Abstract
Efficient intercellular transfer of RNAs, proteins, and lipids as protected exosomal cargo has been demonstrated in the CNS, but distinct physiologic and pathologic roles have not been well defined for this pathway. The capacity to isolate immunochemically human plasma neuron-derived exosomes (NDEs), containing neuron-specific cargo, has permitted characterization of CNS-derived exosomes in living humans. Constituents of the amyloid β-peptide (Aβ)42-generating system now are examined in 2 distinct sets of human neural cells by quantification in astrocyte-derived exosomes (ADEs) and NDEs, enriched separately from plasmas of patients with Alzheimer’s disease (AD) or frontotemporal dementia (FTD) and matched cognitively normal controls. ADE levels of β-site amyloid precursor protein-cleaving enzyme 1 (BACE-1), γ-secretase, soluble Aβ42, soluble amyloid precursor protein (sAPP)β, sAPPα, glial-derived neurotrophic factor (GDNF), P-T181-tau, and P-S396-tau were significantly (3- to 20-fold) higher than levels in NDEs for patients and controls. BACE-1 levels also were a mean of 7-fold higher in ADEs than in NDEs from cultured rat type-specific neural cells. Levels of BACE-1 and sAPPβ were significantly higher and of GDNF significantly lower in ADEs of patients with AD than in those of controls, but not significantly different in patients with FTD than in controls. Abundant proteins of the Aβ42 peptide-generating system in ADEs may sustain levels in neurons. ADE cargo proteins may be useful for studies of mechanisms of cellular interactions and effects of BACE-1 inhibitors in AD.—Goetzl, E. J., Mustapic, M., Kapogiannis, D., Eitan, E., Lobach, I. V., Goetzl, L., Schwartz, J. B., Miller, B. L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease.
Keywords: dementia, amyloid, tau, biomarkers
Astrocytes in the brain and spinal cord associate with neuronal synapses, are distinguished by their prominent network of intermediate filaments, and are rich in glial fibrillary acidic protein (GFAP) and glutamine synthetase (GluSyn) (1). The diversity of astrocyte functional phenotypes may be attributable developmentally to signals from proteins such as sonic hedgehog and later to activation by mediators such as IFN-γ and cGMP-adenosine monophosphate (2, 3). Astrocytes have many neuronal supportive functions including supplying nutrients, regulating extracellular ion concentrations, promoting myelinating activity of oligodendrocytes, and modulating synaptic transmission (4–7).
Astrocytes accumulate at sites of deposition of Aβ peptides in the brain, where they internalize and degrade aggregated peptides in an apparently protective process (8, 9). At high intracellular levels, but in the absence of Aβ plaques, soluble Aβ peptides modify many astrocyte activities, ranging from mitochondrial functions to protein transcription (10–12). However, it has only recently been appreciated that some subsets of astrocytes also contain the amyloid precursor protein (APP) and the β-secretase, termed β-site APP cleaving enzyme 1 (BACE-1), and γ-secretase required for generation of Aβ peptides from APP, and that levels of these components in astrocytes are increased by fibrillary Aβ42 and several inflammatory cytokines (3, 13, 14). In the same studies, microglia contained no proteins of the Aβ42-generating pathway. Mechanisms of regulation of expression of BACE-1 involve several transcriptional elements, such as type 1 specificity protein type 1 (Sp1) and NFAT3, as well as post-transcriptional events (15, 16). BACE-1 stability and function also are influenced by other neural proteins, including ubiquilin-1 and septin-8 (17, 18). A greater understanding of the relative role of these astrocyte pathways in generation of Aβ peptides in the brain has come from recent analyses of human induced pluripotent stem cell–derived neural cells (19). With these methods, astrocytes were shown to secrete high levels of Aβ peptides, and astrocyte-like cells were prominently represented among the highest producers of Aβ peptides.
Exosomes now are being considered as distinct vehicles of intercellular communication in the CNS, but neither physiologic nor pathologic roles have been defined for this pathway (20, 21). Immunochemical isolation of human plasma neuron-derived exosomes (NDEs), containing neuron-specific cargo, has permitted the first characterization of CNS-derived exosomes in living humans (22, 23). We now examine the Aβ42-generating system in astrocyte-derived exosomes (ADEs) and NDEs.
MATERIALS AND METHODS
Patient and control subject selection and evaluation
We retrospectively identified 12 patients with amnestic mild cognitive impairment (aMCI) or early dementia from AD, 14 patients with behavioral variant FTD (bFTD), and 20 age- and gender-matched cognitively normal control subjects, with 10 in the healthy control for AD (AC) group and 10 in the healthy control for FTD (FTC) group, who had donated blood in the Clinical Research Unit of the National Institute on Aging, the Jewish Home of San Francisco, or the Memory and Aging Center of the University of California San Francisco (UCSF). Patients and normal control subjects underwent mental status testing before blood sampling, and most patients had measurements of cerebrospinal fluid (CSF) Aβ1-42, total tau, and P-T181-tau when they first received a diagnosis of dementia. All subjects studied and some patient designates signed a consent form with the approved protocol at each institution.
