Abstract
The use of calcineurin inhibitors (CNIs) to reduce the risk of graft-versus-host disease (GVHD) after hematopoietic cell transplantation (HCT) requires intensive post-transplant toxicity monitoring. Sirolimus-based GVHD prophylaxis is associated with a favorable toxicity profile and requires less intensive monitoring. However, the efficacy of sirolimus-based regimen as compared to CNI-based regimen has not been evaluated in the setting of reduced-intensity conditioning (RIC) double umbilical cord blood (UCB) HCT. We compared outcomes of patients receiving sirolimus/mycophenolate mofetil (MMF; n=37) or cyclosporine (CSA)/MMF (n=123) in an ongoing phase II study of RIC UCB transplantation. In multiple regression analysis, sirolimus/MMF did not influence the risk of grade II–IV or grade III–IV acute GVHD. In addition, there was no association between type of GVHD prophylaxis and hematopoietic engraftment. Infection density analysis found a significantly lower risk of infections with sirolimus/MMF between days +46 and +180 post-HCT as compared to CSA/MMF (3.4 vs. 6.3 per 1000 patient-days, p=0.03); however, no difference was observed before day +45. Sirolimus/MMF use resulted in no thrombotic microangiopathy, fewer instances of elevated serum creatinine >2mg/dL (14% vs. 45%; p<0.01), and similar rates of sinusoidal obstruction syndrome (2.7% vs. 4%; p=0.68) as compared to CSA/MMF. Disease-free survival at 1 year was 51% for sirolimus/MMF and 41% for CSA/MMF (p=0.41), and sirolimus/MMF use did not influence the risk of non-relapse mortality or survival. In conclusion, sirolimus/MMF GVHD prophylaxis was better tolerated and resulted in similar rates of GVHD and survival as compared to CSA/MMF after RIC double UCB transplant.
Keywords: Sirolimus, CSA, RIC, double UCB, transplantation, GVHD
INTRODUCTION
Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor with immunosuppressive properties. Sirolimus has been shown to permit relative expansion of thymic-derived regulatory T-cells (T-regs) and the preferential inhibition of effector T-cell function.1–4 In addition, sirolimus has been found to be effective in preventing graft-versus-host disease (GVHD) both in rodent models5, 6 and in human studies.4, 7–9 Calcineurin inhibitors (CNIs) can successfully reduce the risk of GVHD; however, their use requires intensive post-transplant monitoring of side effects such as nephrotoxicity, electrolyte imbalances, hypertension, posterior reversible encephalopathy syndrome (PRES), and transplant-associated thrombotic microangiopathy (TMA). Given sirolimus’s overall favorable side effect profile, less frequent need for drug level monitoring, and lower cost as compared to CNIs,10, 11 it has been increasingly used for the past decade as an immunosuppressive agent both after solid organ transplantation and allogeneic (allo) hematopoietic cell transplantation (HCT).4, 7, 12–14 In addition, the direct antineoplastic activity of sirolimus via mTOR pathway inhibition makes it a particularly attractive GVHD prophylaxis drug for recipients of alloHCT with hematological malignancies, especially in the reduced-intensity conditioning (RIC) setting where relapse remains the main cause of treatment failure.7, 15 Despite the recently increased use of sirolimus in alloHCT for GVHD prophylaxis, most studies have examined sirolimus in combination with CNIs in mainly adult-related or unrelated donor HCT.8, 16–23 No studies have compared sirolimus- and CNI-based GVHD prophylaxis in recipients of RIC double umbilical cord blood (UCB) HCT. The present study compares the outcomes of GVHD prophylaxis with cyclosporine (CSA)/mycophenolate mofetil (MMF) or sirolimus/MMF after RIC double UCB HCT.
METHODS
Study design and patient eligibility
We performed a secondary analysis of data from the ongoing phase II study of RIC double UCB HCT. The protocol for GVHD prophylaxis was originally CSA/MMF; however, it was amended to use sirolimus/MMF in September 2012, Eligible patients were those ≤75 years old with no available matched sibling donor, who had a Karnofsky score >60%, and adequate organ functions (cardiac left ventricular ejection fraction ≥35%, pulmonary DLCO >30% predicted, liver transaminases <5 times and total bilirubin <3 times the upper limit of normal, serum creatinine ≤2.0 mg/dl, as described).24 Patients with previous alloHCT and those receiving experimental cellular therapies were excluded. The protocol was approved by the University of Minnesota Institutional Review Board. All patients/guardians provided written informed consent. The study was registered at clinicaltrial.gov as NCT00305682.
Treatments
Sirolimus was administered once daily, with 8–12 mg oral loading dose on day −3, followed by 4mg daily dose with target trough levels 3–12 ug/L until day +100, followed by a tapering of the dose by day +180. CSA was administered twice daily, with initial dose of 2.5 mg/kg intravenously (IV) on day −3, then was continued either IV or orally until day +100 to maintain a target trough level between 200 and 400 ng/mL, followed by a tapering of the dose by day +180. All patients received MMF at 1.5 g twice daily between days −3 and +30; MMF was delivered intravenously initially and then orally in the same dose.
