Abstract
Hematopoietic cell transplantation can cure many high-risk diseases but is associated with complexity, cost, and risk. Several areas in transplantation practice were identified in the 2014 Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium (BMT CTN SOSS) as high priorities for further study. We developed a survey for hematopoietic cell transplantation clinicians to identify current practices in BMT CTN SOSS priority areas and to understand, more generally, the variation in approach to transplantation and estimation of transplantation benefit in current medical practice. Of 1439 transplantation clinicians surveyed, 305 responded (20% response rate). Clinicians were well represented by age, experience, geography, and size of practice. We found that several techniques identified in the BMT CTN SOSS, such as maintenance therapy for acute myeloid leukemia or myelodysplastic syndromes after allogeneic transplantation, were already being utilized in practice on and off study, with higher rates of use in higher-volume centers. There was significant variation among clinicians in use of transplantation technologies and approaches to common transplantation scenarios. Appraisals of risks and benefits of transplantation appeared to converge upon similar estimates despite the presentation of different hypothetical scenarios. These results suggest overall equipoise in several BMT CTN SOSS high-priority areas and support the need for better data to inform clinical practice.
Keywords: Hematopoietic cell, transplantation, Practice patterns, Diffusion of innovations, Technology, Survey
INTRODUCTION
Hematopoietic cell transplantation (HCT) is a life-extending or life-saving therapy for many patients with malignant and nonmalignant diseases, but it is 1 of the more costly and complex areas of contemporary medical practice [1]. For almost any condition that is potentially amenable to transplantation, there are a wide array of management approaches that incorporate variations in patient selection criteria, pretransplantation conditioning regimens, donor type, graft source, and prevention and management of infectious and noninfectious post-transplantation complications. Thus, it is important to subject areas of uncertainty or new technology to scientific scrutiny, to identify new standards for the field. The 2014 Blood and Marrow Transplant Network (BMT CTN) State of the Science symposium (SOSS) identified several areas that were deemed to be high priorities for further study [2].
To facilitate the implementation of studies in high-priority areas from the BMT CTN SOSS, we sought to determine the extent to which emerging transplantation techniques in these areas were already being used in current transplantation practice. Further, by studying practice variation, an area highlighted in earlier transplantation studies [3], we hoped to better understand the degree to which transplantation clinicians might differ from each another in their utilization of new technologies, their preferences for approaches to common transplantation problems, and the way in which they estimated the benefits or risks of these therapies. As part of this effort, we hoped to find ways to increase the development and implementation of research to address areas of practice uncertainty.
We developed a survey to identify current practice patterns and preferences among HCT clinicians. Our immediate goal was to inform the BMT CTN about current practices among transplantation clinicians in BMT CTN SOSS priority areas to assist future BMT CTN study planning. For example, if the survey indicated wide variability in the use of a given intervention, that would argue in favor of a study evaluating the utility of that intervention. On the other hand, if the survey found broad off-study use of an intervention, that would suggest that a study comparing that intervention to a placebo might be difficult to complete. Our larger goal was to begin to observe and understand variation in clinicians’ approaches to transplantation in contemporary practice. For example, many of the interventions described in the BMT CTN SOSS are associated with high costs and potential toxicities, leading to their prioritization as topics worthy of study in clinical trials. If our survey found that there were high rates of use of these technologies in practice ahead of well-designed clinical trials, this would suggest that additional ways to assess benefits and risks of these techniques in usual practice are needed. Lastly, by studying treatment preferences among HCT clinicians, we hoped to gain insight into the way that decisions are made in usual practice and identify potential contributors to practice variation that could be addressed in future work.
METHODS
Survey Development
A 3-section survey was developed for HCT clinicians. In the first section, 11 questions collected participant and practice characteristics, including age, experience, center geographic region, and center transplantation volume. A second section of 22 questions asked participants to describe their current use, on or off study, of transplantation technologies identified within high-priority research areas from the 2014 BMT CTN SOSS. Examples included the use of maintenance therapy after allogeneic transplantation for FLT3+ acute myeloid leukemia (AML), the use of autologous transplantation for multiple sclerosis, and the use of pretransplantation risk stratification variables to guide patient selection and decision-making [4-6]. In the third section, questions included 2 hypothetical clinical vignettes: 1 about a 45-year-old woman with intermediate-risk AML considering transplantation and the other about a 30-year-old male with BCR/ABL-negative B cell acute lymphoblastic leukemia (ALL) considering transplantation. Each vignette was associated with 7 or 8 questions that asked participants to indicate their treatment recommendation for the hypothetical patient or variations of the hypothetical patient (eg, the same patient but a different age, or the same patient with a different comorbidity profile) and their estimates of long-term success or toxicity of their selected approach. The survey was designed so that participants would be asked to answer all questions from the first and second sections and then randomized to answer questions from 1 of the 2 vignettes in the third section. The survey was pilot tested by 2 of the investigators (W.A.W. and S.J.L.), as well as by 4 additional transplantation physicians, and revised for clarity. A final copy of the survey is attached in the Appendix. Subsequent distribution of the finalized survey to a larger national sample of transplantation clinicians was approved by the institutional review board at the University of North Carolina. The survey introduction contained the elements of informed consent, and completion of the survey indicated consent.
