Abstract
Purpose
We are developing a computerized system for bladder segmentation on CT urography (CTU), as a critical component for computer-aided detection of bladder cancer.
Methods
The presence of regions filled with intravenous contrast and without contrast presents a challenge for bladder segmentation. Previously, we proposed a Conjoint Level set Analysis and Segmentation System (CLASS). In case the bladder is partially filled with contrast, CLASS segments the non-contrast (NC) region and the contrast-filled (C) region separately and automatically conjoins the NC and C region contours; however, inaccuracies in the NC and C region contours may cause the conjoint contour to exclude portions of the bladder. To alleviate this problem, we implemented a local contour refinement (LCR) method that exploits model-guided refinement (MGR) and energy-driven wavefront propagation (EDWP). MGR propagates the C region contours if the level set propagation in the C region stops prematurely due to substantial non-uniformity of the contrast. EDWP with regularized energies further propagates the conjoint contours to the correct bladder boundary. EDWP uses changes in energies, smoothness criteria of the contour, and previous slice contour to determine when to stop the propagation, following decision rules derived from training. A data set of 173 cases was collected for this study: 81 cases in the training set (42 lesions, 21 wall thickenings, 18 normal bladders) and 92 cases in the test set (43 lesions, 36 wall thickenings, 13 normal bladders). For all cases, 3D hand segmented contours were obtained as reference standard and used for the evaluation of the computerized segmentation accuracy.
Results
For CLASS with LCR, the average volume intersection ratio, average volume error, absolute average volume error, average minimum distance and Jaccard index were 84.2±11.4%, 8.2±17.4%, 13.0±14.1%, 3.5±1.9 mm, 78.8±11.6%, respectively, for the training set and 78.0±14.7%, 16.4±16.9%, 18.2±15.0%, 3.8±2.3 mm, 73.8±13.4% respectively, for the test set. With CLASS only, the corresponding values were 75.1±13.2%, 18.7±19.5%, 22.5±14.9%, 4.3±2.2 mm, 71.0±12.6%, respectively, for the training set and 67.3±14.3%, 29.3±15.9%, 29.4±15.6%, 4.9±2.6 mm, 65.0±13.3%, respectively, for the test set. The differences between the two methods for all five measures were statistically significant (p<0.001) for both the training and test sets.
Conclusions
The results demonstrate the potential of CLASS with LCR for segmentation of the bladder.
1. INTRODUCTION
Bladder cancer is the fourth most common cancer diagnosed in men. The American Cancer Society estimates that bladder cancer will cause 15,210 deaths (10,820 in men, 4,390 in women) in the United States in 2013, with 72,570 new cases (54,610 in men, 17,960 in women) diagnosed (American Cancer Society, 2013). Early detection and treatment of bladder cancer increases patient survivability. If bladder cancers are detected and treated while the cancer is confined within the bladder’s inner lining but has not invaded the muscular bladder wall, the 5-year survival rate is 88%. If the cancer is detected after it has invaded the bladder wall but is still confined to the bladder, the 5-year survival rate drops to 63%; however, only about 50% of the patients are diagnosed before the cancer has invaded the muscular bladder wall (American Cancer Society, 2013).
Multi-detector row CT (MDCT) urography has shown promise of detecting bladder lesions and has become the imaging modality of choice for most urinary track abnormalities since a single exam can be used to evaluate the kidneys, intrarenal collecting systems, and ureters. CT urography (CTU), therefore, may spare the patients from having to undergo other imaging studies (intravenous pyelogram (IVP), ultrasound, conventional abdominal CT, and even MRI), thereby reducing health care costs (Akbar et al., 2004; Caoili et al., 2002; Liu et al., 2005; McCarthy and Cowan, 2002; Noroozian et al., 2004).
Interpretation of a CTU study requires thorough image analysis, often requiring extensive time. On average, 300 slices are generated for each CTU scan at a slice interval of either 1.25 mm or 0.625 mm (range: 200 to 600 slices). The radiologists interpreting the study have to visually determine whether or not lesions are present within the urinary tracts, frequently needing to adjust the brightness and contrast of the images and use zooming from a display workstation. The possibility that multiple lesions may be present requires that the radiologists pay close attention throughout the entire urinary tract. In addition, many different urinary anomalies may be found in a single CTU study. Not only do the radiologists have to identify these anomalies, they must also determine how likely each of them is an urothelial neoplasm. The challenges of analyzing a CTU study leads to a substantial variability among radiologists in detection of bladder cancer, with reported sensitivities ranging from 59% to 92% (Park et al., 2007; Sudakoff et al., 2008). Due to the workload of interpreting CTU studies, the chance for the radiologists’ to miss a subtle lesion may not be negligible, thus any technique that may help radiologists with identification of urothelial neoplasms within the urinary tract will be useful. Computer-aided detection (CAD) used as an adjunct may be the tool that reduces the chance of oversight by the radiologists. We are developing a CAD system that detects bladder cancer in CTU, and a critical part of this system is accurate bladder segmentation that isolates the bladder from the surrounding anatomical structures.
