Abstract
TGF-β plays a central role in the pathogenesis of fibroproliferative disorders. Defining the exact underlying molecular basis is therefore critical for the development of viable therapeutic strategies. Here, we show that expression of the facilitative glucose transporter 1 (GLUT1) is induced by TGF-β in fibroblast lines and primary cells and is required for the profibrotic effects of TGF-β. In addition, enhanced GLUT1 expression is observed in fibrotic areas of lungs of both patients with idiopathic pulmonary fibrosis and mice that are subjected to a fibrosis-inducing bleomycin treatment. By using pharmacologic and genetic approaches, we demonstrate that up-regulation of GLUT1 occurs via the canonical Smad2/3 pathway and requires autocrine activation of the receptor tyrosine kinases, platelet-derived and epidermal growth factor receptors. Engagement of the common downstream effector PI3K subsequently triggers activation of the MEK and mammalian target of rapamycin complex 2, which cooperate in regulating GLUT1 expression. Of note, inhibition of GLUT1 activity and/or expression is shown to impair TGF-β–driven fibrogenic processes, including cell proliferation and production of profibrotic mediators. These findings provide new perspectives on the interrelation of metabolism and profibrotic TGF-β signaling and present opportunities for potential therapeutic intervention.—Andrianifahanana, M., Hernandez, D. M., Yin, X., Kang, J.-H., Jung, M.-Y., Wang, Y., Yi, E. S., Roden, A. C., Limper, A. H., Leof, E. B. Profibrotic up-regulation of glucose transporter 1 by TGF-β involves activation of MEK and mammalian target of rapamycin complex 2 pathways.
Keywords: fibrosis, signaling, metabolism
Documented as a leading cause of morbidity and mortality, fibrotic diseases account for nearly 45% of all deaths in developed countries and represent a group of disorders for which there is no available effective therapy (1–5). These dismal figures have fueled the continued interest in unlocking the underlying molecular basis. The role of TGF-β in the fibrogenic process remains a recurring theme as a result of its involvement in the pathogenesis of virtually all forms of fibroproliferative disorders (6, 7). Recognized as the most potent profibrotic cytokine, TGF-β exerts its effects essentially by stimulating fibroblast proliferation, myofibroblast differentiation, and extracellular matrix remodeling (6, 8).
Activation of the TGF-β receptor complex triggers phosphorylation of the receptor-regulated transcription coregulators (R-Smads), Smad2 and Smad3. After heteromerization with the common mediator, Smad4, R-Smads translocate to the nucleus to modulate gene expression (9, 10). Numerous TGF-β–associated functions are regulated via Smads (7, 8, 11), although non-Smad signaling pathways and/or crosstalk between the two are also critical for TGF-β’s action (7, 12, 13). For example, Smad activation facilitates induction of fibrogenic intermediate effectors, including platelet-derived growth factor (PDGF) (14–16) and CCN family 2/connective tissue growth factor (CTGF) (17, 18). Likewise, non-Smad effectors, such as the p21-activated kinase 2/cAbl (19, 20) and Akt/mammalian target of rapamycin (mTOR) (21) modules, both of which operate downstream of the focal adhesion kinase (22) and PI3K (23), are also known to have potent profibrotic activities. In addition, different members of the MAPK family are critical mediators of TGF-β (24, 25). Of note, our recent work highlighted the importance of an autocrine loop implicating the receptor tyrosine kinases, PDGF and epidermal growth factor receptors, in the regulation of TGF-β–dependent fibrogenesis, both in vitro and in a murine model of lung fibrosis (26, 27). The present study extends these findings to define the role of the glucose metabolic pathway in profibrotic TGF-β signaling and address the associated regulatory mechanisms.
Glucose is a major source of metabolic energy for all mammalian cells and is primarily transported across the plasma membrane by the facilitative glucose transporters (GLUT/SLC2) (28–31) and/or, in some tissues, the active sodium/glucose symporters (SGLT/SLC5) (28, 32, 33). Deregulated expression of GLUTs has been implicated in a number of pathologic conditions. For example, in various types of malignancies, increased glucose uptake and metabolism via enhanced expression of specific GLUTs, particularly GLUT1, has been associated with accelerated tumor cell growth and poor survival (reviewed in refs. 34–37). Moreover, in the context of diabetic nephropathy, up-regulation of GLUT1 has been shown to significantly impact the development of glomerulosclerosis (38). Of interest, although TGF-β has been shown to have an essential role in glucose-induced hypertrophy (39), and earlier studies have reported the induction of GLUT1 expression by TGF-β in fibroblasts and mesangial cells (40, 41), little is known about the generality of such observations or the underlying regulatory mechanisms and physiologic implications. Here, we address these important issues and show that: 1) up-regulation of GLUT1 by TGF-β in fibroblasts is a general phenomenon; 2) GLUT1 induction and activity are critical for profibrotic TGF-β signaling; 3) enhanced GLUT1 expression is observed in the lungs of patients with idiopathic pulmonary fibrosis (IPF) and in the bleomycin mouse model of lung fibrosis; and 4) TGF-β–dependent GLUT1 up-regulation occurs via the canonical Smad2/3 pathway and requires activation of MEK and mTOR complex (mTORC) 2 downstream of PI3K. These findings provide new mechanistic insights into profibrotic TGF-β signaling and present opportunities for potential therapeutic intervention.
MATERIALS AND METHODS
Cell culture
Murine fibroblasts (AKR-2B and Swiss-3T3) and derived cell lines expressing short hairpin RNA (shRNA) specific for different target genes were routinely maintained in high-glucose DMEM (Thermo Fisher Scientific, Waltham, MA, USA) that was supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Fetal (IMR-90) and adult human lung fibroblasts (HuLFs; generously provided by Dr. Daniel Tschumperlin, Mayo Clinic, Rochester, MN, USA) were maintained in Eagle’s minimum essential medium (American Type Culture Collection, Manassas, VA, USA) that contained 10% FBS. Isolation of HuLF was done according to a protocol approved by the Mayo Clinic Institutional Review Board. Cells were routinely grown under reduced serum (0.1% FBS) or serum-free (for HuLF) conditions for 24 h before being subjected to specific test reagents. Matrix protein induction by TGF-β1 (Supplemental Fig. 1) was more robust in the absence of 24-h incubation in reduced serum.
Bleomycin-induced lung fibrosis
The protocol for induction of lung fibrosis in female C57 black mice (The Jackson Laboratory, Bar Harbor, ME, USA) and drug treatment was performed as previously described (26).
Antibodies and other reagents
Anti–phospho-AKT (Ser473; 9271), anti-AKT (9272), anti–S6 kinase (9292), anti–phospho-S6 kinase (9205), anti–platelet-derived growth factor receptor (PDGFR)-β (3175), and anti–phospho-ERK1/2 (9106) were purchased from Cell Signaling Technologies (Beverly, MA, USA), whereas anti-PDGFRα (sc-338), anti-PDGFRβ (sc-1627), and anti-ERK1/2 (sc-94) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-SMAD3 (ab28379) and anti-SMAD2 (Ab63576) were from Abcam (Cambridge, MA, USA), and anti–glyceraldehyde 3-phosphate dehydrogenase (MAB374), anti-phosphotyrosine (clone 4G10; 05-321), anti-ErbB1 (06-847), anti-mTOR (05-1564), anti-Rictor (05-1471), and anti-ErbB2 (06-562) were from Millipore (Billerica, MA, USA). Anti–FLAG-M2 (F1804) was from Sigma-Aldrich (St. Louis, MO, USA), whereas anti-Raptor (A300-506A) was from Bethyl Laboratories (Montgomery, TX, USA). GLUT1 antibodies used for Western blotting (GT12-A) and immunohistochemistry (AB115730) were obtained from Alpha Diagnostic (San Antonio, TX, USA) and Abcam, respectively. GLUT1 blocking peptide (AB202335) was purchased from Abcam, and the glucose transport inhibitors, phloretin and GLUT inhibitor II, were from Sigma-Aldrich and Millipore, respectively. A phospho-Smad3–specific antibody (immunogen: COOH-GSPSIRCSpSVpS) was generated in our laboratory (20), whereas anti–phospho-Smad2 (566415) was from Calbiochem (San Diego, CA, USA). CP673451 was from Medchem Express (Monmouth Junction, NJ, USA), lapatinib from LC Laboratories (Woburn, MA, USA), U0126 from Merck (Whitehouse Station, NJ, USA), LY294002 from Sigma-Aldrich, and both MK2206 and rapamycin from Selleck Chemicals (Houston, TX, USA). Imatinib was purchased from the Mayo Clinic pharmacy, whereas human TGF-β1 was obtained from R&D Systems (Minneapolis, MN, USA).
