Abstract
Context
Decision aids (DAs) prepare patients to make decisions about healthcare options consistent with their preferences. Helping patients choose among available options for colorectal cancer (CRC) screening is important because rates are lower than screening for other cancers. This systematic review describes studies evaluating patient DAs for CRC screening in average-risk adults and their impact on knowledge, screening intentions, and uptake.
Evidence acquisition
Sources included Ovid MEDLINE, Elsevier EMBASE, EBSCO CINAHL Plus, Ovid PsycINFO through July 21, 2015, pertinent reference lists, and Cochrane review of patient DAs. Reviewers independently selected studies that quantitatively evaluated a DA compared to one or more conditions or within a pre–post evaluation. Using a standardized form, reviewers independently extracted study characteristics, interventions, comparators, and outcomes. Analysis was conducted in August 2015.
Evidence synthesis
Twenty-three articles representing 21 trials including 11,900 subjects were eligible. Patients exposed to a DA showed greater knowledge than those exposed to a control condition (mean difference [MD], 18.3 of 100; 95% CI=15.5, 21.1), were more likely to be interested in screening (pooled relative risk [RR], 1.5; 95% CI=1.2, 2.0), and more likely to be screened (pooled RR, 1.3; 95% CI=1.1, 1.4). DA patients had greater knowledge than patients receiving general CRC screening information (pooled MD, 19.3 of 100; 95% CI=14.7, 23.8); however, there were no significant differences in screening interest or behavior.
Conclusions
DAs improve knowledge and interest in screening, and lead to increased screening over no information, but their impact on screening is similar to general CRC screening information.
Context
Colorectal cancer (CRC) is the second leading cause of cancer death among men and women in the U.S.1 CRC incidence and mortality have been declining2 owing to screening and improvements in treatment. Many tests, including fecal occult blood testing (both guaiac- and immunochemical-based), flexible sigmoidoscopy, and colonoscopy, are recommended to screen for CRC.3–7 Despite these recommendations, screening rates for CRC (59.5%) are lower than other recommended cancer screening tests such as breast cancer (72.4%) and cervical cancer (82.9%).8
One of the underlying factors contributing to low screening rates is suboptimal decision making, including low rates of assessing patient preferences.9 Patients have strong preferences for the different CRC screening test attributes such as accuracy, frequency of testing, and required preparation,10–15 but health providers have difficulty correctly identifying those preferences.9 Health providers tend to recommend colonoscopy, although many patients prefer fecal testing for a variety of reasons.16,17 Several organizations discuss the importance of including patient preferences to increase screening rates for CRC5 and allow individuals to make an informed decision when choosing one of the screening options.2,18,19
One way to promote consideration of patient preferences and informed decision making for CRC screening is through the use of patient decision aids. Patient decision aids provide information about options, and help patients to construct, clarify, and communicate the personal values they associate with the different features of the options.20–22 The desired outcome is a preference-concordant, informed decision, resulting from a high-quality shared decision-making process.21,22
A high-quality shared decision-making process is a valued outcome independent of the impact of patient decision aids on actual screening uptake. Previous systematic reviews of decision aids used in RCTs indicate that, in general, compared with standard care, decision aids improve decision quality by improving knowledge, risk perceptions, match between values and choices, and patient–provider communication, and decrease decisional conflict and passive decision making.23 The impact of decision aids on screening behaviors is inconsistent, and varies by topic. The overall purpose of this review was to describe studies that evaluated decision aids for CRC screening and to determine the impact of these aids in average-risk adults. Specifically, the aim was to determine the effect of decision aids compared to control conditions or to general CRC screening information on CRC screening knowledge, screening intention or interest, and uptake.
Evidence Acquisition
Using the Cochrane Handbook24 and Guide,25 a review protocol was developed to help facilitate the review and to finalize study eligibility, information sources, and data elements for abstraction. The protocol was registered online through NIH Research’s international prospective register of systematic reviews (CRD42013002826, www.crd.york.ac.uk/PROSPERO).
A research librarian searched Ovid MEDLINE, Elsevier EMBASE, EBSCO CINAHL Plus, and Ovid PsycINFO from inception through July 21, 2015. The search strategy for Ovid MEDLINE is provided in Appendix A (available online). The search was not limited to English-only publications and conference proceedings were included in order to obtain a broad picture of all existing decision aids for CRC screening. Reference lists of pertinent reviews were manually searched, including the Cochrane review of decision aids for treatment and screening.23 For the final selection of studies, English publications and only published journal articles were included although the aids were not limited to English language.
Trained raters (at least two for each search) independently reviewed each record retrieved from the search to assess for eligibility. Raters were not blinded to author, journal, or funding source. Raters checked titles and abstracts to rate each record as “included,” “excluded,” or “not sure.” After screening titles and abstracts, raters reviewed full texts for all records marked as “included” and “not sure” to assess for final eligibility. Raters first reviewed discordant ratings, and then the research team reviewed any unresolved discrepancies and those records still marked as “not sure.” Included were studies where the decision point was whether or not to be screened (i.e., one screening option compared to no screening), to choose between two or more screening options, or whether or not to continue screening for CRC. The intervention evaluated in the study had to be a decision aid, defined as information provided about the pros and cons of at least two screening options (including no screening) that allows the user to consider trade-offs between options. Trials that compared a decision aid to one or more comparators (e.g., control condition, general CRC screening information, other aid) had to have at least a post-evaluation. Uncontrolled trials or those studies that compared outcomes with a within-subjects design had to report a pre–post evaluation, that is, a comparison of outcomes measured before and after the patient viewed the decision aid.
Data Extraction and Synthesis
Two trained reviewers independently extracted data from each article using a standardized abstraction form and procedure developed by the Task Force on Community Preventive Services26 as a guideline. Reviewers were not blinded to author, journals, or funding source. Reviewers extracted study characteristics including:
study purpose, taken directly from the abstract or text;
study design, categorized into RCT, controlled trial, or uncontrolled trial (within-subjects assessment);
country, the geographic location of the study;
setting, where the recruitment took place and where the intervention was conducted;
sampled population, including sample size, age requirements, and any unique characteristics; and
measured outcomes.