Li et al (Li et al., 2004) segmented the bladder wall from magnetic resonance (MR) cytoscopy in 6 patients and analyzed it for suspected lesions using a partial volume segmentation algorithm. Duan et al (Duan et al., 2010) used two collaborative level set functions and clustering to segment the bladder, also in MR cytoscopy of 6 patients. In a different study, Duan et al (Duan et al., 2012) developed a segmentation method using MR images of 10 patients, which used an adaptive window-setting scheme to detect tumor surfaces. Recently, Han et al (Han et al., 2013) of the same group segmented the bladder wall in T1-weighted MR images using an adaptive Markov random field model and coupled level set information in 6 patients. Li et al (Li et al., 2004) did not report quantitative results for the bladder segmentation. Duan et al (Duan et al., 2010, Duan et al., 2012) and Han et al (Han et al., 2013) evaluated the segmentation performances using radiologists’ subjective ratings without reporting quantitative results. Chai et al (Chai et al., 2012) presented a method for semiautomatic bladder segmentation on cone beam CT by using a population-based statistical bladder shape calculated using spherical harmonics description, then applying principal component analysis. They used 95 scans from 8 patients to train their method, and validated their method using 233 scans from 22 patients. Segmentation performance was measured using the Jaccard index comparing the segmentation with manual segmentation done slice-by-slice, which was 70.5% at the automatic stage and increased to 77.7% at the following semiautomatic stage. Hadjiiski et al developed preliminary bladder segmentation methods for CTU using active contour with 15 patients (Hadjiiski et al., 2009) without quantitative results, and level sets with 70 patients, which was evaluated using quality ratings. (Hadjiiski et al., 2012).
There are challenges to segment bladders in CTU. Bladders may be partially or fully filled with intravenous (IV) contrast material that opacifies a portion of the bladder. The boundaries between the bladder wall and the surrounding soft tissue have very low contrast such that they are often difficult to delineate. In addition, bladders may be imaged in a variety of shapes and sizes. To address these challenges, Hadjiiski et al (Hadjiiski et al., 2013) developed a segmentation package specifically designed based on the characteristics of the bladder in CTU images referred to as Conjoint Level set Analysis and Segmentation System (CLASS) (frequently used acronyms are listed in Table 1). The segmentation performance was qualitatively evaluated using 81 bladders and quantitatively evaluated using 30 bladders by comparing the computer-segmented contours to hand-segmented reference contours and obtained promising results.
Table 1.
Definitions of frequently used acronyms.
| AVDIST | Average minimum distance between two contours |
| C | Contrast-filled |
| CAD | Computer-aided detection |
| CCP | Contour conjoint procedure |
| CLASS | Conjoint level set analysis and segmentation system |
| CTU | CT urography |
| EDWP | Energy-driven wavefront propagation |
| IV | Intravenous |
| L | Contour of the contrast-filled region of the bladder |
| LCR | Local contour refinement |
| MDCT | Multi-detector row CT |
| MGR | Model-guided refinement |
| NC | Non-contrast |
| ROI | Region of Interest |
In this study, we further developed the CLASS method by incorporating a local contour refinement procedure to improve the segmentation accuracy and evaluated its performance using a moderately-sized data set, although it was the largest data set of independent subjects compared to those used in other reported studies. The improvement in the segmentation performance of CLASS with the new refinement technique compared to that of CLASS alone was quantified using manual segmentation as reference standard.
2. MATERIALS AND METHODS
2.1 Bladder segmentation using CLASS
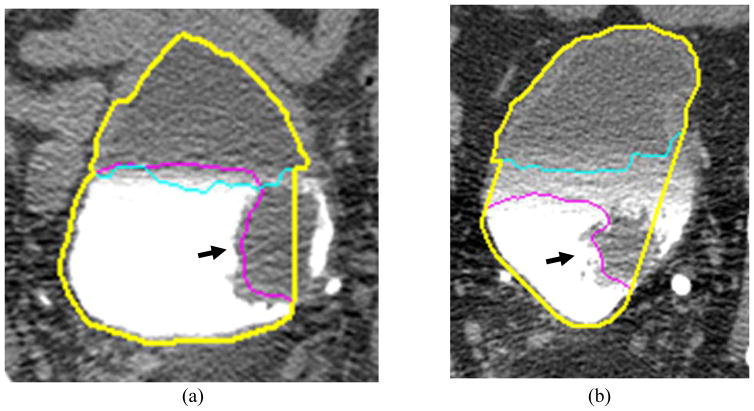
Our previous work on CLASS (Hadjiiski et al., 2013) is described briefly in the following. An axial CTU scan of the bladder is shown in Figure 1. Figures 2 and 3 show two regions of interest (ROI) from different CTU slices that contain the bladder. The bladders shown are partially filled with IV contrast material, and malignant lesions of different sizes can be identified in the lower, contrast-enhanced portion of the bladder. The presence of the two distinct areas that have very different attenuation values: an area filled with IV contrast material and an area without contrast material (Figures 2 and 3), poses a challenge for segmentation that needs to go across the strong boundary.
Figure 1.

An axial slice of a CTU scan in which the bladder is partially filled with IV contrast material. A malignant lesion is present in the contrast-filled region of the bladder, indicated by the bold arrow.
Figure 2.

ROI of a bladder partially filled with IV contrast material, showing the two distinct areas. A malignant mass lesion is present in the contrast-filled region of the bladder (black arrow).
Figure 3.
A large malignant mass lesion is located in the contrast-filled portion of the bladder (black arrow).
We are developing a software package, referred to as the Conjoint Level set Analysis and Segmentation System (CLASS), for segmenting the bladder in CTU, We introduced CLASS previously (Hadjiiski et al., 2013) with limited quantitative assessment of its performance including comparison of its segmentation accuracy to radiologist’s hand outlines in a small data set and their qualitative visual judgment on a larger data set.
