Abstract
To investigate the cytokine profile as biomarkers in the gingival crevicular fluid (GCF) of chronic periodontitis (CP) patients with and without obesity, MEDLINE/PubMed, EMBASE, ScienceDirect, and SCOPUS databases were combined with handsearching of articles published from 1977 up to May 2016 using relevant MeSH terms. Meta-analyses were conducted separately for each of the cytokines: resistin, adiponectin, TNF-α, leptin, IL-6, IL-8, and IL-1β. Forest plots were produced reporting standardized mean difference of outcomes and 95% confidence intervals. Eleven studies were included. Three studies showed comparable levels of leptin among obese and nonobese patients with CP. Four studies reported comparable levels of interleukin- (IL-) 6 and resistin whereas five studies reported comparable levels of adiponectin. Two studies reported similar levels of CRP in patients with periodontitis with and without obesity. One study showed higher levels of tumor necrosis factor-alpha in obese patients with CP. One study showed higher levels of IL-1β and IL-8 in obese patients with CP. The level of localized periodontal inflammation may have a greater influence on the GCF proinflammatory biomarker levels as compared to systemic obesity. Whether patients having chronic periodontitis with obesity have elevated proinflammatory GCF biomarkers levels compared to nonobese individuals remains debatable.
1. Introduction
Obesity is described as a condition associated with expansion in adipocytes and increased infiltration of macrophage cells in the adipose tissues, defining the inflammatory state [1, 2]. Substantial evidence in the form of in vivo and in vitro studies has demonstrated a strong association between chronic periodontitis (CP) and obesity [3–6]. The underlying mechanisms of periodontitis in obesity are not well understood; however, it is suggested that the increased levels of proinflammatory cytokines as biomarkers (such as interleukin- (IL-) 6, IL-1β) in the gingival crevicular fluids (GCF) of obese individuals are associated with poor periodontal health [7].
Cytokines are low molecular weight water-soluble glycoprotein biomarkers secreted by hematopoietic and nonhematopoietic cells in response to infection. Inflammatory biomarkers which are induced during inflammatory responses have been associated with the onset or progression of tissue insult [8, 9]. It is suggested that proinflammatory biomarkers show pleiotropic effect and can target specific cells by controlling activation of cells, cell proliferation, and function in the periodontium [10, 11]. As a result, raised proinflammatory biomarkers levels such as tumor necrosis factor-alpha (TNF-α), IL-1β, IL-6, and IL-8 cause periodontal tissue destruction [12]. The reason for studying these proinflammatory cytokines in obesity is to validate the association of the same cytokines which are actively involved in jeopardizing periodontal tissues by mediating alveolar bone resorption and collagen destruction [12].
Obese patients are associated with a state of elevated systemic inflammatory burden due to increased serum proinflammatory cytokine levels [13, 14]. In addition, studies have shown increased susceptibility of inflammatory periodontal tissue destruction in overweight or obese patients as compared to healthy individuals [15]. Moreover, inflammatory cytokine mediated mechanisms are implicated in periodontal inflammatory conditions [11]. In this context, it may be hypothesized that the GCF cytokine profile in obese patients with CP should be elevated as compared to nonobese individuals with periodontal disease. In the study by Modéer et al. [16] GCF IL-1β and IL-8 levels among obese patient with CP were significantly higher as compared to nonobese CP patients. In contrast, Duzagac et al. [17] showed comparable GCF cytokine profile among obese and nonobese patients with CP. There appears to be a controversy with regard to GCF cytokine profile in chronic periodontitis patients with and without obesity. Therefore, the aim of this study was to systematically review the GCF cytokine profile in CP patients with and without obesity.
2. Methods
2.1. Protocol and Registration
This review was registered at the National Institute for Health Research PROSPERO, International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO/, registration number CRD42015029928). Based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [18], a specific question was constructed. The addressed focused question was “Does the GCF cytokine profile of obese and nonobese patients with chronic periodontitis differ?”
2.2. Selection Criteria
The following eligibility criteria were entailed:
clinical trials (baseline data), cross-sectional and observational (prospective; baseline data) studies in humans (adults and adolescents only) using any type of assay method;
cytokine profile in the GCF of patients with chronic periodontitis with and without obesity.
In vitro studies; animal studies; studies providing analyses of cytokines in fluids other than GCF; studies that reported cytokine profile among obese only with no normal weight controls; letters to the editor; and review papers and unpublished articles were excluded.
