Abstract
The therapeutic effects of wheel running (WR) during abstinence on reinstatement of ethanol seeking behaviors in rats that self-administered ethanol only (ethanol drinking, ED) or ED with concurrent chronic intermittent ethanol vapor experience (CIE-ED) were investigated. Neuronal activation as well as oligodendroglial and neuroinflammatory factors were measured in the medial prefrontal cortex (mPFC) tissue to determine cellular correlates associated with enhanced ethanol seeking. CIE-ED rats demonstrated escalated and unregulated intake of ethanol and maintained higher drinking than ED rats during abstinence. CIE-ED rats were more resistant to extinction from ethanol self-administration, however, demonstrated similar ethanol seeking triggered by ethanol contextual cues compared to ED rats. Enhanced seeking was associated with reduced neuronal activation, and increased number of myelinating oligodendrocyte progenitors and PECAM-1 expression in the mPFC, indicating enhanced oligodendroglial and neuroinflammatory response during abstinence. WR during abstinence enhanced self-administration in ED rats, indicating a deprivation effect. WR reduced reinstatement of ethanol seeking in CIE-ED and ED rats, indicating protection against relapse. The reduced ethanol seeking was associated with enhanced neuronal activation, reduced number of myelinating oligodendrocyte progenitors, and reduced PECAM-1 expression. The current findings demonstrate a protective role of WR during abstinence in reducing ethanol seeking triggered by ethanol contextual cues and establish a role for oligodendroglia-neuroinflammatory response in ethanol seeking. Taken together, enhanced oligodendroglia-neuroinflammatory response during abstinence may contribute to brain trauma in chronic alcohol drinking subjects and be a risk factor for enhanced propensity for alcohol relapse.
Keywords: alcohol, wheel running, reinstatement, medial prefrontal cortex, BrdU, PECAM-1, MBP, MOG, Olig2, NG2
1.0 Introduction
A major factor contributing to the enduring nature of alcohol relapse is the persistence of subjective responses to contextual cues that were paired with alcohol consumption (O’Brien et al., 1998). These responses could be reduced by behavior-based approaches that have the potential to activate areas of the prefrontal cortex (PFC) associated with voluntary inhibitory control of motivational impulses (Phan et al., 2005, Diekhof and Gruber, 2010).
Growing evidence suggests that the rodent medial prefrontal cortex (mPFC) likely represents a functional homolog of the human medial and dorsolateral PFC (Vertes, 2006), and therefore rodent models of relapse (modeled as reinstatement of ethanol seeking) can be used to uncover neural correlates in the PFC that assist with enhanced propensity for relapse (Martin-Fardon and Weiss, 2013). For example, one such model, namely the extinction-reinstatement model elicits drug seeking in response to ethanol-associated environmental stimuli, such as ethanol context and cues after extinguishing responses to these environmental stimuli in a novel (nondrug-paired) context (Shaham et al., 2003). Using this model, it has been demonstrated that enhanced ethanol seeking triggered by ethanol cues, stress and ethanol itself is associated with alterations in neurotransmitter systems and neuronal activation in the extended amygdala, and pharmacological manipulations targeting the dysregulated neurotransmitter systems assisted with reducing propensity for relapse (Ciccocioppo et al., 2003, Zhao et al., 2006). However, neurobiological correlates in the mPFC in response to ethanol seeking triggered by ethanol context and contextual cues is unknown (Weiss et al., 2001), and could help determine risk factors in a brain region implicated in preoccupation/anticipation or craving stage of addiction (Koob and Volkow, 2010).
We have recently demonstrated that unregulated self-administration of ethanol in animals that experienced chronic intermittent ethanol vapor exposure (CIE-ED; a paradigm that produces alcohol dependence-like behavior) produces profound alterations in the birth and survival of oligodendroglial progenitors (OPCs) in the mPFC compared with regulated ethanol drinking (ED, a paradigm that maintains nondependent drinking; (Richardson et al., 2009, Kim et al., 2015, Somkuwar et al., 2015)). Particularly interesting is the long-lasting effect of withdrawal on OPCs in the mPFC, visualized as increases in proliferation and survival of progenitors in CIE-ED animals that also demonstrated enhanced drinking after prolonged abstinence, suggesting a permanent dysregulation in the oligodendroglial niche maintaining oligodendroglial homeostasis (Somkuwar et al., 2015). Protracted abstinence from CIE is also associated with increases in myelin associated proteins in the mPFC indicating additional compensatory changes in myelinating glia (Navarro and Mandyam, 2015). Such alterations in the expression of oligodendroglia and myelinating glia during withdrawal and abstinence in the mPFC may be regulated by neuroinflammatory response during withdrawal, as interactions between oligodendroglia, myelin, endothelial cells and neuroinflammatory proteins have been demonstrated in models of brain injury, including, stroke and ischemia (Pham et al., 2012, Ortega et al., 2015). For example, proliferation and survival of OPCs is regulated by vascular endothelial cells, such that increase in endothelial response enhances OPC proliferation and survival (Arai and Lo, 2009). Notably, withdrawal from chronic ethanol experience enhances generation of inflammatory mediators such as cytokines in the cortex (Whitman et al., 2013, Harper et al., 2015) and increases in cytokines can upregulate expression of endothelial cell adhesion molecules in endothelial cells, that can in concert support leukocyte emigration via blood-brain barrier disruption (Woodfin et al., 2007, Larochelle et al., 2011). The recruitment of leucocytes by endothelial cell adhesion molecules is directly linked to more tissue damage and an increased release of inflammatory mediators, ultimately leading to uncontrolled inflammation (Privratsky et al., 2010).
In this context, platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is known to produce neuroinflammation and subsequent blood brain barrier disruption, and accumulating evidence suggests that it could be used as a potential marker for neurological disorders (Losy et al., 1999, Zaremba and Losy, 2002, Hwang et al., 2005, Kalinowska and Losy, 2006, Woodfin et al., 2007, Privratsky et al., 2010, Cheung et al., 2015, Andrews et al., 2016). However, it is not known whether withdrawal from chronic ethanol experience regulates PECAM-1 and if the alterations in PECAM-1 correlate with withdrawal induced increases in oligodendroglia and myelinating glia.
Accumulating evidence demonstrates that behavioral therapies (e.g. physical activity) that augment cognitive flexibility and alter neuroinflammatory responses can be used to enhance recovery from brain injury in models of stroke and ischemia (Hu et al., 2010, Olver et al., 2015). Notably, clinical and preclinical studies also demonstrate an interaction between physical activity (via wheel running in rodents) and alcohol drinking behaviors. Specifically, aerobic exercise in humans and wheel running activity in animals increase drinking behaviors when access to both are not concurrent (Werme et al., 2002, Ozburn et al., 2008, French et al., 2009, Lisha et al., 2011, Lisha et al., 2013, Leasure et al., 2015), and lead to reward substitution when both are available concurrently (McMillan et al., 1995). However, it is not clear, at least in animal models of relapse, whether wheel running prevents ethanol seeking triggered by ethanol context and contextual cues after extinguishing ethanol responses in a novel context, and whether the behavioral outcomes correlate with running induced neuroadaptations (Deehan et al., 2011, Li et al., 2015).
The current study tested the hypothesis that wheel running during abstinence prevents ethanol seeking in CIE-ED and ED animals that demonstrate enhanced propensity for relapse. The study also tested the subhypothesis that the reduced ethanol seeking with wheel running is associated with running-induced decreases in oligdendroglia, myelinating glia and PECAM-1 and increases in neuronal activation in the mPFC.
2.0 Methods
2.1 Animals
Seventy adult male Wistar rats (Charles River) completed the study. All rats were 8 weeks old at the beginning of the study, and weighed approximately 220–250 g. The rats were maintained in reverse 12h light-12h dark cycle rooms and housed two/cage unless otherwise specified. Food and water were available ad libitum. All experimental procedures were carried out in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996), and were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute.
2.2 Ethanol Self-Administration
The behavioral experiments conducted herein are presented as a detailed schematic in Figure 1a. Seventy adult male Wistar experimentally-naïve rats were given two 14-hour lever-responding training sessions in the operant conditioning boxes (Med Associates Inc, VT), on an fixed-ratio 1 schedule (FR1; one response resulted in one reinforce delivery), where one press on the available lever resulted in the delivery 0.1ml of water to a sipper cup mounted on the wall in between the two levers. The operant conditioning boxes were housed inside sound attenuating chambers. During these sessions, the house-light and white noise were turned off (Context A). Then, rats were trained to respond for 0.1ml of alcohol (10% v/v) over four daily 2-h FR1 sessions; all other conditions remained the same as before. Subsequently, the rats were trained to discriminate between two available levers to obtain on 0.1 ml ethanol (10% v/v) during daily 30-min FR1 sessions. During these sessions, active (right) lever responding resulted in the delivery of ethanol, while responding on the inactive (left) lever was recorded but had no programmed consequence. Each ethanol delivery followed by a 4-sec time-out during which responding on the active lever did not result in the delivery of ethanol. During this time-out period, the cue-light above the active lever remained on; thus the cue-light was paired with the delivery of ethanol. These 30-min discrimination training sessions continued till stable responding was obtained, where stable responding was defined as less than 10% variation in active lever responding for 3 consecutive 30-min FR1 sessions.
Figure 1.
