Abstract
Radiation-induced cardiovascular disease is an emerging problem in a steadily increasing population of survivors of cancer. However, the underlying biology is poorly described, and the late onset, which occurs several years after exposure, precludes adequate investigations in animal and cell culture models. We investigated the role of the 5-lipoxygenase (5-LO)/leukotriene pathway in radiation-induced vascular changes. Use of paired samples of irradiated arteries and nonirradiated internal control arteries from the same patient that were harvested during surgery for cancer reconstruction ≤10 yr after radiotherapy provides a unique human model of chronic radiation–induced vascular changes. Immunohistochemical stainings and perioperative inspection revealed an adventitial inflammatory response, with vasa vasorum expansion and chronic infiltration of CD68+ macrophages. These macrophages stained positive for the leukotriene-forming enzyme 5-LO. Messenger RNA levels of 5-LO and leukotriene B4 receptor 1 were increased in irradiated arterial segments compared with control vessels. These results point to targeting the 5-LO/leukotriene pathway as a therapeutic adjunct to prevent late adverse vascular effects of radiotherapy.—Halle, M., Christersdottir, T., Bäck, M. Chronic adventitial inflammation, vasa vasorum expansion, and 5-lipoxygenase up-regulation in irradiated arteries from cancer survivors.
Keywords: radiotherapy, cardiovascular disease, leukotrienes
Life expectancy for patients with cancer is steadily improving, with an age-adjusted 10-y survival of 70% for breast cancer and 80% for Hodgkin’s lymphoma. Treatment-related long-term complications, such as secondary malignancies and cardiovascular disease (CVD), have therefore become an increasing problem in a rapidly growing population of survivors of cancer (1, 2). Epidemiologic studies have shown that cancer radiotherapy (XRT) increases the risk of localized CVD. Incidence of myocardial infarction increases after left- vs. right-sided breast cancer irradiation (3) and thorax irradiation for Hodgkin’s lymphoma (4). An increased risk of stroke is also seen after previous neck and brain irradiation (5). However, there is still a paucity of evidence regarding the underlying pathophysiology, as the late onset—several years after exposure—precludes adequate investigation in animal and cell culture models. We recently established the Biobank of Irradiated Tissues at Karolinska to study previously irradiated human conduits from the carotid artery vs. internal controls (6). The model provides a unique opportunity to study the long-term effects of irradiation on human arteries, for which we have identified a sustained NF-κB activation several years after XRT (6).
Studies of coronary brachytherapy have indicated that acute radiation-induced vascular injury involves affection of the adventitia, but long-term effects of external radiation on human conduit vessels have remained largely unexplored in human participants (5). Only a few studies in experimental animal models have observed the short-term effects on adventitia after radiation exposure (5, 7–9), but only 1 study has investigated the long-term effects (10). The adventitia is a primary site for early vessel wall response to arterial injury at the luminal side (11), which includes inflammatory cell accumulation and expansion of the vasa vasorum (12).
Leukotrienes are proinflammatory lipid mediators that are derived from the 5-lipoxygenase (5-LO) pathway of arachidonic acid metabolism. We and others have shown the proinflammatory properties of leukotrienes in the pathogenesis and progression of CVD (13, 14), in which 5-LO activity in the adventitia seems to be of particular importance in progressive arterial disease (15, 16). However, the role of the lipoxygenase pathway has not been previously explored in the context of radiation-induced CVD.
On the basis of the above, we hypothesized that 5-LO may drive a persistent chronic inflammation in the vasculature of patients with a medical history of previous XRT. Therefore, we compared irradiated human arteries with intraindividual nonirradiated control arteries, with the aim of unravelling the role of proinflammatory 5-LO activation in radiation-induced vascular changes. Results point to the importance of adventitial chronic inflammation, vasa vasorum expansion, and a therapeutic potential for targeting the 5-LO pathway in cardiovascular prevention after XRT.
MATERIALS AND METHODS
Human tissue specimens
Fifty-six arterial biopsies were harvested during head and neck cancer reconstruction in 28 patients who had been previously irradiated. Before performing microvascular anastomosis, biopsies were harvested from the irradiated branch of the external carotid artery and from the equally sized nonirradiated recipient artery from autologous free tissue transfer (6). Biopsies were freed from the surrounding tissue and surgical material under a dissection in microscope. Care was taken to ensure that the endothelium and adventitia were not damaged during tissue preparation. Samples were collected from Karolinska University Hospital. The study was approved by the Ethical Committee of Stockholm and was performed in agreement with institutional guidelines and the principles of the Declaration of Helsinki. All enrolled participants gave informed consent.