We used institutional guidelines for UCB graft selection with both units 4–6/6 matched to the patient and to each other.24 Minimum required total nucleated cell (TNC) dose at cryopreservation was 1.5×107/kg per unit. The RIC conditioning regimen consisted of fludarabine (Flu) at a dose of 30 mg/m2 daily for 5 days, cyclophosphamide (CY) at a single dose of 50 mg/kg, and TBT at a dose of 200 cGy as a single fraction. Antithymocyte globuline (ATG) at a dose of 15 mg/kg twice daily for 3 days was given to patients receiving no immunosuppressive chemotherapy within 3 months.24, 25 Supportive care has been previously described and did not change over the two time periods of the study.24, 25
Definitions and Endpoints
The primary study endpoint was the incidence of acute GVHD at day +100 graded as previously described.26, 27 Secondary endpoints included neutrophil and platelet engraftment, non-relapse mortality at day +180, relapse, and disease-free (DFS) and overall survival (OS) at 1 year. Exploratory endpoints included infections within post-transplant intervals of days 0 to +45 and days +46 to +180. Bacterial, fungal, and viral infectious episodes were previously described.25 Patient, disease, and transplant characteristics included age, sex, year of transplantation, disease diagnosis, disease risk, hematopoietic stem cell transplant comorbidity index (HCT-CI), prior history of autologous transplantation, recipient CMV serological status, HLA disparity by worst unit matching, ATG use, infused total nucleated cell dose (TNC), and total CD34+ cell dose. Disease risk was defined as either standard or high risk at HCT using America Society for Blood and Marrow Transplantation (ASBMT) 2006 risk scoring schema.28 HCT-CI was assessed before alloHCT.29 Patient outcomes are reported as of April 2015. Neutrophil engraftment was defined as absolute neutrophil counts recovery of >0.5 × 109/L for 3 consecutive measures by day +42, and platelet engraftment as platelet count recovery >20,000 by day +180 and platelet transfusion-free for at least 7 days. DFS was defined as being alive without malignancy relapse or progression after HCT. OS was defined as the time from HCT to death from any cause.
Statistical Analysis
Comparisons between GVHD prophylaxis cohorts were completed with the log rank test. OS and DFS were estimated by Kaplan-Meier curves with 95% confidence intervals (CI) derived from the standard errors.30 Non-relapse mortality (NRM) was analyzed using cumulative incidence treating relapse as a competing risk. Relapse was analyzed using cumulative incidence treating death as a competing risk. Neutrophil and platelet engraftment was analyzed using cumulative incidence treating non-event death as a competing risk.31 Fine and Gray proportional hazards regression was used to assess the independent effect of the indices on NRM, relapse, and engraftment.32 Factors considered in the regression models included HLA disparity considering the worst matched UCB units (4/6 versus 5/6+6/6), age (<60 versus 60+), disease (acute leukemia versus lymphoma versus other), disease risk (standard versus high risk), gender (male versus female), Karnofsky performance status at baseline (60–80% versus 90–100%), HCT-CI (0 versus 1–2 versus ≥3), recipient CMV serostatus (positive versus negative), prior auto transplant (yes versus no), conditioning (ATG versus no ATG), HHV6 reactivation (no versus prior outcome), and grade II–IV acute GVHD as a time-dependent variable. Backward selection was used to build prognostic factor models for all endpoints. A p-value of ≤0.05 was considered significant for remaining in the model; however, GVHD prophylaxis was included in all models. Cumulative density function was used to estimate the infection density per 1000 patient days to account for multiple infections per patient. The Mantel-Haenszel test was used to compare the frequency of bacterial, fungal, and viral infections between two GVHD prophylaxis types for person-years data. The analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
The patient, disease, and transplant characteristics of sirolimus/MMF-treated patients (n=37) and CSA/MMF-treated patients (n=137) are summarized in Table 1. The year of transplant differed between the two groups because alloHCT recipients received sirolimus/MMF GVHD prophylaxis after 2012. Patients in the sirolimus/MMF cohort were older (median age 61 vs. 53 years; p <0.01), but had less comorbid conditions at transplant as compared to the CSA/MMF cohort (27% vs. 55% HCT-CI ≥3; p <0.01). However, the remaining patient, disease, and treatment characteristics were similar. The median follow-up of surviving patients was shorter in the sirolimus/MMF cohort (1.7 years) as compared to the CSA/MMF cohort (7.2 years); thus, 1-year OS and DFS were selected as the primary endpoints.
Table 1.