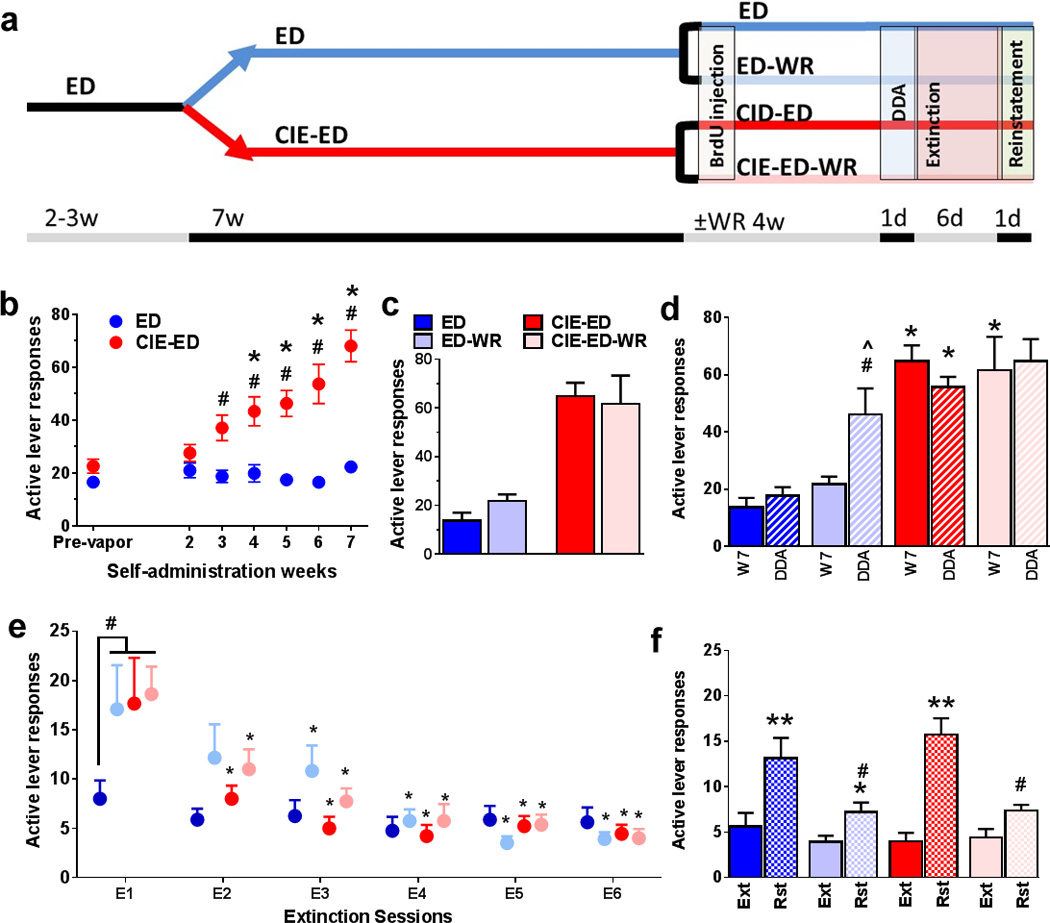
(a) Schematic representation of behavioral paradigm for the entire study. ED animals are always indicated in shades of blue and ED-CIE animals are indicated in shades of red. Animals were either given access to running wheels during withdrawal and abstinence or no wheels in their home cage for the same duration of time indicated by ±WR (b) Ethanol self-administration indicated as active lever responses. (c) Active lever responses averaged from last three days of self-administration prior to separation into ED and ED-WR and CIE-ED and CIE-ED-WR groups. (d) Active lever responses from ED, ED-WR, CIE-ED, and CIE-ED-WR animals when they experience a single drinking session during abstinence. (e) Lever responses during extinction (in context B) on the previously paired active lever (in context A). (f) Reinstatement (active lever presses) triggered by ethanol contextual cues. Data are represented as mean ± S.E.M.; n=6–12 each group. #p<0.05 vs ED in (b), *p<0.05 vs. week 1 in (b). *p<0.05 compared to the respective ED groups; #p<0.05 compared to ED during DDA, and ^p<0.05 vs. week 7 responses in ED-WR animals in (d). #p<0.05 indicates significant effect of wheel and CIE; *p<0.05 vs. day 1 extinction within each group in (e). *p<0.05, **p<0.01 vs. extinction and #p<0.05 versus sedentary groups in (f).
Subsequently, the rats were divided into two groups; one group received chronic intermittent ethanol vapor exposure (CIE; see procedure below) while the other group was exposed to air in their normal housing condition (did not experience ethanol vapors) for a duration of 7 weeks. Henceforth, these rats will be called CIE-ED (n=21) and ED (n=20) rats, respectively. All rats received two 30-min FR1 sessions per week (Tuesdays and Thursdays) during these 7 weeks. Responding was analyzed to determine escalation of self-administration compared to pre-vapor stable responding.
After 7 weeks of CIE, CIE-ED rats were withdrawn from ethanol vapors and both CIE-ED and ED rats were withdrawn from ethanol self-administration. Both CIE-ED and ED rats were divided into two groups and maintained as described for the remainder of the study. One group was maintained under standard housing conditions (referred as the sedentary cohort, or as CIE-ED and ED, respectively). The second group of rats were housed in special cages equipped with running (see wheel running below) and referred to as CIE-ED-WR and ED-WR, respectively.
2.3 Chronic Intermittent Ethanol vapor exposure (CIE)
During CIE, rat cages were housed in specialized chambers and were exposed to alcohol vapors on a 14-h ON / 10-h OFF schedule. Alcohol (95% ethanol) from a large reservoir was delivered to a heated flask at a regulated flow rate using a peristaltic pump (model QG-6, FMI Laboratory, Fluid Metering). The drops of alcohol in the flask were immediately vaporized and carried to the vapor chambers containing the rat cages by controlled air flow (regulated by a pressure gauge). The air pressure and ethanol flow rates were optimized to obtain blood alcohol levels (BALs) between 125 and 250 mg/dl or 27.2 and 54.4 mM (Gilpin et al., 2008a); these BALs are 2–3 times the BAL observed in binge drinking, but not high enough to abolish righting reflex (Ernst et al., 1976, Courtney and Polich, 2009).
2.4 Tail bleeding for determination of BAL
For measuring BALs, tail bleeding was performed on the CIE-ED and ED rats, once a week (every Friday), between hours 13–14 of vapor exposure (Gilpin et al., 2008b). Rats were gently restrained while the tip of the tail was pricked with a clean needle. Tail blood (0.2 ml) was collected and centrifuged at 2000 rpm for 10 min. Plasma (5 µL) was used for measurement of blood alcohol levels (BALs) using an Analox AM1 analyzer (Analox Instruments USA Inc., MA). Single-point calibrations were performed for each set of samples with reagents provided by Analox Instruments (100 mg/dl). When plasma samples were outside the target range (125–250 mg/dl), vapor levels were adjusted accordingly.
2.5 Wheel running
Wheel running experiment was conducted according to our previously published method (Engelmann et al., 2014). Briefly, CIE-ED-WR and ED-WR rats were single housed in cages equipped with running wheels (Nalgene activity wheels, 34.5-cm diameter 9 9.7-cm wide). Age-matched ethanol naïve rats were also housed in identical cages with running wheels for the same duration and served as wheel running controls (WR; n=12). Each of the running wheels were equipped with magnetic switches that were connected to a computer console. Running activity was monitored for the entire duration as number of wheel revolutions collected in 10 min bins with VitalView Software (Minimitter Inc.). Additional set of ethanol naïve control rats were maintained under standard housing conditions for the same duration (ethanol naïve sedentary, n=12) alongside the sedentary CIE-ED and ED rats. These rats did not have access to locked running wheels in their home cages as rodents climb in locked running wheels, a form of physical activity (environmental enrichment) and a potential confound for a sedentary group. The rats were maintained under these housing conditions till they were euthanized.
2.6 Drinking during abstinence (DDA)
After 23 days of abstinence from CIE and ethanol self-administration, CIE-ED and ED rats, both with and without running wheel access, were given one 30 min FR1 session to lever press for ethanol reinforcement (0.1 ml of 10% v/v ethanol) under cue-context conditions identical to that used for training and maintenance. Active and inactive lever responses were recorded.
2.7 Extinction
Following DDA, rats were subject to 6 daily 30-min extinction sessions under a different cue-context combination than that used for training and maintenance (Context B). Specifically, operant boxes different from those used for self-administration were used and the house-light and white noise were turned on, and no cue-lights were available following lever presses. Finally lever response did not result in the delivery of ethanol. Both lever responses were recorded.
2.8 Reinstatement
Following the 6th day of extinction, rats were subject to one session of cued-context reinstatement of ethanol seeking. Specifically, rats were introduced to operant chambers under conditions identical to training and maintenance (no house-light, no white noise; Context A). Active lever responses resulted in the presentation of the cue-light for 4 sec, but did not result in the delivery of ethanol. Both active and inactive lever responses were recorded.
2.9 5’- Bromo 2-deoxyuridine (BrdU) Labeling
Three days following removal from ethanol vapor, all rats, irrespective of treatment group, received an injection of BrdU (150 mg/kg, i.p.). The 3 day time-point was selected based on a previous publication that reported a burst in proliferation in the hippocampal SGZ using a comparable CIE model (Hansson et al., 2010). The single BrdU dose (150 mg/kg) was chosen to maximize labeling of proliferating cells by using a near-saturating concentration of BrdU (saturating concentration in rodents is ∼200 mg/kg). Of note, while BrdU is used for experimentally labeling proliferating cells (Dayer et al., 2003, Mandyam et al., 2007a, Taupin, 2007), injections of BrdU are also indicated to have cytotoxic and teratologic effects (Kolb et al., 1999, Sekerkova et al., 2004, Ogawa et al., 2005, Kuwagata et al., 2007, Duque and Rakic, 2011, Rowell and Ragsdale, 2012), primarily because BrdU is a marker of DNA synthesis and not of cell division per se (Breunig et al., 2007). However, in adult rodent models BrdU cytotoxicity is typically evident at greater than 2 times the currently used dose ((Cameron and McKay, 2001, Eadie et al., 2005); for review, (Taupin, 2007)), suggesting that the BrdU dose used in the current study could be suitable to evaluate proliferation and survival of progenitors in adult rats.