Patients were classified as having aMCI from AD according to the Petersen criteria and had a clinical dementia rating (CDR) global score of 0.5 (24, 25). Those with mild dementia and probable AD had their condition diagnosed by the Dubois criteria and had a CDR global score of 1.0 (26, 27). A CSF level of Aβ1-42 < 192 pg/ml supported their diagnosis of AD (28). The Mini-Mental State Examination and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) were conducted as described (29, 30). Patients with bFTD had been evaluated at the Memory and Aging Center of UCSF. Diagnosis and assessment were based on standard clinical and mental status criteria, including discriminant analyses of neuropsychiatric and other elements that distinguish bFTD from AD (31, 32).
Ten milliliters of venous blood was drawn into 0.5 ml of saline with EDTA or 100 U/ml of heparin, incubated for 10 min at room temperature, and centrifuged for 15 min at 1500 g. Plasma was stored in 0.5 ml aliquots at −80°C. Laboratory studies were performed without knowledge of donor identity.
Exosome isolation from plasma for extraction and ELISA quantification of cargo proteins
One-fourth milliliter aliquots of plasma from frozen stocks each were defrosted, received 0.1 ml of thromboplastin, and were incubated for 60 min at room temperature with continuous mixing before addition of 0.15 ml of Dulbecco’s calcium- and magnesium-free salt solution containing the suggested final concentrations of protease inhibitor cocktail (Roche Applied Sciences, Inc., Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Pierce Halt; Thermo Fisher Scientific, Rockford, IL, USA) (22, 33). After the contents were mixed at room temperature for 10 min, all tubes were centrifuged at 3000 g for 20 min at 4°C. ExoQuick exosome solution (EXOQ; System Biosciences, Inc., Mountain View, CA, USA) was added at 126 µl per tube, followed by incubation for 60 min at room temperature to precipitate total exosomes as described (22). Each exosome pellet was resuspended in 350 μl of distilled water with inhibitor cocktails before immunochemical enrichment of exosomes from neural sources.
To enrich ADEs, exosome suspensions were incubated for 1 h at 4°C with 1.5 µg of mouse anti-human glutamine aspartate transporter (GLAST) (ACSA-1) biotinylated antibody (Miltenyi Biotec, Inc., Auburn, CA, USA) in 50 µl of 3% bovine serum albumin [BSA; 1:3.33 dilution of Blocker BSA 10% solution in DBS−2 (Thermo Fisher Scientific)] per tube with mixing, followed by addition of 10 μl of streptavidin-agarose UltraLink Resin (Thermo Fisher Scientific) in 40 µl of 3% BSA and incubation for 30 min at 4°C. After centrifugation at 400 g for 10 min at 4°C and removal of the supernatant, each pellet was suspended in 100 µl of 0.05 M glycine-HCl (pH 3.0) by gentle mixing for 10 s and centrifuged at 4000 g for 10 min at 4°C. The supernatants then were transferred to clean tubes containing 50 µl of 10% BSA and 10 µl of 1 M Tris-HCl (pH 8.0) and mixed. Then each tube received 0.59 ml of mammalian protein extraction reagent (M-PER; Thermo Fisher Scientific) that had cocktails of protease and phosphatase inhibitors at recommended concentrations. These suspensions were incubated at room temperature for 10 min before storage at −80°C. NDEs were enriched as described (22, 23).
Exosome proteins were quantified by ELISA kits for neurofilament light chain, Aβ42, γ-secretase, and the tetraspanning exosome marker human CD81 (American Research Products-Cusabio, Waltham, MA, USA) with verification of the CD81 antigen standard curve using human purified recombinant CD81 antigen (Origene Technologies, Inc., Rockville, MD, USA); glutamine synthetase (GluSyn; American Research Products-Cloud-Clone Corp., Waltham, MA, USA); neuron-specific enolase (NS-enolase; R&D Corp., Minneapolis, MN, USA); human BACE-1, soluble (s)APPα and sAPPβ (Fivephoton Biochemicals, San Diego, CA, USA); rat/human BACE-1 (IBL-Fivephoton Biochemicals); GFAP (EMD–Millipore Corp., Billerica, MA, USA); and glial-derived neurotrophic factor (GDNF) P-T181-tau, and P-S396-tau (Thermo Fisher Scientific, Carlsbad, CA, USA), according to the suppliers’ directions. The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative values for individual samples were used to normalize their recovery.