Protein extraction, immunoprecipitation, and Western blotting
Unless otherwise indicated, total proteins were extracted in modified RIPA buffer and processed for immunoprecipitation and/or Western blotting as described (26, 27).
Lung biopsy selection and patient clinical features
Samples from 7 patients with normal lung biopsies and 12 patients with IPF were used in this study. All patients authorized the use of their records and samples for research according to the Minnesota Research Authorization Law, and permission for use of these archived tissue biopsies was granted by Mayo Clinic Institutional Review Board. All biopsies were reviewed by pathologists with pulmonary expertise to confirm histologic characteristics, and all patient histories were reviewed by an expert pulmonologist to confirm characteristics of clinical diagnosis (Supplemental Table 1).
Immunohistochemical process
Paraffin-embedded lung tissue sections (5 μm, uncharged) were deparaffinized by incubating for 1 h at 60°C, followed by multiple xylene soaks, and rehydrated through a series of ethanol washes. Antigen retrieval was done by heat treatment under acidic condition (CTS014; R&D Systems) in a steamer for 10 min. GLUT1 staining was achieved by using the 3,3′-diaminobenzidine (DAB)–horseradish peroxidase anti-rabbit cell and tissue staining kit (CTS005; R&D Systems) with hematoxylin counterstain (Gills Formula No. 2, CS401-1D; Thermo Fisher Scientific). Tissue sections were subsequently dehydrated and mounted in a nonaqueous medium. The specificity of GLUT1 staining in murine and human lung is shown in Supplemental Figs. 2 and 3, respectively.
Immunohistochemistry quantification and analysis
For quantitative analyses, slides were scanned with an Aperio Digital Pathology spectral imager (brightfield scanner; Leica Biosystems, Buffalo Grove, IL, USA), which allows detection of the chromogen associated with the presence of GLUT1 antigen (DAB; brown color spectrum); nuclei were counterstained with hematoxylin. Equal size annotations were randomly selected and analyzed in ImageScope using the Positive Pixel Count macro algorithm (version 9.1; Leica Biosystems; hematoxylin is excluded). Percent GLUT1 expression was quantitated on the basis of pixels positive for DAB staining—excluding basal expression values—normalized to the total number of color pixels (hematoxylin + DAB, representing cell density).
For murine specimens, 3 paraffin-embedded lung tissue sections per treatment were scanned at ×20 magnification and analyzed. Values from collective annotations (n = 10 per sample; 150 μm × 150 μm annotation) were used for statistical analyses. Percent GLUT1 expression was calculated in Aperio (N strong positive + N positive/N total) × 100 = % GLUT1 expression). For human specimens, a total of 7 normal and 12 IPF paraffin-embedded lung tissue biopsy sections (clinical data provided in Supplemental Table 1) were scanned at ×40 magnification, and collective annotations (n ≥ 10 per sample; 50 μm × 50 μm) were subjected to statistical analyses. Calculation of percent strong GLUT1 expression was similarly calculated in Aperio.
Quantitative RT-PCR analysis
Total RNA was isolated by using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA). Of RNA, 2 μg were reverse transcribed with Maxima Reverse Transcriptase (Thermo Fisher Scientific), and complementary DNAs (1–5 ng RNA equivalents) were subjected to real-time PCR amplification by using SYBR Premix Ex Taq II (Takara/Clontech, Mountain View, CA, USA) on a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). SMAD4 was used as a normalization control. Primer sets for murine genes used were as follows: GLUT1: forward: 5′-GGCTTCTCCAACTGGACCTC-3′, reverse: 5′-AAGAAGAGCACGAGGAGCAC-3′; CTGF: forward: 5′-CACAGAGTGGAGCGCCTGTTC-3′, reverse: 5′-GATGCACTTTTTGCCCTTCTTAATG-3′; PAI-1 (plasminogen activator inhibitor 1): forward: 5′-TTCCAACCAGCATCCCAGAC-3′, reverse: 5′-CCATGAGACCTTTGTGGGGT-3′; α-SMA (α-smooth muscle actin): forward: 5′-CTGACAGAGGCACCACTGAA-3′, reverse: 5′-CAGAGGCATAGAGGGACAGC-3′; SMAD4: forward: 5′-AGAGTCTAACGCCACCAGC-3′, reverse: 5′-TGAAGCTATCTGCAACAGTCCT-3′.
Relative expression levels of the target genes were determined by the 2−∆∆Ct method (42). For all samples, a total of 3 biologic replicates, each assayed in 2 technical replicates, were used.
RNA interference
Plasmids (pLKO.1-puro) encoding shRNA that targeted ErbB1, ErbB2, PDGFRα, PDGFRβ, and GLUT1 were purchased from the Mayo Clinic Jacksonville RNA Interference Shared Resource. Production of lentivirus and transduction of AKR-2B cells were performed as previously described (26, 27). Stable pools (sh-GLUT1) or clones (sh-ErbB1+2, sh-PDGFRα+β) (26, 27) were generated in the presence of 1.5 μg/ml puromycin.
Plasmid and transfection
The construct encoding a FLAG-tagged constitutively active version of MEK1 (FLAG-MEK1-S218-222D) was generously provided by Dr. Scott Eblen (Medical University of South Carolina, Charleston, SC, USA). Transfection procedures using the TransIT-2020 reagent (Mirus Bio, Madison, WI, USA) were performed according to manufacturer recommendations.
Soft agar assay
Experimental procedures for soft agar assay were as described in Andrianifahanana et al. (26). The potential for anchorage-independent colony formation of TGF-β–treated cells was evaluated by counting colonies >50 μm in diameter by using a Gelcount apparatus (Oxford Optronics, Abingdon, United Kingdom).
Glucose uptake assay
2-Deoxyglucose uptake rates were measured essentially as described for insulin-stmulated 3T3-L1 fibroblasts (43). In brief, AKR-2B cells (3.33 × 105/well) that were plated in 6-well plates overnight were placed under reduced-serum conditions for 24 h and washed twice in 1 ml/well PBS (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4, 0.68 mM CaCl2, 0.49 mM MgCl2, pH 7.4). Cells were subsequently incubated in 1 ml/well PBS that contained 0.1 mM 2-deoxyglucose and 1 μCi/ml 2-deoxyD[3H]glucose for 5 min, washed 3 times in ice-cold PBS that contained 100 μM phloretin, and solubilized in 500 μl 1N NaOH. Whereas 10–15 min [3H]deoxyglucose uptake was previously used for TGF-β1–treated Swiss 3T3 fibroblasts (40), we found that 5 min gave more consistent/reproducible results. Detection of 3H was done by using a Beckman LS6500 scintillation counter (Beckman Coulter, Brea, CA, USA).