Reviewers extracted intervention and comparison characteristics including decision aid name, format, theory used in the development, presented screening options, description of the content, features, and implementation process. Reviewers described comparisons and then categorized comparisons into the following:
no information, usual care, or health education materials not related to CRC screening (e.g., safety belt use, health tips), or use of a pre-test, post-test design;
general CRC screening information, interventions that provided the basics about CRC and screening options, but lacked an opportunity to compare risks and benefits regarding the tests; and
another decision aid, where a version of the decision aid or another decision aid was the comparison.
Data analysis was completed in August 2015. For the quantitative analysis, eligible studies assessed knowledge, screening interest or intention, or screening uptake. All study designs and comparators to the decision aid were included. Study authors were only contacted to obtain data for the quantitative analysis if the data needed were not reported in the study.
Knowledge was analyzed in two ways. First, adequacy of knowledge was considered using the study’s predefined degree of knowledge that was determined to be adequate in making an informed decision. Adequate knowledge was dichotomized as adequate or inadequate. Second, overall accuracy knowledge was considered, expressed as the percentage of correct responses to a knowledge measure assessed during the final assessment.
Screening interest or intention was included for those studies where responses could be dichotomized to intended to be screened or intended not to be screened. Screening uptake was defined as the completion of any test for CRC indicated by self-report, medical chart review, or claims review. Screening uptake was dichotomized as screened and not screened.
The two main comparisons were the decision aid versus a control condition and the decision aid versus general CRC screening information. Because some studies compared decision aids or made multiple comparisons, two additional comparisons were included: a detailed decision aid versus a simple decision aid, and a decision aid with more options versus fewer options. For some of the meta-analyses, the results from the detailed and simple decision aids were combined and compared to a control condition or general CRC screening information. Sample sizes were recalculated as reported elsewhere27 for those studies that used a cluster design and where the intraclass correlation coefficient was provided. To avoid unit of analysis error (over-precise results), the sample sizes and events were reduced to its effective size by calculating the average cluster size in the trial and the design effect for the trial.
Pooled occurrences and relative risks (RRs) for dichotomous outcomes (i.e., adequate knowledge, screening intention/interest) were calculated. Pooled occurrence information can be used to report absolute event rates of the outcomes for decision aid patients versus controls. For pooled occurrences, the Freeman–Tukey arcsine transformation was used to stabilize variances and conducted a meta-analysis using inverse variance weights. Then, estimates and CI boundaries were back-transformed into proportions. Mean differences (MDs) were calculated for continuous measures. The proportion of accurate knowledge responses was transformed into a percentage as suggested by O’Connor et al.20,28 When SDs were not directly reported in the article, the study authors were contacted, or they were imputed from other measures of dispersion (95% CIs or p-values from t-tests), or the baseline measurement reported in the study was used instead.
A sensitivity analysis was performed to assess the robustness of the methods used to impute by excluding any trials with imputed SDs. Study heterogeneity was assessed using the I2 statistic and subsequent chi-square test. Fixed-effect meta-analysis was used if the score was <40%, and random-effects if it was ≥40%.29 Meta-regression was performed to investigate if study characteristics could explain the observed heterogeneity. Among the variables tested as predictors of study heterogeneity were study design, quality, follow-up, and type of comparison used (control condition or general CRC screening information). Study quality was assessed using the study quality form from the Guide to Community Preventive Services.26 The risk of publication bias was assessed through funnel plots and an Egger regression test.30 When the regression slope significantly deviated from the vertical slope, this was considered significant potential for bias. Statistical analyses were performed using RevMan, version 5.0.22 and Stata, version 10. All statistical tests were two-sided and defined the cut off for statistical significance as a p-value <0.05.
This study was funded by the National Cancer Institute, Agency for Healthcare Research and Quality, American Cancer Society, and Cancer Prevention Research Institute of Texas. The funding sources had no role in the design, conduct, collection, management, analysis, or interpretation of the data or in the preparation, review, or approval of the manuscript. The content is the sole responsibility of the authors.
Evidence Synthesis
After removal of duplicate records, the search identified 762 unique records for review (Figure 1). A total of 97 full-text articles were assessed for eligibility, which resulted in 23 studies representing 21 trials included in the qualitative synthesis. For the quantitative synthesis, 20 trials were eligible: seven for accurate knowledge (percentage correct), six for adequate knowledge, 13 for screening intention or interest, and 13 for screening uptake.
Figure 1.
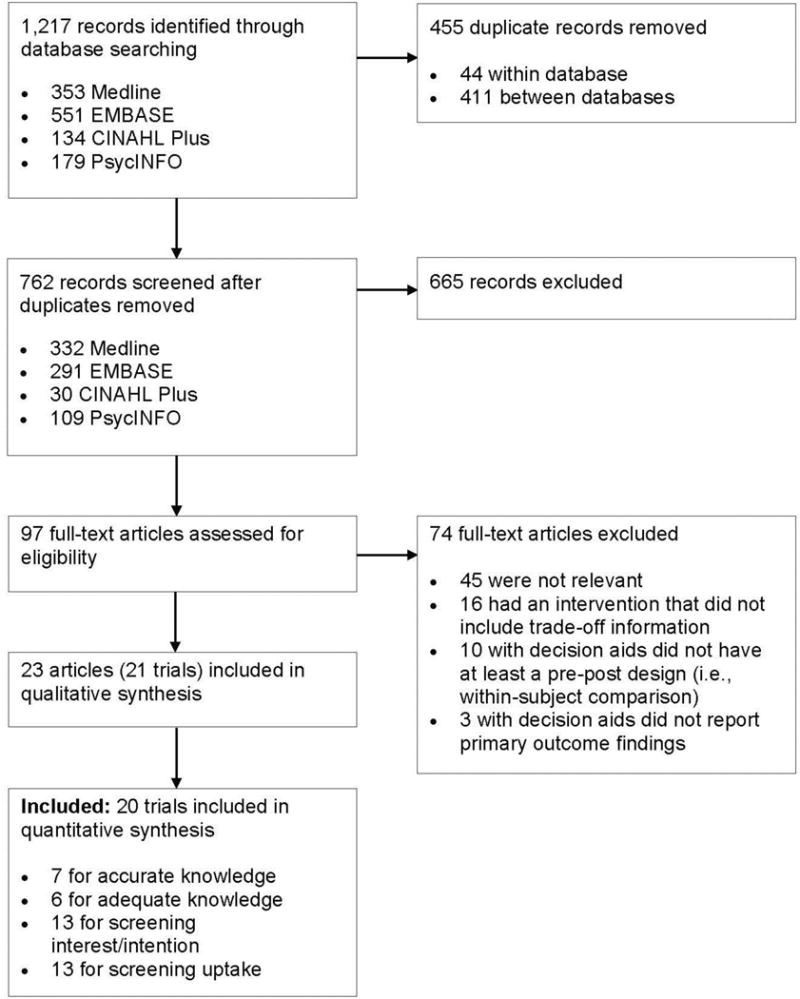
Study flow for the systematic review of colorectal cancer screening decision aid studies.