CLASS consists of four stages: (1) preprocessing and initial segmentation, (2) 3D level set segmentation, (3) 2D level set segmentation, and (4) post-processing. CLASS segments the non-contrast (NC) region and the contrast-filled (C) region by applying the level sets to each region separately, then automatically conjoins them together during post-processing using a procedure called Contour Conjoint Procedure (CCP). More details of CLASS can be found in the literature (Hadjiiski et al., 2013).
2.2 Local Contour Refinement
Although CLASS performs reasonably well in comparison to hand segmentations, achieving a large volume intersection ratio for more than 70% of bladders (Hadjiiski et al., 2013), a number of cases failed because the level set propagation in the C region may stop prematurely due to either substantial non-uniformity of the contrast, or a lesion present in the region. In this study, we designed a new Local Contour Refinement (LCR) method to further improve the segmentation accuracy, especially to improve the segmentation of the C region, and including lesions that may be present.
Local contour refinement consists of two main processes: model-guided refinement (MGR) and energy-driven wavefront propagation (EDWP). MGR propagates the contour of the C region (L) using local moving windows if the level set segmentation stops prematurely. EDWP uses regularized local energies to propagate the occasionally imperfect conjoining contour segments that conjoin the contours of NC and C regions, to the correct bladder boundary. The block diagram of CLASS with LCR is presented in Figure 4. The parameter values for the proposed methods were determined experimentally using cases from the training set. By altering one parameter at a time, we studied the sensitivity of the parameters (i.e., the effect of the change of each parameter on the quality of the automatic 3D contour), both by visual inspection and by the performance measures. We fixed the less sensitive parameters first, and altered the parameters with higher sensitivity to achieve the best segmentation. Once the parameters were fixed based on the training set, the CLASS with LCR was applied to the test set with that fixed parameter set without further changes.
Figure 4.
Block diagram of the CLASS with LCR. MGR is performed on the CLASS contour of the contrast-filled region. The CLASS contour of the non-contrast region and the contour L of contrast-filled region after MGR are joined and refined by EDWP to obtain the final contour of the bladder.
2.2.1 Model-guided refinement
The model-guided refinement (MGR) method was designed to improve the CLASS C contour. Inaccuracies in the NC and C contours may cause the Contour Conjoint Procedure (CCP) to exclude portions of the bladder. To alleviate this problem, we implemented an MGR method to propagate the C region contour, L, if the level set propagation in the C region stops prematurely due to substantial non-uniformity of the contrast. MGR uses the level set contour, the local gradient and contrast as input and incorporates adaptive thresholding to propagate the L contour in 2D for every slice to the correct bladder boundary.
MGR propagates the level set L contour by analysis of three moving windows, all of 3 X 3 pixels, at each point Li along the L contour: one centered at the point Li, the other two positioned at an inner and outer neighborhood of the contour, respectively. The center of the outer window (WO) is located outside the contour in the normal direction, two pixels away from Li. The center of the inner window (WI) is located inside the contour, also in the normal direction, two pixels away from Li (Figure 5). The average intensities of the three windows are calculated as follows:
| (1) |
| (2) |
| (3) |
where IW(Li) is the average intensity of the window W centered at Li, obtained by taking the average of the intensity of the voxels I(P) within the window W. IWO(Li) and IWI(Li) are the average intensity of the WO and WI windows, respectively, calculated in a similar way as IW(Li). NW, NWO, and NWI are the areas of the W, WO, and WI windows, respectively and NW = NWO = NWI =9 pixels.
Figure 5.
Diagram of inner and outer window used for MGR. Li is the given point on contour L that may be propagated. Li+1 and Li−1 are the next and previous points, respectively, on the contour neighboring the point Li. The inner and outer windows are located two pixels away from Li and centered along the normal. The inner window WI is located towards the centroid of the contour L. The outer window WO is located outside the contour.
The normal direction is determined by the normal angle θ defined as:
| (4) |
where (xi,yi) is the coordinate of the given point Li on the contour, and (xi+1,yi+1) and (xi−1,yi−1) are the coordinates of the next Li+1 and previous Li−1 neighboring points on the contour to the point Li, respectively (Figure 5).
The point Li is propagated if the condition in either Equation (5) or Equation (6) is satisfied:
| (5) |
| (6) |
where Th is the optimal threshold determined at the beginning of the MGR by the process explained below. Th is kept constant during the propagation of all Li points for all contours in a case. Equation (5) propagates the contour while the contour is within the C region of the bladder, propagating the contour to areas with positive gradient. Equation (6) propagates the contour even if the non-uniformity of the contrast creates areas of negative gradient. Equation (6) also propagates the contour to encompass the bladder wall once the contour reaches the edge of the C region. If one of these conditions is met, point Li propagates to a point one pixel away in the normal direction, determined by Equation (4) in a single iteration. Multiple iterations are carried out to propagate the contour. The propagation of the contour stops when all points Li stop moving. Smoothness of the contour is maintained by automatically adding points to the contour if the distance between consecutive points on the contour exceeds 6 pixels where the contour becomes too sparse, and removing points from the contour that cause sharp angles, defined as angle less than 90 degrees.