Exosomes from cultured rat neural cells
Rat brain cortical cells from embryos (E 18.5) were cultured on polyethyleneimine-coated plastic dishes to generate neurons (34). Astrocytes were differentiated from rat cortical stem cells in medium with growth factors and replated after 2 wk (35). Neuron and astrocyte media were collected on d 7 and centrifuged at 2500 g for 10 min at 4°C. Two milliliters of ExoQuick-TC were added to 10 ml of medium, followed by incubation overnight at 4°C and centrifugation at 1500 g for 30 min. Exosome pellets were resuspended in 50 µl DBS, from which 5 µl was removed for counts and the rest diluted with 205 µl of M-PER with inhibitors and stored at −80°C as for plasma exosomes.
Exosome counts
Each suspension of extracellular vesicles was diluted 1:200 in PBS. The mean diameter (in nanometers ) and concentration (particles/milliliter) of extracellular vesicles in each suspension were determined with the Nanosight NS500 system with a G532nm laser module and NTA 3.1 nanoparticle tracking software (Malvern Instruments, Malvern, United Kingdom). Camera settings were gain, 366; shutter, 31.48; and frame rate, 24.9825 frames per second; Brownian motion was captured by 5 repeated 20 s video recordings.
Statistical analyses
The statistical significance of differences between means for cross-sectional patient groups and between each patient group and their respective control group was determined with an unpaired Student’s t test including a Bonferroni correction (Prism 6; GraphPad, San Diego, CA, USA). The discriminatory ability of each exosomal protein is presented using receiver operating characteristic (ROC) analyses with confidence intervals estimated based on the binomial exact distribution (STATA 13.1; College Station, TX, USA). Discriminant classifier analyses were performed to evaluate the conjoint ability of exosomal proteins to differentiate the subject groups (STATA 13.1).
RESULTS
Plasma ADEs were significantly less numerous than plasma NDEs in patients with AD and matched AC subjects, based on exosome counts and exosome extract levels of CD81 per milliliter of plasma (Table 1). The relationship between exosome count and CD81 level was the same for ADEs as for NDEs. In patients with FTD, mean ± sem counts of recovered ADEs were 86.0 ± 12.9 × 109/ml of plasma and were not different from those of FTC subjects at 82.5 ± 14.3 × 109/ml or of patients with AD and AC subjects. CD81 levels for patients with FTD and FTC subjects were indistinguishable at 1145 ± 75 and 1498 ± 157 pg/ml, respectively. ADEs had the same size distribution as NDEs, with respective mean ± sem diameter values of 196 ± 10.8 and 165 ± 27.6 nm for patients with AD and 189 ± 21.0 and 173 ± 15.4 nm for matched controls. For patients with FTD and FTC subjects, ADE values also were similar at 224 ± 11.5 and 171 ± 14.8 nm, respectively. CD81-normalized levels of the astrocyte markers GFAP and GluSyn were significantly higher in ADEs than NDEs, whereas levels of the neuronal markers neurofilament light chain (NF-Lch) and neuron-specific (NS) enolase were significantly higher in NDEs than in ADEs (Table 1). The detection of GFAP in NDEs is consistent with its previously observed presence in neurons in relation to aging, hypoxia, and neurodegeneration (36, 37).
TABLE 1.
Distinctive protein markers of plasma ADEs and NDEs
| Analyte (pg/ml) |
||||||
|---|---|---|---|---|---|---|
| Plasma source | Exosome count (×109/ml) | CD81 | GluSyn | GFAP | NF-Lch | NS-enolase |
| ADEs | ||||||
| AD patients | 69.8 ± 14.9 | 1305 ± 100 | 672 ± 145 | 33,533 ± 1976* | 94.8 ± 7.89 | 295 ± 25.7 |
| Matched controls | 88.5 ± 16.1 | 1074 ± 56.1 | 660 ± 137 | 59,708 ± 6869 | 104 ± 4.21 | 291 ± 30.5 |
| NDEs | ||||||
| AD patients | 229 ± 49.2** | 4269 ± 234** | 42 ± 7.03* | 8395 ± 817** | 1412 ± 56.9** | 6024 ± 139** |
| Matched controls | 307 ± 53.4** | 5145 ± 257** | 53 ± 8.49* | 2016 ± 267** | 1075 ± 37.6** | 3383 ± 131** |
Data are the means ± sem of results for 12 subjects per group, except for exosome counts that are for 8 subjects per group. Levels of GluSyn, GFAP, NF-Lch, and NS-enolase were normalized to the levels of CD81 in the same samples. The double asterisks indicate significantly higher or lower values than for the corresponding levels in ADEs, and the single asterisk indicates the level of significance of the difference between ADE GFAP levels in patients with AD and matched controls. In NDEs, levels of CD81 (P = 0.022) and GluSyn (not significant) are lower, whereas those of GFAP, NF-Lch, and NS-enolase are higher (P < 0.001) in patients with AD than in controls. *P < 0.01, **P < 0.001 by 2-sample Student's t test.