Patient characteristics
| Variable | Sirolimus/MMF n=37 N (%) |
CSA/MMF n=123 N (%) |
P-value* | |
|---|---|---|---|---|
| Age (years) | Median (range) | 61 (22–69) | 53 (21–69) | <0.01 |
| Male | 28 (76) | 74 (60) | 0.09 | |
| Year of HCT | <0.01 | |||
| 2006–2009 | 0 (0) | 98 (80) | ||
| 2010–2014 | 37 (100) | 25 (20) | ||
|
HLA disparity (worst match) |
4/6 | 20 (60) | 59 (49) | 0.42 |
| 5/6 | 14 (38) | 53 (43) | ||
| 6/6 | 3 (8) | 11 (9) | ||
|
ATG in conditioning |
17 (46) | 48 (39) | 0.45 | |
| Diagnosis | 0.40 | |||
| Acute leukemia | 17 (46) | 55 (45) | ||
| Lymphoma | 6 (16) | 32 (26) | ||
| Other† | 14 (38) | 36 (29) | ||
| Disease risk | 0.68 | |||
| Standard | 16 (43) | 58 (47) | ||
| High | 21 (57) | 65 (53) | ||
| Prior Auto-HCT | 6 (16) | 26 (21) | 0.51 | |
| HCT-CI | <0.01 | |||
| 0 | 11 (30) | 32 (26) | ||
| 1–2 | 15 (41) | 21 (17) | ||
| ≥ 3 | 10 (27) | 67 (55) | ||
| CMV seropositive | 19 (51) | 82 (67) | 0.05 | |
|
Total TNC (×108/kg) |
Median (range) | 0.4 (0.3–0.8) | 0.4 (0.2–0.7) | 0.16 |
|
Total CD34 (×106/kg) |
Median (range) | 0.5 (0.2–1.8) | 0.4 (0.1–1.3) | 0.34 |
P-value for between-treatment comparisons. Continuous variables were analyzed by general Wilcoxon test. Categorical variables were analyzed by chi-square.
Other includes diagnoses of Myelodysplastic syndromes and plasma cell disorders
Acute and Chronic GVHD
Both GVHD prophylaxis cohorts had similar cumulative incidences of grade II–IV acute GVHD (sirolimus/MMF 41%, 95% CI, 24–57% vs. CSA/MMF 42,% 95% CI, 33–51%; p=0.98; Figure 1) and grade III–IV acute GVHD (22% (95% CI, 9–25%) vs. 14% (95% CI, 8–20%); p=0.22). Acute GVHD organ involvement was similar in both cohorts including skin (80% for sirolimus/MMF and 76% of CSA/MMF patients; p=0.74), lower GI (67% vs. 57%; p=0.52), upper GI (20% vs. 35%; p=0.26), and liver involvement (7% vs. 17%; p=0.33). In multiple regression analysis, the risk of grade II–IV and grade III–IV acute GVHD was similar for sirolimus/MMF and CSA/MMF. The cumulative incidence of chronic GVHD was 9% (95% CI, 0–18%) for sirolimus/MMF cohort and 20% (95% CI, 13–28%) for CSA/MMF cohort (p=0.08; Figure 2). Similarly, the type of GVHD prophylaxis had no influence on chronic GVHD rates in multivariable analysis. In addition, the cumulative incidence of GVHD-free and relapse-free survival (GRFS) at 1-year was 37% (95% CI, 21–53%) for sirolimus/MMF and 23% (95% CI, 16–30%) for CSA/MMF (p=0.3).
Figure 1.
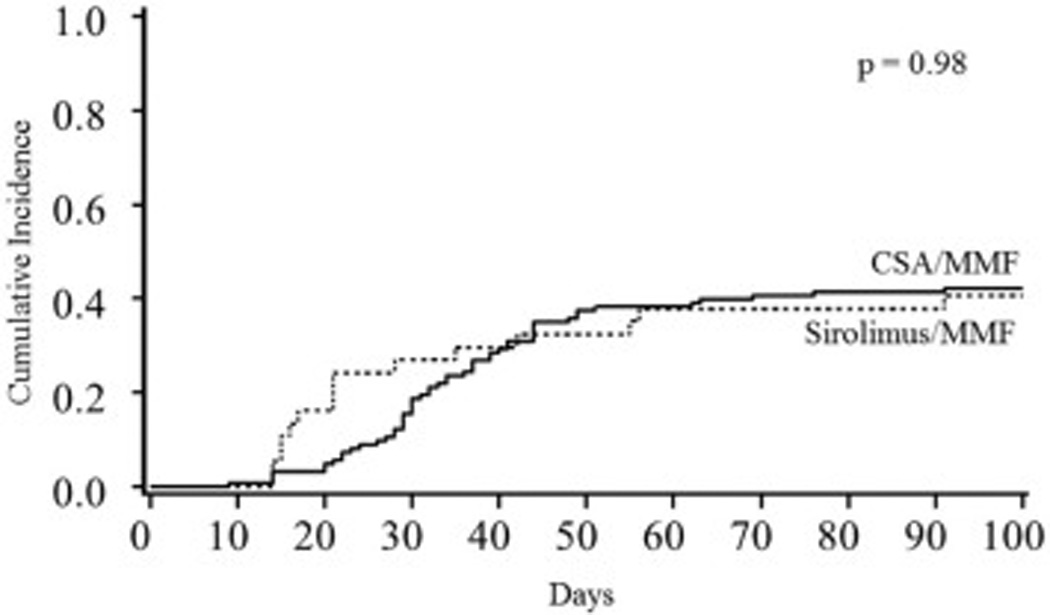
Grade II–IV acute GVHD by GVHD prophylaxis
Figure 2.