Qualitative Synthesis
The 21 trials included a total of 11,900 participants (Appendix Table 1, available online).31–53 Fourteen of the trials were RCTs including two randomized at the group level. Four of the trials were non-RCTs including one group-level design. The remaining three trials were uncontrolled using a pre–post assessment.
Sixteen of the trials took place in the U.S., two in Australia, one in Germany, one in Canada, and one in the Netherlands. Fifteen trials recruited participants from a clinical setting; five trials recruited from community settings such as telephone marketing databases, senior centers, or registries; and one trial recruited from both types of settings. For the trials that recruited participants from a clinical setting, six had the participant view the decision aid at home.
The majority of the trials focused on average-risk adults aged 50–74 years. Two of the trials focused on older adults, with one focusing on adults aged ≥65 years50 and the other on adults aged ≥75 years.40 Three of the trials focused on specific populations, including individuals with low education,47 individuals with low SES,41 and Hispanic or Latino individuals with limited English proficiency.51
Thirteen different decision aids for CRC screening were evaluated in the trials (Appendix Table 2, available online). Seven of the studies evaluated a version of a video called “Communicating Health Options through Interactive Computer Education,” three studies evaluated the Foundation for Informed Medical Decision Making (now Informed Medical Decisions Foundation) video titled, “Colon Cancer Screening: Deciding What’s Right for You,” and 11 aids were developed by the study investigators. Video decision aids were in a variety of formats including VHS, DVD, computer-based, and web-based. Interactive programs were either computer- or web-based. For five studies, the main component of the decision aid was a printed booklet. There was a wide range of theories and frameworks used during development (Appendix Table 2, available online). Theory used in the development of the decision aid was not mentioned for four of the 13 aids.
Seven different screening options were presented in the decision aids. The most common option presented in the aids was fecal occult blood testing, presented as an option in all the decision aids except for a study that compared a colonoscopy decision aid and a CT-colonography decision aid.31 The next most common screening option was colonoscopy, followed by flexible sigmoidoscopy, barium enema, a combination of flexible sigmoidoscopy and fecal occult blood test, and CT-colonography. Twelve of the studies evaluated decision aids that included no screening either explicitly as an option (e.g., no testing, wait and see) or presented it during trade-offs of test attributes, including all four non-U.S. studies.
Three of the studies used pre–post comparisons without a control group. For the controlled trials, eight of the trials compared a decision aid to a control condition that included no information about CRC screening (e.g., video about automobile or drug refill safety, handout on ways to stay healthy), and seven compared a decision aid to general CRC screening information with fewer details or less attractive presentation. Not enough information was provided about the general CRC screening information to compare the content with the decision aid. In addition, seven trials compared it to another aid or another version of the aid. For example, one trial compared an aid with a no screening option to the aid without the option,34 one trial compared a five-option aid to a two-option version,35 and one trial compared a decision aid with and without a personalized risk calculator.45,46 For the controlled trials, three of the 21 trials had more than one comparison condition.45–47,50
Thirteen trials assessed knowledge (Appendix Table 1, available online).31,33–36,40,43,45–51 Seven of the trials assessed interest in screening,34,35,37–39,47,50 and nine assessed intentions for screening.32–34,40,45,46,48–51 Several of the studies measured a variation of interest or intention, including attitudes toward screening (four trials),31,33,45–48 intentions to ask or discuss screening (four trials),33,37,38,51 and stage of readiness (five trials).36,37,41,42,44,53 Fifteen of the trials assessed screening uptake or test completion,31,32,37–39,41–49,51–53 and seven assessed test preference.35,37,38,41,42,44–46,53 Several of the trials measured a variation of behavior or test preference, including if screening was discussed (four trials)33,42,45,46,51 or if a test was ordered (four trials).37,41,42,45,46,53
Many of the studies measured the extent to which the decision aid improved informed decision making by using a combination of measured outcomes. For example, one study defined informed choice as a combination of adequate knowledge and clear values.49 Three other studies defined informed choice as adequate knowledge and attitude toward screening consistent with behavior.31,47,48
Meta-analysis Results
The pooled rates of adequate knowledge, screening interest or intention, and screening uptake are shown in Table 1. Nearly half of the patients exposed to any type of decision aid were deemed to have adequate knowledge (49%, 95% CI=36%, 62%). However, most studies considered adequate knowledge in a subjective fashion with cut offs specified by the authors. The pooled rates of screening interest or intention for patients in any type of decision aid group was 66% (95% CI=54%, 77%), but uptake was only 33% (95% CI=22%, 46%). Patients in the general CRC screening information group showed lower rates of adequate knowledge, but similar screening interest/intention and screening uptake rates. Patients in a control condition group (e.g., usual care, no CRC screening information, other health information) had lower rates for all three outcomes.
Table 1.
Pooled Rates of Adequate Knowledge, Screening Interest/Intention and Uptake for Colorectal Cancer (CRC) Screening.