Survey Distribution and Data Collection
The finalized survey was distributed to an electronic mailing list maintained by the Center for Blood and Marrow Transplant Research (CIBMTR), using Survey Gizmo (www.surveygizmo.com). The intent was to identify practicing transplantation clinicians using the CIBMTR mailing list, and the list was reviewed by 1 of the investigators to remove participants who were not identified as transplantation clinicians. The final sample size of potential participants was 1439. The survey was open from April 24, 2015 through May 26, 2015. Weekly email reminders were sent to all potential participants, and 2 sequential survey drawings ($500 and $250 prizes, respectively) for respondents were held to incentivize participation.
Statistical Methods
Statistical analyses were performed using SAS 9.4. Summaries of subject responses to survey questions with categorical responses were examined using frequency tables, and percentages are reported.
Exploratory analyses were conducted using Fisher's exact tests, with Monte Carlo estimates of the exact P values to compare responses across characteristic categories; unadjusted P values are reported.
RESULTS
Participants
Of the 1439 potential participants invited to complete the survey, we received 305 responses. Eleven individuals declined to fill out the survey after initially responding. Six participants reported spending 0% time on patient care and were excluded from analysis. Fifteen participants did not provide information beyond the “general information” section of the survey. The overall survey response rate was 20%.
Participant characteristics are described in Table 1. Most participants (86%) were from academic centers, with 65% providing care primarily for adult patients. Seventy-seven percent of participants had at least 5 years of experience as a transplantation physician, with an average of 14.8 years of postfellowship experience per participant. A majority of participants (54%) practiced at a BMT CTN core center, and about one-third of participants practiced at centers performing a high volume (>100/year) of either autologous or allogeneic transplantations.
Table 1.
Survey Respondents Self-Report of HCT Physician, HCT Center, and Patients
| HCT Physician Characteristics | n (%) |
|---|---|
| Practice setting | |
| Academic | 262 (85.9%) |
| Community | 43 (14.1%) |
| Age | |
| 30-40 yr | 73 (24.8%) |
| 41-50 yr | 90 (30.6%) |
| 51-60 yr | 79 (26.9%) |
| ≥ 60 yr | 52 (17.7%) |
| Percent effort in clinical duties | |
| <20% | 13 (4.3%) |
| 20%-39% | 59 (19.3%) |
| 40%-59% | 80 (26.2%) |
| 60%-79% | 91 (29.8%) |
| 80%-100% | 62 (20.3%) |
| Duration of practice as HCT physician | |
| ≤uryr | 68 (23.2%) |
| 5-15 yr | 83 (28.3%) |
| 15-25 yr | 69 (23.6%) |
| ≥9 yr | 73 (24.9%) |
| Adult | 190 (64.9%) |
| Pediatric | 93 (31.7%) |
| Adult and pediatric | 10 (3.4%) |
| HCT Center and Patient Characteristics | n (%) |
|---|---|
| BMT CTN center status | |
| Core | 164 (53.8%) |
| Affiliate | 108 (35.4%) |
| Not a BMT CTN center | 33 (10.8%) |
| Geographic location | |
| Mid-Atlantic | 34 (11.2%) |
| Midwest | 85 (27.9%) |
| Northeast | 59 (19.3%) |
| Pacific | 42 (13.8%) |
| Southeast | 48 (15.7%) |
| Southwest | 37 (12.1%) |
| Center auto experience | |
| <20 | 65 (22.2%) |
| 20-50 | 66 (22.5%) |
| 50-100 | 58 (19.8%) |
| >100 | 104 (35.5%) |
| Center allo experience | |
| <20 | 46 (15.7%) |
| 20-50 | 81 (27.7%) |
| 50-100 | 77 (26.3%) |
| >100 | 89 (30.4%) |
Allo indicates allogeneic.
