Abstract
Bile acids are synthesized from cholesterol and are known to be involved with the emulsification and digestion of dietary lipids and fat-soluble vitamins. Outside of this role, bile acids can act as cell signaling effectors through binding and activating receptors on both the cell membrane and nucleus. Numerous reports have investigated these signaling pathways in conditions where the liver is damaged. More recently, effort has been made to investigate the role of bile acids in diseases outside of those associated with liver damage. This review summarizes recent findings on the influences that bile acids can exert in normal neurological function and their contribution to diseases of the nervous system, with the intent of highlighting the role of these metabolites as potential players in neurological disorders.—McMillin, M., DeMorrow, S. Effects of bile acids on neurological function and disease.
Keywords: FXR, TGR5, cholesterol, metabolism, neuroinflammation
Bile acids are the amphipathic end products of cholesterol metabolism and can contribute to hepatic, intestinal, and metabolic disorders. They are predominantly synthesized in the liver and are excreted into the duodenum with the primary function of aiding in the digestion and absorption of dietary lipids and fat-soluble vitamins. They have a secondary role as steroid hormones, modulating various cell signaling cascades via activation of membrane-bound receptors or nuclear receptors following uptake through membrane transporters. This ability of bile acids to act as cell-signaling mediators indicates that they can influence a variety of physiological functions.
When bile acids are secreted into the intestine, >90% are reabsorbed into the portal system via the enterohepatic circulation and are recycled into hepatocytes (1). However, in disease states where there is a disruption of this reuptake, there is a spillover of bile acids into the circulation that can produce a variety of pathological effects. Recent research has indicated that bile acids and bile acid signaling can influence a variety of neuropathological conditions. Therefore, this review focuses on bile acid metabolism and signaling during physiological and pathophysiological states with a specific focus on their roles in neurological function and disease.
BILE ACID METABOLISM
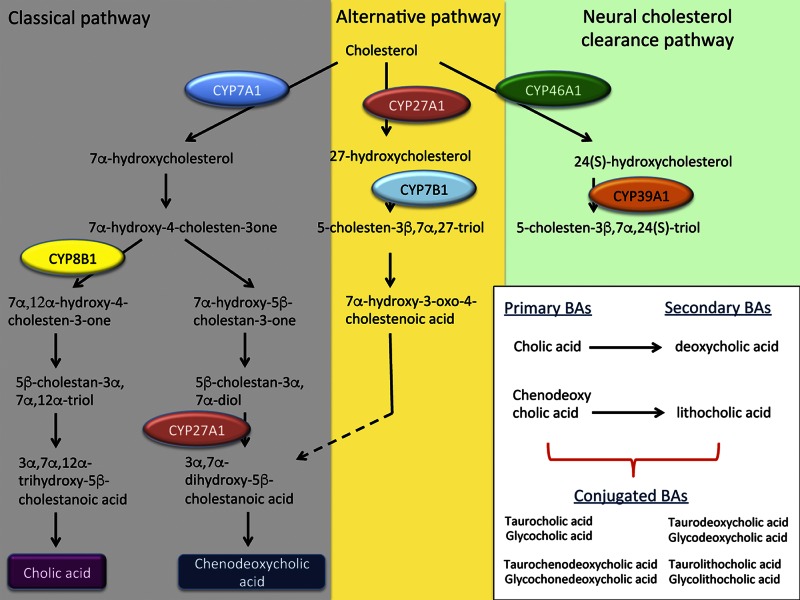
Bile acids are a metabolic product of cholesterol and are synthesized in the liver via cytochrome p450 activity in hepatocytes with multiple metabolic pathways that facilitate this process (2). The first is the classic pathway of bile acid synthesis where cholesterol is metabolized by cholesterol 7α-hydroxylase (CYP7A1) to 7α-hydroxycholesterol and subsequently is hydroxylated by sterol 12α-hydroxylase or sterol 27-hydroxylase (CYP27A1). The second (or alternate) pathway involves the formation of 27-hydroxycholesterol from cholesterol via CYP27A1 and the subsequent hydroxylation by oxysterol 7α-hydroxylase (CYP7B1). The final pathway involves oxidation of cholesterol into 24 and 25-hydroxycholesterol via cholesterol 24-hydroxylase (CYP46A1), which is expressed primarily in the brain (3). A detailed summary of bile acid metabolism follows and an outline of these synthesis pathways is shown in Fig. 1.
Figure 1.
Schematic representation of the classic, alternative, and neural cholesterol clearance pathways of bile acid synthesis. The classic pathway of bile acid synthesis is initiated from cholesterol by CYP7A1, which leads to the formation of 7α-hydroxycholesterol which, after some metabolic reactions, interacts with CYP8B1 or CYP27A1 to form cholic acid or chenodeoxycholic acid. The alternative pathway of bile acid synthesis begins with the metabolism of cholesterol to 27-hydroxycholesterol via CYP27A1. 27-Hydroxysterol is subsequently metabolized by CYP7B1 which, after a few metabolic steps leads to the synthesis of CDCA. The final pathway depicted is the neural cholesterol clearance pathway, which is initiated by the formation of 24(S)-hydroxycholesterol from cholesterol by CYP46A1. 24(S)-hydroxycholesterol subsequently exits the brain and enters the bloodstream where it is metabolized by CYP39A1 in the liver to continue bile acid synthesis.
Classic bile acid synthesis by CYP7A1
The classic or neutral pathway of bile acid synthesis begins with the conversion of cholesterol to 7α-hydroxycholesterol by CYP7A1, followed by sterol 12α-hydroxylation by sterol 12α-hydroxylase (CYP8B1) or sterol 27-hydroxylation via CYP27A1 to form the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA), respectively (4). CYP7A1 is almost exclusively expressed in the liver in humans, primates, and various rodent models (2, 5). Identification of the function of the CYP7A1-mediated bile acid synthesis pathway has been elucidated in CYP7A1 knockout mice and the study of CYP7A1 mutations in humans. CYP7A1 knockout mice demonstrate the importance of bile acid synthesis during development, as these mice exhibit vision and skin abnormalities, fat and vitamin malabsorption, and increased postnatal death compared with wild-type controls (6, 7). Furthermore, CYP7A1-knockout mice that are fed a normal chow diet are found to have hypercholesterolemia and decreased fecal bile acid content (8). Similarly, hypercholesterolemia and reduced bile acid excretion have been observed in patients with homozygous deletion of CYP7A1 by a frameshift mutation caused by a Leu→Arg substitution at codon 413 resulting in a premature stop codon at codon 414 in exon 6 (9). This mutation results in a truncated protein that has no function because of the loss of the heme-binding domain. While primarily influencing nutrition and metabolism, single-nucleotide polymorphisms (SNPs) of the CYP7A1 promoter have been observed in various neurological disorders, including multiple sclerosis, neuromyelitis optica, and idiopathic recurrent transverse myelitis (10). This study found that presentation of neuromyelitis optica, an inflammatory demyelinating disease of the CNS, has a strong and positive association with SNPs in the CYP7A1 promoter. However, as previously mentioned, CYP7A1 is not expressed in the CNS and therefore how these mutations lead to the development of neuromyelitis optica is not understood at this time.
Alternative bile acid synthesis pathway mediated by CYP27A1
The alternative or acidic pathway for bile acid synthesis is driven by the metabolism of cholesterol into 27-hydroxycholesterol by CYP27A1, which then, through subsequent hydroxylation by CYP7B1, and other metabolic steps, leads to the formation of primary bile acids, in particular CDCA. Unlike CYP7A1, CYP27A1 is expressed in mitochondria on most cells throughout the body, and this pathway is responsible for ∼25% of bile acid synthesis in rodents and between 5 and 10% in humans (4). CYP27A1-knockout mice have significantly reduced bile acid levels (11), resulting in decreased cholesterol absorption and de novo cholesterol synthesis. CYP27A1-knockout mice also present with hypertriglyceridemia, hepatomegaly, adrenomegaly, and significant elevations of cholestanol in the brain (12, 13). This elevation of neural cholestanol is also observed in patients with CYP27A1 loss of function mutations presenting with cerebrotendinous xanthomatosis (CTX), a neurological disorder characterized by both behavioral and motor dysfunction and a variety of systemic complications (14).