The optimal threshold Th for the MGR is selected based on an adaptive method and is specific for every case. The Th is selected at the beginning of MGR. First, the mean (μ) and the standard deviation (σ) of the voxel intensities within the volume defined by the 3D level set CLASS contours of the C region are calculated. A set of candidate thresholds Thm are estimated by Thm = μ − nmσ, where nm = 0, 0.5, 1, …, 10. For every candidate threshold, the level set contours on the best slice b (the slice that best represents the bladder region, where the bladder is seen the largest, which was manually selected when the ROI was defined), the slice before (b−1), and the slice after (b+1), are propagated A given point Li on the 2D contours of the three slices is propagated if it satisfies both Equation (5) and Equation (7):
| (7) |
To further prevent leaking, a criterion is set to determine adaptively how much a subsequent contour can expand from the current contours. We first calculated the average minimum distance between two contours (AVDIST) as:
| (8) |
where G and U are two contours being compared. NG and NU denote the number of voxels on G and U, respectively. The function d is the Euclidean distance. For a given voxel along the contour G, the minimum distance to a point along the contour U is determined. The minimum distances obtained for all points along G are averaged. This process is repeated by switching the roles of G and U. AVDIST is then calculated as the average of the two average minimum distances.
For every candidate threshold Thm, the relative contour propagation increase (RCPI) is defined as:
| (9) |
where S(b), S(b−1), S(b+1) are the contours obtained by propagating level set L contours on the best slice b, the slice before b−1, and the slice after b+1, respectfully, using the candidate threshold Thm and Equations (5) and (7). The optimal threshold Th is selected to be the largest Thm that provides less than 40% increase in RCPI from the RCPI obtained from the previous threshold, Thm−1. Th is fixed for the entire case, and is used to obtain the L contours on other slices of the case by propagating their respective level set L contours using Equations (1) through (6), applying methods as mentioned above. Obtaining the optimal threshold by this method helps prevent MGR from leaking. Figure 6 shows an example comparing segmentation results with and without MGR.
Figure 6.
Bladder segmentation using CLASS with and without MGR. (a) CLASS excluded portions of the bladder due to a malignant lesion (black arrow) attached to the bladder wall, but MGR propagated the L contour through the lesion to the correct bladder boundary. (b) MGR resolved a similar problem with a malignant mass lesion preventing the L contour from correctly segmenting the bladder. The thick contour represents CLASS with MGR. The thin contour shows CLASS without MGR.
2.2.2 Energy-driven wavefront propagation (EDWP)
The EDWP method was designed to smooth the transition between the conjoint C and NC contours and further pushes the contour towards the true bladder boundary, as described in the following. Inaccuracies in the contours of the NC and C regions may cause Contour Conjoint Procedure (CCP) to exclude portions of the bladder. In cases where a lesion is present in the C region, MGR may not propagate through the lesion due to the edge of the lesion having similar edge properties as the bladder wall. When the level set contour of the NC region and the contour L of C region after MGR are conjoined, CCP may create erroneous connection between the two contours, as seen by the straight connections between the contours of NC and C regions in Figure 7. This conjoining segment may cut across a lesion or the bladder, excluding other portions of the bladder in the process. The EDWP method combines local energies with a priori knowledge on contrast-enhanced bladder on CT images to guide the contour to search for the true bladder boundary.
Figure 7.
Bladder segmentation using CLASS with MGR. Inaccuracies in the NC region contour and C region contour L may cause CCP to exclude portions of the bladder. MGR was unable to propagate past the lesions (black arrow) in the C region to the correct bladder wall in both (a) and (b). (a) The malignant lesion extends to the NC area, thus MGR did not propagate the L contour through the lesion. (b) The inhomogenous nature of the boundary between the NC and C regions of the bladder prevented MGR from propagating the L contour fully, missing the malignant lesion. The thin upper contour represents the CLASS NC region contour. The thin lower contour represents the L contour with MGR. The thick outer contour is the result of conjoining the NC region contour and L contour without LCR.
EDWP propagates the points on the conjoining contour segment to the bladder boundary by calculating the local energies ER(Zi), EOutR(Zi), and EOrigR(Zi). The local energy ER(Zi) is the energy of a region Ri centered at a given point Zi along the conjoining segment. Ri includes Zi and the points on the conjoining segment that are a maximum of two points away from Zi, i.e. Zi±2, Zi±1, therefore Zi, Zi±1, Zi±2 ∈ Ri. The energy term ER(Zi) is defined as:
| (10) |
where IW(P) is the average intensity of a window defined by Equation (1), but a window size of 5 X 5 pixels is used here. α is the weight assigned to IW(P): α is 4 for the energy of Zi and 1 for the rest of the reference points in Ri. Tα is the total weight, which is 8.
The local energy EOutR(Zi), is the energy of the region obtained by propagating all points in Ri one pixel outside of the contour in their respective normal direction, estimated by the normal angle (Equation (4)). EOutR(Zi) is defined as:
| (11) |
where IW(P), α, and Tα are defined as in Equation (10) above.
EOrigR(Zi) is the local energy of the region centered at the original Zi in the conjoint segment before propagation with EDWP. EOrigR(Zi), is defined as:
| (12) |
where IW(P), α, and Tα are defined as in Equation (10) above. Before the first iteration, ER(Zi) = EOrigR(Zi).