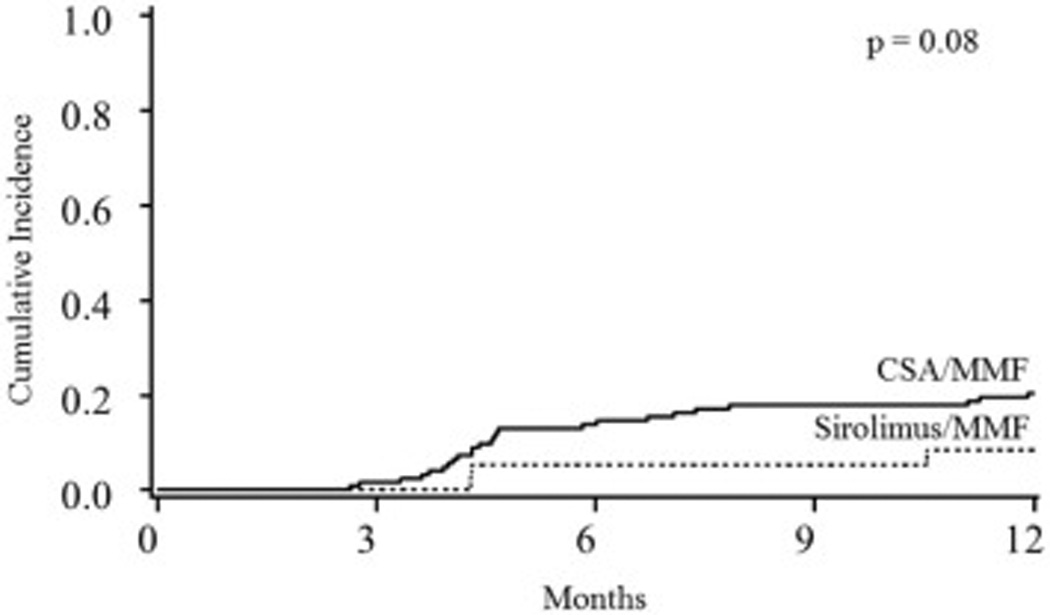
Chronic GVHD by GVHD prophylaxis
Hematopoietic Engraftment
Median time to neutrophil recovery was 17 days in both cohorts, and the cumulative incidence of neutrophil engraftment by day 42 was 81% (95% CI 67–92%) for sirolimus/MMF and 93% (95% CI 88–97%) for CSA/MMF (p=0.06). All but one (6 of 7) patient in the sirolimus/MMF cohort who had not achieved neutrophil recovery by day 42 had HHV-6 reactivation. However, 3 of these patients achieved neutrophil engraftment by day 60 after receiving antiviral therapy with foscanet for HHV-6; the other 3 patients died of graft failure. The only primary graft failure patient without HHV-6 reactivation in sirolimus/MMF cohort achieved neutrophil recovery at day +43. In contrast, 9 patients in CSA/MMF cohort did not achieve neutrophil engraftment by day 42. HHV6 polymerase chain reaction (PCR) testing was performed only 7 of those 9 subjects, and HHV6 reactivation was identified in only 3 patients: 1 patient achieved neutrophil engraftment at day +60 after receiving antiviral therapy with foscanet for HHV-6; 1 patient achieved engraftment at day +69 with continued growth factor, blood product and antibiotic support, and 1 patient died of graft failure. Tree of 4 graft failure patients who tested negative for HHV-6 reactivation died of graft failure, and one had neutrophil recovery at day +55 with continued growth factor, blood product and antibiotic support. Two of the remaining graft failure patients in CSA/MMF cohort who were treated before 2007 and had no HHV-6 PCR testing performed died of graft failure as well. In multivariable analysis, after adjusting for age, total infused CD34+ cell dose, and HHV6 reactivation prior to neutrophil recovery, the GVHD prophylaxis regimen had no influence on neutrophil engraftment by day 42. HHV6 reactivation prior to neutrophil recovery was the only factor significantly associated with risk of graft failure (Table 2). Median time to platelet engraftment was 63 days for sirolimus/MMF and 50 days for CSA/MMF, and the cumulative incidence of platelet engraftment at day 180 was 78% (95% CI 60–97%) for sirolimus/MMF and 72% (95% CI 61–82%) for CSA/MMF (p=0.46). In multivariable analysis adjusting for HHV6 reactivation as a time dependent variable, sirolimus/MMF vs. CSA/MMF was not associated with platelet recovery. HHV6 reactivation (RR=0.5, 95% CI 0.3–0.9; p=0.01) prior to platelet recovery was the only factor adversely affecting platelet engraftment. Median total chimerism at day 21 was 88% (interquartile, 69–96%) for sirolimus/MMF and 83% (interquartile, 52–96%) for CSA/MMF (p=0.98). Similarly, by day +60 the median chimerism was 100% (interquartile, 100-100%) in both cohorts.