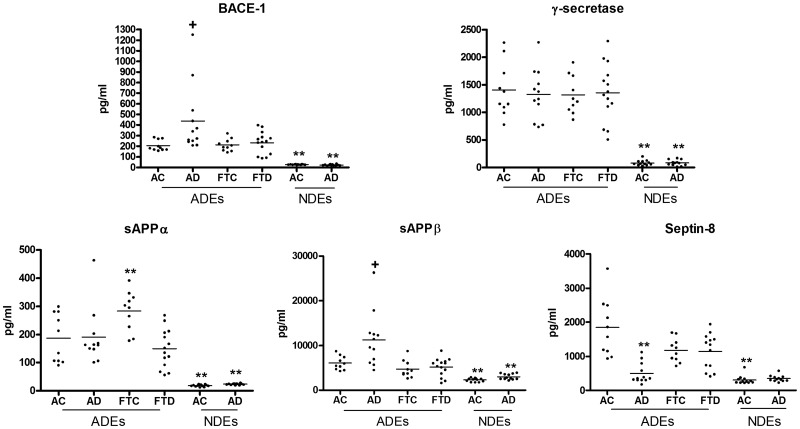
Levels of BACE-1, γ-secretase, sAPPβ, and sAPPα all were a mean of 10-fold higher in ADEs than in NDEs of patients with AD and their matched AC subjects (P < 0.0001; Fig. 1). ADE levels of BACE-1 were significantly higher in patients with AD (398 ± 81.6 pg/ml, mean ± sem) than in matched AC subjects (209 ± 16.4 pg/ml), using a polypeptide antigen-based ELISA, whereas no such difference was found for the lower BACE-1 levels in NDEs. A second ELISA (from IBL), based on a full-length recombinant human BACE-1 antigen, and 2 differently specific antibodies were used to quantify levels in the same subjects. With this ELISA, the mean ± sem of AD level in patients was 1191 ± 61.0 pg/ml (range, 1083–1471) and that for matched controls was 173 ± 22.9 pg/ml (range, 77.3–285; P < 0.0001). In contrast, neither ADE levels nor the much lower NDE levels of γ-secretase differed significantly between patients with AD and matched AC subjects. Neither BACE-1 nor γ-secretase levels in ADEs or NDEs of patients with FTD were different than in matched FTC subjects.
Figure 1.
Components of the Aβ42 peptide-generating system in ADEs and NDEs. Each point depicts the value for one patient or control subject, and the mean for each group of points is shown by a solid horizontal line. The mean ± sem levels in pg/ml are: BACE-1 ADEs: AC, 209 ± 16.4; AD, 398 ± 81.6; FTC, 211 ± 18.0; FTD, 233 ± 27.5; and NDEs: AC, 26.9 ± 0.74; AD, 23.3 ± 1.70. γ-Secretase ADEs: AC, 1406 ± 154; AD, 1329 ± 131; FTC, 1317 ± 109; FTD, 1357 ± 140; and NDEs: AC, 82.1 ± 14.1; AD, 83.4 ± 15.0. sAPPα ADEs: AC, 187 ± 27.4; AD, 191 ± 30.5; FTC, 284 ± 22.2; FTD, 150 ± 18.4; and NDEs: AC, 18.1 ± 1.10; AD, 23.3 ± 0.76. sAPPβ ADEs: AC, 6048 ± 483; AD, 11243 ± 1747; FTC, 4684 ± 605; FTD, 5127 ± 547; and NDEs: AC, 2266 ± 112; AD, 2992 ± 184. Septin-8 ADEs: AC, 1843 ± 269; AD, 497 ± 86.6; FTC, 1183 ± 109; FTD, 1152 ± 144; and NDEs: AC, 308 ± 36.7; AD, 350 ± 26.3. Statistical significance of differences between disease and control groups for ADEs are shown above the disease group clusters of data, and those between values in NDEs and corresponding values in ADEs are shown above the NDE clusters of data. +P < 0.05; * P < 0.01; **P < 0.001.