The local energies ER(Zi), EOutR(Zi), and EOrigR(Zi), are calculated for every iteration of the propagation. The EDWP monitors the changes in the energy to determine when to stop the propagation. The point Zi is propagated if both the following conditions are satisfied:
| (13) |
| (14) |
Equation (13) propagates the conjoining segment through lesions and C region of the bladder until the drop in EOutR(Zi) energy exceeds the criterion, usually the darker tissue background around the bladder wall. Equation (14) stops the propagation in cases where Equation (13) is still satisfied when the conjoint segment is at the bladder wall. Using these conditions allows the segment to propagate through regions of higher energy such as contrast material (Figure 8), and eventually stop at a region of low energy (the darker tissue background around the bladder wall (Figure 8)). If both criteria for propagation are satisfied, Zi propagates to the pixel located one pixel outside of the contour in the normal direction, estimated by Equation (4), to the conjoining segment. After propagating all points along the conjoint segment for a given iteration, smoothness of the contour is maintained by limiting the smoothing energy terms in 2D and 3D as follows.
Figure 8.
Bladder segmentation using CLASS with LCR. (a) Energy-driven wavefront propagation (EDWP) propagated the conjoint contour past the malignant lesion (black arrow) to the correct bladder boundary. (b) Inaccuracy with L contour after MGR caused CCP to exclude portions of the bladder, but EDWP propagated the contour to the correct bladder boundary. Thin contour represents contour without EDWP. Thick contour represents the contour with EDWP.
The 2D smoothness criterion is applied sequentially to all regions Ri, along the conjoint segment. The 2D smoothing energy is defined as:
| (15) |
where the function γ provides the number of iterations in which Zi propagates since the iteration in which a point P among Zi±1 and Zi±2 stopped propagating. Within a region Ri, Zi stops propagating if ESmooth 2D is greater than 4, even if the energy criteria is met. Using this method ensures that Zi does not over-propagate by accounting for the changes in energy at the reference points in Ri.
3D smoothing energy is defined as:
| (16) |
where the function d is the Euclidean distance. PS is the previous slice (j−1) located above the current slice (j). ESmooth 3D measures the minimum distance between Zi on slice j and the points on the previous contour on slice j−1. If this value is greater than 5 voxels, Zi stops propagating. Additional steps to maintain smoothness include adding points to the contour if the contour becomes too sparse defined by the same criteria as MGR, and removing points from the contour that cause sharp angles, defined as angle less than 90 degrees.
Once propagation stops the energy of the conjoining segment follows the relationships below:
| (17) |
| (18) |
where Q is the number of points along the conjoining segment. Figure 8 shows examples after improvement by applying EDWP to the cases in Figure 7.
2.4 Data set
In this study, a data set of 173 patients undergoing CTU who subsequently underwent cystoscopy and biopsy was utilized. The cases were collected retrospectively from the Abdominal Imaging Division of the Department of Radiology at the University of Michigan with approval of the Institutional Review Board. We designated 81 of these cases as the training set, and the other 92 cases as the test set. The cases were assigned to the training or the test sets by balancing the difficulty of the cases between the two sets.
Of the 81 training set bladders, 42 bladders contained focal mass-like lesions (40 malignant and 2 benign), 21 bladders had wall thickening (16 malignant and 5 benign) and 18 were normal. 61 bladders were partially filled with IV contrast material, 8 were completely filled with contrast material, and 12 had no visible contrast material. Of the 92 test set bladders, 43 bladders contained focal mass-like lesions (42 malignant and 1 benign), 36 bladders had wall thickening (23 malignant and 13 benign) and 13 were normal. 85 bladders were partially filled with IV contrast material, 4 were completely filled with contrast material, and 3 had no visible contrast material. The bladder conspicuity was medium to high.
The MDCT urography scans used in this study were acquired with GE Healthcare LightSpeed MDCT scanners. Excretory phase images, obtained 12 minutes after the initiation of the first bolus of a split-bolus intravenous contrast injection and two minutes after the initiation of the second bolus of 175 ml of nonionic contrast material at a concentration of 300 mg iodine per ml, were utilized. The images used were acquired at an interval and slice thickness of 1.25 mm or 0.625 mm using 120 kVp and 120–280 mA. Since patients were not turned prior to image acquisition, dependently layering IV contrast material that had been excreted into the renal collecting systems partially filled the bladder on the CTU images.
2.5 Evaluation methods
Segmentation performance was evaluated by quantitative methods comparing computer segmentation result to the reference standard. The 3D hand-segmented contours for all 173 cases were obtained as reference standard in this study. An experienced radiologist provided manual outlines on the CT slices for all cases using a graphic user interface (GUI). The radiologist outlined the bladder on every 2D CT slice on which the bladder was visible, resulting in a 3D surface contour. There were a total of 16,197 slices manually outlined by the radiologist for the 173 bladders. Several performance metrics (Street et al., 2007) that quantify the similarity of a pair of contours were used for evaluating the system, including the volume intersection ratio, the volume error, the average minimum distance, and the Jaccard index (Jaccard, 1912), between the hand-segmented contours and computer segmented contours.
The volume intersection ratio is the ratio of the intersection between the reference volume and the given volume to the reference volume:
| (19) |
where VG is the volume enclosed by the reference standard contour G and VU is the volume enclosed by the contour U being evaluated. A value of 1 indicates that VU completely overlaps with VG, while a value of 0 means VU and VG do not overlap.
The volume error is the ratio of the difference between the reference volume and the given volume to the reference volume:
| (20) |
where positive error indicates under-segmentation and negative error indicates over-segmentation. Because the over- and under-segmentation tend to mask the actual deviations from the reference standard when the average is taken, the absolute error |E3D| is also calculated.
The average distance, AVDIST, is the average of the distances between the closest points of the two contours already defined in Section 2.2.1, Equation (8). For this calculation, G is the 3D reference contour marked by the radiologist and U is the 3D contour being evaluated.