RNA extraction
Immediately after surgical excision, biopsies were placed in RNA later (Qiagen, Hilden, Germany), frozen, and stored at –80°C until RNA extraction. Demographic data, including XRT dosages, for 19 of 20 patients who donated arterial segments used for RNA extraction are listed in Table 1 (demographic data retrieval was not possible for 1 patient).
TABLE 1.
Patient demographics for mRNA studies
| Age (yr)/sex | XRT dose (Gy) | Time after XRT (wk)a | Current smoking | Prevalent CVD | Control vessels |
|---|---|---|---|---|---|
| 60/maleb | 64 | 500 | No | None | Forearm |
| 77/female | 54 | 5 | Yes | Past MI and CVL | Forearm |
| 47/femaleb | 68 | 7 | No | None | Forearm |
| 68/male | 64 | 48 | No | Hypertension | Forearm |
| 60/maleb | 50 | 6 | No | Hypertension | Fibula |
| 63/male | 68 | 9 | No | None | Fibula |
| 64/male | 64 | 2100 | No | Hypertension | Fibula |
| 48/male | 64 | 146 | No | None | Fibula |
| 59/maleb | 68 | 139 | No | Past CVL | Fibula |
| 49/maleb | 54 | 5 | Yes | None | Forearm |
| 50/female | 60.3 | 22 | No | None | Forearm |
| 50/male | 64 | 90 | No | None | Forearm |
| 59/maleb | 54 | 7 | Yes | None | Forearm |
| 39/femaleb | 54 | 6 | No | None | Forearm |
| 30/male | 68 | 14 | No | None | Forearm |
| 54/male | 68 | 271 | No | None | Fibula |
| 73/female | 64 | 170 | No | None | Fibula |
| 62/male | 68 | 290 | No | Hypertension | Fibula |
| 63/female | 66 | 550 | Yes | None | Thigh |
CVL, cerebrovascular lesion; MI, myocardial infarction.
Time from last XRT session to biopsy.
Samples not analyzed for BLT1 expression as a result of insufficient mRNA quantity.
Extraction of RNA was performed by using the RNeasy Mini kit (Qiagen), including an on-column DNase digestion step. RNA quality was analyzed by microcapillary electrophoresis (Agilent 2100 Bioanalyzer; Agilent Technologies, Santa Clara, CA, USA), whereas RNA quantification was determined by UV spectrophotometry (NanoDrop ND-1000 UV-Vis Spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instructions. cDNA was synthesized from total RNA by using SuperScript II reverse transcriptase (Thermo Fisher Scientific). RNA and cDNA were stored at –80°C.
Gene expression
Leukotriene-associated genes were identified as target genes of interest in the context of expanded vasa vasorum observed in perioperative microscopic photographs of irradiated vs. nonirradiated arteries (Fig. 1). Differential expression in target genes, 5-LO (ALOX5, Hs01095330_m1), leukotriene B4 receptor 1 (BLT1; Hs00609525_m1), and the housekeeping gene, phosphoglycerolkinase 1 (Hs99999906_m1), was analyzed by real-time PCR (TaqMan; Applied Biosystems, Foster City, CA, USA). Selection of housekeeping gene was performed after using a TaqMan Endogenous Control Plate as previously described (6). Relative quantification and ∆Ct values were generated from the Taqman analysis performed in duplicates and results are expressed as 2ΔCt.
Figure 1.
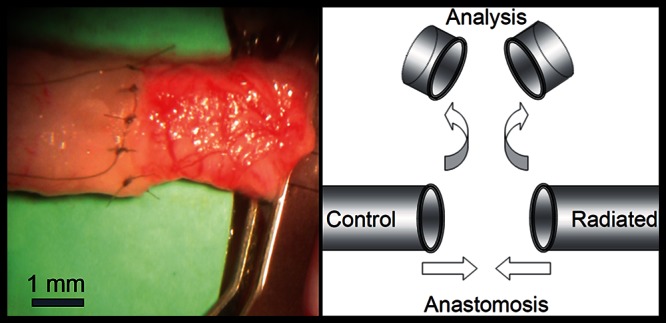
Macroscopic adventitial inflammation. Anastomosis of irradiated (right) and nonirradiated (left) human arteries during autologous free tissue transfer (microvascular surgery).