ADE levels of sAPPβ, but not of sAPPα, were significantly higher in patients with AD than those in the AC subjects (P = 0.0159), with no differences in patients with FTD compared with the controls. ROC plots showed that both BACE-1 and sAPPβ levels in ADEs significantly distinguished patients with AD from controls, whereas only sAPPβ levels in ADEs distinguished AD from patients with FTD (Supplemental Figs. 1, 2). The much lower levels of sAPPβ and sAPPα in NDEs are significantly higher for patients with AD than in the AC subjects (P = 0.0028 and P = 0.0008, respectively). The neural protein septin-8 has been implicated in normal synaptic plasticity and in the regulation of amyloidogenic processing of APP through control of sorting and expression of BACE-1 (18). ADE, but not NDE, levels of septin-8 were significantly lower in patients with AD than in the AC subjects (P < 0.0001), without a difference in ADE levels of patients with FTD than in controls.
To investigate further the respective levels of BACE-1 in ADEs and NDEs, separate astrocyte and neuron cultures were established using well-validated methods for embryonic rat brain cells (34, 35). Mean exosome counts were the same at 1.2 × 108/milliliter for media from both types of cells. The rat BACE-1 ELISA (from IBL) results for those sets of ADEs and NDEs were a mean of 2170 pg/ml/108 ADEs (n = 3 preparations) for astrocyte-conditioned medium and a mean of 369 pg/ml/108 NDEs (n = 3) for neuron-conditioned medium. The relative ratio of ADE/NDE levels of BACE-1 thus were similar for cultured rat cell exosomes and human plasma exosomes.
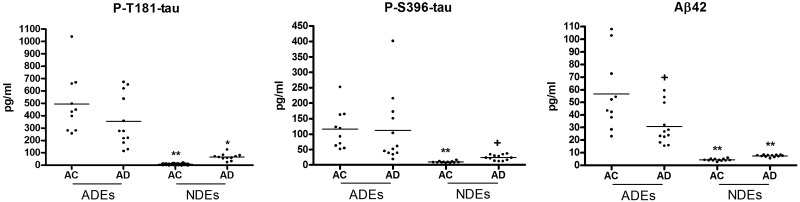
The exosome levels of the neuronal trophic-survival factor GDNF were examined with extracts from the same group of subjects as in Figs. 1 and 2 (Table 2). The ADE levels of GDNF were much higher than those in the NDEs. ADE levels in patients with AD were significantly lower than those in matched controls, whereas no difference in NDE levels of GDNF was observed between the groups.
Figure 2.
Primary pathogenic proteins of AD in ADEs and NDEs. Each point depicts the value for a single patient or control subject, and the mean for each group of points is shown by a solid horizontal line. The mean ± sem levels are: P-T181-tau ADEs: AC, 496 ± 76.0; AD, 355 ± 60.4; and NDEs: AC, 6.55 ± 0.94; AD, 66.0 ± 7.47. P-S396-tau ADEs: AC, 116 ± 20.2; AD, 111 ± 32.1; and NDEs: AC, 9.76 ± 0.75; AD, 24.1 ± 2.63. Aβ42 ADEs: AC, 56.6 ± 9.25; AD, 30.9 ± 4.38; and NDEs AC, 4.43 ± 0.25; AD, 7.58 ± 0.25. Statistical significance of differences between disease and control groups for ADEs are shown above the disease group clusters of data and those between values in NDEs and corresponding values in ADEs are shown above the NDE clusters of data. +P < 0.05; *P < 0.01; **P < 0.001.
TABLE 2.
Differences in GNDF levels of ADEs and NDEs
| Plasma source | GDNF (pg/ml) |
|---|---|
| ADEs | |
| AD patients | 82.3 ± 5.17* |
| Matched controls | 109 ± 7.98 |
| NDEs | |
| AD patients | 7.40 ± 2.50 |
| Matched controls | 5.56 ± 2.28 |
For 3 factors putatively pathogenic in AD, levels of each were significantly higher in ADEs than in NDEs of patients with AD and in the AC control subjects (Fig. 2). For each factor, levels in NDEs were significantly higher (P < 0.0001) in patients with AD than in the AC subjects. In contrast, despite the much higher levels of all 3 factors in ADEs than NDEs, only those of Aβ42 distinguished between patients with AD and AC subjects where paradoxically the former had significantly lower concentrations (P = 0.015; Fig. 2).
DISCUSSION
ADEs were enriched from the total population of human plasma exosomes in patients with AD or FTD and their matched AC and FTC subjects by specific positive immunochemical selection (Table 1). Plasma ADEs had the same size characteristics and similar CD81 exosome marker level per exosome as NDEs, but prominently expressed the expected GFAP and GluSyn astrocyte markers, but not neuronal markers of NDEs. Most strikingly, ADEs of patients and controls had up to 20-fold higher concentrations of BACE-1, γ-secretase, sAPPβ, sAPPα, and GDNF than in NDEs (Fig. 1 and Table 2). ADEs from cultured rat astrocytes also had much higher levels of BACE-1 than NDEs from cultured rat neurons. Of pathogenic importance, levels of BACE-1 and sAPPβ, but not sAPPα, were significantly higher and those of GDNF significantly lower in ADEs of patients with AD than in the AC subjects (Fig. 1 and Table 2). The putatively pathogenic proteins P-T181-tau and P-S396-tau and soluble Aβ42 also were present at much higher levels in ADEs than in NDEs of patients with AD and the AC subjects, but only levels of Aβ42 differed in patients with AD and paradoxically were lower than in AC subjects (Fig. 2). As has been demonstrated, NDE levels of these same 3 proteins all are significantly higher in patients with AD than in their AC subjects (Fig. 2) (33).