| Any decision aid | Control condition (no intervention or materials about a different topic) | General CRC screening information | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Designa | Events | Total N | Rate (95% CI) | Events | Total N | Rate (95% CI) | Events | Total N | Rate (95% CI) |
| Adequate Knowledgeb | ||||||||||
| Griffith (2008)35c | RCT | 21 | 62 | 0.34 (0.23, 0.46) | – | – | – | – | – | – |
| Lewis (2010)40d | UCT | 24 | 46 | 0.52 (0.38, 0.66) | 2 | 46 | 0.05 (0.01, 0.13) | – | – | – |
| Smith (2010)47e | RCT | 200 | 357 | 0.56 (0.51, 0.61) | – | – | – | 32 | 173 | 0.19 (0.13, 0.25) |
| Steckelberg (2011)48 | RCT | 468 | 785 | 0.60 (0.56, 0.63) | – | – | – | 128 | 792 | 0.16 (0.14, 0.19) |
| Trevena (2008)49 | RCT | 28 | 134 | 0.21 (0.15, 0.28) | – | – | – | 8 | 137 | 0.06 (0.03, 0.11) |
| Wolf (2000)50f | RCT | 191 | 269 | 0.71 (0.65, 0.76) | – | – | – | 72 | 133 | 0.54 (0.46, 0.62) |
| Pooled | 0.49 (0.36, 0.62) | 0.05 (0.01, 0.13) | 0.22 (0.08, 0.39) | |||||||
| Screening Interest and/or Intention | ||||||||||
| Dolan (2002)32 | RCT | 37 | 45 | 0.82 (0.69, 0.91) | – | – | – | 27 | 43 | 0.63 (0.48, 0.76) |
| Frosch (2008)33g | CT | 39 | 100 | 0.39 (0.30, 0.49) | – | – | – | 53 | 107 | 0.50 (0.40, 0.59) |
| Griffith (2008)34c | RCT | 59 | 62 | 0.94 (0.88, 0.99) | – | – | – | – | – | – |
| Leone (2013)38 | G–CT | 34 | 56 | 0.61 (0.48, 0.73) | – | – | – | – | – | – |
| Lewis (2008)39h | CT | 31 | 35 | 0.88 (0.75, 0.96) | – | – | – | – | – | – |
| Lewis (2010)40d | UCT | 28 | 46 | 0.61 (0.46, 0.74) | 31 | 46 | 0.67 (0.53, 0.80) | – | – | – |
| Miller (2011)41 | RCT | 40 | 132 | 0.30 (0.23, 0.39) | 28 | 132 | 0.21 (0.15, 0.29) | – | – | – |
| Pignone (2000)42 | RCT | 63 | 125 | 0.50 (0.42, 0.59) | 33 | 106 | 0.31 (0.23, 0.40) | – | – | – |
| Reuland (2012)51d | UCT | 76 | 80 | 0.94 (0.88, 0.98) | 50 | 80 | 0.62 (0.52, 0.73) | – | – | – |
| Smith (2010)47 | CT | 121 | 357 | 0.34 (0.29, 0.39) | – | – | – | 21 | 173 | 0.12 (0.08, 0.18) |
| Steckelberg (2011)48 | RCT | 397 | 785 | 0.51 (0.47, 0.54) | – | – | – | 416 | 792 | 0.53 (0.49, 0.56) |
| Trevena (2008)49 | RCT | 117 | 134 | 0.87 (0.81, 0.92) | – | – | – | 124 | 137 | 0.90 (0.85, 0.95) |
| Wolf (2000)50f | RCT | 175 | 269 | 0.65 (0.59, 0.71) | – | – | – | 79 | 133 | 0.59 (0.51, 0.67) |
| Pooled | 0.66 (0.54, 0.77) | 0.45 (0.24, 0.67) | 0.55 (0.33, 0.75) | |||||||
| Screening Uptake | ||||||||||
| Dolan (2002)32 | RCT | 18 | 45 | 0.40 (0.27, 0.55) | – | – | – | 14 | 43 | 0.33 (0.20, 0.47) |
| Kim (2005)37 | UCT | 34 | 80 | 0.43 (0.32, 0.53) | – | – | – | – | – | – |
| Leone (2013)38 | G-CT | 39 | 240 | 0.16 (0.12, 0.21) | 18 | 174 | 0.11 (0.06, 0.16) | – | – | – |
| Lewis (2008)39 | CT | 20 | 137 | 0.15 (0.09, 0.21) | 4 | 100 | 0.04 (0.01, 0.09) | – | – | – |
| Miller (2011)41 | RCT | 25 | 132 | 0.19 (0.13, 0.26) | 18 | 132 | 0.14 (0.09, 0.20) | – | – | – |
| Pignone (2000)42 | RCT | 46 | 125 | 0.37 (0.29, 0.45) | 28 | 124 | 0.23 (0.16, 0.31) | – | – | – |
| Pignone (2011)43d | G-RCT | 50 | 129 | 0.37 (0.29, 0.45) | 50 | 156 | 0.32 (0.25, 0.40) | – | – | – |
| Reuland (2012)51b | BA | 13 | 68 | 0.20 (0.11, 0.30) | ||||||
| Ruffin (2007)44 | RCT | 56 | 87 | 0.64 (0.54, 0.74) | – | – | – | 33 | 87 | 0.38 (0.28, 0.48) |
| Schroy (2011)45i | RCT | 220 | 549 | 0.40 (0.36, 0.44) | 96 | 276 | 0.35 (0.29, 0.41) | – | – | – |
| Smith (2010)47e | RCT | 211 | 357 | 0.59 (0.54, 0.64) | – | – | – | 130 | 173 | 0.75 (0.68, 0.81) |
| Steckelberg (2011)48 | RCT | 171 | 785 | 0.22 (0.19, 0.25) | – | – | – | 161 | 792 | 0.20 (0.18, 0.23) |
| Trevena (2008)49 | RCT | 7 | 134 | 0.06 (0.02, 0.10) | 9 | 137 | 0.07 (0.03, 0.12) | |||
| Clouston (2014)52d | G-RCT | 740 | 1,112 | 0.67 (0.64, 0.69) | 609 | 1071 | 0.57 (0.54, 0.60) | |||
| Pooled | 0.33 (0.22, 0.46) | 0.23 (0.09, 0.41) | 0.33 (0.12, 0.59) | |||||||
Design: G-RCT=group RCT, G-CT=group controlled trial, UCT=uncontrolled trials with within-subjects comparisons, CT=controlled trial. Lower-case n refers to frequency of occurrence, such as adequate knowledge; upper-case N refers to total sample size.
Adequate knowledge was defined as follows: Griffith (2008),35 responding correctly to all questions. Lewis (2010),40 defined as 67% of the questions responded correctly. Smith (2010)47 and Steckelberg (2011),48 if patient had 50% or above correct responses. Trevena (2008),49 if the patient had a positive score for understanding the potential benefits and for understanding the potential harms of screening. Wolf (2000),50 reported as patients that responded correctly or incorrectly to one question only.
Griffith (2008)35 compared 2-option decision aid to a 5-option decision aid. Here, the results are combined.
Smith (2010)47 compared decision aid with question prompt list to a decision aid without the list and to general CRC screening information. Here, the two decision aid group results are combined. It was noted in the trial that there were no significant differences between the two decision aid groups.