The Jaccard index is defined as the ratio of the intersection between the reference volume and the segmented volume to the union of the reference volume and the segmented volume:
| (21) |
A value of 1 indicates that VU completely overlaps with VG, whereas a value of 0 implies VU and VG are disjoint.
No single measure can completely describe the agreement between the two volumes; however, by combining two performance measures, different aspects of the performance can be assessed. For example, the Jaccard index, the overlap and non-overlap fractions with the reference standard, can be derived from the volume intersection ratio and the volume error (Way et al., 2006).
3. RESULTS
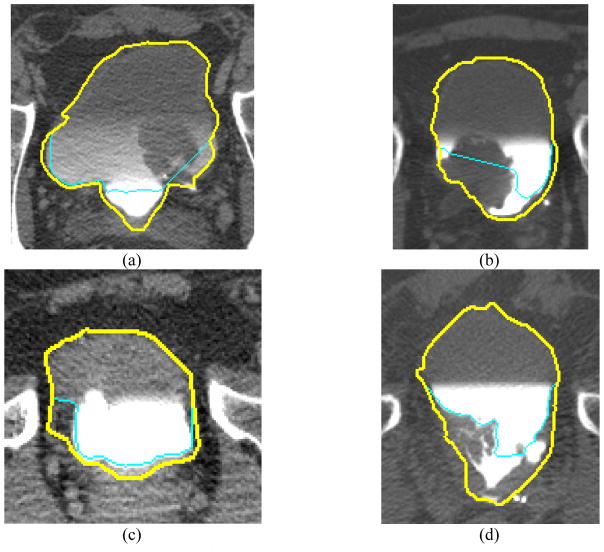
Examples of CLASS NC and C region contours and CLASS with LCR segmentation are shown in Figure 9. The segmentation performance measures averaged over the cases in the training and test sets, respectively, are presented in Tables 2 and 3.
Figure 9.
Bladder segmentation of test cases using CLASS with and without LCR. (a) CLASS missed portions of the malignant lesion and failed to segment the bladder boundary in the C region, whereas LCR segmented the bladder more accurately. (b) The large malignant lesion in the C region was mostly missed by CLASS; however, LCR fully segmented the lesion. (c) Benign wall thickening was missed by CLASS, but LCR segmented the bladder more accurately. (d) A difficult malignant lesion was missed with CLASS, but LCR propagated the contour through the lesion to the bladder boundary. The thin contour represents segmentation results using CLASS. The thick contour represents segmentation result from CLASS with LCR.
Table 2.
Segmentation results from CLASS with and without LCR, averaged over the 81 bladders in the training set.
| Segmentation Method (Training set) | Volume intersection ratio | Volume error | Absolute volume error | Average minimum distance | Jaccard index |
|---|---|---|---|---|---|
| R3D | E3D | |E3D| | AVDIST | JACCARD3D | |
| CLASS with LCR | 84.2±11.4% | 8.2±17.4% | 13.0±14.1% | 3.5±1.9 mm | 78.8±11.6% |
| CLASS without LCR | 75.1±13.2% | 18.7±19.5% | 22.5±14.9% | 4.3±2.2 mm | 71.0±12.6% |
Table 3.
Segmentation results from CLASS with and without LCR, averaged over the 92 bladders in the test set.
| Segmentation Method (Test set) | Volume intersection ratio | Volume error | Absolute volume error | Average minimum distance | Jaccard index |
|---|---|---|---|---|---|
| R3D | E3D | |E3D| | AVDIST | JACCARD3D | |
| CLASS with LCR | 78.0±14.7% | 16.4±16.9% | 18.2±15.0% | 3.8±2.3 mm | 73.8±13.4% |
| CLASS without LCR | 67.3±14.3% | 29.3±15.9% | 29.4±15.6% | 4.9±2.6 mm | 65.0±13.3% |
CLASS with LCR consistently performed better than CLASS for the data set used. In comparison to CLASS only, CLASS with LCR resulted in larger volume intersection ratio, smaller volume error, smaller absolute volume error, smaller average distance error, and larger Jaccard index for both training and test sets. The standard deviations for all 5 measures were smaller with LCR than without LCR for the training set. For the test set, the standard deviations were smaller with LCR than without LCR for the absolute volume error and average distance error. The differences for all of the performance measures between the two methods were statistically significant for both the training and test sets (p<0.001 by two-tailed paired t-test) at alpha level of 0.01 after the Bonferroni correction for the 5 comparisons.
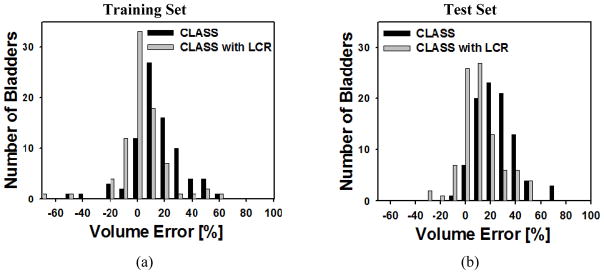
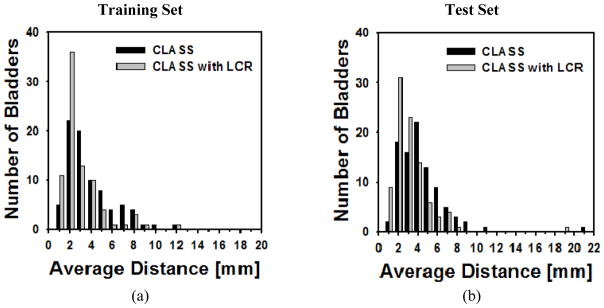
The histograms for volume intersection ratio, volume error, and average distance for both the training and test sets are shown in Figures 10, 11, and 12, respectively.