Previous studies had revealed that 3-days after cessation of CIE and ethanol self-administration, cell proliferation is increased in the mPFC for CIE-ED rats compared to ED and age-matched ethanol and behaviorally naïve rats (Somkuwar et al., 2015). However, the effect of 3-days of running experience concurrently with cessation of CIE and ethanol self-administration on cell proliferation is not known. To measure cellular proliferation of newly born cells, two hours after the BrdU injection, a subset of CIE-ED-WR (n=4), ED-WR (n=6), and ethanol naïve WR (n=6) and sedentary (n=6) rats were sacrificed, and their mPFC sections were probed for BrdU. The remaining CIE-ED-WR (n=9), ED-WR (n=12) and ethanol naïve control WR (n=6) and sedentary (n=6) rats were sacrificed 28 days after BrdU injection to measure the survival of newly born cells in the mPFC. All the CIE-ED (n=8) and ED (n=8) rats that did not have running wheel access during abstinence were also sacrificed 28 days after the BrdU injection. On the 28th day following BrdU administration, the responding for ethanol paired cue and context was evaluated using the 30-min self-administration reinstatement session. The rats were sacrificed 60–90 minutes after this session for measuring neuronal activation (cFos) and progenitor survival (BrdU).
2.10 Brain tissue collection
Rats were killed by rapid decapitation and the brains were isolated, and dissected along the midsagittal plane. The left hemisphere was snap frozen for Western blotting analysis (see below) and the right hemisphere was postfixed in 4% paraformaldehyde for immunohistochemistry. For tissue fixation, the hemispheres were incubated at room temperature for 36 hours and subsequently at 4 °C for 48 hours with fresh paraformaldehyde replacing the old solution every 12 hours. Finally, the hemispheres were transferred to sucrose solution (30% sucrose with 0.1% sodium azide) for cryoprotection and storage till tissue sectioning was conducted (Cohen et al., 2015). Subsequently, the tissue was sliced in 40µm sections along the coronal plane on a freezing microtome. Every ninth section through the PFC (+3.7 to +2.5 mm from bregma; 4 sections per rat) was mounted on Superfrost® Plus slides and dried overnight and used for BrdU analysis. Two sections through the PFC (+3.2 and +2.7 mm from bregma) were mounted as described before and processed for cFos analysis.
2.11 Quantitative Immunohistochemical Analysis for BrdU labelled cells
Quantitative immunohistochemical assay performed using a previously published optical fractionator method (Kim et al., 2015) using mouse monoclonal anti-BrdU (1:400; catalog # MCA2060, Serotec); the sections were counter-stained with Vector Fast Red (a nuclear stain). BrdU labelled cells were quantified in the mPFC with a Zeiss AxioImager Microscope equipped with Stereo Investigator 11.06 (MicroBrightField Bioscience, Williston, VT USA), a three-axis Mac 5000 motorized stage, a Zeiss digital MRc video camera, PCI color frame grabber, and computer work station. mPFC regions were contoured by referencing histological landmarks including corpus collosum, anterior commissure and rhinal fissure, using a 2.5x objective with a 10x eye piece and the above software (Paxinos and Watson, 1997). Cells were visually quantified within the contour using a 40x objective and a 10x eyepiece by an observer blind to the study using the following criteria - cells stained as dark brown to black, with the ability to focus the boundary of the cell within the mounted section thickness. A software generated 180 × 120 µm counting frame was systematically moved through the entire contoured area of the tissue to manually assess and count the BrdU-positive (BrdU+) cells. Mounted section thickness after immunohistochemistry was determined to be ∼28 µm. The two-hour-old BrdU+ cells always appeared in clusters of irregularly shaped dark stained cells. The overlapping-pair arrangement and the number of cells in each cluster were confirmed by focusing on different layers of cells along the Z-axis. Twenty-eight-day old BrdU+ cells were predominantly distributed as single cells or pairs of oval to round, spotty or dark stained cells, rarely seen in clusters, and exhibited cell morphology different from the proliferating time point. Absolute cell counting (complete counting of all immunoreactive cells in the contoured area) was performed in the mPFC; the data are presented as total number of cells per unit area (cells/mm2 based on mounted section thickness) per animal.
2.12 Phenotypic Analysis of BrdU labelled cells
To determine the phenotype of twenty-eight day old BrdU+ cells, Superfrost slides with mPFC sections were immunoprobed for BrdU (sheep primary 1:100; CY3 donkey anti-sheep secondary 1:200), oligodendroglial marker Olig2 (rabbit primary: 1:100, FITC goat anti-rabbit secondary: 1:200 (Mandyam et al., 2007b, Kim et al., 2014)) and myelin marker myelin basic protein (MBP; mouse primary 1:250; CY5 donkey anti-mouse secondary 1:500). Confocal analysis of triple labeled cells was performed with a Zeiss Axiovert 100 M and LSM510 using a previously published method (Kim and Mandyam, 2014, Kim et al., 2014). Briefly, using a 60× oil immersion objective (equipped with a 10× eyepiece), immunoreactive cells were optically sectioned in the z-plane using multitrack scanning (section thickness of 0.45 µm). Colocalization of antibodies was assessed with the confocal system by analysis of adjacent z-sections (using gallery function and orthogonal function for equal penetration of the antibodies). The phenotypic distribution of the surviving BrdU+ cells was calculated as the percent of mPFC BrdU+ cells colabeled with Olig2 (BrdU+Olig2+) or Olig2 and MBP (BrdU+Olig2+MBP+) in each rat. A minimum of 15 BrdU+ cells were analyzed per animal from each of the rats.
2.13 Quantitative analysis of cFos labeled cells
The following primary antibody was used for cFos immunohistochemistry (IHC): (1:1000, catalog # sc-52, Santa Cruz Biotechnology (Recinto et al., 2012)). The sections were pretreated (Mandyam et al., 2004), blocked, and incubated with the primary antibody followed by biotin-tagged secondary antibody. Fos immunoreactive cells were examined and quantified with a Zeiss AxioImager Microscope as described previously. Live video images were used to draw contours delineating the subregions of the PFC (anterior cingulate, prelimbic and infralimbic cortices). The fields of the brain regions for quantification were traced separately at 25x magnification. A 150 × 150 µm frame was placed over the regions of interest using the StereoInvestigator stereology platform followed by analysis using the optical fractionator method. The frame was systematically moved over the tissue to cover the entire contoured area and the labeled cells in each subregion falling entirely within the borders of the contour were marked and analyzed. Immunoreactive cells were quantified (absolute cell counting in the area contoured for analysis) and were summed up for each PFC subregion. Data were then summed up for the entire PFC and are presented in Figure 2.
Figure 2.
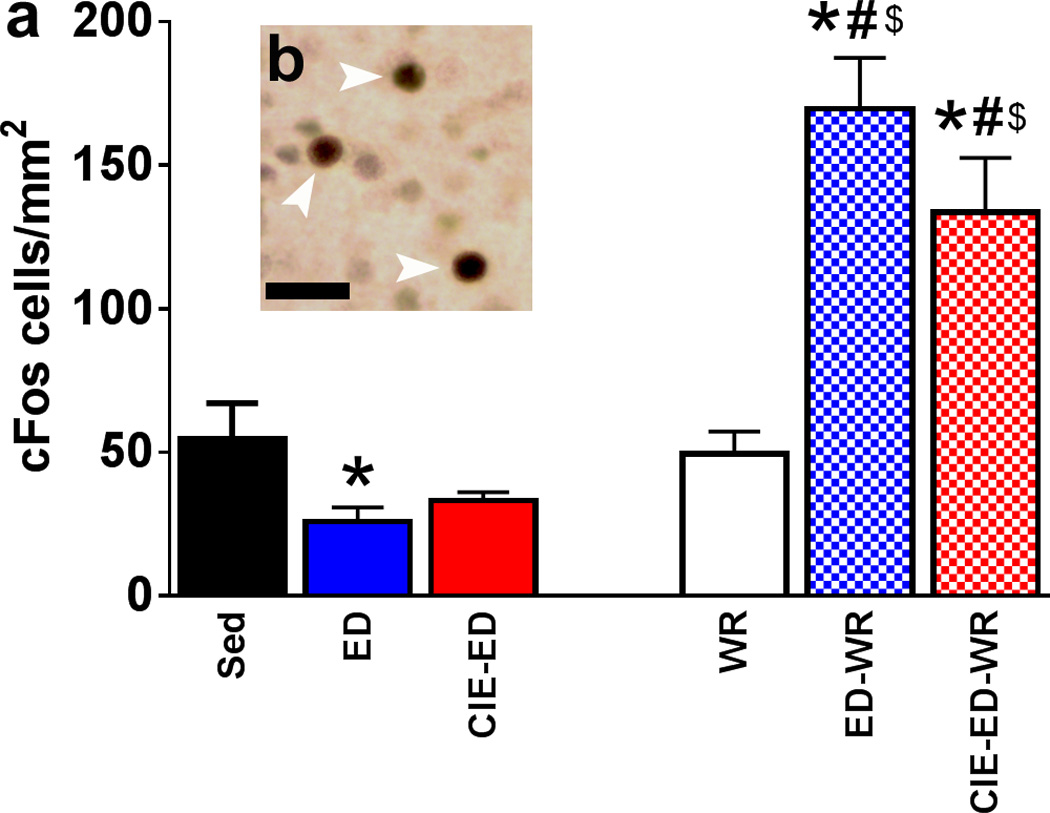
Quantitative analysis (a) and qualitative representation (b) of cFos in all experimental groups. (a) Total number of cFos immunoreactive cells in the mPFC expressed as cells/mm2. (b) DAB stained cFos cells in the mPFC with arrowhead pointing to immunolabeled cells. Scale bar in (b) is 15 um. *p<0.05 vs. sedentary control; #p<0.05 vs. ED and CIE-ED groups respectively; $p<0.05 vs. WR. Data are represented as mean ± S.E.M.; n=6–12 each group.