Maintenance Therapies
Participants were asked whether they recommended various maintenance strategies after transplantation for patients in remission. These strategies included FLT inhibitors for FLT3 internal tandem duplication (ITD)+ AML patients after allogeneic HCT; tyrosine kinase inhibitor maintenance therapy for Philadelphia chromosome (Ph)–positive ALL patients after allogeneic HCT; antibody-based maintenance therapy for Ph-negative ALL patients after allogeneic HCT; hypomethylating agents for myelodysplastic syndrome or AML patients after allogeneic HCT; and maintenance therapy such as rituximab, ibrutinib, or lenalidomide for diffuse large B cell lymphoma patients after autologous HCT. Figure 1 depicts the percentage of respondents who would recommend the use of each of these approaches, on or off clinical trials. In general, there was already significant uptake of maintenance strategies in current practice, though this varied by indication and by the willingness of participants to use these strategies outside of existing clinical studies. However, of the interventions included in the survey, the only 1 that was recommended for use off study by a majority of respondents was the use of tyrosine kinase inhibitor maintenance for Ph+ ALL patients after transplantation (72.9%). In contrast, none of the other interventions were recommended by a majority for use outside of clinical trials.
Figure 1.
Recommendations for various maintenance strategies after transplantation for patients in remission. FLT3 was the designation for the use of FLT inhibitors for FLT3 ITD+ acute myeloid leukemia patients in morphologic remission after allogeneic HCT; BCR/ABL+ for the use of tyrosine kinase inhibitor maintenance therapy for Philadelphia chromosome–positive acute lymphoblastic leukemia patients in remission after allogeneic HCT; BCR/ABL− for the use of antibody-based maintenance therapy for Philadelphia chromosome–negative acute lymphoblastic leukemia patients in remission after allogeneic HCT; HMA for the use of hypomethylating agents for myelodysplastic syndrome or acute myeloid leukemia patients in remission after allogeneic HCT; DLBCL for the use of maintenance therapy such as rituximab, ibrutinib, or lenalidomide for DLBCL in remission after autologous HCT. HMA indicates hypomethylating agent; DLBCL, diffuse large B cell lymphoma.
Emerging Transplantation Techniques
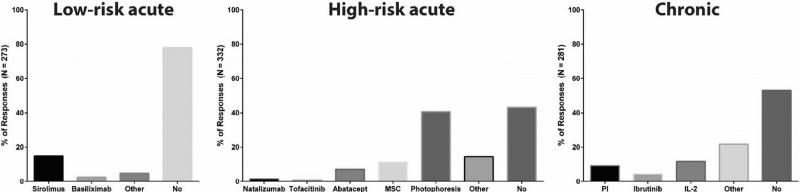
Respondents were queried about their use of newer transplantation strategies and technologies that had been identified as priority areas of study by the 2014 BMT CTN SOSS. For example, participants were asked about the use of newer medications in the initial management of graft-versus-host disease (GVHD), either instead of corticosteroids (low-risk acute GVHD), in addition to corticosteroids (high-risk acute GVHD), or with or without corticosteroids (chronic GVHD) (Figure 2). Although a majority or near majority of participants did not use newer medications, several participants indicated that they had used a variety of techniques, such as sirolimus alone for low-risk acute GVHD (13.6%), extracorporeal photopheresis for high-risk acute GVHD (39.6%), and IL-2 for chronic GVHD (11.4%). The use of extracorporeal photopheresis for high-risk acute GVHD on or outside of clinical trials was very similar between all center volumes (40.5% for <20 allogeneic HCT/year, 47.4% for 20 to 50 allogeneic HCT/year, 40.0% for 50 to 100 allogeneic HCT/year, and 38.3% for >100 allogeneic HCT/year.
Figure 2.
Responses to the use of newer medications in the initial management of graft-versus-host disease (GVHD), whether instead of corticosteroids (low-risk acute GVHD), in addition to corticosteroids (high-risk acute GVHD), or with or without corticosteroids (chronic GVHD). MSC indicates mesenchymal stem cells; PI, proteasome inhibitor.
Other questions asked about the use of additional transplantation strategies, including autologous transplantation for multiple sclerosis, post-transplantation cyclophosphamide for GVHD prophylaxis, haploidentical donor natural killer cells for post-transplantation relapse prevention, cytomegalovirus-specific T cells for refractory cytomegalovirus infection, and screening and prevention strategies for post-transplantation bone density loss. In each situation, substantial minorities of participants reported use of these technologies in current practice (Table 2). Table 3 demonstrates the use of selected transplantation techniques (including a few of the maintenance strategies) categorized by transplantation center volume, using the number of allogeneic transplantations performed per year as a surrogate measure of volume. Higher-volume centers appeared to be associated with a higher use of newer transplantation techniques.
