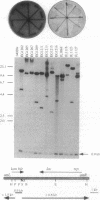
Abstract
The production of antibacterial peptides is a host defense strategy used by various species, including mammals, amphibians, and insects. Successful pathogens, such as the facultative intracellular bacterium Salmonella typhimurium, have evolved resistance mechanisms to this ubiquitous type of host defense. To identify the genes required for resistance to host peptides, we isolated a library of 20,000 MudJ transposon insertion mutants of a virulent peptide-resistant S. typhimurium strain and screened it for hypersensitivity to the antimicrobial peptide protamine. Eighteen mutants had heightened susceptibility to protamine and 12 of them were characterized in detail. Eleven mutants were attenuated for virulence in vivo when inoculated into BALB/c mice by the intragastric route, and 8 of them were also avirulent following intraperitoneal inoculation. The mutants fell into different phenotypic classes with respect to their susceptibility to rabbit defensin NP-1, frog magainin 2, pig cecropin P1, and the insect venom-derived peptides mastoparan and melittin. The resistance loci mapped to eight distinct locations in the genome. Characterization of the mutants showed that one had a defective lipopolysaccharide and another mutant harbored a mutation in phoP, a locus previously shown to control expression of Salmonella virulence genes. Our data indicate that the ability to resist the killing effect of host antimicrobial peptides is a virulence property and that several resistance mechanisms operate in S. typhimurium.
Full text
PDF
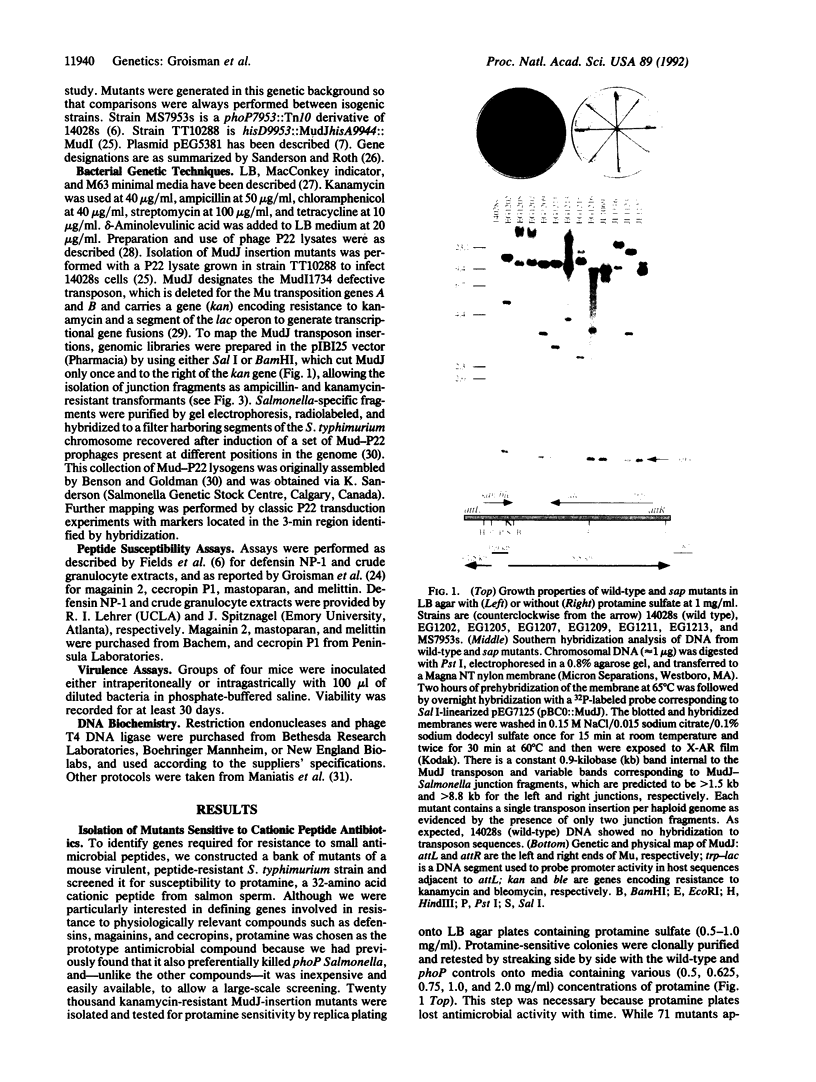
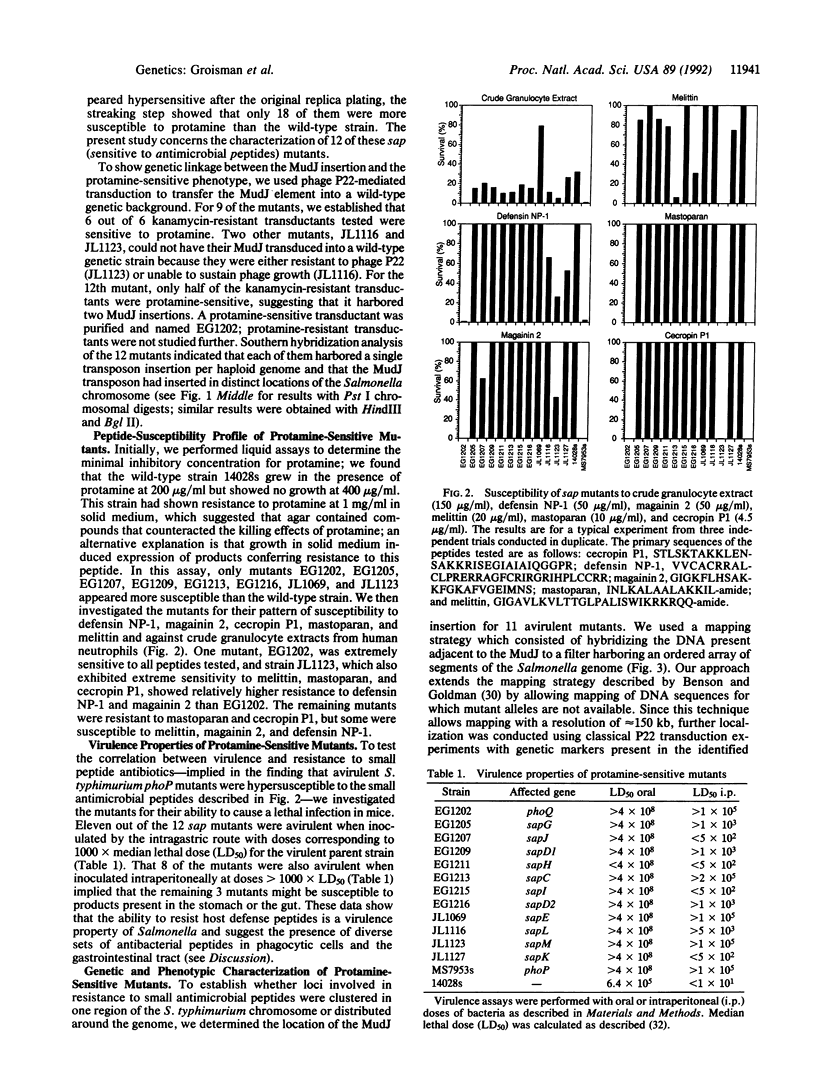
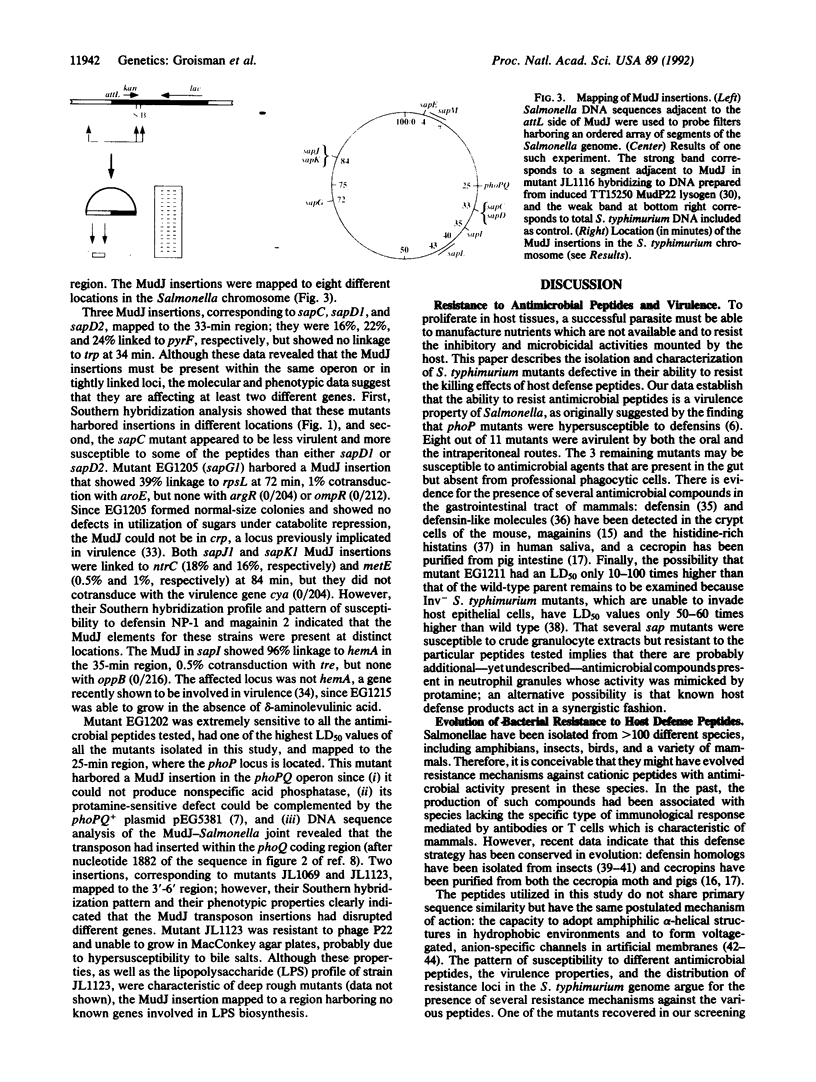