2.14 Western blotting
Western blot procedures optimized for measuring levels of both phosphoproteins and total proteins were employed (Graham et al., 2007, Kim et al., 2014, Galinato et al., 2015). Tissue punches from 500-um thick sections of mPFC were homogenized by sonication in ice-cold buffer (320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1 mM EDTA, 1% SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails II and III diluted 1:100; Sigma, St. Louis, MO), and protein concentration was determined using a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). 20 µg protein samples subjected to gel electrophoresis and transferred to PVDF membranes. The membranes were incubated with the appropriate primary/secondary antibody for detecting several oligovasculature markers (Supplementary content, Table S1). Immunoreactivity was detected using SuperSignal West Dura chemiluminescence detection reagent (Thermo Scientific) and collected using HyBlot CL Autoradiography film (Denville Scientific) and a Kodak film processor. Net intensity values were determined using the ImageJ software. Blots were reprobed for β-Tubulin (1:8000, SCBT sc-53140) for normalization purposes. Protein expression is presented as percent of ethanol naïve sedentary control samples on the same blot to normalize for blot-to-blot variability.
2.15 Statistical analysis
Each parametric measure was compared between groups using appropriate statistical analyses detailed in supplementary content (Table S2). In rats without running wheel access, number of BrdU and cFos cells was correlated with the density of PECAM-1 using Pearson’s product-moment correlation coefficient. Statistical significance was accepted at p < 0.05. Two-way ANOVA was conducted using GraphPad Prizm software, three-way ANOVA was conducted using SPSS version 20. Post-hoc tests were conducted to determine group differences and are indicated in Table S2.
3.0 Results
3.1 Escalation of Ethanol Drinking and Blood Alcohol Levels during CIE
Active lever responding for ethanol (10 % v/v; pre-vapor; t(39)=1.75; p=0.090; Figure 1b), as well as inactive lever responding (t(39)=0.508; p=0.61; Figure S1a) did not differ between the rats separated into the CIE-ED and ED groups prior to the onset of CIE. The amount of ethanol vapor experienced by each CIE-ED rat reached greater than 150 mg/dl of plasma; the BALs were significantly greater in CIE-ED compared to ED rats from week 1–7 of CIE (FweekXtreatment[6,49.2]=92.9, p<0.0001; Figure S1b). Active lever responses for ethanol self-administration significantly increased from week 3–7 of CIE treatment in CIE-ED rats, but not in the ED rats, compared to the respective pre-vapor responding and ED rats (FweekXtreatment[6,234]=14.1, p<0.0001; Ftreatment[1,39]=27.5, p<0.0001; Fweek[6,234]=17.9, p<0.0001; Figure 1b). After week 7, CIE-ED and ED rats were separated into CIE-ED and CIE-ED-WR, and ED and ED-WR groups and their active lever responding did not differ between the groups (last 3 days of self-administration; CIE-ED: t(15)=0.235; p=0.82; ED: t (19)=1.91; p=0.071; Figure 1c). Ethanol experience did not alter body weight (Figure S4).
3.2 Wheel Running during Abstinence
Running activity, measured as number of revolutions per day, did not differ between ED-WR, CIE-ED-WR and WR controls (FdayXtreatment[58,696]=0.818, p=0.96; Ftreatment[2,24]=0.0237, p=0.92; Figure S2).
3.3 Drinking during Abstinence
Following four weeks of abstinence, CIE-ED demonstrated higher drinking (higher active lever responses) compared to ED when rats were given access to ethanol, however, drinking was not different between CIE-ED-WR and ED-WR (FsessionXtreatmentXwheel[1,34]=0.267, p=0.61; FsessionXtreatment[1,34]=4.65, p=0.038; FsessionXwheel[1,34]=4.24, p=0.047; Fsession[1,34]=2.00, p=0.17; Figure 1c). Importantly, drinking during abstinence was enhanced in ED-WR compared to ED (p=0.016), and ED-WR compared to responding prior to abstinence (p=0.0054). Inactive lever responses did not differ between CIE-ED and ED rats, and CIE-ED-WR and ED-WR rats (Figure S1d).
3.4 Extinction and Contextual Cued Reinstatement of Ethanol Seeking
Following drinking during abstinence, the rats were subjected to six sessions of extinction in a novel context (context B, different from self-administration context A). ED-WR, CIE-ED and CIE-ED-WR rats demonstrated enhanced seeking during extinction compared to ED rats (FsessionXtreatmentXwheel[5,160]=3.28, p=0.046; FsessionXwheel[5,160]=1.75, p=0.18; FsessionXtreatment[5,160]=2.15, p=0.13; Fsession[5,160]=25.8, p<0.0001; Figure 1e). CIE-ED and CIE-ED-WR rats extinguished operant responses without significant differences in latency to extinguish. In contrast, ED-WR rats demonstrated enhanced latency to extinguish lever responses compared to ED, CIE-ED and CIE-ED-WR rats (ps<0.05). Lever responses on the previously paired inactive lever (in context A) did not differ between groups (FsessionXtreatmentXwheel[5,165]=2.00, p=0.12; FsessionXwheel[5,165]=0.445, p=0.74; FsessionXtreatment[5,165]=0.544, p=0.67; Fsession[5,165]=1.52, p=0.21; Figure S1d). All rats extinguished prior to reinstatement testing.
Following extinction sessions, the rats were subject to contextual cued reinstatement (in context A where active lever responding with cues is indicative of delivery of alcohol). Reinstatement of ethanol seeking on the previously paired active lever was higher in ED and CIE-ED rats compared to ED-WR and CIE-ED-WR rats (FsessionXtreatmentXwheel[1,33]=3.16, p=0.084; FsessionXtreatment[1,33]=2.28, p=0.14; FsessionXwheel[1,33]=25.4, p<0.0001; Fsession[1,33]=97.3, p<0.0001; Figure 1f). In contrast, responding on the previously paired inactive lever was not altered in any of the groups (FsessionXtreatmentXwheel[1,33]=1.51, p=0.24; FsessionXtreatment[1,33]=0.007, p=0.93; FsessionXwheel[1,33]=0.30, p=0.59; Fsession[1,33]=0.007, p=0.93; Figure S1e).
3.5 Neuronal Activation in the Medial Prefrontal Cortex
Two-way ANOVA demonstrated significant group differences in neuronal activation in mPFC (FtreatmentXwheel[2,43]=13.11, p<0.0001; Ftreatment[2,43]=4.9, p=0.01; Fwheel[1,43]=44.33, p<0.0001; Figure 2). Post hoc analysis revealed that the number of cFos cells were reduced in ED rats compared to sedentary controls (p=0.03), and access to running wheels did not alter the number of cFos cells in WR rats compared to sedentary rats. In contrast, ED-WR and CIE-ED-WR rats showed enhanced number of cFos cells following contextual cued reinstatement compared to WR controls, sedentary controls, ED and CIE-ED rats (ps<0.05).
3.6 Cell Survival and Phenotypic Analysis of Progenitors in the Medial Prefrontal Cortex
Newly born progenitors were labeled with BrdU 72h after CIE cessation and 2-h-old (Figure S3) and 28-day-old (Figure 3) BrdU cells were quantified. The number of 28-day-old BrdU+ cells were increased in ED and CIE-ED rats compared to ED-WR, CIE-ED-WR, sedentary and WR controls (FtreatmentXwheel[2,43]=27.5, p<0.0001; Ftreatment[2,43]=48.1, p<0.0001; Fwheel[1,43]=100, p<0.0001; Figure 3a–g). Post hoc analyses demonstrated higher number of BrdU cells in CIE-ED compared to ED (p=0.028); higher number of BrdU cells in ED compared to ED-WR, and CIE-ED compared to CIE-ED-WR (ps<0.01). Phenotypic analysis of 28-day-old OPCs revealed that a greater proportion of the BrdU+ cells expressed Olig2 (78–89%) compared with expression of Olig2 and MBP (0–15%) or neither of the markers (5–12%; Fphenotype[2,70]=523, p<0.0001; Figure 3n). Phenotypic distribution of BrdU+ cells did not significantly differ between treatment groups (FphenotypeXtreatmentxwheel[4,70]=0.569, p=0.69; FphenotypeXtreatment[5, 70]=1.15, 0.34; FphenotypeXwheel[5, 70]=2.84, p=0.065).
Figure 3.