Table 2.
Multivariable analysis of clinical outcomes
| Variable | Multivariable | |||
|---|---|---|---|---|
| Total N | RR | 95% CI | P-value | |
| Neutrophil Engraftment | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 1.0 | 0.6–1.5 | 0.89 |
| HHV6 prior to ANC engraftment | ||||
| No | 138 | 1.0 | ||
| Yes | 22 | 0.3 | 0.1–0.5 | <0.01 |
| Age | ||||
| <60 | 104 | 1.0 | ||
| ≥60 | 56 | 0.8 | 0.6–1.2 | 0.31 |
| CD34 dose | ||||
| <3.5 cells × 107/kg | 44 | 1.0 | ||
| ≥3.5 cells × 107/kg | 116 | 1.3 | 1.0–1.9 | 0.09 |
| Platelet Engraftment (20K) | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 1.0 | 0.7–1.5 | 0.83 |
| HHV6 prior to platelet engraftment | ||||
| No | 121 | 1.0 | ||
| Yes | 39 | 0.5 | 0.3–0.9 | 0.01 |
| Grade II-IV acute GVHD | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 1.1 | 0.6–1.9 | 0.86 |
| Chronic GVHD | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 0.3 | 0.1–1.1 | 0.07 |
| Gender | ||||
| Male | 102 | 1.0 | ||
| Female | 58 | 0.4 | 0.2–1.0 | 0.06 |
| Non-Relapse Mortality | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 1.0 | 0.4–2.6 | 0.94 |
| Disease risk | ||||
| Standard | 74 | 1.0 | ||
| High | 86 | 2.9 | 1.2–7.3 | 0.02 |
| Relapse | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 0.8 | 0.4–1.7 | 0.60 |
| Disease risk | ||||
| Standard | 74 | 1.0 | ||
| High | 86 | 0.6 | 0.3–1.1 | 0.09 |
| Disease-Free Survival | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 0.8 | 0.5–1.3 | 0.79 |
| CD34 dose | ||||
| <3.5 cells × 107/kg | 44 | 1.0 | ||
| ≥3.5 cells × 107/kg | 116 | 0.7 | 0.5–1.1 | 0.09 |
| CMV serostatus | ||||
| Negative | 59 | 1.0 | ||
| Positive | 101 | 1.5 | 1.0–2.3 | 0.06 |
| Overall Survival | ||||
| GVHD prophylaxis | ||||
| CSA/MMF | 123 | 1.0 | ||
| Sirolimus/MMF | 37 | 0.8 | 0.5–1.5 | 0.57 |
| CD34 dose | ||||
| <3.5 cells × 107/kg | 44 | 1.0 | ||
| ≥3.5 cells × 107/kg | 116 | 0.6 | 0.4–1.0 | 0.04 |
Infection Density
The risks of bacterial, viral, and total serious infections between days 0 to +45 were similar in both cohorts (Figure 3). While the frequency of early fungal infections was low in both cohorts, it was significantly more frequent in the sirolimus/MMF cohort (1.8 vs. 0.2 per 1000 patient-days, p=0.01). In contrast, between days +46 to +180 post-transplantation, the sirolimus/MMF cohort had significantly fewer bacterial infections (3.4 vs. 6.3 per 1000 patient-days, p=0.03) and total serious infections (9.1 vs. 14.3 per 1000 patient-days, p=0.01) events. In addition, between days +46 to +180 post-transplantation, there were no fungal infections in the sirolimus/MMF cohort, and the frequency of viral infections was similar in both cohorts. HHV6 reactivation occurred early post-HCT with a median onset time of 26 days (range, 13–180 days) for all patients: 23 days (range, 13–63) for sirolimus/MMF and 27 days (range, 16–180) for CSA/MMF (p<0.01). The cumulative incidence of HHV6 reactivation was significantly higher in sirolimus/MMF both at early (51% vs. 20%; p<0.01 by day +45) and later (54% vs. 24%; p<0.01 by day +180) post-transplant time periods. In contrast, CMV reactivation among CMV seropositive patients (n=100) occurred later at a median of 46 days (range, 18–152) post-transplant for all CMV seropositive patients: for sirolimus/MMF (n=6), it was 74 days (range, 32–98), and for CSA/MMF (n=51), it was 44 days (range, 18–152; p=0.02). In addition, among CMV seropositive patients, the sirolimus/MMF cohort had significantly less CMV reactivation events in both post-transplant time periods (11% vs. 32%; p=0.02 by day +45 and 33% vs. 62%; p=0.02 by day +180) as compared to the CSA/MMF cohort. Epstein-Barr virus (EBV) reactivation was rare in the sirolimus/MMF cohort (5%, 2 of 37) but slightly more frequent in the CSA/MMF cohort (10%, 13 of 123) between days 0 to +180 post-transplant. All EBV reactivations resolved with administration of rituximab. Overall, infection-related mortality by day +180 was low (11%), with similar incidences in both sirolimus/MMF and CSA/MMF cohorts (14% vs. 11%; p=0.68).