Cell-surface biotinylation
AKR-2B cells (2 × 106/10-cm plate) that were plated overnight and placed under reduced-serum conditions for 24 h were washed 3 times in ice-cold HBSS (Thermo Fisher Scientific) and incubated in HBSS that contained 0.25 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific) for 40 min at 4°C with gentle shaking. Excess biotin was quenched by washing twice in HBSS that contained 5 mM Tris, pH 7.5, for a total of 20 min at 4°C. Cells were harvested in 500 μl affinity purification buffer (50 mM Tris-HCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, pH 7.4) that was supplemented with Complete protease inhibitor cocktail (Sigma-Aldrich) and briefly sonicated. After protein extraction on ice, cleared lysates (equivalent to 2 × 106 cells) were incubated with 150 μl streptavidin-agarose resin slurry (Thermo Fisher Scientific) for 3 h on a rotator at 4°C. Beads were washed twice in affinity purification buffer (5 min at 4°C each wash) and twice in 50 mM Tris-HCl, 3% SDS, 5 mM EDTA, 5 mM EGTA, pH 7.4 (5 min at room temperature each wash) to remove copurifying nonbiotinylated proteins. Biotinylated proteins were eluted by adding 75 μl 4× Laemmli buffer.
Statistical analyses
Statistical analyses were performed by using single-factor ANOVA and/or unpaired 2-tailed Student’s t test; P values are provided in Supplemental Tables 2 and 3. Data are presented as means ± sem.
RESULTS
Profibrotic TGF-β signaling is dependent upon induction of GLUT1
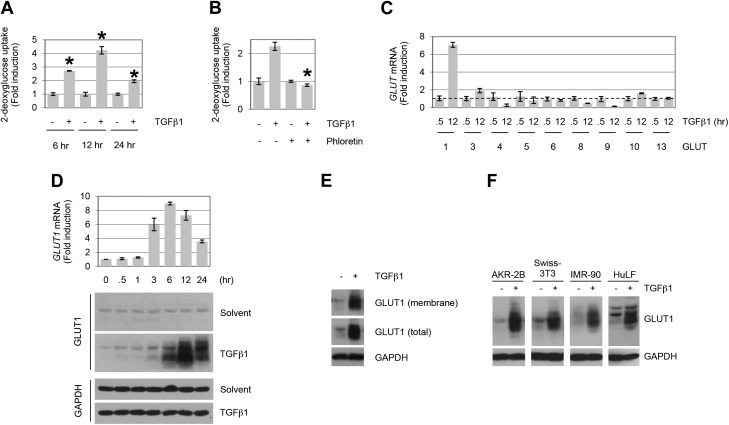
Although the interplay of metabolism and nutrient sensing has significantly impacted the oncologic literature since the pioneering work of Otto Warburg in the 1920s (44, 45), it is only recently that analogous approaches have begun to address this issue in fibrotic diseases (46–48). As the role of TGF-β in the pathogenesis of tissue fibrosis is critical, we initiated studies to investigate the relation between glucose uptake and profibrotic TGF-β signaling. Addition of TGF-β resulted in a time-dependent increase in glucose uptake that was sensitive to the class 1 and 2 glucose transporters inhibitor, phloretin (Fig. 1A, B). Consistent with those findings, quantitative PCR analysis of GLUTs 1–14 showed that, of those expressed in AKR-2B fibroblasts (i.e., GLUTs 1, 3, 4–6, 8–10, and 13), only the class 1 transporter GLUT1 was significantly induced by TGF-β (Fig. 1C, D). Western blot analysis of whole-cell lysates showed increased GLUT1 protein evident by 3–6 h TGF-β treatment (Fig. 1D), associated with the plasma membrane (Fig. 1E), and similarly regulated in an additional murine mesenchymal line as well as human lung fibroblasts (IMR-90 and HuLF; Fig. 1F).
Figure 1.
TGF-β stimulates GLUT1 up-regulation and glucose uptake in fibroblasts. A) AKR-2B cells were treated in the absence (−) or presence (+) of TGF-β1 (10 ng/ml), and 2-deoxyglucose uptake was determined at the indicated times (n = 3). B) Glucose uptake assay in TGF-β1–treated (12 h) AKR-2B fibroblast cells. Results show an increase in 2-deoxyglucose uptake, which is inhibited by the GLUT-specific inhibitor, phloretin (100 μM; n = 3). C) Quantitative RT-PCR analyses of GLUT induction by TGF-β1 in AKR-2B cells (n = 3). GLUT7 expression was not examined, whereas GLUTs 2 and 12 displayed no detectable expression. Dotted line indicates 1 unit on y axis. GLUTs 11 and 14 were not included, as no corresponding murine genes have been reported. The profiles of GLUT expression indicate that GLUT1 and GLUT3 exhibit >2-fold induction by TGF-β1, although only GLUT1 protein showed a parallel increase (data not shown for GLUT3 protein). D) AKR-2B cells were treated for the indicated times with TGF-β1 (10 ng/ml) or solvent (4 mM HCl, 1.0 mg/ml bovine serum albumin), and at the indicated times whole-cell lysates were prepared for quantitative PCR (top) or Western blot analysis (bottom) and blotted for GLUT1 or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data show a time-dependent up-regulation of GLUT1 mRNA and protein by TGF-β1 (n = 3). E) Subcellular localization of GLUT1 in AKR-2B fibroblasts. Cell-surface biotinylation reveals the presence of GLUT1 in the membrane fraction under basal and TGF-β–stimulated (12 h) conditions. F) Profiles of GLUT1 protein expression in human (IMR-90 and HuLF) and murine (AKR-2B and Swiss-3T3) fibroblasts stimulated with TGF-β1 (18 or 24 h for HuLF). Results demonstrate that TGF-β1 (10 ng/ml) triggers GLUT1 up-regulation in various fibroblast cells. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
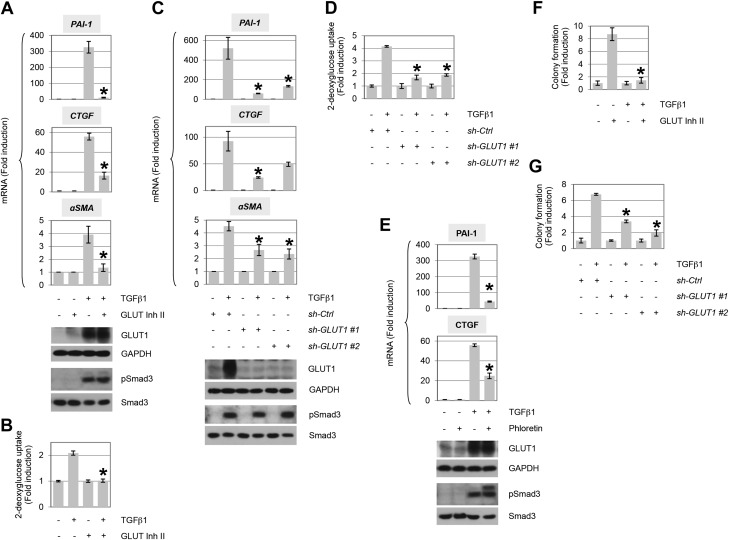
As GLUT1 has previously been determined to provide a regulatory, not facilitative, role in promoting prosclerotic events in diabetes (38), we investigated whether profibrotic TGF-β signaling was similarly dependent. GLUT1 activity was inhibited by treatment with GLUT inhibitor II and TGF-β induction of PAI-1, CTGF, and α-SMA determined by quantitative PCR (Fig. 2A). Whereas Smad3 phosphorylation was unaffected, each of the aforementioned target genes was significantly inhibited coincident with the abrogation of glucose uptake (Fig. 2A, B). In support of these pharmacologic findings, identical results were observed by using 2 distinct shRNAs to GLUT1 (Fig. 2C, D) or use of phloretin as an additional pharmacological inhibitor (Fig. 2E). Analogous findings were observed by Western blotting for extracellular matrix proteins, collagen 1α1 and fibronectin (Supplemental Fig. 1).
Figure 2.