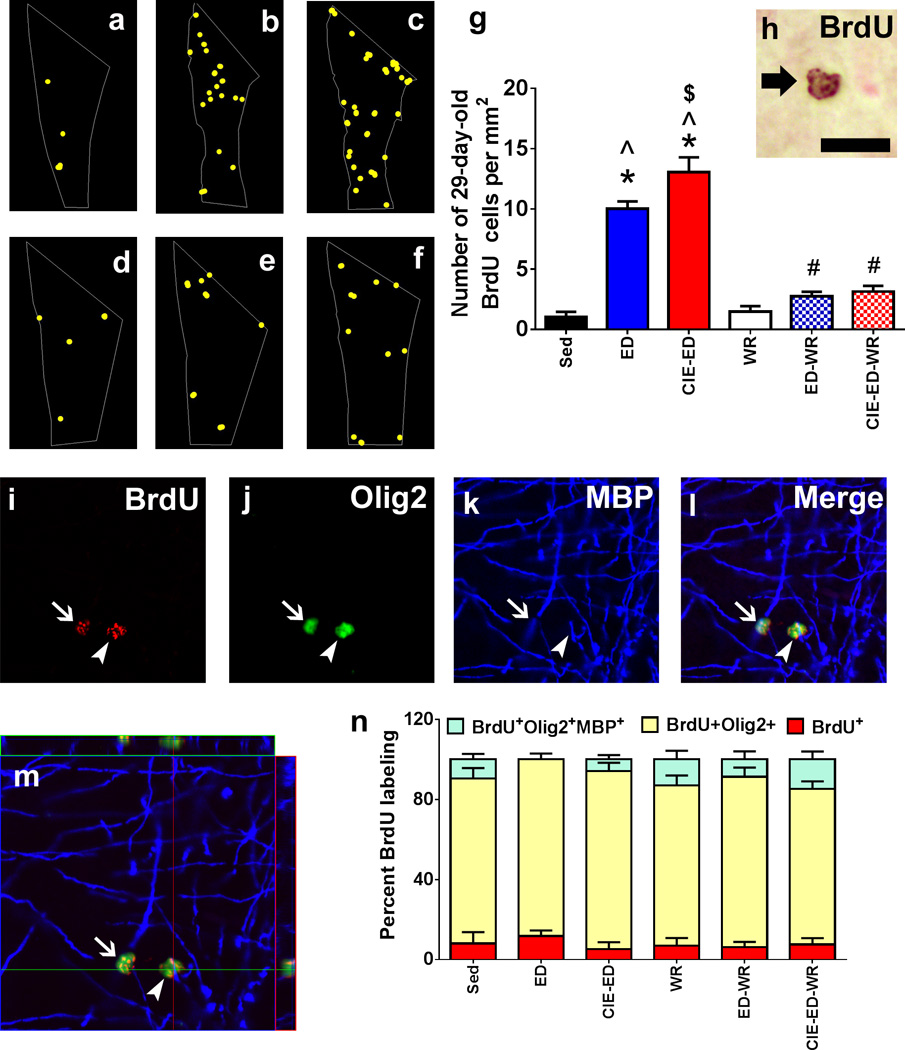
Qualitative map (a–f) and quantitative analysis of BrdU labeled cells in the mPFC acquired via StereoInvestigator software. Map in (a) shows distribution of 28-day-old BrdU cells in sedentary control (a), ED (b), CIE-ED (c), WR (d), ED-WR (e) and CIE-ED-WR (f) animal. One representative section from a matched bregma was chosen to demonstrate cellular distribution in each group. BrdU cells are indicated as yellow dots. (g) Total number of BrdU cells per mm2 in the mPFC. (h) DAB labeled BrdU cells in the mPFC. BrdU cells always appeared in cluster of 2 or more cells overlapping in a counting frame. Arrow in (h) points to two BrdU cells visualized as one on top of the other. (i–m) confocal triple labeling analysis of BrdU labeled cells. (i) BrdU, CY3; (j) Olig-2, FITC; (k) MBP, CY5, (l) merge. (m) Indicates an orthogonal section through the z-stack collected for each cell for qualitative analysis of the phenotype of the cells. Image is indicated in the x-z and y-z axis to demonstrate equal penetration of the all the three antibodies and fluorophores used for labeling. Arrow in (i–l) points to a BrdU/Olig2 double labeled cell placed on top of a myelin fiber, whereas arrowhead in (i–l) points to a BrdU/Olig2/MBP triple labeled cell. Scale bar indicated in (h) is 10 um for (h) and 20 um for (i–m). (n) Stacked bar graph representation of triple labeled population in each experimental group. *p<0.05 vs. sedentary control; ^p<0.05 vs. WR controls, $p<0.05 vs. ED, #p<0.05 vs. respective non-wheel groups. Data are represented as mean ± S.E.M.; n=6–12 each group.
3.7 Biochemical analysis of Oligodendroglia and myelinating glial Markers in the mPFC
Western blot analyses of proteins expressed during developmental stages of OPCs [NG2, PLP, CNPase, MBP, MOG, Olig2] were conducted on mPFC tissue lysates (Figure 4). Two-way ANOVA was performed for each protein indicated above and the detailed results are presented in Table S3. ED and CIE-ED had enhanced expression of NG2 and PLP in the oligodendroglial lineage compared to sedentary control as well as ED-WR and CIE-ED-WR rats (Figure 4b–c). CIE-ED had enhanced expression of MBP and MOG compared to sedentary control and CIE-ED-WR rats (Figure 4d–e). Overall, CIE-ED rats demonstrated an increase in expression of immature oligodendroglial and myelinating glial markers, whereas, ED rats demonstrated an increase in immature oligodendroglial markers (Figure 4f–g).
Figure 4.
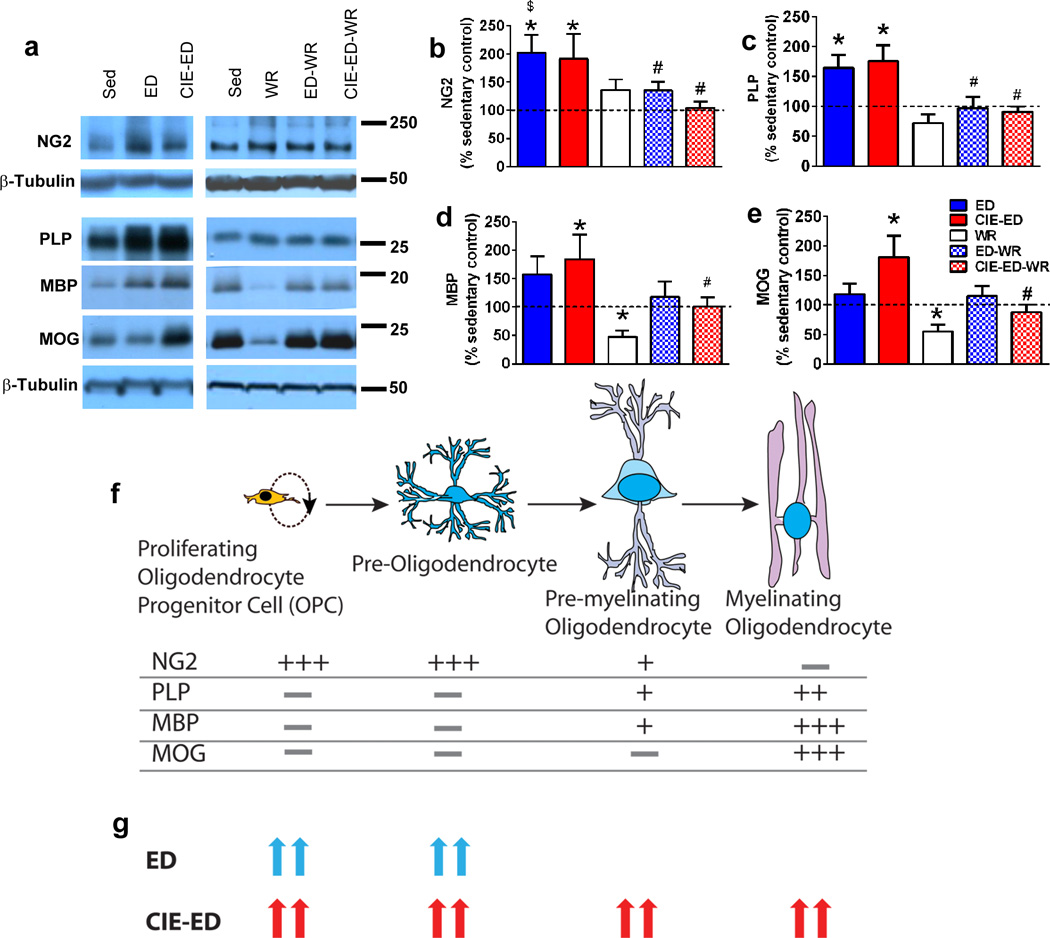
(a) Representative immunoblot of NG2, PLP, MBP and MOG with corresponding blots for β-tubulin which was used as loading control. (b–e) Densitometric analysis of NG2 (b), PLP (c), MBP (d) and MOG (e) indicated as percent change from sedentary control. (f) Cartoon representation of different developmental stages of OPCs and corresponding levels of expression of proteins investigated in our study. The schematic is based on reviews from (Nishiyama et al., 2009, Barateiro and Fernandes, 2014). (g) Summary of the change in expression levels of OPC proteins tested in ED and CIE-ED animals compared to sedentary controls. Data are represented as mean ± S.E.M.; n=6–12 each group. *p<0.05 vs. sedentary control, $p<0.05 vs. WR, #p<0.05 vs. respective sedentary ethanol control groups.
3.8 Expression of PECAM-1 in the mPFC
We used immunohistochemistry to determine qualitative changes in PECAM-1 levels in the mPFC, and staining demonstrated an increase in PECAM-1 expression in the entire mPFC in ED and CIE-ED rats compared to ethanol naïve controls, and WR groups (Figure 5a). Western blotting was performed to determine quantitative differences in PECAM-1.Two-way ANOVA of PECAM-1 demonstrated increased expression in ED and CIE-ED rats (Ftreatment[2,47]=2.61, p=0.03; Fwheel[1,47]=6.0, p=0.01; Figure 5b–c), and post hoc analysis revealed that PECAM-1 expression was greater in CIE-ED and ED compared to sedentary controls, CIE-ED-WR and ED-WR rats (ps<0.05).