Table 2.
Use of Additional Transplantation Strategies
| Response | Post Cy | Donor NK cells | CMV T Cells | DEXA Allo |
|---|---|---|---|---|
| No, do not recommend | 101 of 267 (37.8%) | 243 of 267 (91.0%) | 187 of 266 (70.3%) | 190 of 266 (71.4%) |
| Yes, on/off trials and within trial | 166 of 267 (62.2%) | 24 of 267 (9.0%) | 79 of 266 (29.7%) | 76 of 266 (28.6%) |
Post Cy indicates post-transplantation cyclophosphamide for GVHD prophylaxis; Donor NK cells, haploidentical donor natural killer cells for post-transplantation relapse prevention; CMV T cells, cytomegalovirus-specific T cells for refractory cytomegalovirus infection; DEXA allo, screening and prevention strategies for post-transplantation bone density loss.
Table 3.
Use of Selected Transplantation Techniques and Maintenance Strategies
| Center Size |
||||
|---|---|---|---|---|
| Technologies | <20 | 20-50 | 50-100 | >100 |
| FLT3 | 21 of 39 (53.9%) | 54 of 78 (69.2%) | 43 of 71 (60.6%) | 68 of 81 (84.0%) |
| DLBCL | 9 of 65 (13.9%) | 10 of 61 (16.4%) | 8 of 56 (14.3%) | 20 of 101 (19.8%) |
| IL-2 | 1 of 37 (2.7%) | 7 of 78 (9.0%) | 8 of 70 (11.4%) | 17 of 81 (21.0%) |
| Auto for MS | 6 of 65 (9.2%) | 10 of 61 (16.4%) | 9 of 56 (16.1%) | 36 of 101 (35.6%) |
| Post-Cy | 9 of 38 (23.7%) | 38 of 78 (48.7%) | 24 of 70 (34.3%) | 41 of 81 (50.6%) |
| Donor NKs | 2 of 78 (2.6%) | 4 of 70 (5.7%) | 1 of 38 (2.6%) | 17 of 81 (21.0%) |
The number of allogeneic transplantations performed each year was used as a proxy for center size.
Data provided reflect the responses “Yes, including patients who are on and off clinical trials” and “Yes, only within the context of a clinical trial.”
FLT3 indicates use of FLT inhibitors for FLT3 ITD+ AML patients in morphologic remission after allogeneic HCT; DLBCL, use of maintenance therapy such as rituximab, ibrutinib, or lenalidomide for diffuse large B cell lymphoma patients in remission after autologous HCT; IL-2 for patients with chronic GVHD (with or without steroids); Auto for MS, autologous transplantation for multiple sclerosis.
Transplantation Decision-Making
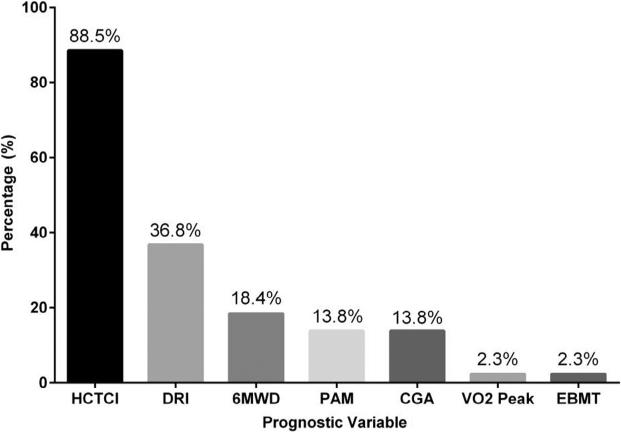
Participants were asked which assessment tools they used to help decide whether to recommend transplantation for older adults. Results are shown in Table 4 and Figure 3. The HCT-specific comorbidity index (HCT-CI) was used frequently by participants for transplantation decision-making (88.5%), whereas other scoring systems of disease risk (Disease Risk Index, 36.8%) or overall risk (Pre-Transplant Assessment of Mortality score, 13.8%) were used less frequently. When categorized by overall center volume (allogeneic transplantations per year), participants from higher-volume centers appeared to endorse a greater use of nearly all of the instruments available for pretransplantation risk assessment.