Clearance of brain cholesterol via CYP46A1
The liver is the primary organ involved with cholesterol metabolism, but the brain is one of the more sensitive organs affected by hypercholesterolemia. For the brain to clear excess cholesterol, CYP46A1, which is highly expressed in the caudate nucleus, amygdala, putamen, cerebral cortex, hippocampus, and frontal lobe, metabolizes cholesterol into 24(S)-hydroxycholesterol (3). Unlike cholesterol, 24(S)-hydroxycholesterol passes through the blood–brain barrier where it enters the circulation and is taken up by the liver to continue through the bile acid synthesis pathway via metabolism by oxysterol 7α-hydroxylase II (CYP39A1) (2). In mice lacking CYP46A1 function, 24(S)-hydroxycholesterol synthesis does not occur in the CNS, leading to significantly reduced serum and brain concentrations of 24(S)-hydroxycholesterol (15). This mutation has been shown to lead to impairments in spatial, associative, and motor learning, and can generate deficits in long-term potentiation in the hippocampus (16).
BILE ACID SIGNALING
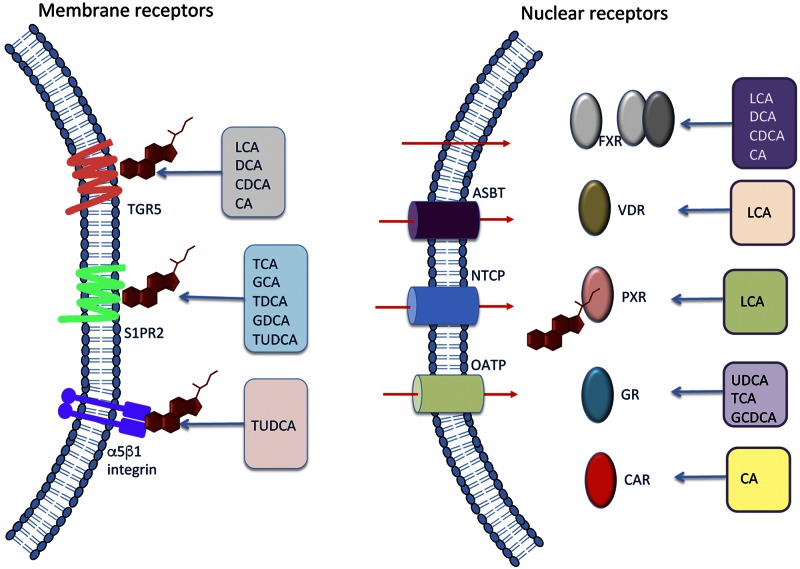
Following the synthesis of primary bile acids in the liver, bile acids are secreted into the gut where they are modified by the bacteria in the intestine to become secondary bile acids. Secondary bile acids can be further modified in the liver or gut via sulphation and glucuronidation and may then be conjugated with glycine or taurine (17). These various enzymatic reactions confer a wide variety of characteristics to bile acids, not only in regard to their lipophilicity and hydrophilicity, but also in their ability to bind and activate receptors. Bile acid signaling occurs through both cell membrane receptors and nuclear receptors (summarized in Fig. 2).
Figure 2.
Bile acid signaling through membrane-bound or nuclear receptors. Certain bile acids can bind the membrane receptors TGR5, S1PR2, and a5B1 integrin. Alternatively, bile acids may exert their effects through nuclear receptors. In order for bile acids to bind nuclear receptors, they need to passively diffuse across the cell membrane or are actively transported by ASBT, NTCP, or OATP. Once inside the cell, bile acids can bind FXR, VDR, PXR, GR, or CAR which facilitates DNA binding and subsequent regulation of transcription. Similar to the membrane bile acid receptors, these nuclear receptors have differential affinity for specific bile acids, which are indicated in this figure.
Farsenoid X receptor, a nuclear bile acid receptor
Identification of bile acids as cell signaling mediators began with the discovery that bile acids are ligands for the nuclear receptor farsenoid X receptor (FXR) (18). Bile acids are taken up into the cell via either passive diffusion or by active transport through transporters such as the apical sodium-dependent bile acid transporter (ASBT), organic anion-transporting polypeptide (OATP), or sodium taurocholate cotransporting polypeptide (NTCP), which have differential expression throughout the gastrointestinal tract and liver (19). Once inside the cell, bile acids can bind and activate the nuclear receptor FXR, which binds DNA, either as an FXR monomer or as a heterodimer bound to other nuclear receptors such as retinoid X receptor. The activated nuclear receptor complex then binds the FXR response element to regulate gene expression (20). One of the downstream targets of FXR signaling is the up-regulation of small heterodimer partner (SHP), which binds to FXR to reduce bile acid synthesis by down-regulating CYP7A1, as well as contributing to the regulation of glucose and lipid metabolism (21). Therefore, FXR-mediated bile acid signaling has been established as one of the primary controllers of bile acid synthesis through feedback inhibition. FXR expression is most prevalent in hepatocytes and in the ileum, though expression has been found at lower levels in many organ systems, including the CNS (22). Recently, it was discovered that FXR is also expressed in the brain, where it is localized primarily in neurons in the cerebral cortex, and is up-regulated during neuroinflammation, indicating that disease states can influence the expression of this receptor in the brain (23).
TGR5, the primary membrane bile acid receptor
Three years after the discovery FXR-mediated bile acid signaling, a report by Maruyama et al. (24) identified a membrane-type receptor for bile acids (M-BAR) that binds and is activated by lithocholic acid (LCA), deoxycholic acid (DCA), and CDCA, increasing intracellular cAMP in HEK293 cells transfected with human M-BAR. Shortly after, a publication by Kawamata et al. (25) investigated the same receptor, but named the receptor Takeda G-protein coupled receptor 5 (TGR5), also known as G-protein-coupled bile acid receptor 1 (GPBAR1), and found expression of this receptor in the brain, liver, lung, and spleen with high expression in monocyte/macrophage cell populations as well. Though not the first publication, the name of the bile acid receptor is now more commonly referred to as TGR5 or GPBAR1 in the literature. Recently, TGR5 expression has been found in neural cell populations, predominantly astrocytes and neurons (26, 27), and can be activated by neurosteroids (26). TGR5 agonists can activate downstream signaling pathways, leading to an increase in intracellular cAMP and calcium and the subsequent induction of reactive oxygen and nitrogen species in primary astrocytes (26). TGR5-knockout mice display normal development and are healthy and fertile with no significant changes in low- and high-density lipoproteins, triglycerides, bilirubin cholesterol, or bile acids in the serum (28). However, TGR5 activation via INT-777, a TGR5 agonist, in RAW264.7 macrophages decreased the expression of the proinflammatory cytokines IL-1β, IL-6, TNFα, and chemokine ligand (CCL)-2 after LPS stimulation compared with untreated RAW264.7 cells stimulated with LPS (29). As TGR5 signaling has been demonstrated to interact with both neurosteroids and bile acids resulting in a reduction in inflammatory signaling suggests that this signaling pathway has the potential to influence multiple neuropathies.