Figure 5.
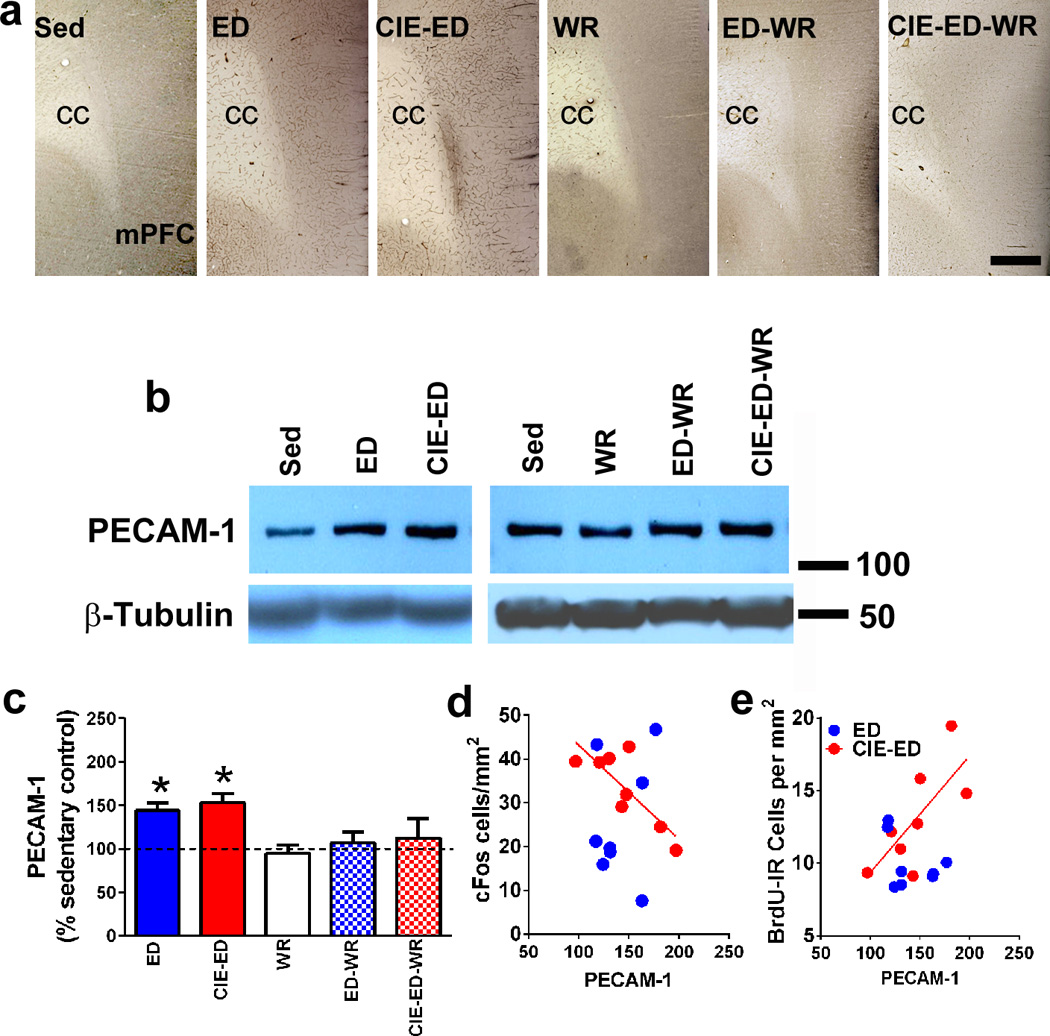
(a) Qualitative images of the mPFC stained with PECAM-1 from sedentary, ED, CIE-ED, WR, ED-WR and CIE-ED-WR rats. Scale bar in CIE-ED-WR panel is 100um applies to all images in (a); cc is corpus callosum. (b) Representative immunoblot of PECAM-1 with corresponding blot for β-tubulin which was used as loading control. (c) Densitometric analysis of PECAM-1 indicated as percent change from sedentary control. *p<0.05 vs. sedentary control. Data are represented as mean ± S.E.M.; n=6–12 each group. (d–e) PECAM-1 expression negatively correlates with the number of cFos cells (d) and positively correlates with the number of BrdU cells (e) in the mPFC in CIE-ED rats.
Linear regression analyses were also conducted between expression of PECAM-1, cFos, BrdU and all the oligodendroglial proteins in ED, CIE-ED, ED-WR and CIE-ED-WR. PECAM-1 expression in CIE-ED rats negatively correlated with cFos cells (r= −0.88, p=0.003) and positively correlated with the number of BrdU cells (r=0.73, p=0.03) (Figure 5d–e). No other correlations reached statistical significance.
4.0 Discussion
The first goal of the study was to identify differences in ethanol drinking behaviors and ethanol seeking behaviors during abstinence from chronic ethanol experience in ED and CIE-ED rats. The second goal was to determine whether wheel running during abstinence increases drinking behavior in ED and CIE-ED rats when ethanol was made available, and whether wheel running preserved extinction learning (a form of behavioral inhibition) performed during abstinence to reduce reinstatement of ethanol seeking behaviors. Here, we report that access to ethanol after a period of abstinence increases drinking in CIE-ED rats compared with ED rats, and this difference in drinking behavior is abolished (via increased drinking in ED-WR and maintained higher drinking in CIE-ED-WR) in animals that had access to running wheels during abstinence. We also report that ED rats reinstate ethanol seeking to a similar degree compared with CIE-ED rats, despite the fact that ED rats demonstrated regulated ethanol intake and did not display elevated drinking when ethanol was made available during abstinence. Notably, wheel running during abstinence reduced reinstatement of ethanol seeking in ED-WR and CIE-ED-WR rats suggesting preserved behavioral inhibition. Additional biochemical and immunohistochemical studies were performed on postmortem tissue from all groups to determine neuroadaptations in the mPFC that were associated with ethanol seeking. Markers of oligodendroglia, myelinating glia and neuroinflammation were enhanced during abstinence, and these effects were more robust in CIE-ED rats compared with ED rats. The PECAM-1 responses correlated with reduced neuronal activation and enhanced oligodendroglial levels in the mPFC in CIE-ED rats. Wheel running during abstinence reduced the number of newly born oligodendroglia and levels of PECAM-1 in the mPFC, and these changes were associated with enhanced neuronal activation. Our data demonstrate an interplay between oligodendroglial and neuroinflammatory responses in the mPFC in regulating neuronal activation in a model of alcohol addiction.
The results confirm that CIE experience reliably increases drinking and produces unregulated and escalated ethanol intake in an operant paradigm compared to animals that experience drinking without CIE (O’Dell et al., 2004). Several preclinical studies show an interaction between wheel running and ethanol drinking, where concurrent access to running wheel and ethanol reduces ethanol drinking, suggesting reward substitution (McMillan et al., 1995, Ehringer et al., 2009, Hammer et al., 2010, Brager and Hammer, 2012, Darlington et al., 2014, Gallego et al., 2015). Contrary to expectations, however, introduction of running wheel access during abstinence from ethanol self-administration did not increase or decrease wheel running in CIE-ED-WR and ED-WR animals, suggesting a lack of reward substitution/withdrawal-induced hypolocomotion in wheel activity (Ozburn et al., 2008, Logan et al., 2012). It is also possible that a ceiling effect rendered further increase in wheel activity unachievable in this age group of rats studied, especially given that age is a factor regulating exercise output (Staples et al., 2015). Reintroduction of ethanol access after abstinence produced higher drinking in CIE-ED rats compared to ED rats, and wheel running during abstinence abolished the difference between the groups by enhancing drinking in ED-WR rats. The increased drinking during abstinence cannot be attributed to the nonspecific arousal effects of ethanol context and cues because responding at the inactive lever remained indistinguishable from those during maintenance of self-administration. More importantly, these findings support an ethanol deprivation effect in ED-WR rats (Werme et al., 2002, Ozburn et al., 2008), which was not observed in CIE-ED-WR rats, suggesting a ceiling effect in CIE-ED-WR rats. This finding is interesting because deprivation effect models certain human aspects of alcoholism, and is observed after several episodes of abstinence post heavy drinking (Heyser et al., 1997, Holly and Wittchen, 1998, Spanagel and Holter, 1999, Werme et al., 2002, Ozburn et al., 2008). Our findings show that that wheel running during abstinence from moderate drinking potentiates deprivation effects, and these behavioral outcomes may enhance the propensity to consume excessive alcohol when alcohol is made available.
Although prior studies have investigated the interactions between running wheel and ethanol in the context of reward substitution and deprivation, no studies have examined the interaction of running wheel with ethanol seeking behaviors. Our findings reveal that CIE-ED animals exhibited greater resistance to extinction compared to ED animals. Wheel running during abstinence eliminated the difference by increasing seeking in ED-WR rats during extinction, suggesting that deprivation effects stimulated by wheel running continued into extinction sessions. However, all animals extinguished self-administration behavior by the last extinction session. Notably, CIE-ED rats and ED rats demonstrated similar ethanol-seeking behaviors triggered by ethanol contextual cues and wheel running prevented ethanol seeking in both groups to a similar extent. These findings support a protective effect of wheel running on propensity for contextual cued reinstatement of ethanol seeking. However, it is important to note that access to a running wheel is a form of environmental enrichment, and the change in seeking behaviors may be due to an enrichment-related effect. Thus, wheel running selectively facilitated inhibition of ethanol seeking, possibly by promoting ethanol-specific extinction learning mechanisms. Given that extinction training is being adopted in clinical behavioral therapy to promote recovery from relapse (Kiefer and Dinter, 2013), our findings suggest that physical exercise can enhance the beneficial effects of extinction training in alcoholic subjects, irrespective of previous intensity of alcohol drinking pattern.