Up-regulation of GLUT1 mediates TGF-β–induced profibrotic phenotype. A) AKR-2B cells were treated in the presence (+) or absence (−) of TGF-β1 (10 ng/ml) and/or the GLUT-specific GLUT inhibitor II (Inh II; 10 μM) for 12 h, and quantitative RT-PCR was performed for PAI-1, CTGF, or α−SMA (n = 3). Western blot of the indicated proteins (bottom). B) TGF-β1–stimulated 2-deoxyglucose uptake in presence or absence of GLUT Inh II (n = 3). C) Analogous study as in panel A, except AKR-2B cells were infected with nontargeting control (ctrl) or shRNA (1 or 2) to GLUT1 (n = 3). D) As in panel C, 2-deoxyglucose uptake in presence or absence of control or GLUT1 shRNA (n = 3). E) Similar study as in panel A, using the GLUT inhibitor phloretin (100 μM; n = 3). F, G) GLUT Inh II (F) or knockdown of GLUT1 by RNA interference (G) reduces soft agar colony formation induced by TGF-β1 (n = 3). GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
TGF-β was initially isolated and characterized by its ability to promote the anchorage-independent growth (AIG) of anchorage-dependent fibroblasts in soft agar (49, 50). To determine whether GLUT1 was similarly required for the fibroproliferative response to TGF-β, AIG was assessed in the presence or absence of GLUT inhibitor II or after shRNA knockdown of GLUT1. As shown in Fig. 2F, G, AIG was significantly reduced by inhibition of GLUT1 activity or expression, respectively. As the preceding data document a critical role for GLUT1 in mediating in vitro profibrotic actions of TGF-β, we next addressed whether in vivo expression of GLUT1 correlated with the degree of pulmonary fibrosis observed in a murine model or human tissue where TGF-β is known to be a primary driver (51–53). Because we have previously demonstrated that treatment with lapatinib plus imatinib significantly ameliorates murine lung fibrosis (26), Fig. 3A–D addresses 2 important questions. First, it documents that GLUT1 levels are increased in fibrotic murine lungs, and, second, it shows that GLUT1 expression is essentially reduced to basal after treatment with antifibrotic agents. Consistent with that observed in the bleomycin model of lung fibrosis, GLUT1 protein was significantly increased in fibrotic foci in human IPF tissue coincident with the degree of fibrosis (Fig. 3E–H).
Figure 3.
GLUT1 expression in mouse and human lung tissue. A–C) Histologic sections of nonfibrotic [saline:saline (SS), top], fibrotic [bleomycin:saline (BS), middle], and fibrosis dual-treated with imatinib (Imat) and lapatinib (Lap) [bleomycin:Imat + Lap (BIL), bottom] (26). Mouse lungs were stained with Masson’s trichrome (A), and GLUT1 antibodies were costained with hematoxylin (B). Color deconvoluted images of mouse lungs stained with GLUT1 (C) indicate the area and intensity of GLUT1 staining. Color-coded legend indicates intensity of GLUT1 expression. D) Quantification of GLUT1 expression in mouse lung tissues was significantly greater in fibrosis vs. normal or dual-treated lungs with Imat + Lap. Mean values of mouse tissues analyzed per condition ± sem (n = 3). E–G) Histologic sections of normal (top) and fibrotic human lungs (bottom) were stained with Masson’s trichrome (E), and GLUT1 antibodies were costained with hematoxylin (F). Arrows indicate the magnified section showing fibroblasts found in normal human lung (top) or fibrotic foci observed in IPF (bottom). Color deconvoluted images are shown of GLUT1-stained human lungs (G). H) Strong GLUT1 expression observed in fibrotic foci was significantly greater than fibroblasts in normal human lung. Mean values of human cases (normal, n = 7; fibrotic, n = 12) ± sem. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
TGF-β induction of GLUT1 requires input from both Smad and non-Smad pathways
The role of TGF-β in the pathogenesis of organ fibrosis is critical as it is considered a master switch as a result of its ability to also induce expression of other profibrotic mediators, including PDGF, endothelin 1, angiotensin II, CTGF, Wnt signaling, and microRNA expression, to name a few (15, 18, 54–59), which we recently extended to the ErbB family of cytokines (26, 27). As induction of GLUT1 protein was relatively late—not occurring until ∼6–9 h after TGF-β treatment (Fig. 1D)—it was next determined whether this reflected a requirement for other paracrine/autocrine acting cytokines. AKR-2B cells were treated with PDGF or ErbB1/2 receptor inhibitors, CP673451 or lapatinib, respectively, and expression of GLUT1 was determined after 12 h of TGF-β stimulation. As shown in Fig. 4A, B, whereas Smad3 phosphorylation was unaffected, inhibition of either PDGF or ErbB receptor activation prevented GLUT1 induction. These pharmacologic findings were further confirmed by using shRNA to either the α and β PDGF receptors (Fig. 4C) or ErbB1 and ErbB2 (Fig. 4D).
Figure 4.
Autocrine activation of receptor tyrosine kinases (RTKs) is required for induction of GLUT1 by TGF-β. Quantitative RT-PCR analysis of 10 ng/ml TGF-β1–stimulated GLUT1 expression (12 h post-treatment) in AKR-2B cells in the presence of the PDGFR-specific inhibitor, CP673451 (A; 2 µM), the ErbB1/2-specific inhibitor, lapatinib (B; 5 µM), shRNA specific for PDGFRα+β (C), or shRNA specific for ErbB1+2 (D; n = 3). Data indicate that both RTK pathways are important for GLUT1 induction by TGF-β. Western blots for the indicated protein to document drug or knockdown efficacy and no effect on Smad3 phosphorylation or expression (bottom). Ctrl, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IP, immunoprecipitation. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
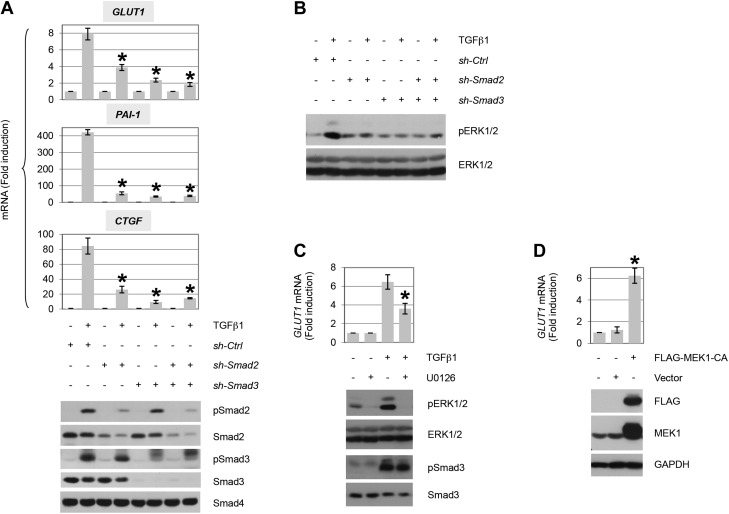
The primary mediators of TGF-β action are the Smad proteins, although non-Smad pathways have been shown to have a critical role often in a cell type–specific manner (7, 8, 11, 12, 60, 61). Whereas the preceding data demonstrate that Smad activation, per se, is not sufficient for GLUT1 induction by TGF-β, as Smad proteins directly regulate PDGF ligands (14–16) and ErbB ligand expression is dependent upon PDGF (15, 26), we further assessed the role of Smads in GLUT1 induction. Cultures were treated with shRNA that targeted Smad2, Smad3, or a non-targeting control, and the effect on GLUT1 induction by TGF-β was determined by quantitative PCR. Knockdown of either Smad2 or Smad3 resulted in a loss of GLUT1 expression (Fig. 5A) similar to that observed by inhibiting PDGF or ErbB receptor signaling (Fig. 4).
Figure 5.