Immunofluorescence
Radiated and nonradiated arteries from 8 patients were immediately fixed in 10% formalin in the operation theater and kept overnight for embedding in paraffin and sectioning the following day. Demographic data, including XRT dosages, for the 8 patients who donated arterial segments used for immunofluorescence are listed in Table 2.
TABLE 2.
Patient demographics for immunofluorescence studies
| Age (yr)/sex | XRT dose (Gy) | Time after XRT (wk)a | Current smoking | Prevalent CVD | Control vessels |
|---|---|---|---|---|---|
| 54/male | 68 | 160 | No | No | Forearm |
| 66/female | 68 | 20 | No | No | Forearm |
| 68/male | 64 | 438 | No | No | Fibula |
| 70/male | 68 | 74 | Yes | No | Fibula |
| 77/female | 50 | 200 | No | No | Forearm |
| 65/male | 64 | 3120 | No | No | Fibula |
| 67/male | 66 | 104 | Yes | Yes | Fibula |
| 64/male | 68 | 416 | No | No | Fibula |
aTime from last XRT session to biopsy.
Sections (5 µm) were cut, deparaffinized, and heat induced for antigen retrieval by using Diva decloaker (Biocar Medical, Casablanca, Morocco). After blocking of endogenous peroxidase (0.3% H2O2, 30 min) and unspecific binding with avidin/biotin blocking kit (Vector Labs, Burlingame, CA, USA), followed by 5% horse sera for 30 min at room temperature, sections were incubated with a primary antibody or matched isotype control at 4°C overnight. The following primary antibodies were used: monoclonal rabbit anti-human arachidonate 5-LO (cat no LS-B2144, 1:200; Life Span BioSciences, Seattle, WA, USA), monoclonal mouse anti-human CD68 (M0876, clone PG-M1, 1:400; Dako, Carpinteria, CA, USA) for analysis of macrophages, mouse anti-human CD3 (PM 110 AA clone PS1, prediluted; BioCare Medical, Concord, CA, USA) for analysis of T cells, and monoclonal mouse anti-human von Willebrand factor (VWF; M0616, clone F8/86, 1:2000; Dako) to visualize the endothelium.
For immunofluorescent stainings, sections were incubated with primary antibodies overnight at 4°C. Negative controls were incubated in 1% bovine serum albumin alone. Sections were subsequently incubated with 2 secondary antibodies (DyLight 594 goat anti-rabbit IgG; DI-1594, 1:300; and DyLight 488 horse anti-mouse IgG; DI-2488, 1:300; Vector Labs). Autofluorescence reduction was obtained with Sudan Black (0.03% in 70% ethanol for 6 min; Sigma-Aldrich, St. Louis, MO, USA). After washing with PBS, nuclei of all sections were counterstained with DAPI (Sigma-Aldrich), and then sections were mounted in fluorescence mounting medium (Dako). Evaluation of immunofluorescence staining was performed by using a Leica DMRB fluorescence microscope with Leica filter cube L4 (Lieca Microsystems, Wetzlar, Germany).
Quantification of 5-LO and CD68 double-positive cells was performed on representative micrographs from the region of interest (adventitia) at ×40 magnification and analyzed in Adobe Photoshop CS (Adobe, San Jose, CA, USA). The amount of positive cells was determined manually and presented as the number of positive cells of the total area in each picture. All sections except negative tissue (one assessor, 0 XRT from 4 patients) were performed by 2 different persons and blinded to the assessor. If cell counting was uncertain to determine from immunofluorescence staining, quantification was performed from 3,3′-diaminobenzidine staining (0 XRT from 3 patients and 2 XRT samples).
Leukotriene B4 analysis in conditioned medium
Irradiated biopsies from 5 patients were collected in physiologic salt solution and transferred directly to the laboratory. After separation of adventitia from the media, tissue was incubated in RPMI 1640 medium (0.1 ml/g of wet tissue; Thermo Fisher Scientific) for 24 h at 37°C as previously described (16). Conditioned media were then collected and stored at −80°C until use. Concentrations of leukotriene B4 (LTB4) were determined by enzyme immune assay (Cayman Chemical, Ann Arbor, MI, USA) according to manufacturer instructions.
Statistics
Wilcoxon’s sign-rank test of paired samples was used to test differences between radiated and nonradiated arteries for RNA levels. Student’s t test was used for cell counting and levels of LTB4. Values of P < 0.05 were considered significant.