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin W. H., Jr, Hall P., Briles D. E. A hemA mutation renders Salmonella typhimurium avirulent in mice, yet capable of eliciting protection against intravenous infection with S. typhimurium. Microb Pathog. 1991 Oct;11(4):289–295. doi: 10.1016/0882-4010(91)90033-7. [DOI] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Yother J., Hall P., Briles D. E. The Salmonella typhimurium locus mviA regulates virulence in Itys but not Ityr mice: functional mviA results in avirulence; mutant (nonfunctional) mviA results in virulence. J Exp Med. 1991 Nov 1;174(5):1073–1083. doi: 10.1084/jem.174.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N. R., Goldman B. S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992 Mar;174(5):1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight M. A., Holland I. B. Structure and function of haemolysin B,P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990 Jun;4(6):873–880. doi: 10.1111/j.1365-2958.1990.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Faye I., Gudmundsson G. H., Lee J. Y., Lidholm D. A. Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur J Biochem. 1991 Oct 1;201(1):23–31. doi: 10.1111/j.1432-1033.1991.tb16252.x. [DOI] [PubMed] [Google Scholar]
- Bulet P., Cociancich S., Dimarcq J. L., Lambert J., Reichhart J. M., Hoffmann D., Hetru C., Hoffmann J. A. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem. 1991 Dec 25;266(36):24520–24525. [PubMed] [Google Scholar]
- Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 1989 Aug;8(8):2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani R. A., Barker J. L., Zasloff M., Chen H. C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarcq J. L., Zachary D., Hoffmann J. A., Hoffmann D., Reichhart J. M. Insect immunity: expression of the two major inducible antibacterial peptides, defensin and diptericin, in Phormia terranovae. EMBO J. 1990 Aug;9(8):2507–2515. doi: 10.1002/j.1460-2075.1990.tb07430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Imai J., Fujiwara M., Yaeshima T., Kawashima T., Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem. 1990 Jul 5;265(19):11333–11337. [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Metcalf J. A., Gallin J. I., Boxer L. A., Lehrer R. I. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and "specific" granule deficiency. J Clin Invest. 1988 Aug;82(2):552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Chiao E., Lipps C. J., Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Heffron F., Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992 Jan;174(2):486–491. doi: 10.1128/jb.174.2.486-491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Saier M. H., Jr Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990 Jan;15(1):30–33. doi: 10.1016/0968-0004(90)90128-x. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juretić D., Chen H. C., Brown J. H., Morell J. L., Hendler R. W., Westerhoff H. V. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989 Jun 5;249(2):219–223. doi: 10.1016/0014-5793(89)80627-1. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J., Keppi E., Dimarcq J. L., Wicker C., Reichhart J. M., Dunbar B., Lepage P., Van Dorsselaer A., Hoffmann J., Fothergill J. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(1):262–266. doi: 10.1073/pnas.86.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Boman A., Sun C. X., Andersson M., Jörnvall H., Mutt V., Boman H. G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Macias E. A., Rana F., Blazyk J., Modrzakowski M. C. Bactericidal activity of magainin 2: use of lipopolysaccharide mutants. Can J Microbiol. 1990 Aug;36(8):582–584. doi: 10.1139/m90-102. [DOI] [PubMed] [Google Scholar]
- McHugh G. L., Miller C. G. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. doi: 10.1128/jb.120.1.364-371.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Pulkkinen W. S., Selsted M. E., Mekalanos J. J. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect Immun. 1990 Nov;58(11):3706–3710. doi: 10.1128/iai.58.11.3706-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. S., Bevins C. L., Brasseur M. M., Tomassini N., Turner K., Eck H., Zasloff M. Antimicrobial peptides in the stomach of Xenopus laevis. J Biol Chem. 1991 Oct 15;266(29):19851–19857. [PubMed] [Google Scholar]