TGF-β induces GLUT1 expression dependent upon Smad pathway activation of MEK. A) Quantitative RT-PCR analysis of GLUT1 and profibrotic gene induction by TGF-β1 (12 h post-treatment) in AKR-2B cells (pools) that stably express control (nontargeting; ctrl) or shRNA targeting Smad2 and/or Smad3 (n = 3). Results show that both Smad2 and Smad3 are required for TGF-β1–dependent GLUT1, PAI-1, and CTGF up-regulation. Western blot analyses confirm efficient knockdown of Smad2/Smad3 and uniform activation of Smad2 and/or Smad3 in appropriate controls (5 h). B) Western blot analysis of ERK1/2 activation by TGF-β1 (5 h post-treatment) in AKR-2B cells (pools) that stably express control (nontargeting) or shRNA targeting Smad2 and/or Smad3. Results indicate that TGF-β1 activates ERK1/2 downstream of the Smad2/3 pathway. C) Quantitative RT-PCR analysis of GLUT1 induction by TGF-β1 (12 h post-treatment) in AKR-2B cells in the absence (−) or presence (+) of the MEK-ERK1/2 inhibitor U0126 (3 μM; n = 3). Prevention of ERK1/2 phosphorylation (5 h) confirms the efficacy of U0126, whereas the lack of effect on pSmad3 documents specificity. D) Quantitative RT-PCR analysis of GLUT1 induction by ectopic expression of a constitutively active MEK1 (54 h post-transfection) in AKR-2B cells (n = 3). Constitutive activation of MEK is shown to trigger up-regulation of GLUT1. TGF-β1 was used at 10 ng/ml. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
Previous studies have documented a role for ERK, a common downstream effector of EGF and PDGF receptors (62, 63), in GLUT1 synthesis (38, 64–66). Because TGF-β induction of GLUT1 was similarly dependent upon both PDGF and ErbB receptor signaling (Fig. 4), and because ERK activation can be observed 9–12 h after TGF-β addition (i.e., requiring the induction of paracrine/autocrine factors, such as PDGF or ErbB ligands) (26), we addressed the following mechanistic questions: first, would ERK play a similar role in GLUT1 expression stimulated by TGF-β?; and second, as PDGF and ErbB receptors interface with a number of targets (67–69), what other signaling pathways might be required?
Because GLUT1 induction was dependent upon Smad2/3 signaling (Fig. 5A), we first investigated whether ERK activation showed a similar requirement. As shown in Fig. 5B, knockdown of Smad2 or Smad3 reduced ERK1/2 phosphorylation by TGF-β. ERK activation is dependent upon the upstream kinase MEK (62, 63). As we previously determined that the MEK inhibitor, U0126, prevented expression of ErbB ligands (26), its effect on GLUT1 expression was examined. Consistent with a requirement for ErbB ligands in TGF-β–stimulated GLUT1 induction (Fig. 4B, D), whereas Smad3 phosphorylation was unaffected, loss in GLUT1 message was observed coincident with the inhibition of ERK1/2 phosphorylation (Fig. 5C). As MEK functioned as a central mediator of GLUT1 induction, we determined whether activation of MEK alone would be sufficient. Consistent with that hypothesis, expression of a constitutively active MEK1 was able to up-regulate GLUT1 (Fig. 5D).
GLUT1 induction by TGF-β is dependent upon mTORC2
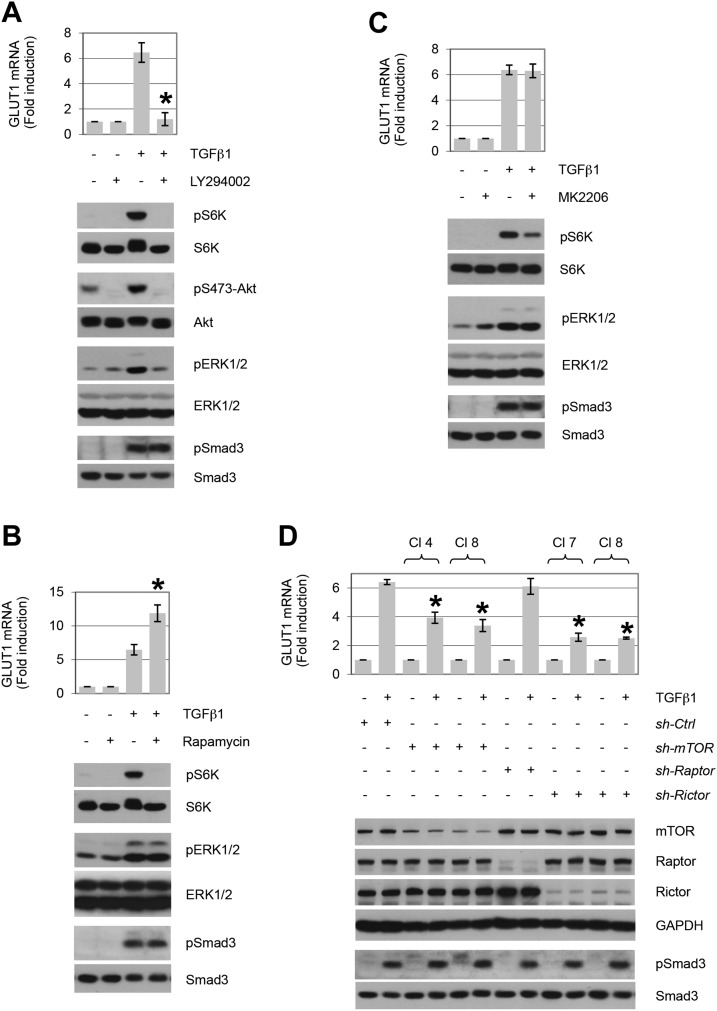
Previous studies have determined that PI3K, Akt, and mTOR reflect critical non-Smad targets that are necessary for TGF-β’s profibrotic actions and epithelial-mesenchymal transition generation (21, 23, 70, 71). As each of these pathways are activated by TGF-β induction of the autocrine/paracrine cytokines, PDGF and ErbB ligands, their role in GLUT1 expression was investigated. AKR-2B cells were treated with PI3K, Akt, or mTOR inhibitors and the induction of GLUT1 mRNA in response to TGF-β was examined by quantitative PCR. Whereas inhibition of PI3K significantly diminished GLUT1 expression (Fig. 6A), loss of mTOR phosphorylation of S6 kinase with rapamycin or Akt-dependent mTOR activity was without effect (Fig. 6B, C).
Figure 6.
Up-regulation of GLUT1 by TGF-β requires the cooperation of multiple PI3K-dependent signaling pathways. Quantitative RT-PCR analysis of TGF-β1–stimulated GLUT1 expression (12 h post-treatment) in AKR-2B cells in the presence (+) of the PI3K-specific inhibitor, LY294002 (A; 20 µM), mTORC1-specific inhibitor, rapamycin (B; 10 nM), the Akt-specific inhibitor, MK22006 (C; 300 nM), or shRNA specific clones (CI) for mTOR, Raptor, or Rictor (D; n = 3). Western blot analyses were performed on samples harvested at 5 h (A–C) or 12 h (D) post–TGF-β treatment. Data show that inhibition of PI3K abrogates induction of GLUT1 by TGF-β (A). Whereas interfering with mTORC1 (B, D; sh-Raptor) or Akt (C) activity does not prevent TGF-β1–induced GLUT1 expression, knockdown of mTOR and Rictor is inhibitory. TGF-β1 was used at 10 ng/ml. Ctrl, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Asterisks denote statistical significance. Supplemental Tables 2 and 3 provide P values for indicated data points.
mTOR exists in 2 complexes referred to as mTORC1 and mTORC2 (72). Whereas mTORC1 is inhibited by rapamycin as a result of the rapamycin-sensitive Raptor component, we previously determined that the analogous constituent Rictor in mTORC2 renders AKR-2B cells insensitive to both acute and prolonged (24 h) rapamycin treatment (21). Because mTORC2 has also been shown to be necessary for profibrotic TGF-β signaling (21), we further examined whether mTOR had any role in GLUT1 induction by TGF-β by using shRNA to mTOR, Raptor, or Rictor. As shown in Fig. 6D, knockdown of either mTOR or Rictor significantly decreased GLUT1 expression, whereas absence of Raptor, as expected (Fig. 6B), had no demonstrable effect. A model that integrates GLUT1 expression with the known Smad and non-Smad components that regulate the fibroproliferative actions of TGF-β is presented in Fig. 7.