RESULTS
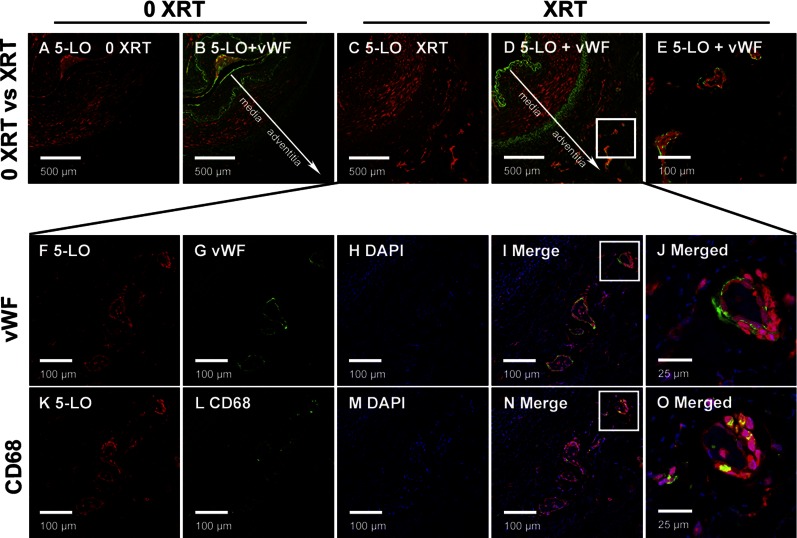
Irradiated parts of arteries exhibited an obvious adventitial vasa vasorum expansion at macroscopic perioperative inspection, which was not observed in arterial segments that were derived from nonirradiated areas of the same participant (Fig. 1). Immunohistochemical staining, together with double labeling immunofluorescence, confirmed this observation. VWF staining showed a morphologically intact endothelium toward the lumen but also revealed vasa vasorum expansion in the adventitia of 7 of 8 irradiated biopsies, whereas adventitial VWF staining were observed in none of the nonirradiated control arterial segments from the same participants (Fig. 2).
Figure 2.
Chronic vascular inflammation and adventitial vasa vasorum expansion in irradiated human arteries. Immunofluorescent staining on the arterial wall is positive for 5-LO in irradiated arteries. A–E) 5-LO–positive cells localized around the expanded vasa vasorum in the adventitia of irradiated (XRT) (C–E), but not in nonirradiated (0 XRT) (A, B) arteries. K–O) Invasion of macrophages into the adventitia was observed in irradiated arteries only. F–J) VWF staining was confined to vasa vasorum in the adventitia of irradiated arteries and not observed in the adventitia of nonirradiated controls from the same patients. Blue fluorescence, DAPI; red fluorescence, 5-LO; green fluorescence, VWF/CD68.
In addition, immunofluorescent analysis revealed 5-LO positivity in irradiated arterial segments (Fig. 2). Of interest, 5-LO–positive cells localized around the expanded vasa vasorum in the adventitia of irradiated arteries. Likewise, invasion of T cells (data not shown) and macrophages (Fig. 2) were observed in the adventitia of irradiated segments only. Staining for 5-LO was strongest in CD68+ cells that localized in and around the vaso vasorum in previously irradiated adventitia (Fig. 2).
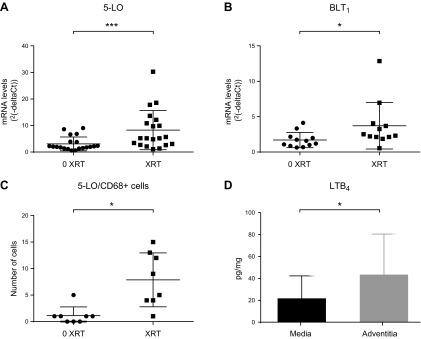
Levels of 5-LO mRNA and mRNA that encode the high-affinity LTB4 receptor BLT1 were significantly up-regulated in irradiated arterial segments compared with nonirradiated arterial biopsies from the same patient (P < 0.001; Fig. 3A; P = 0.034; Fig. 3B). Semiquantification of immunofluorescent staining revealed a significantly increased number of 5-LO and CD68+ cells in irradiated vs. control arterial segments (Fig. 3C).
Figure 3.