With respect to wheel running-induced neuroadaptations in ethanol-experienced subjects, concurrent or delayed access to wheel running inhibits ethanol-induced reduction in hippocampal neurogenesis, and expression of hippocampal trophic factors, suggesting a neuroprotective effect (Crews et al., 2004, Maynard and Leasure, 2013, Gallego et al., 2015). This protection could assist with reduction in certain somatic signs of ethanol withdrawal in animals that experienced wheel running during or after ethanol exposure (Leasure and Nixon, 2010, Devaud et al., 2012, McCulley et al., 2012). However, other than the current study, no studies have examined the neuroadaptations by wheel running in the mPFC in ethanol-experienced subjects during protracted abstinence. Such studies could support the role of mPFC in inhibitory control of motivational impulses in reducing ethanol-seeking behaviors (Phan et al., 2005, Diekhof and Gruber, 2010, Chen et al., 2013, Mihindou et al., 2013). For example, studies employing the extinction-reinstatement model have demonstrated an increase in neuronal activation (via cFos labeling) in the mPFC in response to ethanol seeking triggered by ethanol itself and not footshock stress (Wedzony et al., 2003, Zhao et al., 2006, Dayas et al., 2007), suggesting that mPFC plays a role in incentive motivation in response to ethanol and does not regulate behavioral responses to stress. Notably, blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) studies in abstinent human subjects revealed that alcohol cues triggered enhanced brain activity in the PFC, suggesting that these responses could be interpreted as neuronal correlates of craving or incentive motivation (George et al., 2001, Karch et al., 2015, Kose et al., 2015). Our findings demonstrate that neuronal activity was reduced in the mPFC in ED and strong trend in CIE-ED rats that demonstrated enhanced ethanol seeking triggered by ethanol contextual cues. These results are distinct from the findings reported in ED and CIE-ED rats when ethanol seeking was triggered by ethanol priming, suggesting recruitment of brain regions other than the mPFC in response to ethanol itself (Zhao et al., 2006, Dayas et al., 2007). It is also likely that reduced cFos expression in the mPFC in ED and CIE-ED rats is correlated with reduced behavioral inhibition in these animals as evidenced by enhanced ethanol seeking triggered by contextual cues (Rhodes and Killcross, 2004, Rhodes and Killcross, 2007, Chen et al., 2013, Mihindou et al., 2013). Wheel running enhanced cFos expression in the mPFC and reduced ethanol seeking triggered by contextual cues, suggesting that wheel running could be reversing ethanol-induced hypoactivity in the mPFC as seen with other drugs of abuse (Chen et al., 2013, Zlebnik et al., 2014). Supporting a previous finding, wheel running did not alter cFos expression in running controls in the mPFC, suggesting that cFos response to running experience per se is different in areas of the mPFC compared to other brain regions associated with reward and reinstatement (Zlebnik et al., 2014). Thus, wheel running might modulate inhibitory associations formed during extinction by contextual cues and promote ethanol-specific extinction learning mechanisms by activating distinct neuronal ensembles (Pfarr et al., 2015). These findings support the role of the mPFC in recovery and reinstatement of an extinguished conditioned response, and also implicate the mPFC in some aspects of the recall of extinction learning.
Other forms of plasticity in the mPFC may support the reduced neuronal activity (cFos) in response to ethanol seeking in ED and CIE-ED rats. For example, synaptic plasticity and neural connectivity in the brain are associated with cellular processes such as myelination of axons, elimination of and pruning of synapses and neuroimmune responses (Pascual et al., 2014, Jacobsen et al., 2016). Recent work in animals that experienced binge ethanol exposure demonstrate that ethanol-induced cytokine responses disrupt myelin associated proteins (Pascual et al., 2014), however, it is unclear how excessive cytokine production may alter myelin integrity. Work from research conducted in tissue inflammation and injury models demonstrates a relationship between cytokines and endothelial cell adhesion molecules, where PECAM-1 supports leukocyte emigration and enhances the release of proinflammatory factors (Privratsky et al., 2010). Notably, endothelial cells also regulate OPCs and myelinating glia (Arai and Lo, 2009). We therefore determined the alteration in the expression of PECAM-1, a marker for neuroinflammatory response and blood brain barrier disruption (Kalinowska and Losy, 2006, Woodfin et al., 2007). As predicted, both ED and CIE-ED rats had excessive expression of PECAM-1 in the mPFC. More importantly, higher levels of PECAM-1 predicted lower levels of cFos in CIE-ED rats, demonstrating a negative relationship between the two proteins. Wheel running completely abolished/prevented the increases in PECAM-1 expression, and these changes were associated with enhanced neuronal activity in the mPFC. This is an important finding because PECAM-1 is enhanced at the site of endothelial injury and is a marker for ischemic response and neuroinflammation (Rosenblum et al., 1994, Hwang et al., 2005, Kalinowska and Losy, 2006), effects that could be occurring in ethanol experienced subjects (Whitman et al., 2013, Harper et al., 2015). Therefore, it appears that wheel running provided neuroprotection by inhibiting expression of factors implicated in brain trauma.
It is demonstrated that PECAM-1 in the adult brain and periphery supports the proliferation and differentiation of cells and maintains an anti-apoptotic environment (Bergom et al., 2005, Ohab and Carmichael, 2008). Given the exaggerated response in PECAM-1 expression in ED and CIE-ED rats, we examined the number and phenotype of newly born OPCs in the mPFC. We have recently demonstrated that CIE-ED rats have enhanced proliferation of OPCs during early withdrawal and enhanced survival of OPCs during protracted abstinence compared to ED rats, and these changes are associated with altered trophic factors regulating the gliogenic niche (Somkuwar et al., 2015). In the current report, we show that CIE-ED and ED rats exhibit enhanced survival of OPCs, and particularly enhanced myelinating OPCs and these changes were associated with enhanced ethanol seeking in both groups. More importantly, in CIE-ED rats, levels of PECAM-1 positively correlated with survival of newly generated OPCs (BrdU), indicating a strong relationship between neuroinflammatory response and oligodendroglial response during abstinence post chronic ethanol experience. These findings support previous observations of enhanced oligodendrogenesis in response to brain trauma (as seen in models of stroke and ischemia (Pham et al., 2012, Jing et al., 2015)), and indicate glial and inflammatory remodeling in response to brain injury generated by chronic ethanol experience (Tanaka et al., 2003, Jiang et al., 2011).
Notably, acute wheel running during withdrawal did not alter the proliferative burst in OPCs, however, continued running during abstinence reduced the survival of these progenitors. For example, running completely blocked the responses in newly born OPCs, and PECAM-1 and supported enhanced neuronal activation in the mPFC. These findings suggest a novel relationship between neuroinflammatory/oligodendroglial effects of wheel running in the mPFC and enhancement in inhibitory control and extinction learning mechanism to assist with reduced ethanol seeking behaviors. In sum, although correlational, these findings support the role of an oligodendroglial and neuroinflammatory response in the mPFC in modulating neuronal activity and reinstatement of ethanol seeking in the context of chronic ethanol intake.
Supplementary Material
Highlights.