Figure 3.
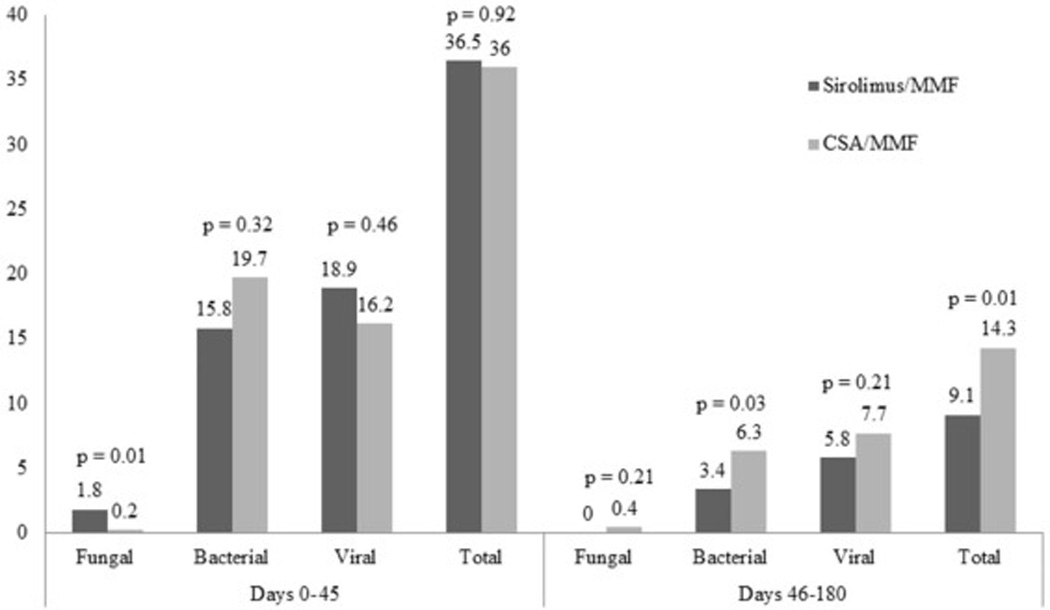
Infection density (Number per 1000 patient days)
Nephrotoxicity and Organ Injury after HCT
Sirolimus/MMF GVHD prophylaxis was well tolerated with no events of idiopathic pulmonary syndrome (IPS), PRES or TMA. In addition, the rate of sinusoidal obstruction syndrome (SOS) was low in both sirolimus/MMF and CSA/MMF cohorts (2.7% vs. 4%; p=0.68). However, sirolimus/MMF led to a significantly lower cumulative incidence of nephrotoxicity defined as elevated serum creatinine >2 mg/dL (sirolimus/MMF 14% vs. CSA/MMF 45%; p<0.01) within 180 days of alloHCT. Moreover, none of the subjects in the sirolimus/MMF cohort experienced fatal organ toxicity, whereas serious end organ failure and toxicities were the second most common cause of death in the CSA/MMF cohort (21%).
Survival and DFS
The cumulative incidence of NRM at 6 months was 22% for sirolimus/MMF and 27% for CSA/MMF (p=0.51). The cumulative incidence of relapse at 1-year was 27% and 37%, respectively (p=0.29). In multiple regression analysis, after adjusting for disease risk, sirolimus/MMF was not predictive of NRM or relapse. High-risk disease resulted in a 2.9-fold increase in the risk of NRM (RR=2.9, 95% CI 1.2–7.3; p=0.02). The point estimates of 1-year DFS and OS for sirolimus/MMF and CSA/MMF cohorts were 51% (95% CI 34–66%) vs. 41% (95% CI 33–50%; p=0.41) and 58% (95% CI 40–73%) vs. 54% (95% CI 45–63%; p=0.61), respectively. In multiple regression analysis, after adjusting for CMV serostatus and total infused CD34+ dose, the GVHD prophylaxis regimen was not an independent predictor of DFS or OS. Survival was best for patients receiving higher than the median dose of total infused CD34+ cells, which decreased the risk of both treatment failure (inverse of DFS; RR=0.7; 95% CI 0.5–1.1; p=0.02) and overall mortality (inverse of OS; RR=0.6; 95% CI 0.4–1.0; p=0.04). Causes of death at 1-year for the entire cohort included relapse of the underlying malignancy (46%), infections (17%), serious end organ toxicities (16%), acute GVHD (8%), graft failure (6%), and other causes (7%).