S1PR2 and α5β1 integrin can act as membrane-bound receptors for bile acids
More recent reports have identified that bile acids can signal through a second G-protein-coupled receptor, sphingosine 1-phosphate receptor (S1PR)2 which is expressed in the heart, lung, liver, and brain (30). Studer et al. (31) demonstrated that treatment of primary rat hepatocytes with taurocholic acid (TCA), taurodeoxycholic acid, tauroursodeoxycholic acid (TUDCA), glycocholic acid, or glycodeoxycholic acid causes the activation of ERK1/2 and AKT, which could be inhibited by treatment with JTE-013, an S1PR2 antagonist. They also showed that JTE-013 inhibits the expression of SHP, suggesting that S1PR2-mediated signaling can influence FXR signaling (31). In rats with elevated bile acids caused by ligation of the common bile duct, antagonism of S1PR2 was found to alleviate portal hypertension (32). In the brain, S1PR2-knockout mice have been found to have normal neurological development, but between 3 and 7 wk of age develop spontaneous and sporadic seizures due to hyperexcitability of pyramidal neurons (33). In addition, in PC12 cells and in dorsal root ganglion neurons, nerve growth factor was found to promote cell membrane translocation of sphingosine kinase 1 to the cell membrane where it would phosphorylate both S1PR1 and S1PR2 and promote neurite extension (34). Taken together, these findings show that S1PR2 signaling via bile acids may have a role in neurological function.
The integrin α5β1 is a receptor for fibronectin and plays a substantial role in angiogenesis. In the brain α5β1 integrin has been shown to influence brain development by inhibiting neuronal migration and disrupting cortical lamination (35). Studies of primary mouse neurons found that fibronectin promotes neurite outgrowth, although this effect was inhibited when neutralizing antibodies to α5β1 integrin subunits were used (36). In regard to the brain, α5β1 integrin plays a role in promoting the pathology associated with ischemic stroke (37). Although not reported to have strong agonist activity for all bile acids, α5β1 integrin has been reported to induce kinase activity in response to TUDCA (38). As TUDCA is used to treat various neurological disorders, which will be outlined later is this review, the influence of α5β1 integrin during neurological diseases should be better identified in future studies.
Nuclear receptors can be activated by bile acids
Bile acids can activate a variety of nuclear receptors besides FXR, including pregnane X receptor (PXR), vitamin D receptor (VDR), constitutive androstane receptor (CAR), and glucocorticoid receptor (GR). PXR is involved with detecting the presence and elimination of xenobiotics in the body by regulating the expression of numerous cytochrome p450 enzymes that aid in their detoxification (39). PXR has been shown to interact with bile acids and can bind LCA, although the IC50 is 10 μM, which are levels that would only occur during pathological liver states like cholestasis (40). In the brain, PXR is expressed in mouse hippocampal neurons and has been shown to promote apoptosis caused by nonylphenol treatment by inducing caspase 3 activity (41). PXR also promotes neurosteroid synthesis and therefore may play an indirect role in mitigating neuroinflammation and neurotransmission (42).
VDR is expressed in the intestine, bone, and kidneys and in a variety of cells, including β cells, adipocytes, vascular smooth muscle cells, and monocytes (43). The natural ligand for VDR is 1,25-dihydroxyvitamin D3 though the bile acid LCA has also been identified as an agonist at concentrations lower than those required for PXR (44). CYP3A4, a cytochrome p450 enzyme involved in the oxidation and detoxification of bile acids, can be directly up-regulated by VDR activity (45). CYP7A1 is also up-regulated in VDR-knockout mice indicating that VDR activity reduces bile acid synthesis and therefore is a negative-feedback mechanism for bile acid synthesis (46). In the brain, VDR activation may influence development and metabolism and generates protection from various disease models, including Parkinson’s disease and Alzheimer’s disease (47, 48). At this time, the interaction between bile acids and VDR during normal and neuropathological states is not well classified and requires further study.
Although bile-acid–mediated activation of CAR is not as well classified as other nuclear receptors, CAR can be activated in response to CA (49). CAR is expressed in several tissues in the body, with the brain, kidney, liver, and adrenals having high expression of this receptor (50). In humans, cerebral expression has been found primarily in the caudate nucleus, indicating that CAR may influence procedural and associative learning, as well as being involved with Parkinson’s disease and other neurological disorders (50). Crosstalk between CAR-mediated signaling and other nuclear bile acid receptors has been demonstrated. For example, CAR-knockout mice have reduced FXR expression, suggesting that FXR gene expression may be regulated by CAR activity (51). At this time, bile acid–mediated CAR signaling in the brain has not been investigated; however, CAR signaling has been shown to increase expression of the ABC-transporter P-glycoprotein at the blood–brain barrier, indicating that CAR-mediated signaling may play a role in blood–brain barrier permeability (52).
The GR is well known to suppress neuroinflammation and can reduce the production and secretion of the proinflammatory cytokines IL-1β and TNFα, as well as reduce the pathology associated with multiple sclerosis, Parkinson’s disease, and other neuroinflammatory diseases (53, 54). Takigawa et al. (55) report that feeding a 0.3% (wt/vol) UDCA diet to mice for 4 wk up-regulated GR and silencing GR using GR small interfering RNA in liver mononuclear cells promoted IFNγ production in response to LPS or concanavalin-A challenge when compared to control liver mononuclear cells. In a recent study, TCA and glycochenodeoxycholic acid (GCDCA) were shown to lead to an increase in GR activity through the use of a GR competitive activity assay in a mouse hypothalamic cell line, indicating that bile acids can directly induce GR signaling (56).
BILE ACIDS IN THE BRAIN AND THEIR INFLUENCE ON NEUROPATHOLOGY
The concept of bile acid signaling in the brain is a novel idea that is gaining traction, with implications in the fields of neuroscience and hepatology. Although bile acid signaling in the gastrointestinal tract is well characterized, there are few studies investigating bile acid signaling mechanisms in the brain, which limits our knowledge and understanding in this area. In addition, publications reporting these phenomena are mostly descriptive, with many describing the effects of bile acid supplementation on reducing inflammation and apoptosis during neuropathological states but not investigating the specific mechanisms that confer these effects. That being said, each year, more studies are being published in this area that support the notion that bile acids and bile acid signaling are potential therapeutic agents/targets, indicating that there is a great need to better understand the role of bile acid metabolism and signaling in the brain during normal and pathological states. Therefore, the remainder of the review reports what is known about neural bile acid signaling within the confines of what is currently published, with the goal of demonstrating the importance of these signaling pathways in neurological function and disease.
Evidence of bile acid signaling in brain
A few early studies have demonstrated that bile acids can be found in the brain, and their presence may be a result of de novo bile acid synthesis via cholesterol oxidation. Specifically, LCA could be detected in brain during experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis in the guinea pig, whereas levels were undetectable in normal guinea pigs (57). Furthermore, incubation of the bile acid precursor 3-keto-5β-cholanoic acid with minced guinea pig brain demonstrated that neural de novo synthesis of LCA can occur (58). Using liquid chromatography/mass spectrometry, Mano et al. (59) found that CA, CDCA, and DCA were present in the cytosolic fraction of rat brain homogenates, with CDCA being the most highly expressed at 92.1%. Given that CDCA makes up only 13.8% of the bile acids measured in the serum, this disparity provides further indirect evidence of de novo synthesis in the brain (59). Furthermore, elevation of total bile acid levels has been observed during neuropathological states in humans, such as hepatic encephalopathy, where elevation of bile acids are observed in brain tissue and cerebrospinal fluid (CSF) of patients with acute liver failure but not in control patients (60).