2.3. Search Strategy
Two reviewers (Z. A. and T. A.) searched the following electronic databases: (1) MEDLINE, (2) PubMed, (3) EMBASE, (4) ISI Web of Knowledge, (5) ScienceDirect, and (6) SCOPUS from 1977 up to May 2016 for appropriate articles addressing the focused question. A structured approach to literature search algorithm was used to explore databases, in which Boolean operators and the asterisk were used as truncation (“Periodontitis” [MeSH terms] OR “Chronic Periodontitis” [MeSH terms] OR “Periodontal Diseases” [MeSH terms] AND (“Cytokines” [MeSH terms] OR “Adipokines” [MeSH terms] OR “Adipocytokines” OR “Biomarkers” OR “Pro-inflammatory”) AND (“Gingival Crevicular Fluid” OR “Crevicular Fluid” [MeSH terms] OR “Sulcular Fluid”) AND (“Obesity” [MeSH terms] OR “Obese” OR “Body Mass Index [MeSH terms]” OR “Adiposity” OR “Body Weight” OR “Waist circumference” OR “Waist-Hip Ratio”).
2.4. Screening and Selection
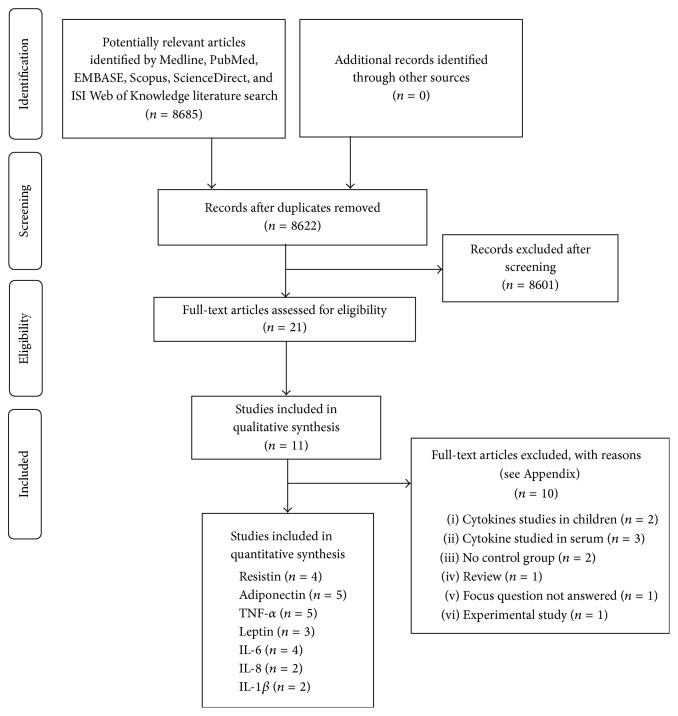
Two reviewers (Z. A. and F. V.) independently screened titles and abstracts for eligible papers. If information relevant to the eligibility criteria was not available in the abstract or if the title was relevant but the abstract was not available, the paper was selected for full reading of the text. Next, full-text papers that fulfilled the eligibility criteria were identified and included in the review. Following that, reference lists of original studies were handsearched to identify articles that could have been missed during the electronic search. Handsearching of the following journals was performed: Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Journal of Dental Research, Journal of Periodontology and Implant Dentistry, Clinical Oral Investigations, Brazilian Dental Journal, Saudi Medical Journal, Journal of Indian Society of Periodontology, Cytokine, Journal of Investigative and Clinical Dentistry, Disease Markers, and International Journal of Pediatric Obesity. Papers that fulfilled all of the selection criteria were processed for data extraction. Figure 1 describes the screening process according to PRISMA guidelines for flow diagram [18].
Figure 1.
PRISMA flow diagram for studies retrieved through the searching and selection process.
2.5. Data Extraction and Quality Assessment
Two reviewers (Z. A. and F. V.) undertook this independently. The information from the accepted studies was tabulated according to the study designs, subject characteristics, sample characteristics, cytokines investigated, and main outcomes. Data collected were based on the focused question outlined for the present systematic review. Baseline data that compared the levels of cytokines among obese and nonobese patients in prospective studies were also included in the review. The reviewers crosschecked all extracted data. Any disagreement was resolved by discussion until consensus was reached. The quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment scale for Observational Studies [27] (Table 3), a validated scale for evaluating the quality of observational and nonrandomized studies. This scale uses a star system to evaluate the studies on three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for included studies respectively.