- Moore K. S., Bevins C. L., Tomassini N., Huttner K. M., Sadler K., Moreira J. E., Reynolds J., Zasloff M. A novel peptide-producing cell in Xenopus: multinucleated gastric mucosal cell strikingly similar to the granular gland of the skin. J Histochem Cytochem. 1992 Mar;40(3):367–378. doi: 10.1177/40.3.1552176. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Xu T., McMillian F. M., Levitz S. M., Diamond R. D., Offner G. D., Troxler R. F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988 Jun 5;263(16):7472–7477. [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Lualdi J. C. A novel mouse gene family coding for cationic, cysteine-rich peptides. Regulation in small intestine and cells of myeloid origin. J Biol Chem. 1990 Jun 15;265(17):9831–9837. [PubMed] [Google Scholar]
- Romeo D., Skerlavaj B., Bolognesi M., Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988 Jul 15;263(20):9573–9575. [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Novotny M. J., Morris W. L., Tang Y. Q., Smith W., Cullor J. S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992 Mar 5;267(7):4292–4295. [PubMed] [Google Scholar]
- Shigenaga T., Muta T., Toh Y., Tokunaga F., Iwanaga S. Antimicrobial tachyplesin peptide precursor. cDNA cloning and cellular localization in the horseshoe crab (Tachypleus tridentatus). J Biol Chem. 1990 Dec 5;265(34):21350–21354. [PubMed] [Google Scholar]
- Wolff A., Moreira J. E., Bevins C. L., Hand A. R., Fox P. C. Magainin-like immunoreactivity in human submandibular and labial salivary glands. J Histochem Cytochem. 1990 Nov;38(11):1531–1534. doi: 10.1177/38.11.2212614. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]