Increased activation of 5-LO and LTB4–BLT1 axis in irradiated human arteries. A, B) Messenger RNA levels of 5-LO (n = 20 in each group) (A) and BLT1 (n = 12 in each group) (B) in irradiated (XRT) human arteries compared with nonirradiated (0 XRT) from the same patient. Relative gene expression results are expressed as 2–ΔCt using phosphoglycerate kinase 1 as housekeeping gene. C) Semiquantification of 5-LO and CD68+ cells was performed in 8 paired arterial biopsies after immunofluorescence staining. D) Levels of LTB4 detected in conditioned medium derived from either the adventitial or medial layer derived from irradiated arteries (n = 5 in each group). Wilcoxon’s sign-rank test of paired samples was used to test differences between XRT and 0 XRT arteries (A–C), and Student’s t test for comparisons between media and adventitia (D). Data values presented with sd. *P ≤ 0.05; ***P ≤ 0.001.
Conditioned medium derived from irradiated arterial segments contained readily measurable levels on LTB4 released from the adventitia, whereas LTB4 levels released from the media were significantly lower (P = 0.046; Fig. 3D).
DISCUSSION
Results of the present study point to a hitherto undescribed link between previous irradiation and chronic vascular inflammation by means of 5-LO and adventitial vasa vasorum expansion. These observations implicate the 5-LO pathway as a potential therapeutic target for cardiovascular prevention in previously irradiated survivors of cancer.
By simultaneously harvesting irradiated and nonradiated arterial biopsies from the same patient, we have been able to eliminate interindividual differences in gene and protein expression patterns and, thereby, through this unique human model, to study the sole effect of radiation years after exposure (6). Here, we report that during the collection of this material, we discovered a macroscopic adventitial inflammation in irradiated, but not in nonirradiated, arterial segments. This visual impression was confirmed by using immunohistochemical/immunofluorescent analysis. These findings suggest that XRT leads to a persistent vasa vasorum expansion, which, in turn, may drive a susceptibility for future significant vascular lesions and cardiovascular events (12, 17).
The presented material, to our knowledge, is the only human data in which gene and protein expression patterns have been studied in conduit arteries years after radiation exposure. Our previous transcriptome-wide gene expression analysis in this material indicated activation of gene networks that are involved in angiogenesis, coagulation, and inflammation after arterial irradiation (6). The current study provides the first evidence of chronic up-regulation of the 5-LO pathway in the human cardiovascular system years after XRT.
5-LO activation is a key enzymatic step in the formation of several lipid mediators (18). Of interest, mRNA levels that encode the BLT1 receptor, which is the high-affinity receptor for the proinflammatory 5-LO–derived lipid mediator LTB4 (19), were significantly up-regulated in irradiated arterial segments. The importance of an adventitial LTB4–BLT1 axis in the latter context is further supported by the release of LTB4 from irradiated arteries in the present study, which, by means of BLT1 receptor signaling, may sustain vascular inflammatory circuits after XRT. However, it should be pointed out that in addition to the LTB4-BLT1 pathway, it cannot be excluded that other 5-LO–derived lipid mediators, such as cysteinyl-leukotrienes and/or resolvins (20), are also altered by XRT.
Of importance, 5-LO expression prevailed in CD68+ cells, which localized in and around adventitial vasa vasorum. In addition, the number of 5-LO and CD68+ positive cells was significantly increased in irradiated compared with control arterial segments. Taken together, these observations suggest that chronic 5-LO up-regulation in macrophages may drive a vascular inflammatory response from the adventitia several years after irradiation. The latter notion was further supported by an increased formation of 5-LO–derived LTB4 in the adventitia compared with the media in vessels that were derived from irradiated areas.
The preferential adventitial 5-LO activation that was observed in irradiated arteries in the present study strikingly resembles previous observations of human coronary vessels with subclinical atherosclerosis (17). In the latter study, medial and intimal 5-LO expression were only observed in advanced coronary atherosclerotic lesions, in which adventitial 5-LO expression was also further increased (17). Likewise, 5-LO–positive macrophages prevail in the adventitia of human abdominal aortic aneurysms (16). The increased number of adventitial cells in irradiated vessels, which exhibited both 5-LO and CD68 positivity in the present study, supports the major role of adventitial macrophage 5-LO activation, and, for the first time, extends its pathophysiology to XRT-associated vascular inflammation. The causal role of 5-LO activation in adventitial macrophages has been evoked from experimental studies of 5-LO–deficient mice in different models of cardiovascular pathologies, such as abdominal aortic aneurysms (15). Taken together, those observations support the adventitial 5-LO expression observed in previously irradiated arteries in the present study as a potential susceptibility for future development of significant vascular lesions and cardiovascular events.