Table 3.
Quality assessment using Newcastle-Ottawa scale of the included studies.
| Investigators | Selection | Comparability | Exposure | Total score |
|---|---|---|---|---|
| Duzagac et al. [17] | ☆☆☆☆ | ☆ | ☆☆☆ | 8 |
| Gonçalves et al. [19] | ☆☆☆☆ | ☆☆ | ☆☆ | 8 |
| Pradeep et al. [20] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Pradeep et al. [21] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Öngöz Dede et al. [22] | ☆☆☆ | ☆☆☆ | 6 | |
| Patel and Raju [23] | ☆☆☆☆ | ☆ | ☆ | 6 |
| Fadel et al. [24] | ☆☆☆ | ☆☆ | ☆ | 6 |
| Zimmermann et al. [6] | ☆☆☆☆ | ☆☆ | ☆ | 7 |
| Pradeep et al. [25] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Pradeep et al. [26] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Modéer et al. [16] | ☆☆☆ | ☆ | ☆☆ | 6 |
2.6. Statistical Analyses
Meta-analyses were conducted separately for each of the cytokines: resistin, adiponectin, TNF-α, leptin, IL-6, IL-8, and IL-1β. Heterogeneity among the included studies for each cytokine outcome was assessed using the Q-statistic and I 2 statistic [28]. Outcome measures for each inflammatory mediator were combined with a random-effects model utilizing the DerSimonian-Laird method due to its robustness in comparison to fixed-effects models in the case of small sample sizes [29]. Forest plots were produced reporting standardized mean difference (SMD) of outcomes and 95% confidence intervals (CI). The pooled effect was considered significant if P value was <0.05. All above statistical analyses were carried out by a specialized statistical software (MedCalc Software—B-8400 Ostend v. 15.11.04, Belgium).
3. Results
3.1. Study Selection
From an original yield of 8685 articles, a total of 21 studies were accepted for full-text review. After full-text review, 10 more studies were excluded that did not fulfil the inclusion criteria (see Appendix with reasons for exclusion). A total of 11 studies [6, 16, 17, 19–26] were included in the present systematic review. All studies were performed at either university clinics [6, 16, 17, 19–23, 25, 26] or health care centres [24]. The kappa value for interreviewer agreement was (95% confidence interval): 0.82 (0.78–0.87).
3.2. Qualitative Results of Studies
Eleven studies [6, 16, 17, 19–26] included in the present review enlisted eight cross-sectional [6, 16, 20, 21, 23–26] and three prospective intervention studies [17, 19, 22] (Table 1). The total number of participants in the included studies ranged between 40 and 104 individuals with mean age ranging between 14.5 and 51.5 years. These studies reported number of female participants, which ranged between 19 and 57 individuals. The number of obese CP and nonobese CP patients ranged between 10 and 52 individuals, respectively. Two studies [16, 24] collected GCF only while other six studies [6, 17, 19, 23, 25, 26] collected both GCF and blood samples. Two studies [20, 21] collected GCF and tear fluid for the evaluation of cytokine levels. All studies [6, 16, 17, 19–26] employed commercial enzyme-linked immunosorbent assay (ELISA) for the detection of cytokine levels.
Table 1.
General characteristics of included studies.