Bile acids Influence neurotransmission and physiology
Outside of the bile acid receptors that were detailed earlier in the review, bile acids have been reported to interact with receptors involved with neurotransmission. Schubring et al. (61) report that CA, DCA, and CDCA are able to inhibit NMDA and GABA currents with CDCA showing the lowest IC50 for both receptors. These effects were generated through activity on these ligand-gated ion channels rather than on bile acid receptors. In addition, isolated histaminergic neurons treated with UDCA had suppressed GABA current and spontaneous inhibitory postsynaptic currents (62). These bile-acid–mediated effects on neurotransmission can translate to effects on respiration as DCA influences respiratory patterns, whereas CDCA, LCA, and CA decrease the inspiratory time and expiratory time in brainstem medulla slices from bile-acid–injected Sprague-Dawley rats (63). Deletion of FXR has also been found to impair memory and motor coordination and leads to changes in GABA, glutamate, norepinephrine, and serotonin neurotransmission (64).
Bile acids may also have neurological effects outside of neurotransmission. Specifically, in conditions where bile acids are elevated, such as in patients with obstructive jaundice caused by gallstones, there is inhibition of hepatic glucocorticoid clearance that is significant enough to disrupt the hypothalamic–pituitary–adrenal (HPA) axis (65). Similarly, a suppression of the HPA axis has been demonstrated following bile duct ligation (BDL) (66, 67). The mechanism by which extrahepatic biliary obstruction leads to a suppressed HPA axis involves an increase in serum bile acid levels, which are able to induce the opening of the blood–brain barrier (68). It allows bile acids to gain entry to the brain where they can be taken up via the bile acid transporter ASBT, which is expressed in the hypothalamus (56, 68). Once inside the neurons of the hypothalamus, bile acids, in particular TCA and GCDA, bind to and activate GR, leading to a decrease in the expression of corticotropin-releasing hormone, a key regulatory molecule of the HPA axis (56). Furthermore, suppression of release of corticotropin-releasing hormone from the hypothalamus after BDL surgery was alleviated in rats that were fed a diet enriched with the bile acid sequestrant cholestyramine (56). In addition, CA has been found to induce signaling in the ventral midbrain and promote neurogenesis in zebrafish (69). Taken together, these studies support that bile acids have the capability of influencing neurotransmission, neuroendocrine responses, physiology, and neurogenesis indicating an important role of these signaling mediators in neurological function.
Hepatic encephalopathy
Hepatic encephalopathy is the generation of a metabolically induced, functional disturbance of the brain that is a result of acute or chronic liver injury (70). Associated with this disease state is an elevation of serum and cranial ammonia that results in astrocyte swelling, cerebral edema, dysregulation of neurotransmission and increased neuroinflammation, although some of these processes may occur without a direct correlation to ammonia (71). Elevations of circulating and cranial levels of bile acids have been observed in patients with acute liver failure. Bron et al. (60) reported in 1977 that circulating bile acid concentrations were 85.9 ± 8.4 μM in patients with acute liver failure compared with 5.7 ± 0.4 μM in patients without liver failure. They further found that levels of bile acids were not detectable in the CSF or brain tissue of normal patients, but were found to be 3.1 ± 0.5 μM in CSF and 8.9 ± 2.1 nmol/g of brain tissue in patients with acute liver failure. They concluded that these concentrations of bile acids were not high enough to effect cerebral edema directly. However, with the current understanding that bile acids can signal through receptor-mediated mechanisms, the notion that they can induce some effects in the brain at these concentrations is highly feasible. Indeed, serum and cranial bile acids are elevated in mice with hepatic encephalopathy induced by acute liver failure and that these bile acids contribute to neurological decline. In addition, inhibition of FXR-mediated signaling in the frontal cortex confers partial protection against the neurological complications of hepatic encephalopathy (23). Elevations of cranial bile acids during hepatic encephalopathy are also observed during chronic liver injury. In a bile-duct–ligation model of cholestasis in rats, hepatic encephalopathy develops beyond 3 wk after surgery, and these rats have increased concentrations of LCA in the brain compared with sham-operation rats (72).
Many of the therapeutic strategies to treat hepatic encephalopathy, such as lactulose, neomycin, metronidazole, and vancomycin, are targeted at the gut microbiota with the aim of reducing ammonia production, but as a secondary effect would influence the conjugation of secondary bile acids. Therefore, current therapies may also alter the bile acid pool and generate protective effects by altering bile acid signaling. In support of this notion, feeding mice a diet enriched in particular bile acids, which does not alter the total bile acid pool, but alters the balance of individual bile acids, changed the susceptibility to the development of hepatic encephalopathy (23). Specifically, mice fed a diet enriched with DCA or CA had a significantly quicker neurological decline than did control-fed or UDCA-fed mice (23). Therefore, there is evidence that elevation of cranial bile acids and a change in specific bile acids in the bile acid pool contribute to the neurological decline associated with hepatic encephalopathy, although more clinical studies that manipulate bile acids are necessary to determine whether these effects translate to patients with hepatic encephalopathy.
Cerebrotendinous xanthomatosis
Cerebrotendinous xanthomatosis (CTX) is an autosomal-recessive loss-of-function mutation in the CYP27A1 gene that leads to dysregulation of lipid homeostasis, decreased bile acid synthesis, and increased cholesterol and cholestanol accumulation in the brain. This mutation was first identified in 1937, and the prevalence of the disease is estimated to be between 3 and 5 cases per 100,000 (73). The loss of CYP27A1 leads to an elevation of serum cholestanol in patients with CTX (74). Patients with CTX display neurological symptoms such as dementia, ataxia, epilepsy, behavioral changes, and a variety of other systemic complications affecting the cardiovascular, skeletal, pulmonary, and gastrointestinal organ systems (14). Current therapies designed to manage CTX include bile acid replacement therapy to restore bile acid synthesis and reduce cholestanol levels. Bile acid replacement therapy involves the administration of bile acids such as CDCA, UDCA, CA, or TCA (14). Supplementation with CDCA or CA has been demonstrated to reduce plasma cholestanol concentrations and the nonneurological complications associated with CTX, although only CDCA supplementation has been determined to reduce the neurological pathology associated with this disease state (14, 75). Unfortunately, although CDCA supplementation may be one of the more efficacious treatments for reducing serum cholestanol concentrations in patients with CTX, mortality can still be relatively high in those undergoing CDCA supplementation (76). Therefore, other treatments strategies may be necessary to improve outcomes in patients with CTX, with CDCA supplementation with pravastatin or other statins showing some promise (77).
Multiple sclerosis
Multiple sclerosis is an autoimmune disorder of the nervous system that is associated with T-cell entry into the brain, after a breakdown of the blood–brain barrier. These T cells subsequently attack the myelin, resulting in neurological deficits. In the EAE model of multiple sclerosis, LCA has been measured in brain homogenates from guinea pigs (57). In addition, the researchers determined that LCA was present in the brain of a patient with multiple sclerosis (78). Unfortunately, the specific role that this LCA elevation has in the pathology of multiple sclerosis was not elucidated in these reports. More recently, the use of agonists for bile acid receptors has been proposed for the management of multiple sclerosis, as bile acid signaling can modulate inflammation. Indeed, Lewis et al. (79) developed a novel agonist for TGR5 that reduces proinflammatory cytokine production from mouse monocytes stimulated with LPS, and this therapeutic was found to reduce EAE severity in mice. FXR also seems to play a role in the progression of EAE, as mice with genetic knockout of FXR have a more severe grade of EAE compared to wild-type EAE mice (80). In addition, oral administration of obeticholic acid, a synthetic FXR agonist, was found to reduce the severity of EAE in mice compared to vehicle-treated EAE mice (80). These newer reports investigating bile acid signaling during multiple sclerosis indicate that this signaling pathway may be a potential therapeutic target for the management of this disorder.
Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disorder associated with memory and cognition deficits that are related to the deposition of amyloid-β (Aβ) plaques and the hyperphosphorylation and subsequent aggregation of tau (τ) protein (81). Evidence that bile acids can influence this disorder is supported by the suppression of Aβ-induced apoptosis by TUDCA in neuron cell cultures and primary rat neurons through the inhibition of the E2F-1/p53/Bax pathway (82, 83). In the APP/PS1 mouse double-knockout model of Alzheimer’s disease, feeding of a TUDCA-enriched diet for 6 mo was found to reduce the number of Aβ aggregates and improve memory compared with control-diet–fed mice (84). Using this same knockout model, researchers found that intraperitoneal injection of TUDCA reduced Aβ deposition, glycogen synthase kinase 3β activity, phosphorylation of τ, and neuroinflammation (85). A joint therapy involving the oral administration of baicalin, jasminoidin, (components of the Chinese herb preparation qingkailing), and the bile acid CA was reported to reduce ibotenic acid–induced cognitive deficits and neuronal lesions in rats, as assessed by Morris water maze performance and histology assessments in hippocampus brain sections (86). Although these studies demonstrate that Alzheimer’s disease can be influenced by bile acid supplementation, more studies are needed to determine the involvement of bile acid transporters and receptors, as well as the subsequent mechanisms of this protective effect.
Parkinson’s disease
Parkinson’s disease is a progressive neurodegenerative disorder characterized by tremor, dyskinesia, and speech changes in patients. In the brain, α-synuclein aggregates and the formation of Lewy bodies are the neurological changes that characterize this disease. Parkinson’s disease can be associated with a mutation in leucine-rich repeat kinase 2 (LRRK2)G2019S that is involved in 5–6% of familial and 1–2% of sporadic cases of Parkinson’s disease (87). This mutation leads to increased activity of LRRK2 kinase and results in neuronal dysfunction and a subsequent loss of neurons (88, 89). The loss of neurons caused by LRRK2G2019S in Drosophila can be reduced if they are fed UDCA via a mechanism involving the restoration of adenosine 5′-triphosphate levels back to normal levels, indicating that this bile acid may be a therapeutic agent for Parkinson’s disease (90). In transgenic nemotodes with induced α-synuclein expression or deletion of parkin, exposure to TUDCA increased survival by 25.0 to 23.8%, respectively, because of reduced apoptosis, which supports a protective role of TUDCA in nematode models of Parkinson’s disease (91).
Traumatic brain injury
Traumatic brain injury (TBI) is the term for a class of neurological traumas that involve penetrating and nonpenetrating injuries, resulting in chronic neurological disease and dysfunction. The link between the brain and the liver during TBI has been identified for more than a decade. Kalsotra et al. (92) identified changes in cytochrome p450 activity in the liver and kidneys by observing controlled cortical impact in rats. In addition, a nationwide study of the 1 yr survival of patients from Taiwan indicated that patients with TBI who have liver cirrhosis have much higher mortality (52.18%) vs. patients with TBI without liver cirrhosis (30.61%) (93). One of the main contributing factors to the progression of TBI is apoptosis of neurons and glia (94). No studies have investigated whether there are elevations of bile acids in the brain during TBI; however, supplementation with bile acids such as TUDCA may help alleviate the pathologic effects of TBI. Indeed, TUDCA has been shown to play a direct role in reducing apoptosis by reducing p53 expression and modulating B-cell lymphoma-2 expression in neuroblastoma cells (95). TUDCA has also been shown to inhibit apoptosis of neurons through suppression of the release of apoptosis-inducing factor from mitochondria (96). Therefore, it appears that certain bile acids may have the capability to mitigate neuronal apoptosis and inflammation associated with TBI, although more research is needed in this area to determine whether this effect is receptor mediated.
Other neuropathies influenced by bile acids
Several other neurological diseases such as stroke and amyotrophic lateral sclerosis can be improved by bile acid supplementation, although the mechanism of action is poorly understood. TUDCA was found to reduce lesion size, TUNEL-positive brain cells, mitochondria swelling, and cleavage of caspase 3 in focal cerebral ischemia in rats caused by middle cerebral artery occlusion (97). Further investigation of this bile acid showed that TUDCA reduced lesion volumes by nearly 50% in collagenase-induced hemorrhagic stroke in rats caused by a decrease in caspase 3 activity and apoptosis (98). Supplementation with TUDCA was found to be safe and effective in patients with amyotrophic lateral sclerosis who were being treated with riluzole (99). In a cell line model of amyotrophic lateral sclerosis in NSC-34 cells expressing wild-type human superoxide dismutase 1 or G93A mutant human superoxide dismutase 1, 50 μM GUDCA treatment was found to reduce caspase 9 activity and matrix metalloproteinase 9 expression, showing that GUDCA supplementation may be beneficial for the management of amyotrophic lateral sclerosis (100).
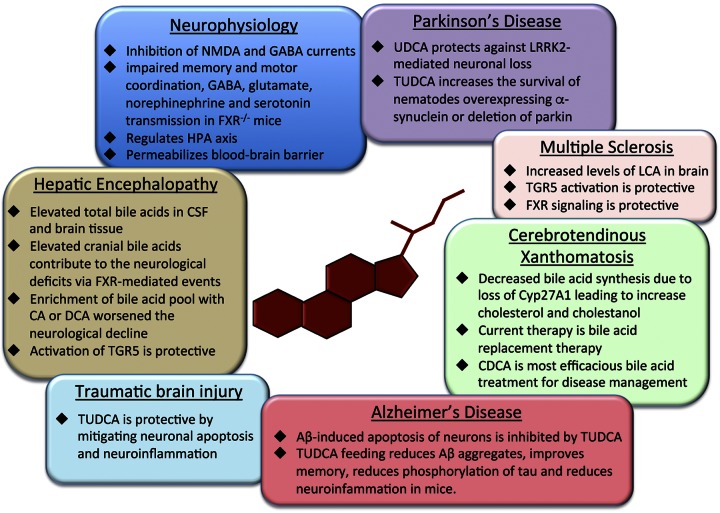
PERSPECTIVES AND CONCLUSIONS
Bile acid signaling is becoming increasingly important in numerous pathological states outside of the gastrointestinal tract. Elevations of bile acids have been found in neurological disorders and many neurological diseases are improved by supplementation with TUDCA and other bile acids. In the brain, cholesterol clearance via CYP27A1 and CYP46A1 is the only well-classified role of bile acid signaling. That being said, investigations into specific bile acid content and subsequent physiological effects on bile acids in the brain may help identify new pathological features of neuropathies that are currently not understood. An overview of the role of bile acids during neuropathies is outlined in Fig. 3. The purpose of this review was to highlight what is currently known about bile acids and their signaling during neurological states, although there remains a paucity of information regarding bile acid signaling mechanisms in many of these disorders. We acknowledge that bile acid signaling or bile acid supplementation may not be applicable for all neurological disorders, but better understanding of bile acid signaling and its effects in the brain will improve our understanding of this axis and may lead to the development of novel treatment strategies for the management of neurological disorders.
Figure 3.
Summary of the effects of bile acid–mediated signaling on normal brain physiology and neurologic disorders.
AUTHOR CONTRIBUTIONS
M. McMillin and S. DeMorrow jointly crafted the concept of this review; M. McMillin wrote the first draft; and S. DeMorrow designed and drew the figures and critically reviewed, edited, and approved the final draft.
ACKNOWLEDGMENTS
This work was completed with support from the U.S. Veterans Health Administration and with the resources and the use of facilities at the Central Texas Veterans Health Care System (Temple, TX, USA). The views are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs (VA). This study was funded by U. S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK082435, and VA Merit award BX002638-01 from the VA Biomedical Laboratory Research and Development Service (to S.D.). The authors declare no conflicts of interest.