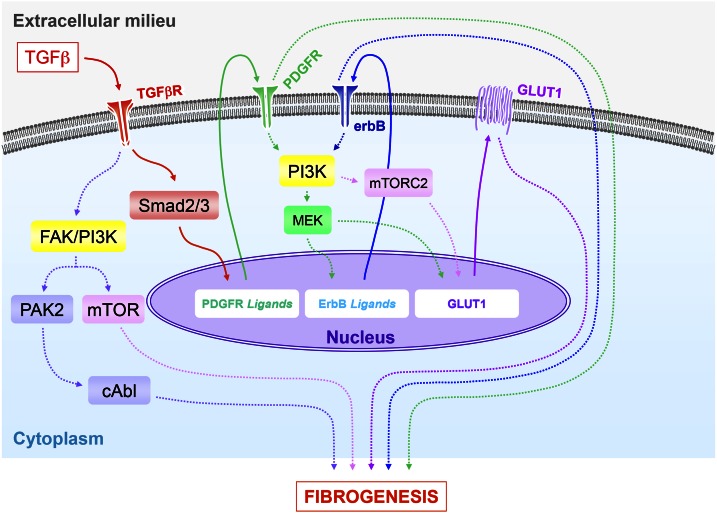
Figure 7.
Proposed model for the profibrotic regulation of GLUT1 expression by TGF-β. This model incorporates the cooperative action of multiple signaling pathways in regulating GLUT1 induction by TGF-β and their subsequent integration in profibrotic TGF-β signaling. Engagement of the TGF-β receptor complex activates the canonical Smad pathway, which leads to up-regulation of PDGFR ligands. Subsequent PDGFR phosphorylation promotes activation of MEK/ERK and induction of ErbB ligand expression and cognate receptor activation (26, 27). Activation of the MEK/ERK and mTORC2 pathways downstream of PI3K promotes up-regulation of GLUT1, whose activity is necessary for the profibrotic effects of TGF-β. The Smad-dependent pathways (PDGFR, ErbB, and GLUT1), in concert with the noncanonical TGF-β–activated signaling modules, p21-activated kinase 2 (PAK2)/cAbl and PI3K/AKT/mTOR (19–23, 25, 85), all contribute to the fibrogenic program directed by TGF-β.
DISCUSSION
The present study provides new insights into the molecular basis of TGF-β–driven fibrogenesis, a process that is central to the pathogenesis of fibrotic disorders (6, 7). Our results highlight the importance of enhanced glucose uptake (via GLUT1 up-regulation) in mediating profibrotic TGF-β signaling and expose the complex interplay of multiple signaling pathways regulating GLUT1 induction (Fig. 7). These findings offer opportunities for the development of new strategies for therapeutic intervention.
Pathogenic up-regulation of GLUTs (GLUT1 in most cases) and increased glucose uptake and metabolism have been associated with a number of diseases, including diabetic nephropathy (38, 73–75) and various types of malignancies (34–37). Our current data support a role for TGF-β–driven GLUT1 up-regulation in the promotion of lung fibrogenesis, for which the proposed regulatory mechanisms are consistent with our previous observations (26, 27). In contrast, a 2013 publication that focused on erythrocytes and inflammatory cells from normal patients and those with IPF by using GLUT1 immunofluorescence suggested GLUT1 is absent in fibroblasts, as their anti-GLUT1 antibody did not react with fibroblasts in either normal or IPF samples (76). Our immunohistochemistry results, however, indicate increased fibroblast expression of GLUT1 in IPF fibrotic foci vs. normal human fibroblasts (Fig. 3). This is supported by multiple negative controls, which confirm the specificity of our GLUT1 and secondary antibodies in fibrotic and normal tissues (Supplemental Figs. 2 and 3). Moreover, our findings are in agreement with a recent report that emphasized the role of glycolytic reprogramming in mediating myofibroblast differentiation in lung fibroblasts and bleomycin-induced lung fibrosis in mice (77). Thus, approaches to interfere with cellular pathways that contribute to enhanced glycolysis may hold promise in treating lung fibrosis and other fibroproliferative disorders.
As glucose transport across the plasma membrane is the first rate-limiting step in glucose metabolism, mechanisms that regulate GLUT expression and subcellular distribution in the contexts of various pathologies have been the subject of intensive investigation. Multiple proliferative and prosurvival signaling pathways emanating from growth factor receptors [e.g., PI3K-Akt, hypoxia-inducible factor 1α (HIF1α), Ras, c-Myc, and p53] have been implicated in the regulation of various GLUTs, including GLUT1 (reviewed in refs. 34, 38, 75, 78). Our study extends these observations to the cooperative action of the MEK-ERK1/2 and mTORC2 pathways in mediating GLUT1 up-regulation by TGF-β; although inhibition of either significantly reduces GLUT1 expression, it is not sufficient to prevent induction (Figs. 5C and 6D). Thus, as depicted in Fig. 7, there are multiple Smad-dependent and -independent pathways regulated by TGF-β that are capable of impacting GLUT1 either directly or via induction of other profibrotic mediators. Although this clearly depicts the inherent complexity in defining the operative signaling pathways, it similarly indicates new avenues for potential therapies. Of note, as mTORC2 has recently been reported to stimulate GLUT1 plasma membrane translocation subsequent to β3-adrenoreceptor stimulation in brown fat cells (79), these findings further emphasize the various cell type–dependent mechanisms by which this critical transporter is regulated. In that regard, elevated expression of HIF1α has been commonly associated with GLUT1 up-regulation (80, 81), and TGF-β has previously been shown to stimulate the production and/or activity of HIF1α (82–84). However, we were unable to observe induction of this transcription factor by TGF-β, either at the mRNA or protein level (data not shown).
Our current findings further document the myriad interconnected pathways and mechanisms that mediate profibrotic TGF-β signaling. Moreover, they provide support to the relatively underappreciated role of metabolism in fibroproliferative disorders (47), as glucose transport reflects an activity that impacts, essentially, all cellular functions. Our demonstration that TGF-β regulates both GLUT1 expression and plasma membrane translocation via both Smad-dependent and -independent pathways (Fig. 7) provides the opportunity for therapeutic interventions at multiple locales and targets.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants GM-55816 and GM-54200 from the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences, the Caerus Foundation, and the Mayo Foundation (to E.B.L.). A majority of IPF cases were obtained through the NIH National Heart Lung and Blood Lung Tissue Research Consortium (Contract No. 268201100020C-7-0-1). The authors declare no conflicts of interest.
Glossary
- AIG
anchorage-independent growth
- CTGF
connective tissue growth factor
- DAB
3,3′-diaminobenzidine
- FBS
fetal bovine serum
- GLUT
glucose transporter
- HIF1α
hypoxia-inducible factor 1α
- HuLF
human lung fibroblast
- IPF
idiopathic pulmonary fibrosis
- mTOR
mammalian target of rapamycin
- mTORC
mammalian target of rapamycin complex
- PAI-1
plasminogen activator inhibitor 1
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- shRNA
short hairpin RNA
- α-SMA
α-smooth muscle actin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Andrianifahanana and E. B. Leof designed the research and wrote the paper; M. Andrianifahanana, D. M. Hernandez, X. Yin, J.-H. Kang, M.-Y. Jung, and Y. Wang performed research; A. H. Limper confirmed clinical patient diagnoses; E. S. Yi and A. C. Roden provided pathologic analysis; and all authors analyzed the data and revised the paper.