| Author et al., year | Study design; setting; country | Number of patients | Mean age/age range in years | Gender (M/F) | Periodontitis diagnostic criteria | GCF sample site | Sample characteristics: [sample type; collection tool; and storage temperature] |
|---|---|---|---|---|---|---|---|
| Duzagac et al. [17], 2015 | Prospective non-RCT; university clinic; Turkey | 45 | Group 1: 40.66 (28–52) Group 2: 41.06 (28–51) Group 3: 39.66 (30–52) |
19/26 | PD ≥ 4 mm in ≥30% sites, BOP in ≥50% of sites, CAL > 2 mm in ≥20% sites, and radiographic evidence of bone loss | NA | GCF; paper strips; −80°C |
|
| |||||||
| Gonçalves et al. [19], 2015 | Prospective non-RCT; university clinic; Brazil | 40 | Group 1: 50.0 (±4.5) Group 2: 48.5 (±9.3) |
21/19 | PD and CAL ≥ 4 mm in >30% teeth | Two noncontiguous deep sites (PD and CAL ≥ 5 mm with BoP) | GCF; paper strips; −80°C |
|
| |||||||
| Pradeep et al. [20], 2015 | Cross-sectional; university clinic; India | 40 | Group 1: 32.5 (25–40) Group 2: 30.9 (25–40) Group 3: 31.0 (25–40) Group 4: 31.4 (25–40) |
20/20 | PD ≥ 5 mm, GI > 1, and CAL ≥ 3 mm with clinical signs of inflammation | NA | GCF; paper strips; −70°C |
|
| |||||||
| Pradeep et al. [21], 2016 | Cross-sectional; university clinic; India | 40 | Group 1: 33.8 (±3.9) Group 2: 35.1 (±3.9) Group 3: 35.6 (±3.7) Group 4: 34.3 (±4.06) |
20/20 | PD ≥ 5 mm, GI > 1, and CAL ≥ 3 mm with clinical signs of inflammation | NA | GCF; paper strips; −70°C |
|
| |||||||
| Öngöz Dede et al. [22], 2016 | Prospective non-RCT; university clinic; Turkey | 90 | Group 1: 47.13 (34–60) Group 2: 38.47 (26–51) Group 3: 35.73 (25–55) Group 4: 31.53 (25–53) Group 5: 41.33 (25–50) Group 6: 29.60 (25–33) |
43/47 | GI ≥ 2, PD and CAL ≥ 5 mm, and bone loss affecting >30% teeth | ≥5 mm CAL, ≥6 mm PD, and ≥30% bone loss | GCF; paper strips; −40°C |
|
| |||||||
| Patel and Raju [23], 2014 | Cross-sectional; university clinic; India | 90 | Group 1: NA (23–54) Group 2: NA (23–54) Group 3: NA (23–54) |
45/45 | GI > 1, PD ≥ 5 mm, CAL ≥ 3 mm, and evidence of radiographic bone loss | CAL ≥ 3 mm | GCF; micropipettes; 1 μL; −70°C |
|
| |||||||
| Fadel et al. [24], 2014 | Cross-sectional; obesity clinic; Sweden | 55 | Group 1: 15.0 (±1.0) Group 2: 16.0 (±2.0) |
29/26 | NA | NA | GCF; paper strips; −80°C |
|
| |||||||
| Zimmermann et al. [6], 2013 | Cross-sectional; University clinic; Brazil | 78 | Group 1: 51.5 (±7.6) Group 2: 47.8 (±7.7) Group 3: 43.2 (±7.4) Group 4: 42.9 (±7.2) |
21/57 | ≥30% sites with PD and CAL ≥ 4 mm and ≥4 noncontagious teeth with ≥1 site with PD and CAL ≥ 5 mm | Two noncontiguous deep sites (PD and CAL ≥ 5 mm with BoP) | GCF; paper strips; 4 μL; −80°C |
|
| |||||||
| Pradeep et al. [25], 2013 | Cross-sectional; university clinic; India | 40 | Group 1: 31.6 (25–45) Group 2: 32.8 (25–45) Group 3: 33.2 (25–45) Group 4: 31.4 (25–45) |
20/20 | PD ≥ 5 mm, GI > 1, and CAL ≥ 3 mm with clinical signs of inflammation | NA | GCF; paper strips; −70°C |
|
| |||||||
| Pradeep et al. [26], 2012 | Cross-sectional; university clinic; India | 40 | Group 1: 36.8 (25–45) Group 2: 35.2 (25–45) Group 3: 35.2 (25–45) Group 4: 32.4 (25–45) |
20/20 | PD ≥ 5 mm, GI > 1, and CAL ≥ 3 mm with clinical signs of inflammation | NA | GCF; paper strips; −70°C |
|
| |||||||
| Modéer et al. [16], 2011 | Cross-sectional; university clinic; Sweden | 104 | Group 1: 14.5 (11.0–17.9) Group 2: 14.5 (10.9–17.1) |
58/46 | ≥1 sites with PD > 4 mm and alveolar bone loss ≥ 2 mm | NA | GCF; paper strips; −70°C |
RCT: randomized clinical trial, M/F: male to female ratio, GCF: gingival crevicular fluid, PD: pocket depth, CAL: clinical attachment loss, BoP: bleeding on probing, GI: gingival index, and NA: not available.