Figure 10.
Histogram of percent volume intersection ratio for (a) the training set and (b) the test set for CLASS and CLASS with LCR. The improvement by LCR was statistically significant (p<0.001) for both the training and test sets.
Figure 11.
Histogram of the volume error for (a) the training set and (b) the test set for CLASS and CLASS with LCR. The improvement by LCR was statistically significant (p<0.001) for both the training and test sets.
Figure 12.
Histogram of the average minimum distance for (a) the training set and (b) the test set for CLASS and CLASS with LCR. The improvement by LCR was statistically significant (p<0.001) for both the training and test sets.
Of the 81 cases in the training set, CLASS with LCR had 65 bladders with a volume intersection ratio greater than 80% whereas CLASS had 34 bladders (Figure 10(a)). There were 63 bladders whose absolute volume error for the training set was less than 20% for CLASS with LCR, compared to 41 bladders for CLASS only (Figure 11(a)). Forty-seven bladders in the training set had an average distance less than 3 mm for CLASS with LCR, compared to 27 bladders for CLASS only (Figure 12(a)).
Of the 92 test cases, CLASS with LCR had 51 bladders with a volume intersection ratio greater than 80% whereas CLASS had only 18 bladders (Figure 10(b)). There were 60 bladders whose absolute volume error for the test set was less than 20% for CLASS with LCR, compared to 28 bladders for CLASS only (Figure 11(b)). Forty bladders in the test set had an average distance less than 3 mm for CLASS with LCR, compared to 20 bladders for CLASS only (Figure 12(b)).
4. DISCUSSION
In this study, CLASS with the new LCR method was applied to a data set containing bladders in CTUs having a wide range of image quality. Most of the bladders were partially filled with excreted contrast material; however, some bladders were entirely filled with excreted contrast material and others did not contain any contrast-enhanced urine due to variation in timing for image acquisition. CLASS with LCR performed better than CLASS for every performance measure. The LCR more than doubled the number of bladders with volume intersection ratio greater than 80%, absolute volume error less than 20%, and average distance less than 3 mm for the test set. The number of bladders with the above mentioned performance measures also increased for each measure in the training set. Figures 8 and 9 show examples of difficult bladder cases successfully improved by LCR. The bladders in Figure 8 and Figures 9(a) and 9(b) contained large malignant lesions in the contrast-enhanced area that CLASS had missed. The benign wall thickening in Figure 9(c) was missed by CLASS. The case in Figure 9(d) contains inhomogeneous malignant lesion which was also missed by CLASS. In each of these cases, CLASS could not accurately segment the C portion of the bladder due to the strong edge between the large abnormality and the contrast agent, resulting in segmentation excluding portions of the bladder and the lesion. LCR propagated the conjoint contour to the proper bladder boundary, enclosing the previously mentioned features, thus achieving a more accurate segmentation.
It is difficult to perform direct comparison to the previous methods by other investigators summarized in the Introduction due to the differences in the data sets and their varying difficulty. A relative comparison was performed to only one of the studies (Chai et al., 2012), in which quantitative results were reported. Chai et. al. (Chai et al., 2012) achieved Jaccard indices of 70.5% and 77.7% for their automatic and semiautomatic methods, respectively, using 95 scans of 8 patients for training, and using 233 scans of 22 patients for testing. In comparison, the Jaccard indices of our CLASS with LCR method were 78.8% and 73.8% for the training (81 patients) and test set (92 patients), respectively. CLASS with LCR achieved accuracy comparable to that of Chai et. al. (Chai et al., 2012) while using a larger independent data set.
There are cases for which LCR still could not segment the bladder accurately. LCR did not enhance the non-contrast filled region contour from CLASS; therefore, the method was not able to reliably stop the NC region contour at the bladder boundary when a complex background is present, as shown in the upper boundary of the example in Figure 13(a). LCR did, however, propagate the C region contour L properly to the correct lower bladder boundary, compared to CLASS. In addition, LCR does not solve the problem if errors in the NC region contour cause the initial conjoining segment used for EDWP to cut across the bone. As shown in Figure 13(b), LCR propagated the contour L to include a lesion and portions of the contrast-filled region of the bladder that CLASS had missed; however, the inaccuracy in the NC region contour created an initial conjoint segment through the bone. LCR was not able to identify this error, and propagated the contour segment within the bone, due to the similar appearance of the bone and the bone marrow to a C region containing a lesion, causing leaking in the segmentation. Better criteria to prevent leakage into adjacent normal tissue and bone are needed. It may also be necessary to develop additional local refinement methods to improve segmentation accuracy.
Figure 13.
Bladder segmentation using CLASS with LCR. (a) shows leaking in the NC contour due to complex boundary. (b) shows segmentation leaking into the bone. The thin contour represents segmentation results using CLASS. The thick contour represents segmentation result from CLASS with LCR.
5. CONCLUSION
The results show that the LCR method can significantly improve the segmentation of bladders by CLASS on CTU scans. LCR propagates the CLASS contour of the contrast-filled region of the bladder and propagates the conjoint contour of the contrast and non-contrast filled region to the correct bladder boundaries using both 2D and 3D information. Further work is underway to prevent leaking of the segmentation to the adjacent organs or bone edges. This study is a step toward the development of a reliable and efficient system for segmentation of bladders, which is a critical component of a CAD system for detection of urothelial lesions imaged with CT urography.