The potential mechanisms that elevated BACE-1 in ADEs of patients with AD compared to AC subjects remain to be elucidated. However, it is of interest that ADE levels of septin-8 were significantly lower in patients with AD than in AC subjects (Fig. 1). Septin-8 is a member of a family of conserved GTPases implicated in synaptic vesicular trafficking and plasticity. Each of the 4 transcript variants of septin-8, termed TV1-4, alters levels of BACE-1 in neural cells differently by post-translational mechanisms and coordinately affects levels of sAPPβ and Aβ peptide (18). A decreased level of total septin-8 protein in ADEs of patients with AD correlates best with lower levels of TV1 mRNA documented in autopsy brain tissue from patients with AD and a consequently higher level of BACE-1. The overall network of factors regulating BACE-1 expression is quite complex, and further studies are needed, however, as Sp1 and NFAT3 transcriptionally enhance levels of BACE-1 and ubiquilin-1 stabilizes BACE-1 protein post-translationally (15–17).
Neurons are exposed for long periods to greater amounts of BACE-1, γ-secretase, and sAPPβ of the Aβ42-generating system from ADEs than are exported in their NDEs, so that ADEs are likely to be a major vehicle for astrocyte maintenance of the neuronal machinery to produce Aβ42. The higher ADE levels of BACE-1 and sAPPβ in patients with AD than in AC subjects also may explain in part the higher levels of the Aβ42-generating system in neurons affected by AD. Elevated levels of the Aβ42-generating system in ADEs, without observable amyloid plaques on astrocytes, further suggests that astrocytes are much more efficient than neurons at exosome-mediated elimination or proteolytic destruction of Aβ42-generating proteins, or that only a small subset of astrocytes load ADEs with Aβ42-generating system cargo. Optimal astrocyte–neuron interactions through ADE trafficking may require astrocyte phenotypic changes to a previously postulated state of activation. If an activated astrocyte–neuron axis represents a central pathway in the pathogenesis of AD, ADEs may provide a valuable window for further research on neural cellular interactions.
The modest differences in ADE levels of BACE-1 and sAPPβ between patients with AD and AC subjects in the peptide antigen ELISAs do not suggest significant value in quantifying these ADE constituents as a means for detecting preclinical AD. However, such small differences may lead to major increases in amyloid peptide deposits in the central nervous system after decades of augmented generation and accumulation. Such is true for deposition of amyloid-like deposits of some mucopolysaccharides, where only modest differences are detected between synthesis rates in mild cases as contrasted with controls (38, 39). Nonetheless, ADEs will be a valuable target for testing drugs directed to inhibiting BACE-1 or γ-secretase, if results of further investigations validate a role for an activated astrocyte-neuron axis in proteinopathic dementias.
ACKNOWLEDGMENTS
This work was supported by a grant from the Biomarkers Across Neurodegenerative Diseases 2 program of the Michael J. Fox Foundation for Parkinson’s Research, the Alzheimer’s Association, Alzheimer’s Research UK and the Weston Brain Institute, the U.S. National Institues of Health (NIH) National Institute on Aging (NIA) Intramural Program (to M.M., D.K. and E.E.), and NIH/NIA grant P30 AG028383 (to E.L.A.). The authors thank Judith H. Goetzl (Jewish Home of San Francisco) for expert preparation of the illustrations. E.J.G. has filed a provisional patent application with the U.S. Patent Office for the methodology of immunochemical isolation and analytical applications of astrocyte-derived exosomes. The remaining authors declare no competing financial interests.
