442. doi: 10.1097/CHI.0b013e3181642992. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Pittaway M., Mogg K., Bradley B.P., Pine D.S. Attention training towards positive stimuli in clinically anxious children. Dev. Cogn. Neurosci. 2013;4:77–84. doi: 10.1016/j.dcn.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Bradley B.P., Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of distress versus fear diagnostic categorization. Psychol. Med. 2014;44(3):607–616. doi: 10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Werker J.F., Tees R.C. The organization and reorganization of human speech perception. Annu. Rev. Neurosci. 1992;15(1):377–402. doi: 10.1146/annurev.ne.15.030192.002113. [DOI] [PubMed] [Google Scholar]
- Werthmann J., Roefs A., Nederkoorn C., Mogg K., Bradley B.P., Jansen A. Can(not) take my eyes off it: attention bias for food in overweight participants. Health Psychol. 2011;30(5):561–569. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- Werthmann J., Field M., Roefs A., Nederkoorn C., Jansen A. Attention bias for chocolate increases chocolate consumption—an attention bias modification study. J. Behav. Ther. Exp. Psychiatry. 2014;45(1):136–143. doi: 10.1016/j.jbtep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Werthmann J., Jansen A., Vreugdenhil A.C.E., Nederkoorn C., Schyns G., Roefs A. Food through the child’s eye: an eye-tracking study on attentional bias for food in healthy-weight children and children with obesity. Health Psychol. 2015;34(12):1123–1132. doi: 10.1037/hea0000225. [DOI] [PubMed] [Google Scholar]
- White L.K., Henderson H.A., Pérez-Edgar K.E., Walker O.L., Degnan K.A., Shechner T., Fox N.A. Developmental relations between behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Dev. 2016 doi: 10.1111/cdev.12696. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.R., Ponesse J.S., Schachar R.J., Logan G.D., Tannock R. Development of inhibitory control across the life span. Dev. Psychol. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Yeomans M.R., Javaherian S., Tovey H.M., Stafford L.D. Attentional bias for caffeine-related stimuli in high but not moderate or non-caffeine consumers. Psychopharmacology (Berl.) 2005;181(3):477–485. doi: 10.1007/s00213-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: a review of attentional processing of emotional information. Cogn. Emotion. 2010;24(1):3–47. [Google Scholar]
- Zvielli A., Bernstein A., Koster E.H.W. Dynamics of attentional bias to threat in anxious adults: bias towards and/or away? PLoS One. 2014;9(8):e104025. doi: 10.1371/journal.pone.0104025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvielli A., Bernstein A., Koster E.H.W. Temporal dynamics of attentional bias. Clin. Psychol. Sci. 2015;3(5):772–788. [Google Scholar]