Wolf (2000)50 compared a decision aid with absolute risk information to decision aid with relative risk information and to a control. Here, the decision aid group results are combined.
Frosch (2008),33 numbers presented include patients receiving prostate or colorectal cancer information. Removal of this study did not change the magnitude of the pooled estimate (0.69%; 95% CI 0.56, 0.82).
Lewis (2008)39 control group was not asked about screening intention and therefore no data is presented.
Schroy (2011)45 compared a decision aid with a personalized risk assessment to a decision aid without the personalized risk assessment and to a control condition. Here, the two decision aid group results are combined.
All comparisons described in this section can be found in Table 2.
Table 2.
Efficacy of Colorectal Cancer (CRC) Screening Decision Aids (DA) Compared to Various Comparator Interventions
| Accurate knowledge (proportion of accurate responses out of 100) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DA | vs | Control condition | ||||||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI) | ||||
| Schroy (2011)45a | 90.0 | 14.7 | 549 | 71.7 | 21.7 | 276 | 18.3 (15.5, 21.2) | |||
| DA | vs | General CRC information screening | ||||||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI) | ||||
| Frosch (2008)33 | 30.1 | 22.3 | 61 | 18.1 | 16.6 | 60 | 12.0 (5.0, 19.0) | |||
| Smith (2010)47 | 54.2 | 27.8 | 357 | 34.2 | 14.3 | 173 | 20.0 (16.4, 23.6) | |||
| Steckelberg (2011)48 | 53.8 | 28.8 | 785 | 31.3 | 15.0 | 792 | 22.5 (20.2, 24.8) | |||
| Pooled | 19.3 (14.7, 23.8) | |||||||||
| Detailed DA | vs | Simple DA | ||||||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI) | ||||
| Jerant (2007)36 | 67.9 | 22.8 | 24 | 63.7 | 20.9 | 25 | 4.2 (−8.1, 16.5) | |||
| Schroy (2012)46 | 89.2 | 15.8 | 280 | 90.8 | 13.3 | 269 | −1.7 (−4.1, 0.78) | |||
| Pooled | −1.4 (−3.8, 0.96) | |||||||||
| DA with more options | vs | DA with fewer options | ||||||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI) | ||||
| Griffith (2008)34 | 46.0 | 22.8 | 57 | 46.0 | 20.9 | 49 | 0.0 [−8.3, 8.3] | |||
| Griffith (2008)35 | 70.0 | 22.8 | 25 | 66.7 | 20.9 | 37 | 3.3 [−7.9, 14.5] | |||
| Pooled | 1.2 (−5.5, 7.9) | |||||||||
| Adequate knowledge (proportion of patients with adequate knowledge)b | ||||||||||
| DA | vs | General CRC screening information | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| Smith (2010)47 | 200 | 357 | 32 | 173 | 3.0 (2.2, 4.2) | |||||
| Steckelberg (2011)48 | 468 | 785 | 128 | 792 | 3.7 (3.1, 4.4) | |||||
| Trevena (2008)49 | 28 | 134 | 8 | 137 | 3.6 (1.7, 7.6) | |||||
| Wolf (2000)50 | 191 | 269 | 72 | 133 | 1.3 (1.1, 1.6) | |||||
| Pooled | 2.6 (1.4, 5.1) | |||||||||
| DA with more options | vs | DA with fewer options | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| Griffith (2008)35 | 9 | 25 | 12 | 37 | 1.1 (0.55, 2.2) | |||||
| Screening interest/intention | ||||||||||
| DA | vs | Control condition | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| Miller (2011)41 | 40 | 132 | 28 | 132 | 1.4 (0.94, 2.2) | |||||
| Pignone (2000)42 | 63 | 125 | 33 | 106 | 1.6 (1.2, 2.3) | |||||
| Pooled | 1.5 (1.2, 2.0) | |||||||||
| DA | vs | General CRC screening information | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| Dolan (2002)32 | 37 | 45 | 27 | 43 | 1.3 (1.0, 1.7) | |||||
| Frosch (2008)33 | 39 | 100 | 53 | 107 | 0.79 (0.58, 1.1) | |||||
| Smith (2010)47 | 121 | 357 | 21 | 173 | 2.8 (1.8, 4.3) | |||||
| Steckelberg (2011)48 | 397 | 785 | 416 | 792 | 0.96 (0.87, 1.1) | |||||
| Trevena (2008)49 | 117 | 134 | 124 | 137 | 0.96 (0.89, 1.1) | |||||
| Wolf (2000)50 | 175 | 269 | 79 | 133 | 1.1 (0.93, 1.3) | |||||
| Pooled | 1.1 (0.94, 1.3) | |||||||||
| DA with more options | vs | DA with fewer options | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| Griffith (2008)35 | 24 | 25 | 35 | 37 | 1.0 (0.91, 1.1) | |||||
| Screening uptake | ||||||||||
| DA | vs | Control condition | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| 16–24 weeks | ||||||||||
| Clouston (2014)52c | 740 | 1,112 | 609 | 1,071 | 1.2 (1.1, 1.3) | |||||
| Lewis (2008)34 | 20 | 137 | 4 | 100 | 3.7 (1.3, 10.4) | |||||
| Miller (2011)41 | 25 | 132 | 18 | 132 | 1.4 (0.80, 2.4) | |||||
| Pignone (2000)42 | 46 | 125 | 28 | 124 | 1.6 (1.1, 2.4) | |||||
| Pooled | 1.5 (1.1, 2.0) | |||||||||
| 52 weeks | ||||||||||
| Leone (2013)38 | 39 | 240 | 18 | 174 | 1.6 (0.93, 2.7) | |||||
| Pignone (2011)43c | 50 | 129 | 50 | 156 | 1.2 (0.88, 1.7) | |||||
| Schroy (2011)45a | 220 | 549 | 96 | 276 | 1.2 (0.95, 1.4) | |||||
| Pooled | 1.2 (1.0, 1.4) | |||||||||
| All subgroups | Pooled | 1.3 (1.1, 1.4) | ||||||||
| DA | vs | General CRC screening information | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| 4 weeks | ||||||||||
| Trevena (2008)49 | 7 | 134 | 9 | 137 | 0.80 (0.30, 2.1) | |||||
| 8–12 weeks | ||||||||||
| Dolan (2002)32 | 18 | 45 | 14 | 43 | 1.2 (0.70, 2.2) | |||||
| Smith (2010)47 | 211 | 357 | 130 | 173 | 0.79 (0.70, 0.89) | |||||
| Pooled | 0.91 (0.60, 1.4) | |||||||||
| 24 weeks | ||||||||||
| Ruffin (2007)44 | 56 | 87 | 33 | 87 | 1.7 (1.2, 2.3) | |||||
| Steckelberg (2011)48 | 171 | 785 | 161 | 792 | 1.1 (0.89, 1.3) | |||||
| Pooled | 1.3 (0.84, 2.1) | |||||||||
| All subgroups | Pooled | 1.1 (0.78, 1.5) | ||||||||
| Detailed DA | vs | Simple DA | ||||||||
| n | N | n | N | Risk ratio (95% CI) | ||||||
| 52 weeks | ||||||||||
| Schroy (2011)45 | 104 | 280 | 116 | 269 | 0.86 (0.70, 1.1) | |||||
Notes: Boldface in the rightmost column indicates statistical significance (p<0.05)
Lower case n refers to frequency of occurrence, such as adequate knowledge; upper case N refers to total sample size.