Glossary
- AC
healthy control for AD
- AD
Alzheimer’s disease
- ADAS-cog
Alzheimer’s disease assessment scale-cognitive subscale
- ADE
astrocyte-derived exosome
- aMCI
amnestic mild cognitive impairment
- APP
amyloid precursor protein
- Aβ
amyloid β-peptide
- BACE-1
β-site amyloid precursor protein-cleaving enzyme 1
- bFTD
behavioral variant of FTD
- BSA
bovine serum albumin
- CDR
clinical dementia rating
- CSF
cerebrospinal fluid
- FTC
healthy control for FTD
- FTD
frontotemporal dementia
- GDNF
glial-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GLAST
glutamine aspartate transporter
- GluSyn
glutamine synthetase
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- M-PER
mammalian protein extraction reagent
- NDE
neuron-derived exosome
- NFAT
nuclear factor of activated T cells
- NS-enolase
neuron-specific enolase
- sAPP
soluble APP
- Sp1
specificity protein type 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. J. Goetzl developed and applied exosome concepts and methods, designed the experiments, and drafted the manuscript; M. Mustapic counted the exosomes and edited the manuscript; D. Kapogiannis selected and evaluated the patients with AD and edited the manuscript; E. Eitan cultured rat neural cells; I. V. Lobach conducted all statistical analyses; L. Goetzl designed the experiments and edited the manuscript; J. B. Schwartz selected and evaluated the control subjects and edited the manuscript; and B. L. Miller selected and evaluated patients with FTD and edited the manuscript.
REFERENCES
- 1.Hochstim C., Deneen B., Lukaszewicz A., Zhou Q., Anderson D. J. (2008) Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmer W. T., Abrahamsson T., Chierzi S., Lui C., Zaelzer C., Jones E. V., Bally B. P., Chen G. G., Théroux J. F., Peng J., Bourque C. W., Charron F., Ernst C., Sjöström P. J., Murai K. K. (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854 [DOI] [PubMed] [Google Scholar]
- 3.Hartlage-Rübsamen M., Zeitschel U., Apelt J., Gärtner U., Franke H., Stahl T., Günther A., Schliebs R., Penkowa M., Bigl V., Rossner S. (2003) Astrocytic expression of the Alzheimer’s disease beta-secretase (BACE1) is stimulus-dependent. Glia 41, 169–179 [DOI] [PubMed] [Google Scholar]
- 4.Walz W. (2000) Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 36, 291–300 [DOI] [PubMed] [Google Scholar]
- 5.Santello M., Volterra A. (2009) Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158, 253–259 [DOI] [PubMed] [Google Scholar]
- 6.Pascual O., Casper K. B., Kubera C., Zhang J., Revilla-Sanchez R., Sul J. Y., Takano H., Moss S. J., McCarthy K., Haydon P. G. (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 [DOI] [PubMed] [Google Scholar]
- 7.Ishibashi T., Dakin K. A., Stevens B., Lee P. R., Kozlov S. V., Stewart C. L., Fields R. D. (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 9.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., Paul S. M. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 10, 719–726 [DOI] [PubMed] [Google Scholar]
- 10.Abramov A. Y., Canevari L., Duchen M. R. (2004) Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 24, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matos M., Augusto E., Oliveira C. R., Agostinho P. (2008) Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 156, 898–910 [DOI] [PubMed] [Google Scholar]
- 12.Multhaup G., Huber O., Buée L., Galas M. C. (2015) Amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Aβ42, and tau in nuclear roles. J. Biol. Chem. 290, 23515–23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J., O’Connor T., Vassar R. (2011) The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J. Neuroinflammation 8, 150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong H. S., Hwang E. M., Sim H. J., Cho H. J., Boo J. H., Oh S. S., Kim S. U., Mook-Jung I. (2003) Interferon gamma stimulates beta-secretase expression and sAPPbeta production in astrocytes. Biochem. Biophys. Res. Commun. 307, 922–927 [DOI] [PubMed] [Google Scholar]
- 15.Christensen M. A., Zhou W., Qing H., Lehman A., Philipsen S., Song W. (2004) Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Mol. Cell. Biol. 24, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei Z., Yan P., Tan X., Zheng S., Situ B. (2015) Transcriptional regulation of BACE1 by NFAT3 leads to enhanced amyloidogenic processing. Neurochem. Res. 40, 829–836 [DOI] [PubMed] [Google Scholar]
- 17.Natunen T., Takalo M., Kemppainen S., Leskelä S., Marttinen M., Kurkinen K. M., Pursiheimo J. P., Sarajärvi T., Viswanathan J., Gabbouj S., Solje E., Tahvanainen E., Pirttimäki T., Kurki M., Paananen J., Rauramaa T., Miettinen P., Mäkinen P., Leinonen V., Soininen H., Airenne K., Tanzi R. E., Tanila H., Haapasalo A., Hiltunen M. (2016) Relationship between ubiquilin-1 and BACE1 in human Alzheimer’s disease and APdE9 transgenic mouse brain and cell-based models. Neurobiol. Dis. 85, 187–205 [DOI] [PubMed] [Google Scholar]