Protracted abstinence enhances ethanol seeking triggered by contexual cues
Enhanced ethanol seeking is associated with oligodendrogenesis and neuroinflammatory responses in the mPFC
Wheel running during abstinence prevents ethanol seeking triggered by contexual cues
Reduced seeking is associated with reduced oligodendrogenesis and neuroinflammatory responses in the mPFC
Acknowledgments
The study was supported by funds from the National Institute on Alcoholism and Alcohol Abuse and National Institute on Drug Abuse (NIDA; AA020098, AA06420 and DA034140 to CDM, and Summer research internship from NIDA to JAQ). We appreciate the technical support of Ilham Polis and Maury Cole for assistance with operant self-administration studies and ethanol vapor chambers. We thank Drs. Charles Stiles and John Alberta, Harvard Medical School, for generously providing the Olig2 and pOlig2 antibodies and Dr. Peter J. Newman, BloodCenter of Wisconsin for rat PECAM-1 antibody. This is manuscript number 29265 from The Scripps Research Institute. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews AM, Lutton EM, Merkel SF, Razmpour R, Ramirez SH. Mechanical Injury Induces Brain Endothelial-Derived Microvesicle Release: Implications for Cerebral Vascular Injury during Traumatic Brain Injury. Front Cell Neurosci. 2016;10:43. doi: 10.3389/fncel.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro A, Fernandes A. Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta. 2014;1843:1917–1929. doi: 10.1016/j.bbamcr.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Bergom C, Gao C, Newman PJ. Mechanisms of PECAM-1-mediated cytoprotection and implications for cancer cell survival. Leukemia & lymphoma. 2005;46:1409–1421. doi: 10.1080/10428190500126091. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Hammer SB. Impact of wheel running on chronic ethanol intake in aged Syrian hamsters. Physiol Behav. 2012;107:418–423. doi: 10.1016/j.physbeh.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cheung K, Ma L, Wang G, Coe D, Ferro R, Falasca M, Buckley CD, Mauro C, Marelli-Berg FM. CD31 signals confer immune privilege to the vascular endothelium. Proc Natl Acad Sci U S A. 2015;112:E5815–E5824. doi: 10.1073/pnas.1509627112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Cohen A, Soleiman MT, Talia R, Koob GF, George O, Mandyam CD. Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology (Berl) 2015;232:453–463. doi: 10.1007/s00213-014-3685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Darlington TM, McCarthy RD, Cox RJ, Ehringer MA. Mesolimbic transcriptional response to hedonic substitution of voluntary exercise and voluntary ethanol consumption. Behav Brain Res. 2014;259:313–320. doi: 10.1016/j.bbr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Palmatier MI, Cain ME, Kiefer SW. Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behav Neurosci. 2011;125:184–193. doi: 10.1037/a0022627. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Walls SA, McCulley WD, 3rd, Rosenwasser AM. Voluntary wheel running attenuates ethanol withdrawal-induced increases in seizure susceptibility in male and female rats. Pharmacol Biochem Behav. 2012;103:18–25. doi: 10.1016/j.pbb.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci. 2010;30:1488–1493. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15205–15217. doi: 10.1523/JNEUROSCI.3092-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chro n ic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2014;219:657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst AJ, Dempster JP, Yee R, Dennis C, Nakano L. Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. J Stud Alcohol. 1976;37:347–356. doi: 10.15288/jsa.1976.37.347. [DOI] [PubMed] [Google Scholar]
- French MT, Popovici I, Maclean JC. Do alcohol consumers exercise more? Findings from a national survey. Am J Health Promot. 2009;24:2–10. doi: 10.4278/ajhp.0801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: sex differences and hippocampal BDNF expression. Physiol Behav. 2015;138:28–36. doi: 10.1016/j.physbeh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter. 2008a;9 doi: 10.1002/0471142301.ns0929s44. Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008b;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheel running and constant light exposure. Alcohol Clin Exp Res. 2010;34:1651–1658. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Breese GR. Withdrawal from Chronic Alcohol Induces a Unique CCL2 mRNA Increase in Adolescent But Not Adult Brain--Relationship to Blood Alcohol Levels and Seizures. Alcohol Clin Exp Res. 2015;39:2375–2385. doi: 10.1111/acer.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Holly A, Wittchen HU. Patterns of use and their relationship to DSM-IV abuse and dependence of alcohol among adolescents and young adults. European addiction research. 1998;4:50–57. doi: 10.1159/000018928. [DOI] [PubMed] [Google Scholar]
- Hu X, Zheng H, Yan T, Pan S, Fang J, Jiang R, Ma S. Physical exercise induces expression of CD31 and facilitates neural function recovery in rats with focal cerebral infarction. Neurological research. 2010;32:397–402. doi: 10.1179/016164110X12670144526309. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Kim DW, Yoo KY, Jung BK, Song JH, Jung JY, Choi SY, Kang TC, Lee JY, Kwon YG, Won MH. Ischemia-induced changes of platelet endothelial cell adhesion molecule-1 in the hippocampal CA1 region in gerbils. Brain Res. 2005;1048:251–257. doi: 10.1016/j.brainres.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Jacobsen JH, Hutchinson MR, Mustafa S. Drug addiction: targeting dynamic neuroimmune receptor interactions as a potential therapeutic strategy. Curr Opin Pharmacol. 2016;26:131–137. doi: 10.1016/j.coph.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Jiang L, Shen F, Degos V, Schonemann M, Pleasure SJ, Mellon SH, Young WL, Su H. Oligogenesis and oligodendrocyte progenitor maturation vary in different brain regions and partially correlate with local angiogenesis after ischemic stroke. Translational stroke research. 2011;2:366–375. doi: 10.1007/s12975-011-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, Li W, Ruan Y, Huang L. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015;35:1249–1259. doi: 10.1038/jcbfm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. European journal of neurology. 2006;13:1284–1290. doi: 10.1111/j.1468-1331.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Karch S, Keeser D, Hummer S, Paolini M, Kirsch V, Karali T, Kupka M, Rauchmann BS, Chrobok A, Blautzik J, Koller G, Ertl-Wagner B, Pogarell O. Modulation of Craving Related Brain Responses Using Real-Time fMRI in Patients with Alcohol Use Disorder. PLoS One. 2015;10:e0133034. doi: 10.1371/journal.pone.0133034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Dinter C. New approaches to addiction treatment based on learning and memory. Current topics in behavioral neurosciences. 2013;13:671–684. doi: 10.1007/7854_2011_147. [DOI] [PubMed] [Google Scholar]
- Kim A, Mandyam CD. Methamphetamine affects cell proliferation in the medial prefrontal cortex: a new niche for toxicity. Pharmacol Biochem Behav. 2014;126:90–96. doi: 10.1016/j.pbb.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2015;220:1705–1720. doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19:2337–2346. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose S, Steinberg JL, Moeller FG, Gowin JL, Zuniga E, Kamdar ZN, Schmitz JM, Lane SD. Neural correlates of impulsive aggressive behavior in subjects with a history of alcohol dependence. Behav Neurosci. 2015;129:183–196. doi: 10.1037/bne0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwagata M, Ogawa T, Nagata T, Shioda S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2’-deoxyuridine in mice. Neurotoxicology. 2007;28:780–789. doi: 10.1016/j.neuro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Neighbors C, Henderson CE, Young CM. Exercise and Alcohol Consumption: What We Know, What We Need to Know, and Why it is Important. Frontiers in psychiatry. 2015;6:156. doi: 10.3389/fpsyt.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Meng L, Huang K, Wang H, Li D. Environmental enrichment blocks reinstatement of ethanol-induced conditioned place preference in mice. Neurosci Lett. 2015;599:92–96. doi: 10.1016/j.neulet.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Lisha NE, Martens M, Leventhal AM. Age and gender as moderators of the relationship between physical activity and alcohol use. Addict Behav. 2011;36:933–936. doi: 10.1016/j.addbeh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisha NE, Sussman S, Fapa F, Leventhal AM. Physical activity and alcohol use disorders. The American journal of drug and alcohol abuse. 2013;39:115–120. doi: 10.3109/00952990.2012.713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, McCulley WD, 3rd, Seggio JA, Rosenwasser AM. Effects of withdrawal from chronic intermittent ethanol vapor on the level and circadian periodicity of running-wheel activity in C57BL/6J and C3H/HeJ mice. Alcohol Clin Exp Res. 2012;36:467–476. doi: 10.1111/j.1530-0277.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losy J, Niezgoda A, Wender M. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J Neuroimmunol. 1999;99:169–172. doi: 10.1016/s0165-5728(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007a;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. Journal of Neuroscience Research. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007b;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Modeling relapse in animals. Current topics in behavioral neurosciences. 2013;13:403–432. doi: 10.1007/7854_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ME, Leasure JL. Exercise enhances hippocampal recovery following binge ethanol exposure. PLoS One. 2013;8:e76644. doi: 10.1371/journal.pone.0076644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley WD, 3rd, Walls SA, Khurana RC, Rosenwasser AM, Devaud LL. Running wheel activity protects against increased seizure susceptibility in ethanol withdrawn male rats. Pharmacol Biochem Behav. 2012;100:485–489. doi: 10.1016/j.pbb.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- Mihindou C, Guillem K, Navailles S, Vouillac C, Ahmed SH. Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol Psychiatry. 2013;73:271–279. doi: 10.1016/j.biopsych.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015;293:35–44. doi: 10.1016/j.neuroscience.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Muneoka KT, Shioda S. Neuropathological examination of fetal rat brain in the 5-bromo-2’-deoxyuridine-induced neurodevelopmental disorder model. Congenital anomalies. 2005;45:14–20. doi: 10.1111/j.1741-4520.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- Olver TD, Ferguson BS, Laughlin MH. Molecular Mechanisms for Exercise Training-Induced Changes in Vascular Structure and Function: Skeletal Muscle, Cardiac Muscle, and the Brain. Progress in molecular biology and translational science. 2015;135:227–257. doi: 10.1016/bs.pmbts.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Ortega SB, Noorbhai I, Poinsatte K, Kong X, Anderson A, Monson NL, Stowe AM. Stroke induces a rapid adaptive autoimmune response to novel neuronal antigens. Discovery medicine. 2015;19:381–392. [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–424. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Pla A, Minarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol Alcohol. 2014;49:187–192. doi: 10.1093/alcalc/agt164. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci. 2015;35:10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, Guo S, Kim KW, Lo EH, Arai K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25:2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum WI, Murata S, Nelson GH, Werner PK, Ranken R, Harmon RC. Anti-CD31 delays platelet adhesion/aggregation at sites of endothelial injury in mouse cerebral arterioles. Am J Pathol. 1994;145:33–36. [PMC free article] [PubMed] [Google Scholar]
- Rowell JJ, Ragsdale CW. BrdU birth dating can produce errors in cell fate specification in chick brain development. J Histochem Cytochem. 2012;60:801–810. doi: 10.1369/0022155412458588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Ilijic E, Mugnaini E. Bromodeoxyuridine administered during neurogenesis of the projection neurons causes cerebellar defects in rat. J Comp Neurol. 2004;470:221–239. doi: 10.1002/cne.11016. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A, Quigley JA, Edwards S, Mandyam CD. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Staples MC, Somkuwar SS, Mandyam CD. Developmental effects of wheel running on hippocampal glutamate receptor expression in young and mature adult rats. Neuroscience. 2015;305:248–256. doi: 10.1016/j.neuroscience.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, Bienkowski P. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37:2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta neurologica Scandinavica. 2002;106:292–298. doi: 10.1034/j.1600-0404.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Hedges VL, Carroll ME, Meisel RL. Chronic wheel running affects cocaine-induced c-Fos expression in brain reward areas in rats. Behav Brain Res. 2014;261:71–78. doi: 10.1016/j.bbr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