Four studies reported similar levels of IL-6 [6, 17, 19, 24] and five studies reported similar levels of adiponectin [6, 16, 17, 19, 24] between obese and nonobese individuals with CP, whereas 3 studies reported comparable levels of GCF leptin among CP patients with and without obesity [6, 19, 24]. One study [6] showed higher levels of TNF-α in obese CP subjects as compared to nonobese CP subjects. However, in four studies [16, 17, 19, 24], TNF-α was comparable among CP patients with and without obesity. Fadel et al. [24] showed similar levels of IL1-β, IL-8, and plasminogen activator inhibitor-1 (PAI-1) in obese and nonobese patients with CP. Modéer et al. [16] showed higher levels of IL-1β and IL-8 in obese CP patients and similar levels of PAI-1 in obese CP patients in comparison to nonobese patients with CP. Resistin concentration was found to be similar between obese and nonobese patients with CP in four studies [6, 19, 23, 24]. Two studies [25, 26] reported similar levels of CRP in periodontitis patients with and without obesity. Overall, a total of 8 studies [6, 16, 17, 19, 23–26] showed comparable cytokine levels among CP subjects with and without obesity, whereas a total of 5 studies [6, 16, 20, 25, 26] showed significantly raised cytokine levels in obese CP as compared to nonobese CP subjects (Table 2).
Table 2.
Cytokine profile in the crevicular fluid among study groups.
| Author et al., year | Study groups | Type of assay | Cytokines evaluated | Main outcomes |
|---|---|---|---|---|
| Duzagac et al. [17], 2015 | Group 1: OBCP (n = 15) Group 2: NBCP (n = 15) Group 3: NBNP (n = 15) |
ELISA | Adiponectin, IL-6, TNF-α, and IL-10 | GCF concentrations of adiponectin, IL-6, TNF-α, and IL-10 were comparable in OBCP and NBCP |
|
| ||||
| Gonçalves et al. [19], 2015 | Group 1: OBCP (n = 20) Group 2: NBCP (n = 20) |
ELISA | Resistin, adiponectin, leptin, TNF-α, and IL-6 | GCF concentrations of adiponectin, leptin, IL-6, TNF-α, and resistin were comparable in OBCP and NBCP |
|
| ||||
| Pradeep et al. [20], 2015 | Group 1: OBCP (n = 10) Group 2: NBCP (n = 10) Group 3: OBNP (n = 10) Group 4: NBNP (n = 10) |
ELISA | Lipocalin-2 | GCF lipocalin-2 concentrations were higher in OBCP compared to NBCP |
|
| ||||
| Pradeep et al. [21], 2016 | Group 1: OBCP (n = 10) Group 2: NBCP (n = 10) Group 3: OBNP (n = 10) Group 4: NBNP (n = 10) |
ELISA | Vaspin | GCF vaspin levels were comparable in OBCP and NBCP |
|
| ||||
| Öngöz Dede et al. [22], 2016 | Group 1: OBCP (n = 15) Group 2: NBCP (n = 15) Group 3: OBG (n = 15) Group 4: NBG (n = 15) Group 5: OBNP (n = 15) Group 6: NBNP (n = 15) |
ELISA | 8-OHdG | GCF 8-OHdG levels were comparable in OBCP and NBCP |
|
| ||||
| Patel and Raju [23], 2014 | Group 1: OBCP (n = 30) Group 2: NBCP (n = 30) Group 3: NBNP (n = 30) |