DISCUSSION
Our study describes the early outcomes of patients receiving sirolimus/MMF as GVHD prophylaxis in the context RIC double UCB transplantation. Our main observation was that the efficacy of GVHD prophylaxis with a sirolimus/MMF regimen is similar to the widely used CSA/MMF regimen. We observed a similar frequency of infections, incidence of hematopoietic engraftment, NRM, relapse, and survival for both types of GVHD prophylaxis. In addition, the sirolimus/MMF regimen was associated with a lower risk of nephrotoxicity as compared with the CSA/MMF regimen. We observed that, similar to the CSA/MMF regimen, the sirolimus/MMF regimen resulted in an incidence of grade II–IV acute GVHD of 41%.25 The Stanford program studied the sirolimus/MMF GVHD regimen after matched-related donor alloHCT and found an unacceptably high incidence of grade II–IV acute GVHD (54.5%).33 This discrepancy may be at least in part explained by the intrinsically lower risk of GVHD in UCB transplantation and by the higher dose of MMF we used. Although, encouragingly low rates of GVHD has been observed in one report when sirolimus was combined with tacrolimus, this combination regimen required very close monitoring of both drug levels in order to avoid TMA,13 which was not observed with the sirolimus/MMF regimen. The similar risk of GVHD and ease of administration as compared to CSA/MMF suggest that the sirolimus/MMF regimen is worth further investigation for GVHD prophylaxis in alloHCT.
In our study, there was an overall lower risk of serious infections in the sirolimus/MMF cohort. Yet, we observed frequent HHV6 reactivation in the early post-transplant period that was associated with delayed hematopoietic recovery. As we did not systematically monitor for HHV-6 reactivation either in the sirolimus/MMF or CSA/MMF cohorts and only ordered the test in response to symptoms (e.g., fever, rash, delayed hematopoietic recovery), we cannot rule out the presence of assessment bias. While most patients responded to antiviral therapy with foscarnet and achieved successful hematopoietic engraftment, the risk of prolonged cytopenias cannot be ignored. Thus, future studies are warranted to monitor HHV-6 reactivation starting early post-transplantation and to assess prophylactic and preemptive treatment strategies.
We observed that about half of the patents in the CSA/MMF cohort experienced renal toxicity; in contrast, the sirolimus/MMF cohort had a very low risk of renal toxicity as measured by creatinine elevation above 2.0 mg/dL. Whereas sirolimus in combination with CNI results in a 10–20% risk of transplant-associated TMA,14, 34–36 we did not observe an increased risk of transplant-associated TMA or SOS with sirolimus/MMF immune suppression. This observation is consistent with a report using the sirolimus/MMF regimen in the haploidentical HCT setting.37 The longer half-life and favorable toxicity profile of sirolimus/MMF as compared to CNI-based regimens ensure the continuous delivery of GVHD prophylaxis with minimal risk of drug dose interruptions, adjustments, or need for frequent level monitoring.
Despite a lower risk of total serious infections in the sirolimus/MMF cohort, we observed no difference in infection-related or overall NRM with either GVHD prophylaxis regimen. Likewise, the sirolimus/MMF regimen did not significantly influence the risk of relapse either, despite some reports indicating potential antineoplastic activity of sirolimus via inhibition of the mTOR pathway, particularly in lymphoid malignancies.7, 12, 38 We acknowledge that the retrospective nature of our analysis and modest number of patients in sirolimus group are the potential limitations of our study, and we believe that larger patient population and longer follow-up is required to determine whether sirolimus has any effect on the risk of relapse after HCT. Overall, sirolimus/MMF yielded survival similar to CSA/MMF, and a low infused total CD34+ dose was the only factor associated with treatment failure and increased mortality in this study.