Initial studies have suggested that increased leukotriene formation is induced by irradiation, on the basis of clinical (21) as well as experimental in vivo (22) findings. 5-LO inhibitors have also exhibited beneficial effects in different animal models of radiation-induced pneumonitis and microvascular dysfunction (22, 23). Given that antileukotrienes recently have been explored for CVD prevention (24, 25), the current data support the 5-LO pathway as a potential target for prevention of radiation-induced CVD in a steadily increasing population of survivors of cancer.
The observational nature of these data in a restricted number of patients with a certain variation in terms of, for example, time passed since XRT, are limitations of the present study that should be acknowledged. However, this is one of a few studies that has been able to describe chronic vascular alterations after XRT in humans. The unique model of paired individual controls has also proved to be powerful, showing consistent results by analysis of radiation exposure only (6, 26). Among methodologic considerations, it should be mentioned that, for example, liquid chromatographic–tandem mass spectrometry analysis may reveal further alterations of 5-LO pathways in irradiated adventitia in addition to LTB4, which was measured by ELISA in the present study. Finally, the heterogeneous composition of biopsies is another possible limitation, but via immunohistochemistry, immunofluorescence, and conditional medium analysis, the localization of 5-LO expression and activity has been further reinforced.
In summary, our results suggest a maintained chronic inflammatory activity localized in the adventitia years after XRT as well as its association with an increased 5-LO expression in adventitial macrophages localized around a vasa vasorum expansion in previously irradiated arterial segments, as depicted in Fig. 4. This chronic inflammation may remain silent in the arterial adventitia for several years after exposure before progressive vascular injury and cardiovascular events appear. In summary, the 5-LO pathway may represent a potential therapeutic target for cardiovascular prevention post-XRT.
Figure 4.
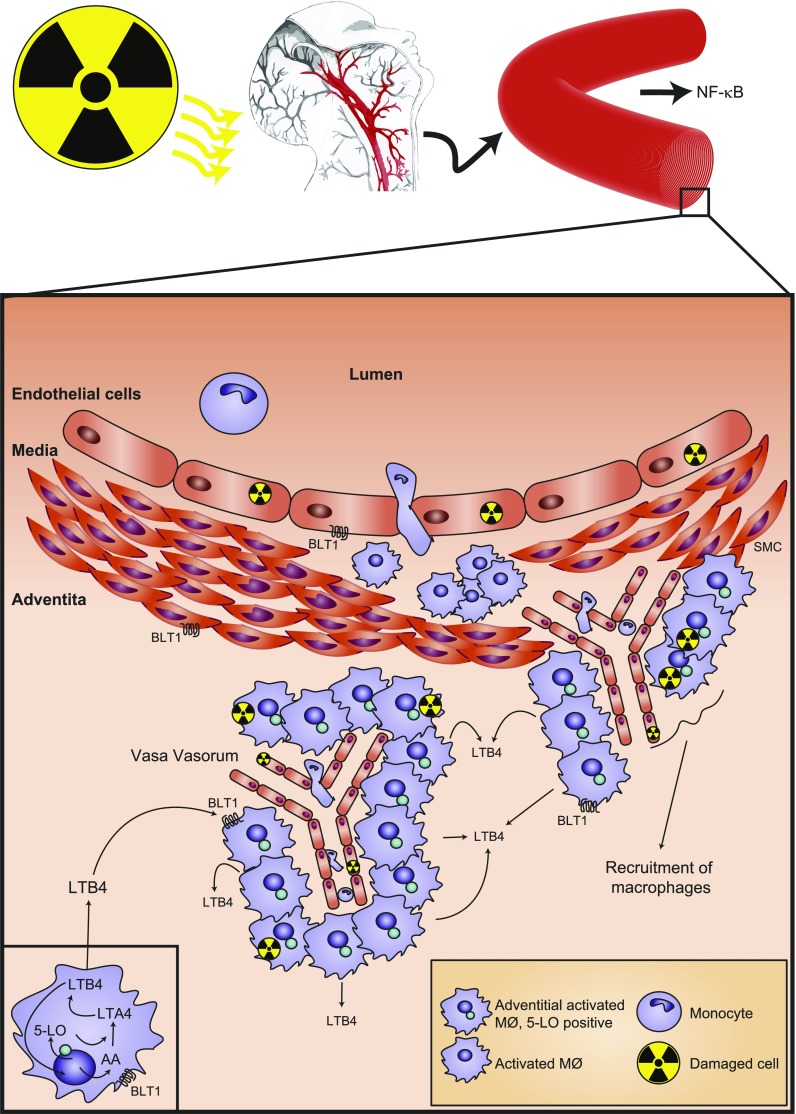
Schematic representation of inflammatory circuits in previously radiated arteries. Previously radiated arterial segments exhibited an increased adventitial inflammatory response that was characterized by macrophage accumulation in the vicinity of an expanded of vasa vasorum. Previous studies have associated radiation with activation of the NF-κB pathway (6). The present study identified increased levels of 5-LO in radiated vs. nonradiated arterial segments, and that this enzyme was localized to perivascular adventitial macrophages. The 5-LO enzyme metabolizes arachidonic acid (AA) the LT precursor, LTA4, which is then further metabolized to the chemotactic and proinflammatory LTB4. In radiated arteries, adventitial LTB4 was increased compared with the release from arterial medium. LTB4 transduces proinflammatory effects by means of its high-affinity BLT1 receptor, expressed on macrophages, smooth muscle cells (SMCs), and endothelial cells. Blocking 5-LO/LTB4 signaling may represent a possible therapeutic adjunct to prevent late adverse cardiovascular effects of XRT.
ACKNOWLEDGMENTS