ELISA | Resistin | GCF resistin levels were comparable in OBCP and NBCP |
|
| ||||
| Fadel et al. [24], 2014 | Group 1: OBCP (n = 27) Group 2: NBCP (n = 28) |
ELISA | IL-1β, IL-6, IL-8, TNF-α, leptin, resistin, PAI-1, adiponectin, and adipsin | GCF levels of IL-1β, IL-6, IL-8, TNF-α, leptin, resistin, PAI-1, adiponectin, and adipsin were comparable in OBCP and NBCP |
|
| ||||
| Zimmermann et al. [6], 2013 | Group 1: OBCP (n = 20) Group 2: NBCP (n = 20) Group 3: OBNP (n = 18) Group 4: NBNP (n = 20) |
ELISA | Resistin, adiponectin, leptin, TNF-α, and IL-6 | GCF concentrations of resistin, adiponectin, leptin, and IL-6 were comparable in OBCP and NBCP TNF-α level was higher in OBCP compared to NBCP |
|
| ||||
| Pradeep et al. [25], 2013 | Group 1: OBCP (n = 10) Group 2: NBCP (n = 10) Group 3: OBNP (n = 10) Group 4: NBNP (n = 10) |
ELISA | MCP-4, hsCRP | GCF levels of MCP-4 were higher in OBCP as compared to NBCP GCF levels of hsCRP were comparable between OBCP and NBCP |
|
| ||||
| Pradeep et al. [26], 2012 | Group 1: OBCP (n = 10) Group 2: NBCP (n = 10) Group 3: OBNP (n = 10) Group 4: NBNP (n = 10) |
ELISA | Progranulin, hsCRP | GCF levels of PGRN were higher in OBCP compared to NBCP GCF levels of hsCRP were comparable between OBCP and NBCP |
|
| ||||
| Modéer et al. [16], 2011 | Group 1: OBCP (n = 52) Group 2: NBCP (n = 52) |
ELISA | Adiponectin, PAI-1, IL-1β, IL-8, and TNF-α | IL-1β and IL-8 were higher in OBCP compared to NBCP PAI-1, TNF-α, and adiponectin were comparable in OBCP and NBCP |
OBCP: obese with periodontitis, NBCP: nonobese with periodontitis, OBNP: obese with no periodontitis; OBG: obese with gingivitis; NBG: nonobese with gingivitis, NBNP: nonobese with no periodontitis, GCF: gingival crevicular fluid, ELISA: enzyme-linked immunosorbent assay, TNF-α: tumor necrosis factor-alpha, IL: interleukin, hsCRP: high sensitivity c-reactive protein, 8-OhdG: 8-hydroxy-deoxyguanosine, ICAM: intercellular adhesion molecule, PAI-1: plasminogen activator inhibitor-1, and MCP-4: monocyte chemoattractant protein-4.
3.3. Quantitative Results of the Studies
3.3.1. TNF-α and IL-6
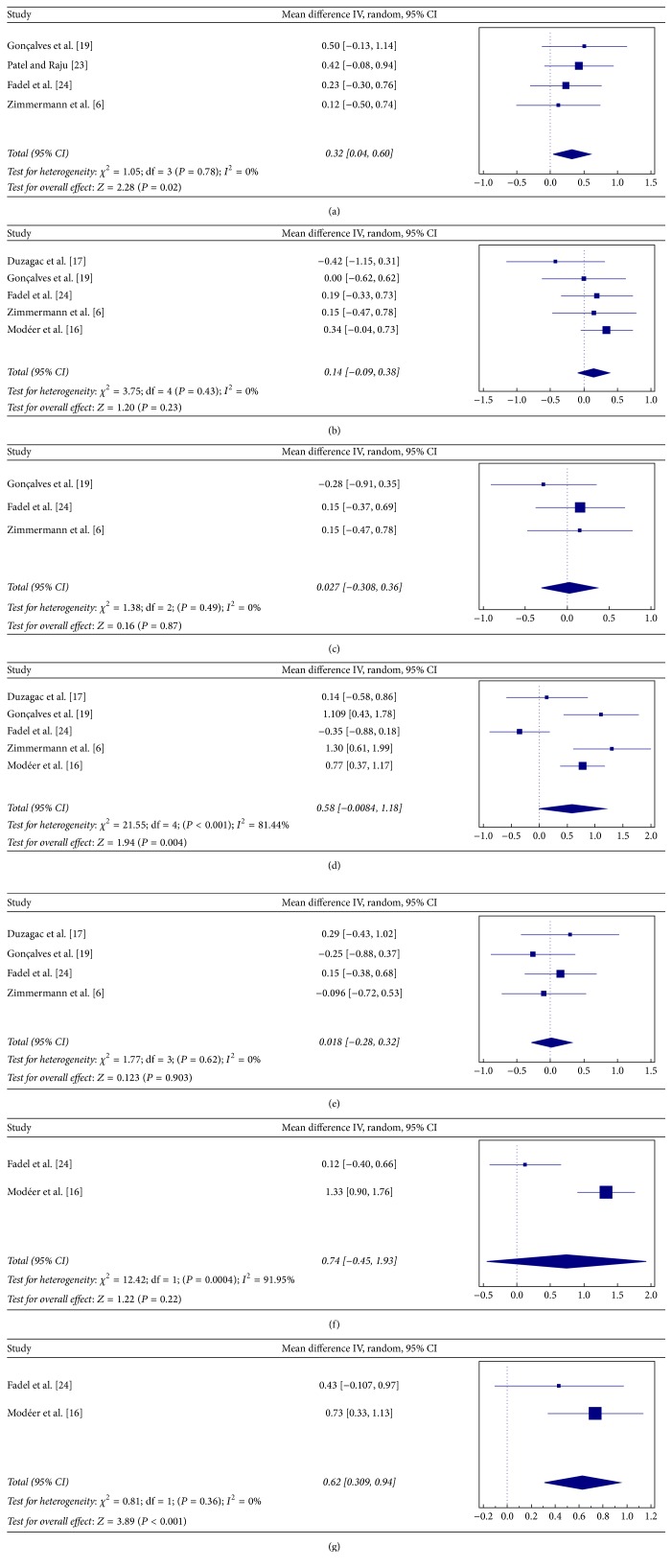
The overall mean difference in TNF-α levels between obese CP and nonobese CP patients was significant (SMD = 0.58; Z = 1.94; and P = 0.004; Figure 2(d)). The variability in differences in TNF-α levels was also significant (Q-value = 21.55; P < 0.001; and I 2 = 81.44%). IL-6 showed no significant difference in the GCF of obese CP and nonobese CP groups in all the four studies [6, 17, 19, 24] (SMD = 0.018; Z = 0.12; and P = 0.903; Figure 2(e)). The heterogeneity in levels of IL-6 between studies was also not significant (Q-value = 1.77; P = 0.62; and I 2 = 0%).