Schroy (2011)45 compared DA with personalized risk assessment to a DA without personalized risk assessment and to a control condition with no information. Here, the two DA groups’ results are combined.
Adequate knowledge was defined by authors as follows: Smith (2010)47 and Steckelberg (2011)48 if patient had 50% or above correct responses. Trevena (2008)49 if the patient had a positive score for understanding the potential benefits and for understanding the potential harms of screening. Wolf (2000)50 reported patients that responded correctly or incorrectly to one question only. Griffith (2008)35 defined as responding to all the questions correctly.
To avoid unit of analysis error (over-precise results), the sample sizes and events were reduced to its effective size by calculating the average cluster size in the trial and the design effect for the trial as reported elsewhere.27
Accuracy of knowledge was reported as the proportion of correct responses (percentage correct) or adequate knowledge (threshold specified a priori by the authors). Patients in the decision aid group improved their knowledge compared with either those patients in a control condition or receiving general CRC screening information (MD=18.3%, 95% CI=15.5%, 21.2% and MD=19.3%, 95% CI=14.7%, 23.8%, respectively). Patients in the decision aid group were more likely to have adequate knowledge after the intervention compared with the group receiving general CRC screening information (RR=2.6, 95% CI=1.4, 5.1). There were no differences in knowledge between different versions of decision aids (detailed versus simple or more options versus fewer options).
More patients in the decision aid group expressed interest or intention to undergo screening compared with those patients in a control condition (RR=1.5, 95% CI=1.2, 2.0). The absolute group benefit was 14% (95% CI=4%, 24%), that is, in the decision aid group, 40 of 100 people reported interest in screening versus 26 of 100 for the control group. The number needed to treat was eight (95% CI=4, 21), interpreted as eight people had to be exposed to the decision aid in order for one additional person to report interest on being screened. Similar rates of screening interest or intention were observed in the patients assigned to the decision aid group compared to the general CRC screening information group (RR=1.1, 95% CI=0.93, 1.3).
Patients in the decision aid group were 1.3 times more likely to complete screening at 16–52 weeks compared with the patients in control conditions (e.g., usual care, no CRC screening information, other health information) (95% CI=1.1, 1.4). The absolute group benefit was 8% (95% CI=6%, 11%), that is, in the decision aid group, 47 of 100 people completed their screening over 16–52 weeks, versus 40 of 100 for the control group. The number needed to treat was ten (95% CI=7, 25), interpreted as ten people had to be exposed to the decision aid in order for one additional person to get screened. No other statistically significant differences were observed.
When comparing rates of screening completion by follow-up time for patients receiving the decision aid versus those patients receiving only general CRC screening information, no differences were observed at 4, 8–12, or 24 weeks. No other associations were found between treatment effects and study design, quality, or follow-up. Publication bias was not observed.
Discussion
This review found that decision aids for CRC screening improve patients’ knowledge by about 20% compared with control conditions and general CRC screening information. This increase in knowledge is higher than the 13% difference reported in a Cochrane systematic review of decision aids for various healthcare conditions.21 Decision aids are associated with greater intentions to be screened and screening uptake compared to control conditions. However, compared to general CRC information, intentions and uptake are not statistically significant when decision aids are used.
It is important to not lose sight of the rationale for using of patient decision aids, that is, to improve the decision-making process and match between preferences and choices.21,22 Similar to studies of decision aids in general, current evaluations of CRC screening decision aids are limited in their attention to the decision-making process. This review showed greater knowledge among patients receiving decision aids compared with standard CRC screening information, and knowledge is one key component of a high-quality decision.
The wide range of theories and frameworks used to guide development of the decision aids does not allow for a detailed examination of the impact of specific theories on patient outcomes. More research is needed to better understand how the theoretic underpinnings of decisions aids influence their effectiveness.54 Similarly, more research is needed to determine which patient groups are most likely to benefit from decision support compared with general information about CRC screening. For example, the development, use, and evaluation of decision aids with lower-literacy populations is recommended.21 It is noteworthy that four of the trials in this review focused on vulnerable populations with mixed effects of the decision aid on screening uptake.41,45–47,51 Clearly, there is a need for more research on the role of patient decision aids on CRC screening decisions for vulnerable populations where the burden of CRC may be highest.
Decision making about screening for CRC is an interesting and challenging prevention model for decision aid developers. There is a both persuasive component and a preference-sensitive component to the decision. In general, there is strong evidence from randomized trials and modeling studies supporting several different screening tests and schedules in decreasing CRC incidence and mortality.4 Although presenting a no-screening option to patients may be ethically justifiable and advocated in standards for decision aid developers,55 it can be argued that the net benefit of CRC screening warrants promotion of screening. This persuasive emphasis is in contrast to the question of which screening test is best for a given patient. Here, the evidence is equivocal, and a values-based decision is generally recognized as the desired outcome.