REFERENCES
- 1.Pinzani M., Vizzutti F. (2008) Fibrosis and cirrhosis reversibility: clinical features and implications. Clin. Liver Dis. 12, 901–913 [DOI] [PubMed] [Google Scholar]
- 2.Sivakumar P., Ntolios P., Jenkins G., Laurent G. (2012) Into the matrix: targeting fibroblasts in pulmonary fibrosis. Curr. Opin. Pulm. Med. 18, 462–469 [DOI] [PubMed] [Google Scholar]
- 3.Todd N. W., Luzina I. G., Atamas S. P. (2012) Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn T. A. (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn T. A., Ramalingam T. R. (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakerakanti S., Trojanowska M. (2012) The role of TGF-β receptors in fibrosis. Open Rheumatol. J. 6, 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi R. A., Leof E. B. (2007) TGF-beta signaling: a tale of two responses. J. Cell. Biochem. 102, 593–608 [DOI] [PubMed] [Google Scholar]
- 8.Prud’homme G. J. (2007) Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Invest. 87, 1077–1091 [DOI] [PubMed] [Google Scholar]
- 9.De Caestecker M. (2004) The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 15, 1–11 [DOI] [PubMed] [Google Scholar]
- 10.Ross S., Hill C. S. (2008) How the Smads regulate transcription. Int. J. Biochem. Cell Biol. 40, 383–408 [DOI] [PubMed] [Google Scholar]
- 11.Feng X.-H., Derynck R. (2005) Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y. E. (2009) Non-Smad pathways in TGF-beta signaling. Cell Res. 19, 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu Y., Gudey S. K., Landström M. (2012) Non-Smad signaling pathways. Cell Tissue Res. 347, 11–20 [DOI] [PubMed] [Google Scholar]
- 14.Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. (1986) Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc. Natl. Acad. Sci. USA 83, 2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor L. M., Khachigian L. M. (2000) Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J. Biol. Chem. 275, 16709–16716 [DOI] [PubMed] [Google Scholar]
- 16.Daniel T. O., Gibbs V. C., Milfay D. F., Williams L. T. (1987) Agents that increase cAMP accumulation block endothelial c-sis induction by thrombin and transforming growth factor-β. J. Biol. Chem. 262, 11893–11896 [PubMed] [Google Scholar]
- 17.Chen Y., Blom I. E., Sa S., Goldschmeding R., Abraham D. J., Leask A. (2002) CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 62, 1149–1159 [DOI] [PubMed] [Google Scholar]
- 18.Phanish M. K., Wahab N. A., Colville-Nash P., Hendry B. M., Dockrell M. E. (2006) The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem. J. 393, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkes M. C., Leof E. B. (2006) Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J. Biol. Chem. 281, 27846–27854 [DOI] [PubMed] [Google Scholar]
- 20.Wilkes M. C., Murphy S. J., Garamszegi N., Leof E. B. (2003) Cell-type-specific activation of PAK2 by transforming growth factor β independent of Smad2 and Smad3. Mol. Cell. Biol. 23, 8878–8889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahimi R. A., Andrianifahanana M., Wilkes M. C., Edens M., Kottom T. J., Blenis J., Leof E. B. (2009) Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-β. Cancer Res. 69, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong M., Wilkes M. C., Penheiter S. G., Gupta S. K., Edens M., Leof E. B. (2011) Non-Smad transforming growth factor-β signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 286, 17841–17850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkes M. C., Mitchell H., Penheiter S. G., Doré J. J., Suzuki K., Edens M., Sharma D. K., Pagano R. E., Leof E. B. (2005) Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 65, 10431–10440 [DOI] [PubMed] [Google Scholar]
- 24.Kolosova I., Nethery D., Kern J. A. (2011) Role of Smad2/3 and p38 MAP kinase in TGF-β1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J. Cell. Physiol. 226, 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K., Wilkes M. C., Garamszegi N., Edens M., Leof E. B. (2007) Transforming growth factor beta signaling via Ras in mesenchymal cells requires p21-activated kinase 2 for extracellular signal-regulated kinase-dependent transcriptional responses. Cancer Res. 67, 3673–3682 [DOI] [PubMed] [Google Scholar]
- 26.Andrianifahanana M., Wilkes M. C., Gupta S. K., Rahimi R. A., Repellin C. E., Edens M., Wittenberger J., Yin X., Maidl E., Becker J., Leof E. B. (2013) Profibrotic TGFβ responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J. 27, 4444–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrianifahanana M., Wilkes M. C., Repellin C. E., Edens M., Kottom T. J., Rahimi R. A., Leof E. B. (2010) ERBB receptor activation is required for profibrotic responses to transforming growth factor beta. Cancer Res. 70, 7421–7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L. Q., Cheung L. S., Feng L., Tanner W., Frommer W. B. (2015) Transport of sugars. Annu. Rev. Biochem. 84, 865–894 [DOI] [PubMed] [Google Scholar]
- 29.Mueckler M., Thorens B. (2013) The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 34, 121–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorens B. (1996) Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am. J. Physiol. 270, G541–G553 [DOI] [PubMed] [Google Scholar]
- 31.Thorens B., Mueckler M. (2010) Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 298, E141–E145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright E. M., Loo D. D., Hirayama B. A., Turk E. (2004) Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda) 19, 370–376 [DOI] [PubMed] [Google Scholar]
- 33.Wright E. M., Loo D. D., Panayotova-Heiermann M., Lostao M. P., Hirayama B. H., Mackenzie B., Boorer K., Zampighi G. (1994) ‘Active’ sugar transport in eukaryotes. J. Exp. Biol. 196, 197–212 [DOI] [PubMed] [Google Scholar]
- 34.Macheda M. L., Rogers S., Best J. D. (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell. Physiol. 202, 654–662 [DOI] [PubMed] [Google Scholar]
- 35.Pereira K. M., Chaves F. N., Viana T. S., Carvalho F. S., Costa F. W., Alves A. P., Sousa F. B. (2013) Oxygen metabolism in oral cancer: HIF and GLUTs (Review). Oncol. Lett. 6, 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szablewski L. (2013) Expression of glucose transporters in cancers. Biochim. Biophys. Acta 1835, 164–169 [DOI] [PubMed] [Google Scholar]
- 37.Zhao F. Q. (2014) Biology of glucose transport in the mammary gland. J. Mammary Gland Biol. Neoplasia 19, 3–17 [DOI] [PubMed] [Google Scholar]
- 38.Heilig C. W., Deb D. K., Abdul A., Riaz H., James L. R., Salameh J., Nahman N. S. Jr (2013) GLUT1 regulation of the pro-sclerotic mediators of diabetic nephropathy. Am. J. Nephrol. 38, 39–49 [DOI] [PubMed] [Google Scholar]
- 39.Wu L., Derynck R. (2009) Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev. Cell 17, 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitagawa T., Masumi A., Akamatsu Y. (1991) Transforming growth factor-beta 1 stimulates glucose uptake and the expression of glucose transporter mRNA in quiescent Swiss mouse 3T3 cells. J. Biol. Chem. 266, 18066–18071 [PubMed] [Google Scholar]
- 41.Inoki K., Haneda M., Maeda S., Koya D., Kikkawa R. (1999) TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 55, 1704–1712 [DOI] [PubMed] [Google Scholar]
- 42.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 43.Kozma L., Baltensperger K., Klarlund J., Porras A., Santos E., Czech M. P. (1993) The ras signaling pathway mimics insulin action on glucose transporter translocation. Proc. Natl. Acad. Sci. USA 90, 4460–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburg O. (1956) On respiratory impairment in cancer cells. Science 124, 269–270 [PubMed] [Google Scholar]