Figure 2.
Forest plots presenting standard mean difference (SMD) of GCF cytokine levels between chronic periodontitis (CP) with and without obesity for (a) resistin; (b) adiponectin; (c) leptin; (d) TNF-α; (e) IL-6; (f) IL-8; and (g) IL-1β.
3.3.2. Resistin, Adiponectin, and Leptin
Obese participants with CP showed significantly higher resistin levels than nonobese CP subjects (SMD = 0.32; Z = 2.28; and P = 0.02; Figure 2(a)). The heterogeneity in resistin between studies was not significant (Q-value = 1.05; P = 0.78; and I 2 = 0%). On the other hand, adiponectin levels showed no significant difference in both overall mean difference (SMD = 0.14; Z = 1.20; and P = 0.23; Figure 2(b)) and heterogeneity (Q-value = 3.75; P = 0.43; and I 2 = 0%) between the studies. These three studies [6, 19, 24] also showed no significant difference in leptin levels among obese and nonobese patients with CP, with mean difference (SMD = 0.027; Z = 0.16; and P = 0.87; Figure 2(c)) and heterogeneity being not significant (Q-value = 1.38; P = 0.49; and I 2 = 0%).
3.3.3. IL-8 and IL1-β
The overall mean difference in IL-8 levels between obese and nonobese patients with CP showed no significant difference (SMD = 0.74; Z = 1.22; and P = 0.22; Figure 2(f)). The heterogeneity in GCF levels of IL-8 between studies was however significant (Q-value = 12.42; P = 0.0004; and I 2 = 91.95%). Obese participants with CP were found to have significantly higher GCF levels of IL1-β than nonobese CP (SMD = 0.628; Z = 3.895; and P < 0.001; Figure 2(g)). However, the heterogeneity for IL1-β between the studies was not significant (Q-value = 0.81; P = 0.36; and I 2 = 0%).
4. Discussion
The present systematic review assessed the GCF cytokine profile in CP patients with and without obesity. Eight studies [6, 16, 17, 19, 23–26] reported similar levels of cytokine (resistin, adiponectin, leptin, IL-6, IL-8, IL-10, IL1β, TNF-α, CRP, and PAI-1) among CP patients with and without obesity, while 5 studies [6, 16, 20, 25, 26] showed significantly higher levels of cytokine (IL-8, IL-1β, TNF-α, progranulin, MCP-4, and lipocalin) in obese CP patients as compared to nonobese CP subjects. Similarly, quantitative analysis showed IL-8, IL-1β, TNF-α, and resistin to be significantly higher in obese CP patients; however, adiponectin, leptin, and IL-6 were found comparable among obese and nonobese CP subjects. In periodontal inflammation, immune cells such as macrophages, leukocytes, and fibroblasts produce proinflammatory cytokines such as matrix metalloproteinases (MMPs), IL-1β, and receptor activator of NF-κB ligand (RANKL) in response to bacterial challenge. These mediators play an essential role in extracellular matrix degradation and osteoclast differentiation and activation, therefore leading to collagen and bone destruction [30–32].