Table 4.
Use of Prognostic Variables More Common in Higher Volume Centers for Pretransplantation Risk Stratification
| Center Size |
||||
|---|---|---|---|---|
| Prognostic Variable | <20 | 20-50 | 50-100 | >100 |
| CGA | 1 of 42 (2.4%) | 3 of 80 (3.8%) | 9 of 71 (12.7%) | 12 of 87 (13.9%) |
| PAM | 3 of 42 (7.1%) | 6 of 80 (7.5%) | 3 of 71 (4.2%) | 12 of 87 (13.8%) |
| HCT-CI | 14 of 42 (33.3%) | 50 of 80 (62.5%) | 55 of 71 (77.5%) | 77 of 87 (88.5%) |
| DRI | 4 of 42 (9.5%) | 17 of 80 (21.3%) | 17 of 71 (23.9%) | 32 of 87 (36.8%) |
| EBMT | 4 of 42 (9.5%) | 4 of 80 (5.0%) | 6 of 71 (8.5%) | 2 of 87 (2.3%) |
| 6MWD | 3 of 42 (7.1%) | 5 of 80 (6.3%) | 10 of 71 (14.1%) | 16 of 87 (18.4%) |
| VO2 Peak | 1 of 42 (2.4%) | 2 of 80 (2.5%) | 3 of 71 (4.2%) | 2 of 87 (2.3%) |
The number of allogeneic transplantations performed each year was used as a proxy for center size.
CGA indicates comprehensive geriatric assessment; PAM, Pre-transplant Assessment of Mortality score; DRI, Disease Risk Index; EBMT, European Group for Blood and Marrow Transplantation risk score; 6MWD, 6-minute walk distance; VO2 peak, peak oxygen uptake.
Figure 3.
Common prognostic variables in highest volume centers (>100 allogeneic transplantations per year) for pretransplantation risk stratification. Participants could check as many tools as they wished. HCT-CI indicates HCT-related comorbidity index; DRI, Disease Risk Index; 6MWD, 6-minute walk distance; PAM, Pretransplant Assessment of Mortality score; CGA, comprehensive geriatric assessment; VO2 peak, peak oxygen uptake; EBMT, European Group for Blood and Marrow Transplantation risk score.
Finally, participants were asked to make transplantation recommendations for hypothetical patients described in clinical vignettes and to provide estimates of risk associated with their preferred management approaches. For each vignette, subsequent questions modified 1 or more details of the hypothetical patient and asked participants to reassess decision-making and risk. Participants were randomized to answer questions associated with only 1 of the vignettes. The first vignette described a 45-year-old female with intermediate-risk AML (normal cytogenetics, FLT3 ITD negative, NPM1 negative) who was in remission, had a matched sibling donor available, had no major comorbid illnesses, and could perform all of her instrumental activities of daily living. In this clinical scenario, the total number of respondents was 93. Most respondents recommended a myeloablative conditioning allogeneic transplantation (n = 74, 79.6%), though some recommended a reduced-intensity transplantation (n = 4, 4.3%) or no transplantation (n = 15, 16.1%) (Figure 4). When asked to provide risk estimates associated with their preferred choice, respondents indicated similar estimates of treatment-related mortality (TRM) (13% to 19%) regardless of transplantation strategy chosen. Median overall survival estimates at 2 years for those recommending transplantation were 61% for those recommending myeloablative transplantation and 71% for those recommending reduced-intensity transplantation. Participants were then asked what they would recommend if the same hypothetical patient had an HCT-CI score of 4, based on diabetes mellitus requiring insulin, forced expiratory volume in 1 second of 66% to 80%, and a body mass index of 36. This led to a wider distribution of recommended transplantation techniques, with most recommending a reduced-intensity transplantation but a sizable minority of participants recommending a myeloablative transplantation or no transplantation at all. Toxicity estimates remained consistent across recommended strategies, with an estimated TRM of 23% among those recommending myeloablative transplantation and 22% for those recommending reduced-intensity transplantation. When informed that the patient in the original vignette did not have a matched sibling or matched unrelated donor identified, the majority of participants (n = 51, 55%) would recommend no transplantation, whereas fewer participants (n = 42, 45%) would recommend a transplantation, with conditioning and donor source preference varying widely among those recommending a transplantation, including myeloablative mismatched unrelated donor (n = 15), myeloablative haploidentical transplantation (n = 10), myeloablative cord blood (n = 9), reduced-intensity mismatched unrelated donor (n= 2), reduced-intensity haploidentical (2), and reduced-intensity cord blood (n = 3).