Glossary
- Aβ
amyloid β
- ASBT
apical sodium-dependent bile acid transporter
- BDL
bile duct ligation
- CA
cholic acid
- CAR
constitutive androstane receptor
- CCL
chemokine ligand
- CDCA
chenodeoxycholic acid
- CSF
cerebrospinal fluid
- CTX
cerebrotendinous xanthomatosis
- CYP7A1
cholesterol 7α-hydroxylase
- CYP7B1
oxysterol 7α-hydroxylase
- CYP8B1
sterol 12α-hydroxylase
- CYP27A1
sterol 27-hydroxylase
- CYP46A1
cholesterol 24-hydroxylase
- DCA
deoxycholic acid
- EAE
experimental autoimmune encephalomyelitis
- FXR
farsenoid X receptor
- GCDCA
glycochenodeoxycholic acid
- GPBAR1
G-protein-coupled bile acid receptor 1
- GR
glucocorticoid receptor
- HPA
hypothalamic–pituitary–adrenal axis
- LCA
lithocholic acid
- LRRK2
leucine-rich repeat kinase 2
- M-BAR
membrane-type receptor for bile acids
- NTCP
sodium taurocholate cotransporting polypeptide
- OATP
organic anion-transporting polypeptide
- PXR
pregnane X receptor
- S1P
sphingosine 1-phosphate
- S1PR
sphingosine 1-phosphate receptor
- SHP
small heterodimer partner
- SNPs
single nucleotide polymorphisms
- TBI
traumatic brain injury
- TCA
taurocholic acid
- TGR5
Takeda G-protein coupled receptor 5
- TUDCA
tauroursodeoxycholic acid
- UDCA
ursodeoxycholic acid
- VDR
vitamin D receptor
REFERENCES
- 1.Dawson P. A., Lan T., Rao A. (2009) Bile acid transporters. J. Lipid Res. 50, 2340–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorbek G., Lewinska M., Rozman D. (2012) Cytochrome P450s in the synthesis of cholesterol and bile acids: from mouse models to human diseases. FEBS J. 279, 1516–1533 [DOI] [PubMed] [Google Scholar]
- 3.Lund E. G., Guileyardo J. M., Russell D. W. (1999) cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA 96, 7238–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell D. W. (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72, 137–174 [DOI] [PubMed] [Google Scholar]
- 5.Uno Y., Hosaka S., Yamazaki H. (2014) Identification and analysis of CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 in cynomolgus macaques. J. Vet. Med. Sci. 76, 1647–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishibashi S., Schwarz M., Frykman P. K., Herz J., Russell D. W. (1996) Disruption of cholesterol 7alpha-hydroxylase gene in mice: I, postnatal lethality reversed by bile acid and vitamin supplementation. J. Biol. Chem. 271, 18017–18023 [DOI] [PubMed] [Google Scholar]
- 7.Schwarz M., Lund E. G., Setchell K. D., Kayden H. J., Zerwekh J. E., Björkhem I., Herz J., Russell D. W. (1996) Disruption of cholesterol 7alpha-hydroxylase gene in mice: II, bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J. Biol. Chem. 271, 18024–18031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson S. K., Lear S. R., Deane S., Dubrac S., Huling S. L., Nguyen L., Bollineni J. S., Shefer S., Hyogo H., Cohen D. E., Shneider B., Sehayek E., Ananthanarayanan M., Balasubramaniyan N., Suchy F. J., Batta A. K., Salen G. (2003) Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J. Lipid Res. 44, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 9.Pullinger C. R., Eng C., Salen G., Shefer S., Batta A. K., Erickson S. K., Verhagen A., Rivera C. R., Mulvihill S. J., Malloy M. J., Kane J. P. (2002) Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 110, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H. J., Park H. Y., Kim E., Lee K. S., Kim K. K., Choi B. O., Kim S. M., Bae J. S., Lee S. O., Chun J. Y., Park T. J., Cheong H. S., Jo I., Shin H. D. (2010) Common CYP7A1 promoter polymorphism associated with risk of neuromyelitis optica. Neurobiol. Dis. 37, 349–355 [DOI] [PubMed] [Google Scholar]
- 11.Rosen H., Reshef A., Maeda N., Lippoldt A., Shpizen S., Triger L., Eggertsen G., Björkhem I., Leitersdorf E. (1998) Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J. Biol. Chem. 273, 14805–14812 [DOI] [PubMed] [Google Scholar]
- 12.Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. (2000) Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia: reversal by cholic acid feeding. J. Biol. Chem. 275, 39685–39692 [DOI] [PubMed] [Google Scholar]
- 13.Båvner A., Shafaati M., Hansson M., Olin M., Shpitzen S., Meiner V., Leitersdorf E., Björkhem I. (2010) On the mechanism of accumulation of cholestanol in the brain of mice with a disruption of sterol 27-hydroxylase. J. Lipid Res. 51, 2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie S., Chen G., Cao X., Zhang Y. (2014) Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 9, 179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund E. G., Xie C., Kotti T., Turley S. D., Dietschy J. M., Russell D. W. (2003) Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278, 22980–22988 [DOI] [PubMed] [Google Scholar]
- 16.Kotti T. J., Ramirez D. M., Pfeiffer B. E., Huber K. M., Russell D. W. (2006) Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. USA 103, 3869–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann A. F. (1999) The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 159, 2647–2658 [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 [DOI] [PubMed] [Google Scholar]
- 19.Dawson P. A. (2011) Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 201, 169–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L., Yang L., Wang Z., Huang W. (2015) Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 5, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calkin A. C., Tontonoz P. (2012) Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookout A. L., Jeong Y., Downes M., Yu R. T., Evans R. M., Mangelsdorf D. J. (2006) Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillin M., Frampton G., Quinn M., Ashfaq S., de los Santos M. III, Grant S., DeMorrow S. (2016) Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am. J. Pathol. 186, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719 [DOI] [PubMed] [Google Scholar]
- 25.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. (2003) A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278, 9435–9440 [DOI] [PubMed] [Google Scholar]
- 26.Keitel V., Görg B., Bidmon H. J., Zemtsova I., Spomer L., Zilles K., Häussinger D. (2010) The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 27.McMillin M., Frampton G., Tobin R., Dusio G., Smith J., Shin H., Newell-Rogers K., Grant S., DeMorrow S. (2015) TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 135, 565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassileva G., Golovko A., Markowitz L., Abbondanzo S. J., Zeng M., Yang S., Hoos L., Tetzloff G., Levitan D., Murgolo N. J., Keane K., Davis H. R. Jr., Hedrick J., Gustafson E. L. (2006) Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. 398, 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pols T. W., Nomura M., Harach T., Lo Sasso G., Oosterveer M. H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., Auwerx J., Schoonjans K. (2011) TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14, 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang A. H., Ishii I., Chun J. (2002) In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta 1582, 197–203 [DOI] [PubMed] [Google Scholar]
- 31.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M., Pandak W. M., Dent P., Spiegel S., Shi R., Xu W., Liu X., Bohdan P., Zhang L., Zhou H., Hylemon P. B. (2012) Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 55, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kageyama Y., Ikeda H., Watanabe N., Nagamine M., Kusumoto Y., Yashiro M., Satoh Y., Shimosawa T., Shinozaki K., Tomiya T., Inoue Y., Nishikawa T., Ohtomo N., Tanoue Y., Yokota H., Koyama T., Ishimaru K., Okamoto Y., Takuwa Y., Koike K., Yatomi Y. (2012) Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents. Hepatology 56, 1427–1438 [DOI] [PubMed] [Google Scholar]