In conclusion, our findings show that the sirolimus/MMF regimen is a valid alternative to the standard CSA/MMF GVHD prophylaxis after RIC double UCB transplantation and therefore warrants future investigation.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, J.E.W, T.E.D), the Children’s Cancer Research Fund (J.E.W., T.E.D), Leukemia and Lymphoma Society Scholar in Clinical Research Award, grant R6029-07 (C.G.B.). We would like to acknowledge Michael Franklin, MS, for assistance in editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship: N.B performed research, analyzed data, and wrote the paper. C.G.B. designed research, performed research, analyzed data, and wrote the paper. J.R. performed research and wrote the paper. T.E.D. analyzed data and wrote the paper. A.L., M.A., S.H., P.A.J., M.L.M., M.R.V., B.R.B., D.J.W., and J.E.W performed research and wrote the paper.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. Journal of immunology. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 2.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akimova T, Kamath BM, Goebel JW, Meyers KE, Rand EB, Hawkins A, et al. Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant. 2012;12(12):3449–3461. doi: 10.1111/j.1600-6143.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peccatori J, Forcina A, Clerici D, Crocchiolo R, Vago L, Stanghellini MT, et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia. 2015;29(2):396–405. doi: 10.1038/leu.2014.180. [DOI] [PubMed] [Google Scholar]
- 5.Blazar BR, Taylor PA, Sehgal SN, Vallera DA. Rapamycin prolongs survival of murine recipients of fully allogeneic donor grafts when administered during the graft-versus-host disease process. Annals of the New York Academy of Sciences. 1993;685:73–85. doi: 10.1111/j.1749-6632.1993.tb35854.x. [DOI] [PubMed] [Google Scholar]
- 6.Blazar BR, Taylor PA, Snover DC, Sehgal SN, Vallera DA. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. Journal of immunology. 1993;151(10):5726–5741. [PubMed] [Google Scholar]
- 7.Armand P, Kim HT, Sainvil MM, Lange PB, Giardino AA, Bachanova V, et al. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. British journal of haematology. 2016;173(1):96–104. doi: 10.1111/bjh.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2004;10(5):328–336. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 9.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foroutan N, Rasekh HR, Salamzadeh J, Jamshidi HR, Nafar M. Budget impact analysis of conversion from cyclosporine to sirolimus as immunosuppressive medication in renal transplantation therapy. ClinicoEconomics and outcomes research : CEOR. 2013;5:545–553. doi: 10.2147/CEOR.S51446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurgensen JS, Ikenberg R, Greiner RA, Hosel V. Cost-effectiveness of modern mTOR inhibitor based immunosuppression compared to the standard of care after renal transplantation in Germany. The European journal of health economics : HEPAC : health economics in prevention and care. 2015;16(4):377–390. doi: 10.1007/s10198-014-0579-3. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(35):5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone marrow transplantation. 2011;46(5):659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teachey DT, Grupp SA, Brown VI. Mammalian target of rapamycin inhibitors and their potential role in therapy in leukaemia and other haematological malignancies. British journal of haematology. 2009;145(5):569–580. doi: 10.1111/j.1365-2141.2009.07657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 17.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(8):920–926. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbeck LL, Kiel PJ, Kalsekar I, Vargo C, Baute J, Sullivan CK, et al. Prophylaxis with sirolimus and tacrolimus +/− antithymocyte globulin reduces the risk of acute graft-versus-host disease without an overall survival benefit following allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(6):916–922. doi: 10.1016/j.bbmt.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W, Smith D, Mitchell A, et al. Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus as acute graft-versus-host disease prophylaxis for unrelated hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(11):1734–1744. doi: 10.1016/j.bbmt.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098–1105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura R, Palmer JM, O'Donnell MR, Stiller T, Thomas SH, Chao J, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leukemia research. 2012;36(9):1152–1156. doi: 10.1016/j.leukres.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez HF, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97(12):1882–1889. doi: 10.3324/haematol.2012.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Simon JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013;98(4):526–532. doi: 10.3324/haematol.2012.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejanyan N, Rogosheske J, DeFor T, Lazaryan A, Esbaum K, Holtan S, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(5):926–933. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 27.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ASBMT. American Society for Blood and Marrow Transplantation RFI. 2006 [Google Scholar]
- 29.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan ELMP. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 31.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in medicine. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Fine JPG, R J. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 33.Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone marrow transplantation. 2012;47(4):581–588. doi: 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11(7):551–557. doi: 10.1016/j.bbmt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Khaled SK, Palmer J, Stiller T, Senitzer D, Maegawa R, Rodriguez R, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as GVHD prophylaxis after unrelated-donor PBSC transplant. Bone marrow transplantation. 2013;48(2):278–283. doi: 10.1038/bmt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Martin P, Alarcon-Payer C, Lopez-Fernandez E, Moratalla L, Romero A, Sainz J, et al. Transplantation-Associated Thrombotic Microangiopathy in Patients Treated With Sirolimus and Cyclosporine as Salvage Therapy for Graft-Versus-Host Disease. The Annals of pharmacotherapy. 2015 doi: 10.1177/1060028015593369. [DOI] [PubMed] [Google Scholar]
- 37.Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Post-transplantation Cyclophosphamide and Sirolimus after Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-based Myeloablative Conditioning and Peripheral Blood Stem Cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(8):1506–1514. doi: 10.1016/j.bbmt.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Costa LJ. Aspects of mTOR biology and the use of mTOR inhibitors in non-Hodgkin's lymphoma. Cancer treatment reviews. 2007;33(1):78–84. doi: 10.1016/j.ctrv.2006.10.004. [DOI] [PubMed] [Google Scholar]