Figure 4.
Breakdown of responses for vignette involving 45-year-old female with intermediate-risk AML (CN, FLT3/NPM1-) in remission after induction therapy with no comorbidities, matched sibling, performs all instrumental activities of daily living and continues to work. Allo indicates allogeneic; RIC, reduced-intensity; TRM, treatment-related mortality.
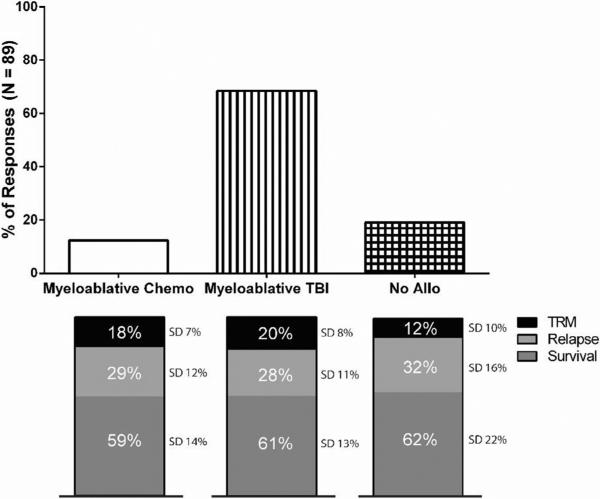
The second vignette described a hypothetical 30-year-old male with standard-risk, Ph-negative B ALL in first complete remission after “adult-like” induction chemotherapy, having no comorbidities and with a matched sibling donor identified. In this clinical scenario, the total number of respondents was 89. A majority of participants (n = 61, 68.5%) recommended a myeloablative, total body irradiation–based transplantation, though a minority of participants recommended a myeloablative, chemotherapy-based transplantation (n = 11, 12.4%) or no transplantation at all (n = 17, 19.1%) (Figure 5). Overall survival estimates at 2 years were remarkably similar regardless of strategy choice (59% to 62%). When the age of the hypothetical patient was changed to 45 from 30, most participants again recommended myeloablative total body irradiation–based transplantation, though the estimates provided for TRM (20% versus 17%) and overall survival (61% versus 59%) for this choice were nearly identical despite the increased age of the hypothetical patient. When informed that the patient in the original vignette did not have a matched sibling or matched unrelated donor identified, the majority of participants (n = 55, 62%) participants would recommend no transplantation, whereas fewer participants (n = 34, 38%) would recommend a transplantation, whether from a mismatched unrelated donor (n = 18), haploidentical donor (n = 11), or cord blood (n = 5).
Figure 5.
Breakdown of responses for vignette involving 30-year-old male standard-risk BCR/ABL- B-ALL in first complete remission after “adult-like” induction with matched sibling and HCT-CI = 0. TBI indicates total body irradiation; allo, allogeneic.
DISCUSSION
We surveyed a large group of HCT clinicians in the United States about current transplantation practices and preferences and obtained a broadly representative sample of individuals from different parts of the country, different size centers, and with varying ages and levels of experience. We enriched our questions for topics that were addressed in the 2014 BMT CTN SOSS, since these topics were felt to represent the future of transplantation and potential topics for clinical trials. From our survey results, several themes emerged.
First, there is a diffusion of new transplantation technology into clinical practice and not always within the context of a clinical trial. For example, a majority of survey participants expressed a willingness to use tyrosine kinase inhibitors off study. However, far fewer favored the use of FLT3 inhibitors or hypomethylating agents off study. Some survey participants reported current use of other cutting-edge practices, such as the use of autologous transplantation for multiple sclerosis or donor natural killer cells after transplantation. In general, it appeared that the use of newer transplantation techniques was most prevalent in survey respondents who reported practicing in a large-volume transplantation center, suggesting that transplantation center size may be a determinant of the diffusion of transplantation innovations, perhaps because they are more likely to have clinical trials testing these interventions. We found that center size also appeared to be associated with a greater use of risk stratification variables as part of transplantation decision-making for older adults. This suggests that the diffusion of pretransplantation evaluative processes (and potentially other processes in the peri- or post-transplantation periods) may occur in a manner that is similar to the spread of specific transplantation technologies.