- 33.MacLennan A. J., Carney P. R., Zhu W. J., Chaves A. H., Garcia J., Grimes J. R., Anderson K. J., Roper S. N., Lee N. (2001) An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur. J. Neurosci. 14, 203–209 [DOI] [PubMed] [Google Scholar]
- 34.Toman R. E., Payne S. G., Watterson K. R., Maceyka M., Lee N. H., Milstien S., Bigbee J. W., Spiegel S. (2004) Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J. Cell Biol. 166, 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetti G., Escuin S., van der Flier A., De Arcangelis A., Hynes R. O., Georges-Labouesse E. (2010) Integrin alpha5beta1 is necessary for regulation of radial migration of cortical neurons during mouse brain development. Eur. J. Neurosci. 31, 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonge D. A., de Burgh H. T., Docherty R., Humphries M. J., Craig S. E., Pizzey J. (2012) Fibronectin supports neurite outgrowth and axonal regeneration of adult brain neurons in vitro. Brain Res. 1453, 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts J., de Hoog L., Bix G. J. (2015) Mice deficient in endothelial α5 integrin are profoundly resistant to experimental ischemic stroke. J. Cereb. Blood Flow Metab. 0271678X15616979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gohlke H., Schmitz B., Sommerfeld A., Reinehr R., Häussinger D. (2013) α5 β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology 57, 1117–1129 [DOI] [PubMed] [Google Scholar]
- 39.Lehmann J. M., McKee D. D., Watson M. A., Willson T. M., Moore J. T., Kliewer S. A. (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 102, 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staudinger J. L., Goodwin B., Jones S. A., Hawkins-Brown D., MacKenzie K. I., LaTour A., Liu Y., Klaassen C. D., Brown K. K., Reinhard J., Willson T. M., Koller B. H., Kliewer S. A. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 98, 3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litwa E., Rzemieniec J., Wnuk A., Lason W., Krzeptowski W., Kajta M. (2016) RXRα, PXR and CAR xenobiotic receptors mediate the apoptotic and neurotoxic actions of nonylphenol in mouse hippocampal cells. J. Steroid Biochem. Mol. Biol. 156, 43–52 [DOI] [PubMed] [Google Scholar]
- 42.Frye C. A., Koonce C. J., Walf A. A. (2014) The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats. Front. Syst. Neurosci. 8, 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norman A. W. (2006) Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542–5548 [DOI] [PubMed] [Google Scholar]
- 44.Adachi R., Shulman A. I., Yamamoto K., Shimomura I., Yamada S., Mangelsdorf D. J., Makishima M. (2004) Structural determinants for vitamin D receptor response to endocrine and xenobiotic signals. Mol. Endocrinol. 18, 43–52 [DOI] [PubMed] [Google Scholar]
- 45.Jurutka P. W., Thompson P. D., Whitfield G. K., Eichhorst K. R., Hall N., Dominguez C. E., Hsieh J. C., Haussler C. A., Haussler M. R. (2005) Molecular and functional comparison of 1,25-dihydroxyvitamin D(3) and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J. Cell. Biochem. 94, 917–943 [DOI] [PubMed] [Google Scholar]
- 46.Schmidt D. R., Holmstrom S. R., Fon Tacer K., Bookout A. L., Kliewer S. A., Mangelsdorf D. J. (2010) Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 285, 14486–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang W., Park H. H., Lee K. Y., Lee Y. J., Kim H. T., Koh S. H. (2015) 1,25-dyhydroxyvitamin D3 attenuates L-DOPA-induced neurotoxicity in neural stem cells. Mol. Neurobiol. 51, 558–570 [DOI] [PubMed] [Google Scholar]
- 48.Durk M. R., Han K., Chow E. C., Ahrens R., Henderson J. T., Fraser P. E., Pang K. S. (2014) 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J. Neurosci. 34, 7091–7101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang T. K. (2009) Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 11, 590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamba J. K., Lamba V., Yasuda K., Lin Y. S., Assem M., Thompson E., Strom S., Schuetz E. (2004) Expression of constitutive androstane receptor splice variants in human tissues and their functional consequences. J. Pharmacol. Exp. Ther. 311, 811–821 [DOI] [PubMed] [Google Scholar]
- 51.Beilke L. D., Aleksunes L. M., Holland R. D., Besselsen D. G., Beger R. D., Klaassen C. D., Cherrington N. J. (2009) Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab. Dispos. 37, 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemmen J., Tozakidis I. E., Bele P., Galla H. J. (2013) Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation. Brain Res. 1501, 68–80 [DOI] [PubMed] [Google Scholar]
- 53.Schweingruber N., Reichardt S. D., Lühder F., Reichardt H. M. (2012) Mechanisms of glucocorticoids in the control of neuroinflammation. J. Neuroendocrinol. 24, 174–182 [DOI] [PubMed] [Google Scholar]
- 54.Sun X. C., Ren X. F., Chen L., Gao X. Q., Xie J. X., Chen W. F. (2016) Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 155(Pt A), 94–103 [DOI] [PubMed] [Google Scholar]
- 55.Takigawa T., Miyazaki H., Kinoshita M., Kawarabayashi N., Nishiyama K., Hatsuse K., Ono S., Saitoh D., Seki S., Yamamoto J. (2013) Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G427–G438 [DOI] [PubMed] [Google Scholar]
- 56.McMillin M., Frampton G., Quinn M., Divan A., Grant S., Patel N., Newell-Rogers K., DeMorrow S. (2015) Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol. Endocrinol. 29, 1720–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naqvi S. H., Herndon B. L., Kelley M. T., Bleisch V., Aexel R. T., Nicholas H. J. (1969) Detection of monohydroxy “bile” acids in the brains of guinea pigs afflicted with experimental allergic encephalomyelitis. J. Lipid Res. 10, 115–120 [PubMed] [Google Scholar]
- 58.Mujtaba Naqvi S. H., Nicholas H. J. (1970) Conversion of 3-keto-5-beta-cholanoic acid to lithocholic acid by guinea pig brain tissue, in vitro. Steroids 16, 297–316 [DOI] [PubMed] [Google Scholar]
- 59.Mano N., Goto T., Uchida M., Nishimura K., Ando M., Kobayashi N., Goto J. (2004) Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J. Lipid Res. 45, 295–300 [DOI] [PubMed] [Google Scholar]
- 60.Bron B., Waldram R., Silk D. B., Williams R. (1977) Serum, cerebrospinal fluid, and brain levels of bile acids in patients with fulminant hepatic failure. Gut 18, 692–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schubring S. R., Fleischer W., Lin J. S., Haas H. L., Sergeeva O. A. (2012) The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABA(A) receptors. Neurosci. Lett. 506, 322–326 [DOI] [PubMed] [Google Scholar]
- 62.Yanovsky Y., Schubring S. R., Yao Q., Zhao Y., Li S., May A., Haas H. L., Lin J. S., Sergeeva O. A. (2012) Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS One 7, e42512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C., Wang X., Cong Y., Deng Y., Xu Y., Chen A., Yin Y. (2014) Effects of bile acids and the bile acid receptor FXR agonist on the respiratory rhythm in the in vitro brainstem medulla slice of neonatal Sprague-Dawley rats. PLoS One 9, e112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang F., Wang T., Lan Y., Yang L., Pan W., Zhu Y., Lv B., Wei Y., Shi H., Wu H., Zhang B., Wang J., Duan X., Hu Z., Wu X. (2015) Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front. Behav. Neurosci. 9, 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeilly A. D., Macfarlane D. P., O’Flaherty E., Livingstone D. E., Mitić T., McConnell K. M., McKenzie S. M., Davies E., Reynolds R. M., Thiesson H. C., Skøtt O., Walker B. R., Andrew R. (2010) Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J. Hepatol. 52, 705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quinn M., Ueno Y., Pae H. Y., Huang L., Frampton G., Galindo C., Francis H., Horvat D., McMillin M., Demorrow S. (2012) Suppression of the HPA axis during extrahepatic biliary obstruction induces cholangiocyte proliferation in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G182–G193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swain M. G., Patchev V., Vergalla J., Chrousos G., Jones E. A. (1993) Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J. Clin. Invest. 91, 1903–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinn M., McMillin M., Galindo C., Frampton G., Pae H. Y., DeMorrow S. (2014) Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 46, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theofilopoulos S., Wang Y., Kitambi S. S., Sacchetti P., Sousa K. M., Bodin K., Kirk J., Saltó C., Gustafsson M., Toledo E. M., Karu K., Gustafsson J. A., Steffensen K. R., Ernfors P., Sjövall J., Griffiths W. J., Arenas E. (2013) Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nat. Chem. Biol. 9, 126–133 [DOI] [PubMed] [Google Scholar]