Acknowledgments
This work is supported by USPHS Grant R01CA134688.
References
- American Cancer Society. What are the key statistics about bladder cancer? 2013 www.cancer.org.
- Akbar SA, Mortele KJ, Baeyens K, Kekelidze M, Silverman SG. Multidetector CT urography: Techniques, clinical applications, and pitfalls. Seminars in Ultrasound CT and MRI. 2004;25:41–54. doi: 10.1053/j.sult.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Caoili EM, Cohan RH, Korobkin M, Platt JF, Francis IR, Faerber GJ, Montie JE, Ellis JH. Urinary tract abnormalities: Initial experience with multi-detector row CT urography. Radiology. 2002;222:353–60. doi: 10.1148/radiol.2222010667. [DOI] [PubMed] [Google Scholar]
- Chai XF, van Herk M, Betgen A, Hulshof M, Bel A. Automatic bladder segmentation on CBCT for multiple plan ART of bladder cancer using a patient-specific bladder model. Phys Med Biol. 2012;57:3945–62. doi: 10.1088/0031-9155/57/12/3945. [DOI] [PubMed] [Google Scholar]
- Duan C, Liang Z, Bao S, Zhu H, Wang S, Zhang G, Chen JJ, Lu H. A coupled level set framework for bladder wall segmentation with application to MR cystography. IEEE Trans Med Imaging. 2010;29:903–15. doi: 10.1109/TMI.2009.2039756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan CJ, Yuan KH, Liu FH, Xiao P, Lv GQ, Liang ZR. An Adaptive Window-Setting Scheme for Segmentation of Bladder Tumor Surface via MR Cystography. IEEE T Inf Technol Biomed. 2012;16:720–9. doi: 10.1109/TITB.2012.2200496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiiski L, Chan H-P, Law Y, Cohan RH, Caoili EM, Cho HC, Zhou C, Wei J. Segmentation of Urinary Bladder in CT Urography (CTU) using CLASS. Proc SPIE. 2012;8315:83150J1–J7. [Google Scholar]
- Hadjiiski LM, Chan H-P, Cohan RH, Caoili EM, Law Y, Cha K, Zhou C, Wei J. Urinary bladder segmentation in CT urography (CTU) using CLASS. Medical Physics. 2013;40 doi: 10.1118/1.4823792. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiiski LM, Sahiner B, Chan H-P, Caoili EM, Cohan RH, Zhou C. Automated segmentation of urinary bladder and detection of bladder lesions in multi-detector row CT urography. Proc SPIE. 2009;7260:72603R1–R7. [Google Scholar]
- Han H, Li L, Duan C, Zhang H, Zhao Y, Liang Z. A unified EM approach to bladder wall segmentation with coupled level-set constraints. Medical Image Analysis. 2013;17:1192–205. doi: 10.1016/j.media.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard P. The distribution of the flora in the alpine zone. New phytologist. 1912;11:37–50. [Google Scholar]
- Li L, Wang Z, Li X, Wei X, LAH, Huang W, Rizvi S, HM, Harrington DP, Liang Z. A new partial volume segmentation approach to extract bladder wall for computer aided detection in virtual cystoscopy. Proc SPIE. 2004;5369:199–206. [Google Scholar]
- Liu WC, Mortele KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: Prevalence and impact on Imaging costs. American Journal of Roentgenology. 2005;185:1051–6. doi: 10.2214/AJR.04.0218. [DOI] [PubMed] [Google Scholar]
- McCarthy CL, Cowan NC. Multidetector CT urography (MD-CTU) for urothelial imaging. Radiology (P) 2002:225–237. [Google Scholar]
- Noroozian M, Cohan RH, Caoili EM, Cowan NC, Ellis JH. Multislice CT urography: State of the art. British Journal of Radiology. 2004;77:S74–S86. doi: 10.1259/bjr/13478281. [DOI] [PubMed] [Google Scholar]
- Park SB, Kim JK, Lee HJ, Choi HJ, Cho K-S. Hematuria: portal venous phase multi detector row CT of the bladder--a prospective study. Radiology. 2007;245:798–805. doi: 10.1148/radiol.2452061060. [DOI] [PubMed] [Google Scholar]
- Street E, Hadjiiski L, Sahiner B, Gujar S, Ibrahim M, Mukherji SK, Chan H-P. Automated Volume Analysis of Head and Neck Lesions on CT Scans Using 3D Level Set Segmentation. Medical Physics. 2007;34:4399–408. doi: 10.1118/1.2794174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakoff GS, Dunn DP, Guralnick ML, Hellman RS, Eastwood D, See WA. Multidetector computerized tomography urography as the primary imaging modality for detecting urinary tract neoplasms in patients with asymptomatic hematuria. Journal of Urology. 2008;179:862–7. doi: 10.1016/j.juro.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Way TW, Hadjiiski LM, Sahiner B, Chan H-P, Cascade PN, Kazerooni EA, Bogot N, Zhou C. Computer-aided diagnosis of pulmonary nodules on CT scans: segmentation and classification using 3D active contours. Medical Physics. 2006;33:2323–37. doi: 10.1118/1.2207129. [DOI] [PMC free article] [PubMed] [Google Scholar]