Interestingly, our results from the hypothetical vignettes showed a consistency around outcomes estimation, such as TRM and overall survival, in different treatment scenarios. For example, survey respondents reported an average 2-year overall survival estimate of 61% and TRM estimate of 19% for a 45-year-old female with intermediate-risk AML receiving a myeloablative allogeneic transplantation in first remission. Very similar estimates to the above were provided by respondents for overall survival (61%) and TRM (20%) for a 30-year-old male with standard risk Ph-negative ALL receiving a cyclophosphamide/total body irradiation transplantation in first complete remission. There may be a cognitive anchoring bias in the way that transplantation physicians estimate the success of their interventions, such that estimates of efficacy and toxicity converge around similar numbers, perhaps adjusted slightly 1 way or the other for positive or negative risk factors. These estimates may or may not correspond with findings from the published literature, such as the Medical Research Council trial [7]. It is possible that clinicians are factoring in improvements in supportive care and other factors affecting outcomes that have been made since the previous studies were published. In any case, despite the consistency in outcomes estimation, there was continued variation in the preferences expressed by transplantation clinicians for different transplantation techniques. For given scenarios, some respondents recommended transplantation and others did not, and some recipients expressed preferences for different conditioning intensities and different graft sources. These findings suggest continued controversy and lack of consensus in key areas of transplantation practice.
We believe that our findings point to the following strategies that could be used to better understand and facilitate optimal practice patterns in the field of HCT.
Clinical Trial Development
As a cooperative trial group dedicated specifically to transplantation, the BMT CTN represents a powerful mechanism for addressing key transplantation questions using rigorous methodology. In planning BMT CTN studies, however, investigators must recognize that there is variability among physicians concerning their perception of the evidence supporting the application of emerging therapies. Indeed, our results demonstrate that several techniques proposed at the 2014 BMT CTN SOSS as worthy of investigation are already in use at many centers. This creates a risk that studies aimed at evaluating these interventions may fail to accrue as planned. On the other hand, it is possible that some centers may wish to use an emerging technology in the absence of a concurrent well-designed clinical trial, but the center may remain willing to enroll onto a clinical trial testing this question if 1 is developed. Surveys, such as that presented here, may prove useful in clinical trial planning.
A separate issue is whether or not the results of studies alter behavior at centers. For example, the BMT CTN 0201 study showed equivalent survival after allogeneic transplantation when peripheral blood or bone marrow was used as a source of stem cells, with a higher risk of chronic GVHD after peripheral blood stem cell grafts [8]. A subsequent analysis confirmed that peripheral blood stem cell grafts were associated with inferior long-term health-related quality of life and reduced rates of return to work, compared with those after bone marrow grafts [9]. Nonetheless, for several reasons, the field had already moved towards an increased use of peripheral blood grafts before publication and dissemination of the BMT CTN 0201 results, and it is not clear that these results will significantly alter this trend [10]. Understanding factors that influence the presence or absence of adoption of BMT CTN clinical trials results in usual practice is an important area for further study.
Real-time Registry Use
Transplantation technology evolves sufficiently rapidly that many emerging practices will not be formally studied in large randomized clinical trials. At the same time, while local innovation has long been an important component of progress in the field, uneven adoption of practices with lower-level evidence creates risks that beneficial practices will not disseminate completely or that practices that expose subsets of patients to undue toxicity or harm will not be identified and avoided. The registry maintained by the CIBMTR may be able, in some circumstances, to monitor emerging transplantation practices. Although some of these studies may be underpowered to draw definitive conclusions about effectiveness, potential safety signals may be identified that lead to larger studies. For example, 2 phase 2 studies, published in early 2015, suggested a potential benefit for a thiotepa, busulfan, and cyclophosphamide conditioning regimen followed by autologous transplantation in the management of central nervous system lymphoma [11,12]. The extent to which this technique has disseminated into routine transplantation practice, and whether observed toxicities in a real-world setting are similar to or different than those reported in the studies, is not known. Depending on available resources for enhanced data collection, the CIBMTR might be able to investigate a question like this in relatively rapid fashion. Safety signals or triggers for further analysis after the initial investigation would need to be determined. At the same time, factors associated with faster or slower rates of innovation uptake could be identified to facilitate implementation of “best practices” as these are determined. It is likely that a group of individuals would need to be appointed to periodically determine which emerging technology questions are of the most urgent priority to the field to minimize reporting burden upon centers.