- 45.Warburg O., Wind F., Negelein E. (1927) The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackwell T. S., Tager A. M., Borok Z., Moore B. B., Schwartz D. A., Anstrom K. J., Bar-Joseph Z., Bitterman P., Blackburn M. R., Bradford W., Brown K. K., Chapman H. A., Collard H. R., Cosgrove G. P., Deterding R., Doyle R., Flaherty K. R., Garcia C. K., Hagood J. S., Henke C. A., Herzog E., Hogaboam C. M., Horowitz J. C., King T. E. Jr., Loyd J. E., Lawson W. E., Marsh C. B., Noble P. W., Noth I., Sheppard D., Olsson J., Ortiz L. A., O’Riordan T. G., Oury T. D., Raghu G., Roman J., Sime P. J., Sisson T. H., Tschumperlin D., Violette S. M., Weaver T. E., Wells R. G., White E. S., Kaminski N., Martinez F. J., Wynn T. A., Thannickal V. J., Eu J. P. (2014) Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am. J. Respir. Crit. Care Med. 189, 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottmann R. M., Kulkarni A. A., Smolnycki K. A., Lyda E., Dahanayake T., Salibi R., Honnons S., Jones C., Isern N. G., Hu J. Z., Nathan S. D., Grant G., Phipps R. P., Sime P. J. (2012) Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am. J. Respir. Crit. Care Med. 186, 740–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L., Roh Y. S., Song J., Zhang B., Liu C., Loomba R., Seki E. (2014) Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology 59, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses H. L., Branum E. L., Proper J. A., Robinson R. A. (1981) Transforming growth factor production by chemically transformed cells. Cancer Res. 41, 2842–2848 [PubMed] [Google Scholar]
- 50.Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. (1981) New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc. Natl. Acad. Sci. USA 78, 5339–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamson I. Y., Bowden D. H. (1974) The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 77, 185–197 [PMC free article] [PubMed] [Google Scholar]
- 52.Moseley P. L., Hemken C., Hunninghake G. W. (1986) Augmentation of fibroblast proliferation by bleomycin. J. Clin. Invest. 78, 1150–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sime P. J., O’Reilly K. M. (2001) Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin. Immunol. 99, 308–319 [DOI] [PubMed] [Google Scholar]
- 54.Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M. O., MacDougald O. A., Distler O., Schett G., Distler J. H. (2012) Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 3, 735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leask A. (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 106, 1675–1680 [DOI] [PubMed] [Google Scholar]
- 56.Verrecchia F., Mauviel A. (2007) Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 13, 3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie T., Liang J., Guo R., Liu N., Noble P. W., Jiang D. (2011) Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol. Genomics 43, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruna A., Darken R. S., Rojo F., Ocaña A., Peñuelas S., Arias A., Paris R., Tortosa A., Mora J., Baselga J., Seoane J. (2007) High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11, 147–160 [DOI] [PubMed] [Google Scholar]
- 59.Rosenbloom J., Castro S. V., Jimenez S. A. (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 152, 159–166 [DOI] [PubMed] [Google Scholar]
- 60.Derynck R., Akhurst R. J. (2007) Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 9, 1000–1004 [DOI] [PubMed] [Google Scholar]
- 61.Massagué J., Blain S. W., Lo R. S. (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103, 295–309 [DOI] [PubMed] [Google Scholar]
- 62.Morrison D. K., Davis R. J. (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19, 91–118 [DOI] [PubMed] [Google Scholar]
- 63.Ramos J. W. (2008) The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol. 40, 2707–2719 [DOI] [PubMed] [Google Scholar]
- 64.Harris V. M., Bendre S. V., Gonzalez De Los Santos F., Fite A., El-Yaman El-Dandachli A., Kurenbekova L., Abou-Samra A. B., Buggs-Saxton C. (2012) GnRH increases glucose transporter-1 expression and stimulates glucose uptake in the gonadotroph. J. Endocrinol. 212, 139–147 [DOI] [PubMed] [Google Scholar]
- 65.Nose A., Mori Y., Uchiyama-Tanaka Y., Kishimoto N., Maruyama K., Matsubara H., Iwasaka T. (2003) Regulation of glucose transporter (GLUT1) gene expression by angiotensin II in mesangial cells: involvement of HB-EGF and EGF receptor transactivation. Hypertens. Res. 26, 67–73 [DOI] [PubMed] [Google Scholar]
- 66.Santalucía T., Christmann M., Yacoub M. H., Brand N. J. (2003) Hypertrophic agonists induce the binding of c-Fos to an AP-1 site in cardiac myocytes: implications for the expression of GLUT1. Cardiovasc. Res. 59, 639–648 [DOI] [PubMed] [Google Scholar]
- 67.Tallquist M., Kazlauskas A. (2004) PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 15, 205–213 [DOI] [PubMed] [Google Scholar]
- 68.Yarden Y. (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37, S3–S8 [DOI] [PubMed] [Google Scholar]
- 69.Yarden Y., Sliwkowski M. X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 [DOI] [PubMed] [Google Scholar]
- 70.Bhowmick N. A., Ghiassi M., Bakin A., Aakre M., Lundquist C. A., Engel M. E., Arteaga C. L., Moses H. L. (2001) Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamouille S., Derynck R. (2007) Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 178, 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guertin D. A., Sabatini D. M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 73.Garud M. S., Kulkarni Y. A. (2014) Hyperglycemia to nephropathy via transforming growth factor beta. Curr. Diabetes Rev. 10, 182–189 [DOI] [PubMed] [Google Scholar]
- 74.Rizvi S., Raza S. T., Mahdi F. (2014) Association of genetic variants with diabetic nephropathy. World J. Diabetes 5, 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schena F. P., Gesualdo L. (2005) Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 16(Suppl 1), S30–S33 [DOI] [PubMed] [Google Scholar]
- 76.El-Chemaly S., Malide D., Yao J., Nathan S. D., Rosas I. O., Gahl W. A., Moss J., Gochuico B. R. (2013) Glucose transporter-1 distribution in fibrotic lung disease: association with [18¹⁸F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest 143, 1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie N., Tan Z., Banerjee S., Cui H., Ge J., Liu R. M., Bernard K., Thannickal V. J., Liu G. (2015) Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 192, 1462–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barron C. C., Bilan P. J., Tsakiridis T., Tsiani E. (2016) Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism 65, 124–139 [DOI] [PubMed] [Google Scholar]
- 79.Olsen J. M., Sato M., Dallner O. S., Sandström A. L., Pisani D. F., Chambard J. C., Amri E. Z., Hutchinson D. S., Bengtsson T. (2014) Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol. 207, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marín-Hernández A., Gallardo-Pérez J. C., Ralph S. J., Rodríguez-Enríquez S., Moreno-Sánchez R. (2009) HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev. Med. Chem. 9, 1084–1101 [DOI] [PubMed] [Google Scholar]
- 81.Masoud G. N., Li W. (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 5, 378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basu R. K., Hubchak S., Hayashida T., Runyan C. E., Schumacker P. T., Schnaper H. W. (2011) Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am. J. Physiol. Renal Physiol. 300, F898–F905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanna C., Hubchak S. C., Liang X., Rozen-Zvi B., Schumacker P. T., Hayashida T., Schnaper H. W. (2013) Hypoxia-inducible factor-2α and TGF-β signaling interact to promote normoxic glomerular fibrogenesis. Am. J. Physiol. Renal Physiol. 305, F1323–F1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueno M., Maeno T., Nomura M., Aoyagi-Ikeda K., Matsui H., Hara K., Tanaka T., Iso T., Suga T., Kurabayashi M. (2011) Hypoxia-inducible factor-1α mediates TGF-β-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L740–L752 [DOI] [PubMed] [Google Scholar]
- 85.Daniels C. E., Wilkes M. C., Edens M., Kottom T. J., Murphy S. J., Limper A. H., Leof E. B. (2004) Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]