In this review, the tension between a persuasive emphasis (i.e., the decision to be tested) and preference sensitivity (i.e., which test to have) was reflected in the decision context selected by the researchers, and the choice of measured outcomes. When the focus of the decision was to choose between test options, shared decision making was encouraged in order to increase screening uptake. Primary outcomes measured included test preference, screening uptake, and concordance between preference and test completed. Of note, all of the studies where the decision was to choose between test options were conducted in the U.S., where preventive services recommendations strongly endorse CRC screening for eligible patients.4 If the decision was whether to be screened or to continue screening, informed decision making was encouraged in order to promote informed choice. Here, the majority of the studies defined informed choice as having adequate knowledge and a match between values or attitude toward screening and screening behavior. Most of these studies were conducted outside the U.S., where CRC screening is not universally endorsed.
Limitations
There are several limitations to this systematic review. First, all study designs and study quality were included in the meta-analysis. Though this can influence findings, the sensitivity analysis indicated that the findings were robust across study design or quality. Second, a broad definition of a decision aid was used and no rating of the aids used in the trials against international standards for content, development, and evaluation of decision aids was conducted.55,56 Third, the outcomes in the meta-analysis were measured using a variety of scales. For example, studies were included that measured screening uptake in various ways—self-report, medical chart review, and medical claims review. Fourth, owing to the nature of the study, the review was constrained to the data published or provided by the authors. Finally, adequacy of patients’ knowledge was based on the authors’ a priori criteria given there is no consensus on essential facts a patient should know to make an informed screening choice.
Conclusions
When compared with general CRC screening information, decision aids appear to lead to greater patient knowledge of CRC and screening options, while having a similar impact on screening rates. As part of a high-quality shared-decision making experience, decisions aids have value independent of their impact on screening rates. Further improvements in colorectal cancer screening rates may be achieved by aligning patient and provider preferences. Focused efforts to integrate patient decision aids in routine clinical practice57,58 appear highly justified given the potential to favorably impact both patient cognitive outcomes and screening rates.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, NIH, Agency of Healthcare Research and Quality, or Cancer Prevention Research Institute of Texas.
This study was supported in part by Award Number R21CA132669 to Dr. Robert J. Volk from the National Cancer Institute. It was also supported for Dr. Suzanne K. Linder by Award Number R24HS022134 from the Agency for Healthcare Research and Quality and Award Number RP140020 from the Cancer Prevention Research Institute of Texas. Dr. Daniel S. Reuland was supported by a Research Scholar Grant RSG-13-165-01-CPPB from the American Cancer Society.
Dr. Pignone is a member of the U.S. Preventive Services Task Force, which issues guidelines on CRC screening. This work does not necessarily represent policy of the USPSTF. Dr. Pignone is a Medical Editor for Healthwise, a non-profit developer and provider of patient decision support materials, including ones for CRC screening.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors contributed to the conceptualization of the study, data acquisition and methods, interpretation of the study findings, revision of the manuscript for critical content, and final approval of the manuscript. We thank Dr. Gary Deyter for careful review and editing of the manuscript.
No financial disclosures were reported by the authors of this paper.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.American Cancer Society. Colorectal Cancer Facts & Figures 2011–2013. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 3.Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010;8(1):8–61. doi: 10.6004/jnccn.2010.0003. http://dx.doi.org/10.1097/mog.0b013e32833d1733. [DOI] [PubMed] [Google Scholar]
- 4.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. http://dx.doi.org/10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 5.McFarland EG, Levin B, Lieberman DA, et al. Revised colorectal screening guidelines: joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology. 2008;248(3):717–720. doi: 10.1148/radiol.2483080842. http://dx.doi.org/10.1148/radiol.2483080842. [DOI] [PubMed] [Google Scholar]
- 6.Qaseem A, Denberg TD, Hopkins RH, Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. http://dx.doi.org/10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 7.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64(1):30–51. doi: 10.3322/caac.21212. http://dx.doi.org/10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 8.Brown ML, Klabunde CN, Cronin KA, White MC, Richardson LC, McNeel TS. Challenges in meeting Healthy People 2020 objectives for cancer-related preventive services, National Health Interview Survey, 2008 and 2010. Prev Chronic Dis. 2014;11:E29. doi: 10.5888/pcd11.130174. http://dx.doi.org/10.5888/pcd11.130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. J Gen Intern Med. 2001;16(12):822–830. doi: 10.1111/j.1525-1497.2001.10337.x. http://dx.doi.org/10.1046/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolan JG. Patient priorities in colorectal cancer screening decisions. Health Expect. 2005;8(4):334–344. doi: 10.1111/j.1369-7625.2005.00348.x. http://dx.doi.org/10.1111/j.1369-7625.2005.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 Suppl 1):S10–16. doi: 10.1097/MLR.0b013e31817d932e. http://dx.doi.org/10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 12.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10(5):415–430. doi: 10.1111/j.1524-4733.2007.00196.x. http://dx.doi.org/10.1111/j.1524-4733.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RL, Schwartz A. A survey of individual preference for colorectal cancer screening technique. BMC Cancer. 2004;4:76. doi: 10.1186/1471-2407-4-76. http://dx.doi.org/10.1186/1471-2407-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14(7):432–437. doi: 10.1046/j.1525-1497.1999.00018.x. http://dx.doi.org/10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shokar NK, Vernon SW, Weller SC. Cancer and colorectal cancer: knowledge, beliefs, and screening preferences of a diverse patient population. Fam Med. 2005;37(5):341–347. [PubMed] [Google Scholar]
- 16.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23(2):169–174. doi: 10.1007/s11606-007-0480-1. http://dx.doi.org/10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. http://dx.doi.org/10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. http://dx.doi.org/10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 19.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. http://dx.doi.org/10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor AM, Stacey D, Rovner D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2001;(3):CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 21.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. http://dx.doi.org/10.1002/14651858.cd001431.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S1. doi: 10.1186/1472-6947-13-S2-S1. http://dx.doi.org/10.1186/1472-6947-13-s2-s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. http://dx.doi.org/10.1002/14651858.cd001431.pub3. [DOI] [PubMed]