The authors thank Linda Haglund (Department of Medicine, Karolinska Institutet) for excellent technical support. M.H. is supported by Funds of Radiumhemmet (Grant 111122), the Swedish Society of Medicine (Grant SLS-408771), and the Stockholm County Council (Grant 20140170). M.B. is supported by the Swedish Research Council (Grant 2014-2312), the Swedish Heart and Lung Foundation (Grants 20150600 and 20150683), and the Stockholm County Council (Grant 20140222).
Glossary
- BLT1
leukotriene B4 receptor 1
- CVD
cardiovascular disease
- 5-LO
5-lipoxygenase
- LTB4
leukotriene B4
- VWF
von Willebrand factor
- XRT
radiotherapy
AUTHOR CONTRIBUTIONS
M. Halle and M. Bäck conceived the study design and designated the methods; M. Halle and T. Christersdottir conducted data collections; M. Halle and M. Bäck performed data analysis; M. Halle and T. Christersdottir conducted statistical analysis and created figures with input from all authors; and all authors wrote the paper.
REFERENCES
- 1.Altena R., Perik P. J., van Veldhuisen D. J., de Vries E. G., Gietema J. A. (2009) Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 10, 391–399 [DOI] [PubMed] [Google Scholar]
- 2.Herrmann J., Yang E. H., Iliescu C. A., Cilingiroglu M., Charitakis K., Hakeem A., Toutouzas K., Leesar M. A., Grines C. L., Marmagkiolis K. (2016) Vascular toxicities of cancer therapies: the old and the new - an evolving avenue. Circulation 133, 1272–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby S. C., Ewertz M., McGale P., Bennet A. M., Blom-Goldman U., Brønnum D., Correa C., Cutter D., Gagliardi G., Gigante B., Jensen M. B., Nisbet A., Peto R., Rahimi K., Taylor C., Hall P. (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998 [DOI] [PubMed] [Google Scholar]
- 4.Mulrooney D. A., Yeazel M. W., Kawashima T., Mertens A. C., Mitby P., Stovall M., Donaldson S. S., Green D. M., Sklar C. A., Robison L. L., Leisenring W. M. (2009) Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer C., Henderson R. D., O’Sullivan J. D., Read S. J. (2011) Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 42, 2410–2418 [DOI] [PubMed] [Google Scholar]
- 6.Halle M., Gabrielsen A., Paulsson-Berne G., Gahm C., Agardh H. E., Farnebo F., Tornvall P. (2010) Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J. Am. Coll. Cardiol. 55, 1227–1236 [DOI] [PubMed] [Google Scholar]
- 7.Fajardo L-G L. F., Prionas S. D., Kaluza G. L., Raizner A. E. (2002) Acute vasculitis after endovascular brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 714–719 [DOI] [PubMed] [Google Scholar]
- 8.Kollum M., Cottin Y., Chan R. C., Kim H. S., Bhargava B., Vodovotz Y., Waksman R. (2001) Delayed re-endothelialization and T-cell infiltration following intracoronary radiation therapy in the porcine model. Int. J. Radiat. Oncol. Biol. Phys. 50, 495–501 [DOI] [PubMed] [Google Scholar]
- 9.Busseuil D., Zeller M., Cottin Y., Maingon P., Barillot I., Martin L., Allouch P., Lalande A., Vergely C., Briot F., Piard F., Wolf J. E., Rochette L. (2003) Intramural neovascularization and haemorrhages are major long-term effects of intravascular gamma-radiation after stenting. Int. J. Radiat. Biol. 79, 787–792 [DOI] [PubMed] [Google Scholar]
- 10.Powers B. E., Thames H. D., Gillette E. L. (1999) Long-term adverse effects of radiation inhibition of restenosis: radiation injury to the aorta and branch arteries in a canine model. Int. J. Radiat. Oncol. Biol. Phys. 45, 753–759 [DOI] [PubMed] [Google Scholar]