- 70.Cash W. J., McConville P., McDermott E., McCormick P. A., Callender M. E., McDougall N. I. (2010) Current concepts in the assessment and treatment of hepatic encephalopathy. QJM 103, 9–16 [DOI] [PubMed] [Google Scholar]
- 71.Butterworth R. F. (2013) The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat. Rev. Gastroenterol. Hepatol. 10, 522–528 [DOI] [PubMed] [Google Scholar]
- 72.Tripodi V., Contin M., Fernández M. A., Lemberg A. (2012) Bile acids content in brain of common duct ligated rats. Ann. Hepatol. 11, 930–934 [PubMed] [Google Scholar]
- 73.Lorincz M. T., Rainier S., Thomas D., Fink J. K. (2005) Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch. Neurol. 62, 1459–1463 [DOI] [PubMed] [Google Scholar]
- 74.Björkhem I., Hansson M. (2010) Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem. Biophys. Res. Commun. 396, 46–49 [DOI] [PubMed] [Google Scholar]
- 75.Ginanneschi F., Mignarri A., Mondelli M., Gallus G. N., Del Puppo M., Giorgi S., Federico A., Rossi A., Dotti M. T. (2013) Polyneuropathy in cerebrotendinous xanthomatosis and response to treatment with chenodeoxycholic acid. J. Neurol. 260, 268–274 [DOI] [PubMed] [Google Scholar]
- 76.Pilo-de-la-Fuente B., Jimenez-Escrig A., Lorenzo J. R., Pardo J., Arias M., Ares-Luque A., Duarte J., Muñiz-Pérez S., Sobrido M. J. (2011) Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur. J. Neurol. 18, 1203–1211 [DOI] [PubMed] [Google Scholar]
- 77.Kuriyama M., Tokimura Y., Fujiyama J., Utatsu Y., Osame M. (1994) Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J. Neurol. Sci. 125, 22–28 [DOI] [PubMed] [Google Scholar]
- 78.Mujtaba Naqvi S. H., Ramsey R. B., Nicholas H. J. (1970) Detection of lithocholic acid in multiple sclerosis brain tissue. Lipids 5, 578–580 [DOI] [PubMed] [Google Scholar]
- 79.Lewis N. D., Patnaude L. A., Pelletier J., Souza D. J., Lukas S. M., King F. J., Hill J. D., Stefanopoulos D. E., Ryan K., Desai S., Skow D., Kauschke S. G., Broermann A., Kuzmich D., Harcken C., Hickey E. R., Modis L. K. (2014) A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS One 9, e100883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho P. P., Steinman L. (2016) Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 113, 1600–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selkoe D. J., Hardy J. (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodrigues C. M., Solá S., Silva R., Brites D. (2000) Bilirubin and amyloid-beta peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol. Med. 6, 936–946 [PMC free article] [PubMed] [Google Scholar]
- 83.Ramalho R. M., Ribeiro P. S., Solá S., Castro R. E., Steer C. J., Rodrigues C. M. (2004) Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J. Neurochem. 90, 567–575 [DOI] [PubMed] [Google Scholar]
- 84.Nunes A. F., Amaral J. D., Lo A. C., Fonseca M. B., Viana R. J., Callaerts-Vegh Z., D’Hooge R., Rodrigues C. M. (2012) TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol. Neurobiol. 45, 440–454 [DOI] [PubMed] [Google Scholar]
- 85.Dionísio P. A., Amaral J. D., Ribeiro M. F., Lo A. C., D’Hooge R., Rodrigues C. M. (2015) Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol. Aging 36, 228–240 [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., Li P., Wang Y., Liu J., Zhang Z., Cheng W., Wang Y. (2013) Ameliorative effects of a combination of baicalin, jasminoidin and cholic acid on ibotenic acid-induced dementia model in rats. PLoS One 8, e56658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonifati V. (2006) Parkinson’s disease: the LRRK2-G2019S mutation: opening a novel era in Parkinson’s disease genetics. Eur. J. Hum. Genet. 14, 1061–1062 [DOI] [PubMed] [Google Scholar]
- 88.Greggio E., Jain S., Kingsbury A., Bandopadhyay R., Lewis P., Kaganovich A., van der Brug M. P., Beilina A., Blackinton J., Thomas K. J., Ahmad R., Miller D. W., Kesavapany S., Singleton A., Lees A., Harvey R. J., Harvey K., Cookson M. R. (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 23, 329–341 [DOI] [PubMed] [Google Scholar]
- 89.Smith W. W., Pei Z., Jiang H., Dawson V. L., Dawson T. M., Ross C. A. (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 9, 1231–1233 [DOI] [PubMed] [Google Scholar]
- 90.Mortiboys H., Furmston R., Bronstad G., Aasly J., Elliott C., Bandmann O. (2015) UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology 85, 846–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ved R., Saha S., Westlund B., Perier C., Burnam L., Sluder A., Hoener M., Rodrigues C. M., Alfonso A., Steer C., Liu L., Przedborski S., Wolozin B. (2005) Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J. Biol. Chem. 280, 42655–42668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalsotra A., Turman C. M., Dash P. K., Strobel H. W. (2003) Differential effects of traumatic brain injury on the cytochrome p450 system: a perspective into hepatic and renal drug metabolism. J. Neurotrauma 20, 1339–1350 [DOI] [PubMed] [Google Scholar]
- 93.Cheng C. Y., Ho C. H., Wang C. C., Liang F. W., Wang J. J., Chio C. C., Chang C. H., Kuo J. R. (2015) One-year mortality after traumatic brain injury in liver cirrhosis patients: a ten-year population-based study. Medicine (Baltimore) 94, e1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raghupathi R., Graham D. I., McIntosh T. K. (2000) Apoptosis after traumatic brain injury. J. Neurotrauma 17, 927–938 [DOI] [PubMed] [Google Scholar]
- 95.Ramalho R. M., Borralho P. M., Castro R. E., Solá S., Steer C. J., Rodrigues C. M. (2006) Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer’s disease mutant neuroblastoma cells. J. Neurochem. 98, 1610–1618 [DOI] [PubMed] [Google Scholar]
- 96.Gaspar J. M., Martins A., Cruz R., Rodrigues C. M., Ambrósio A. F., Santiago A. R. (2013) Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience 253, 380–388 [DOI] [PubMed] [Google Scholar]
- 97.Rodrigues C. M., Spellman S. R., Solá S., Grande A. W., Linehan-Stieers C., Low W. C., Steer C. J. (2002) Neuroprotection by a bile acid in an acute stroke model in the rat. J. Cereb. Blood Flow Metab. 22, 463–471 [DOI] [PubMed] [Google Scholar]
- 98.Rodrigues C. M., Sola S., Nan Z., Castro R. E., Ribeiro P. S., Low W. C., Steer C. J. (2003) Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc. Natl. Acad. Sci. USA 100, 6087–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elia A. E., Lalli S., Monsurrò M. R., Sagnelli A., Taiello A. C., Reggiori B., La Bella V., Tedeschi G., Albanese A. (2016) Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 23, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaz A. R., Cunha C., Gomes C., Schmucki N., Barbosa M., Brites D. (2015) Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 51, 864–877 [DOI] [PubMed] [Google Scholar]