- 24.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. [Google Scholar]
- 25.Cochrane Public Health Group. Guide for developing a cochrane protocol. Updated November 2011. [Google Scholar]
- 26.Zaza S, Wright-De Aguero LK, Briss PA, et al. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):44–74. doi: 10.1016/s0749-3797(99)00122-1. http://dx.doi.org/10.1016/S0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 27.Chapter 16: Special topics in statistics. In: Higgins JPT, Deeks JJ, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 28.O’Connor A. Decisional conflict scale user manual. 2012 Oct 15; 2010. [Google Scholar]
- 29.Chapter 9: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 30.Chapter 10: Addressing reporting biases. In: Sterne JAC, Egger M, Moher D, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 31.de Haan MC, de Wijkerslooth TR, Stoop E, Bossuyt P, Fockens P, Thomeer M, et al. Informed decision-making in colorectal cancer screening using colonoscopy or CT-colonography. Patient Educ Couns. 2013;91(3):318–325. doi: 10.1016/j.pec.2013.01.004. http://dx.doi.org/10.1016/j.pec.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22(2):125–139. doi: 10.1177/0272989X0202200210. http://dx.doi.org/10.1177/02729890222063017. [DOI] [PubMed] [Google Scholar]
- 33.Frosch DL, Legare F, Mangione CM. Using decision aids in community-based primary care: a theory-driven evaluation with ethnically diverse patients. Patient Educ Couns. 2008;73(3):490–496. doi: 10.1016/j.pec.2008.07.040. http://dx.doi.org/10.1016/j.pec.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith JM, Fichter M, Fowler FJ, Lewis C, Pignone MP. Should a colon cancer screening decision aid include the option of no testing? A comparative trial of two decision aids. BMC Med Inform Decis Mak. 2008;8:10. doi: 10.1186/1472-6947-8-10. http://dx.doi.org/10.1186/1472-6947-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008;8:4. doi: 10.1186/1472-6947-8-4. http://dx.doi.org/10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerant A, Kravitz RL, Rooney M, Amerson S, Kreuter M, Franks P. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Educ Couns. 2007;66(1):67–74. doi: 10.1016/j.pec.2006.10.009. http://dx.doi.org/10.1016/j.pec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Whitney A, Hayter S, et al. Development and initial testing of a computer-based patient decision aid to promote colorectal cancer screening for primary care practice. Bmc Med Inform Decis Mak. 2005;5:36. doi: 10.1186/1472-6947-5-36. http://dx.doi.org/10.1186/1472-6947-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leone LA, Reuland DS, Lewis CL, et al. Reach, usage, and effectiveness of a Medicaid patient navigator intervention to increase colorectal cancer screening, Cape Fear, North Carolina, 2011. Prev Chronic Dis. 2013;10:E82. doi: 10.5888/pcd10.120221. http://dx.doi.org/10.5888/pcd10.120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CL, Brenner AT, Griffith JM, Pignone MP. The uptake and effect of a mailed multi-modal colon cancer screening intervention: a pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. http://dx.doi.org/10.1186/1748-5908-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis CL, Golin CE, DeLeon C, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: development and results of an uncontrolled trial. BMC Med Inform Decis Mak. 2010;10:54. doi: 10.1186/1472-6947-10-54. http://dx.doi.org/10.1186/1472-6947-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller DP, Jr, Spangler JG, Case LD, Goff DC, Jr, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40(6):608–615. doi: 10.1016/j.amepre.2011.02.019. http://dx.doi.org/10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. http://dx.doi.org/10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 43.Pignone M, Winquist A, Schild LA, et al. Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: the CHOICE trial. Cancer. 2011;117(15):3352–3362. doi: 10.1002/cncr.25924. http://dx.doi.org/10.1002/cncr.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffin MTt, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. http://dx.doi.org/10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Schroy PC, 3rd, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31(1):93–107. doi: 10.1177/0272989X10369007. http://dx.doi.org/10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroy PC, 3rd, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43(6):573–583. doi: 10.1016/j.amepre.2012.08.018. http://dx.doi.org/10.1016/j.amepre.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ. 2010;341:c5370. doi: 10.1136/bmj.c5370. http://dx.doi.org/10.1136/bmj.c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steckelberg A, Hulfenhaus C, Haastert B, Muhlhauser I. Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomised controlled trial. BMJ. 2011;342(7810):1–7. doi: 10.1136/bmj.d3193. http://dx.doi.org/10.1136/bmj.d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trevena LJ, Irwig L, Barratt A. Randomized trial of a self-administered decision aid for colorectal cancer screening. J Med Screen. 2008;15(2):76–82. doi: 10.1258/jms.2008.007110. http://dx.doi.org/10.1258/jms.2008.007110. [DOI] [PubMed] [Google Scholar]
- 50.Wolf AM, Schorling JB. Does informed consent alter elderly patients’ preferences for colorectal cancer screening? Results of a randomized trial. J Gen Intern Med. 2000;15(1):24–30. doi: 10.1046/j.1525-1497.2000.01079.x. http://dx.doi.org/10.1046/j.1525-1497.2000.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reuland DS, Ko LK, Fernandez A, Braswell LC, Pignone M. Testing a Spanish-language colorectal cancer screening decision aid in Latinos with limited English proficiency: results from a pre-post trial and four month follow-up survey. BMC Med Inform Decis Mak. 2012;12:53. doi: 10.1186/1472-6947-12-53. http://dx.doi.org/10.1186/1472-6947-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clouston K, Katz A, Martens PJ, et al. Does access to a colorectal cancer screening website and/or a nurse-managed telephone help line provided to patients by their family physician increase fecal occult blood test uptake?: Results from a pragmatic cluster randomized controlled trial. BMC Cancer. 2014;14:263. doi: 10.1186/1471-2407-14-263. http://dx.doi.org/10.1186/1471-2407-14-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duren-Winfield V, Onsomu EO, Case DL, Pignone M, Miller D. Health literacy and computer-assisted instruction: Usability and patient preference. J Health Commun. 2015;20(4):491–498. doi: 10.1080/10810730.2014.976322. http://dx.doi.org/10.1080/10810730.2014.976322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durand MA, Stiel M, Boivin J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ Couns. 2008;71(1):125–135. doi: 10.1016/j.pec.2007.12.004. http://dx.doi.org/10.1016/j.pec.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Joseph-Williams N, Newcombe R, Politi M, et al. Toward Minimum Standards for Certifying Patient Decision Aids: A Modified Delphi Consensus Process. Med Decis Making. 2013;34(6):699–710. doi: 10.1177/0272989X13501721. http://dx.doi.org/10.1177/0272989X13501721. [DOI] [PubMed] [Google Scholar]
- 56.Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. http://dx.doi.org/10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. doi: 10.1186/1748-5908-1-16. http://dx.doi.org/10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Legare F, Ratte S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010;(5):CD006732. doi: 10.1002/14651858.CD006732.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