- 11.Michel J. B., Thaunat O., Houard X., Meilhac O., Caligiuri G., Nicoletti A. (2007) Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler. Thromb. Vasc. Biol. 27, 1259–1268 [DOI] [PubMed] [Google Scholar]
- 12.Mulligan-Kehoe M. J., Simons M. (2014) Vasa vasorum in normal and diseased arteries. Circulation 129, 2557–2566 [DOI] [PubMed] [Google Scholar]
- 13.Nagy E., Andersson D. C., Caidahl K., Eriksson M. J., Eriksson P., Franco-Cereceda A., Hansson G. K., Bäck M. (2011) Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation 123, 1316–1325 [DOI] [PubMed] [Google Scholar]
- 14.Bäck M. (2009) Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr. Pharm. Des. 15, 3116–3132 [DOI] [PubMed] [Google Scholar]
- 15.Zhao L., Moos M. P., Gräbner R., Pédrono F., Fan J., Kaiser B., John N., Schmidt S., Spanbroek R., Lötzer K., Huang L., Cui J., Rader D. J., Evans J. F., Habenicht A. J., Funk C. D. (2004) The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat. Med. 10, 966–973 [DOI] [PubMed] [Google Scholar]
- 16.Houard X., Ollivier V., Louedec L., Michel J. B., Bäck M. (2009) Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 23, 1376–1383 [DOI] [PubMed] [Google Scholar]
- 17.Spanbroek R., Grabner R., Lotzer K., Hildner M., Urbach A., Ruhling K., Moos M. P., Kaiser B., Cohnert T. U., Wahlers T., Zieske A., Plenz G., Robenek H., Salbach P., Kuhn H., Radmark O., Samuelsson B., Habenicht A. J. (2003) Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc. Natl. Acad. Sci. USA 100, 1238–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäck M., Dahlén S. E., Drazen J. M., Evans J. F., Serhan C. N., Shimizu T., Yokomizo T., Rovati G. E. (2011) International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol. Rev. 63, 539–584 [DOI] [PubMed] [Google Scholar]
- 20.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. (2005) Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole A. T., Slater K., Sokal M., Hawkey C. J. (1993) In vivo rectal inflammatory mediator changes with radiotherapy to the pelvis. Gut 34, 1210–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panés J., Mollà M., Casadevall M., Salas A., Sans M., Conill C., Anderson D. C., Roselló-Catafau J., Granger D. N., Piqué J. M. (2000) Tepoxalin inhibits inflammation and microvascular dysfunction induced by abdominal irradiation in rats. Aliment. Pharmacol. Ther. 14, 841–850 [DOI] [PubMed] [Google Scholar]
- 23.Gross N. J., Holloway N. O., Narine K. R. (1991) Effects of some nonsteroidal anti-inflammatory agents on experimental radiation pneumonitis. Radiat. Res. 127, 317–324 [PubMed] [Google Scholar]
- 24.Ingelsson E., Yin L., Bäck M. (2012) Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J. Allergy. Clin. Immunol. 129, 702–707.e2 [DOI] [PubMed] [Google Scholar]
- 25.Bäck M., Hansson G. K. (2015) Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 12, 199–211 [DOI] [PubMed] [Google Scholar]
- 26.Christersdottir Björklund T., Reilly S. J., Gahm C., Bottazzi B., Mantovani A., Tornvall P., Halle M. (2013) Increased long-term expression of pentraxin 3 in irradiated human arteries and veins compared to internal controls from free tissue transfers. J. Transl. Med. 11, 223 [DOI] [PMC free article] [PubMed] [Google Scholar]