- 18.Kurkinen K. M., Marttinen M., Turner L., Natunen T., Makinen P., Haapalinna F., Sarajarvi T., Gabbouj S., Kurki M., Paananen J., Koivisto A. M., Rauramaa T., Leinonen V., Tanila H., Soininen H., Lucas F. R., Haapasalo A., Hiltunen M. (2016) SEPT8 modulates beta-amyloidogenic processing of APP via affecting the sorting and accumulation of BACE1. J. Cell Sci. 129, 2224–2238 [DOI] [PubMed] [Google Scholar]
- 19.Liao M. C., Muratore C. R., Gierahn T. M., Sullivan S. E., Srikanth P., De Jager P. L., Love J. C., Young-Pearse T. L. (2016) Single-cell detection of secreted Aβ and sAPPα from human IPSC-derived neurons and astrocytes. J. Neurosci. 36, 1730–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajendran L., Bali J., Barr M. M., Court F. A., Krämer-Albers E. M., Picou F., Raposo G., van der Vos K. E., van Niel G., Wang J., Breakefield X. O. (2014) Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 34, 15482–15489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budnik V., Ruiz-Cañada C., Wendler F. (2016) Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetzl E. J., Boxer A., Schwartz J. B., Abner E. L., Petersen R. C., Miller B. L., Kapogiannis D. (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapogiannis D., Boxer A., Schwartz J. B., Abner E. L., Biragyn A., Masharani U., Frassetto L., Petersen R. C., Miller B. L., Goetzl E. J. (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 29, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen R. C. (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 [DOI] [PubMed] [Google Scholar]
- 25.Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R. Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 [DOI] [PubMed] [Google Scholar]
- 27.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 [DOI] [PubMed] [Google Scholar]
- 28.Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irizarry M. C., Webb D. J., Bains C., Barrett S. J., Lai R. Y., Laroche J. P., Hosford D., Maher-Edwards G., Weil J. G. (2008) Predictors of placebo group decline in the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer’s disease. J. Alzheimers Dis. 14, 301–311 [DOI] [PubMed] [Google Scholar]
- 30.Cano S. J., Posner H. B., Moline M. L., Hurt S. W., Swartz J., Hsu T., Hobart J. C. (2010) The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 81, 1363–1368 [DOI] [PubMed] [Google Scholar]
- 31.Rascovsky K., Hodges J. R., Knopman D., Mendez M. F., Kramer J. H., Neuhaus J., van Swieten J. C., Seelaar H., Dopper E. G., Onyike C. U., Hillis A. E., Josephs K. A., Boeve B. F., Kertesz A., Seeley W. W., Rankin K. P., Johnson J. K., Gorno-Tempini M. L., Rosen H., Prioleau-Latham C. E., Lee A., Kipps C. M., Lillo P., Piguet O., Rohrer J. D., Rossor M. N., Warren J. D., Fox N. C., Galasko D., Salmon D. P., Black S. E., Mesulam M., Weintraub S., Dickerson B. C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T. W., Manes F., Grafman J., Cappa S. F., Freedman M., Grossman M., Miller B. L. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., Ogar J. M., Rohrer J. D., Black S., Boeve B. F., Manes F., Dronkers N. F., Vandenberghe R., Rascovsky K., Patterson K., Miller B. L., Knopman D. S., Hodges J. R., Mesulam M. M., Grossman M. (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J. (2015) Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11, 600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson M. P., Barger S. W., Begley J. G., Mark R. J. (1995) Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 46, 187–216 [DOI] [PubMed] [Google Scholar]
- 35.Chojnacki A., Weiss S. (2008) Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat. Protoc. 3, 935–940 [DOI] [PubMed] [Google Scholar]
- 36.Hol E. M., Roelofs R. F., Moraal E., Sonnemans M. A., Sluijs J. A., Proper E. A., de Graan P. N., Fischer D. F., van Leeuwen F. W. (2003) Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol. Psychiatry 8, 786–796 [DOI] [PubMed] [Google Scholar]
- 37.Bi B., Salmaso N., Komitova M., Simonini M. V., Silbereis J., Cheng E., Kim J., Luft S., Ment L. R., Horvath T. L., Schwartz M. L., Vaccarino F. M. (2011) Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J. Neurosci. 31, 9205–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tieu P. T., Bach G., Matynia A., Hwang M., Neufeld E. F. (1995) Four novel mutations underlying mild or intermediate forms of alpha-L-iduronidase deficiency (MPS IS and MPS IH/S). Hum. Mutat. 6, 55–59 [DOI] [PubMed] [Google Scholar]
- 39.Ruth L., Eisenberg D., Neufeld E. F. (2000) alpha-L-iduronidase forms semi-crystalline spherulites with amyloid-like properties. Acta Crystallogr. D Biol. Crystallogr. 56, 524–528 [DOI] [PubMed] [Google Scholar]