Clinical Decision Support
The results of our hypothetical vignettes suggest that transplantation technique preferences vary among clinicians, but estimates of the efficacy of these practices may converge around cognitive anchors that may or may not correspond to published data. This is not surprising in a field where there are many possible permutations of patient-, disease-, and transplantation-related variables for any given clinical scenario. Certain combinations of variables may be more or less familiar to clinicians based on personal experience. Further, clinicians may be challenged to readily identify published data that corresponds to a particular clinical scenario encountered in practice. To the extent that data do exist, patient-centered outcomes such as quality of life and functional status are often not included. These deficiencies may create barriers to achieving a balanced appraisal of the risks and benefits of a management approach that is being considered. Thus, there may be a role for electronic platforms that facilitate the spread of personal experience and published data throughout the transplantation community, such as the recently launched American Society for Blood and Marrow Transplantation Clinical Case Forum [13]. There may also be a role for entities such as the CIBMTR to develop point-of-care risk calculators that can guide clinical decision-making, similar to Adjuvant Online (https://adjuvantonline.com/), which is used for breast cancer [14,15]. These calculators could integrate known risk indices, such as the HCT-CI, and emerging risk factors as these are identified, so that clinician estimates of efficacy or toxicity would vary appropriately based on the literature, rather than converging around similar estimates for most scenarios as we observed in the vignettes. If the CIBMTR incorporates patient-reported outcomes into routine data collection, these risk calculators could become even more relevant for patient counseling [16-18]. Of note, the CIBMTR currently has an online survival calculator for allogeneic HCT, using the center-specific survival analysis model to generate estimates for 1-year overall survival based on a set of user-inputted individual patient characteristics. Calculators like this, as they become further developed and more widely available, may prove an invaluable resource for transplantation clinicians.
CONCLUSION
In our survey of HCT clinicians, we identified prevalent but uneven uptake of newer technologies in transplantation practice, variation among clinicians in transplantation preferences, and potential challenges in point of care risk/ benefit estimation in routine practice. These observations highlight the value of networks such as the BMT CTN dedicated to the conduct of prospective, controlled clinical trials; warrant further study to confirm these findings; and support approaches to facilitate advancements in transplantation outcomes throughout the community.
ACKNOWLEDGMENTS
Financial disclosure: There is nothing to disclose.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2016.07.014.
REFERENCES
- 1.Gratwohl A, Sureda A, Baldomero H, et al. Economics and outcome after hematopoietic stem cell transplantation: a retrospective cohort study. EBioMedicine. 2015;2:2101–2109. doi: 10.1016/j.ebiom.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Anasetti C, Antin JH, et al. Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium 2014. Biol Blood Marrow Transplant. 2015;21:202–224. doi: 10.1016/j.bbmt.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–2170. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 4.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation: a study from the CIBMTR. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorror ML, Maris MB, Storb R, et al. Hematopoietic Cell Transplantation (HCT)-Specific Comorbidity Index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parimon T, Au DH, Martin PJ, et al. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144:407–414. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 7.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 8.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Logan B, Westervelt P, et al. 5 year results of BMT CTN 0201: unrelated donor bone marrow is associated with better psychological well-being and less burdensome chronic gvhd symptoms than peripheral blood. Blood. 2015;126:270. [Abstract] [Google Scholar]
- 10.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13:1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched related donor allogeneic HCT. Blood. 2014;124:1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YB, Batchelor T, Li S, et al. Phase 2 trial of high-dose rituximab with high-dose cytarabine mobilization therapy and high-dose thiotepa, busulfan, and cyclophosphamide autologous stem cell transplantation in patients with central nervous system involvement by non-Hodgkin lymphoma. Cancer. 2015;121:226–233. doi: 10.1002/cncr.29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barba P, Burns LJ, Litzow MR, et al. Success of an international learning health care system in hematopoietic cell transplantation: the American Society of Blood and Marrow Transplantation clinical case forum. Biol Blood Marrow Transplant. 2016;22:564–570. doi: 10.1016/j.bbmt.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer BC, Ahn KW, Hu ZH, et al. Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 2016;34:1864–1871. doi: 10.1200/JCO.2015.65.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Storer B, Wang H, et al. Providing personalized prognostic information for adult leukemia survivors. Biol Blood Marrow Transplant. 2013;19:1600–1607. doi: 10.1016/j.bbmt.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover A, Irwin DE, Chen RC, et al. Integrating patient-reported outcome measures into routine cancer care: cancer patients’ and clinicians’ perceptions of acceptability and value. EGEMS (Wash DC) 2015;3:1169. doi: 10.13063/2327-9214.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer. 2016;122:91–98. doi: 10.1002/cncr.29717. [DOI] [PMC free article] [PubMed] [Google Scholar]