The premise of increased proinflammatory cytokines in obesity is such that the metabolic cells such as adipocytes initiate inflammation by triggering inflammatory signalling pathways [33]. This mediates a modest, low-level induction of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 which occurs in response to excess nutrients. Overtime, this low-grade inflammation may induce the infiltration and activation of immune cells which is characterized by increase in the number of macrophages, mast cells, and T-lymphocytes that results in proinflammatory changes in the tissue environment and the inflammatory pathways and proinflammatory cytokines continue to reinforce. The inflammatory state therefore becomes maintained (chronic) and unresolved [33].
There are several explanations which can be posed regarding the similarity in cytokine levels in the GCF of CP patients with and without obesity. The studies [6, 16, 17, 19–26], which were included in this systematic review, were conducted with an aim to assess the level of cytokines in GCF as risk indicator for periodontal inflammation in obese patients. In the studies included [6, 16, 17, 19–26], the depth of periodontal pockets from which GCF was collected was not standardized among obese and nonobese patients. For instance, collection of GCF sampled sites from PD ≥ 5 mm was reported in some studies [6, 19, 22], whereas other studies did not report probing PD of GCF sampled sites [16, 17, 23–25] (Table 1). This may characterize a bias as level of periodontal inflammation in diseased periodontal pockets is known to influence GCF cytokine levels [34, 35]. Therefore it is hypothesized that the severity of localized periodontal inflammation on GCF cytokine levels in nonobese subjects could have exceeded the impact of obesity on GCF cytokine levels in obese CP patients. Moreover, in nearly half of the studies, the exclusion of patients with systemic diseases was not reported [6, 16, 23, 24]. It may therefore be speculated that the similarity in GCF cytokine profiles in obese and nonobese patients with CP could be associated with covert systemic conditions (such as diabetes) in the otherwise systemically healthy individuals [36, 37]. It may also be proposed that the balance between proinflammatory and anti-inflammatory mediators in periodontal tissues of nonobese subjects may be shifted towards a hyperinflammatory state that could impair the host response against pathogens and periodontal deterioration [38].
It is reported that leptin (expressed from adipocytes) shows an inverse relation with periodontal inflammation demonstrating a protective role in periodontal disease [39–41]. Interestingly, in the present review, GCF leptin levels were found comparable among obese and nonobese subjects [6, 19, 24]. A possible explanation for this may be derived from the fact that leptins release is stimulated by TNF-α, which is increased in CP patients as compared to healthy periodontium [42]. As all subjects included (obese and nonobese) in the studies reviewed had CP, the comparable stimulatory effect of TNF-α on leptin could have resulted in its similar levels in obese and nonobese subjects with CP. Therefore it may be hypothesized that periodontal inflammation may have a greater influence on GCF cytokine levels (including leptin) rather than the increased systemic inflammatory burden due to obesity.
It is well recognized that tobacco smoking has been shown to be deleterious for periodontal health [43, 44]. Studies on GCF assay have also shown cytokine concentrations to be low in habitual tobacco smokers owing to its immunosuppressant state as compared to nonsmokers [45]. Worthy of note, however, is that the subjects included in the studies fulfilling our inclusion criteria were nonsmokers. This again suggests that the intensity of periodontal inflammation alone may mainly be responsible for the increased GCF cytokine concentrations in subjects with periodontitis with and without obesity [46]. Weight management in obese subjects has shown a reduction in systemic inflammatory burden as expressed by lower levels of serum cytokines [47, 48]. It is also reported that weight control could reduce the amounts of MMP-8, MMP-9, and IL-1β in GCF of obese subjects with healthy periodontium [49]. The effect of periodontal therapy on obesity has been reported in recent study [50]; however, the effect of weight control on GCF cytokine profile in obese patients with CP still needs to be assessed. Moreover, the effect of periodontal treatment including recent adjunctive therapies (such as laser and photodynamic therapy) on the levels of proinflammatory needs to be explored [51, 52].
The review of included studies suggests that considerable heterogeneity existed in the studies reviewed (methodology, cytokines assessed, systemic health of subjects, and cytokine collection sites). Therefore, in light of the systematic review and assessment of available data, it remains arguable whether patients having chronic periodontitis with obesity have elevated proinflammatory GCF cytokine levels compared to nonobese individuals.
5. Conclusion
The present review suggests that the level of localized periodontal inflammation may have a greater influence on the GCF proinflammatory biomarker levels as compared to systemic obesity. Whether patients having chronic periodontitis with obesity have elevated proinflammatory GCF biomarkers levels compared to nonobese individuals remains debatable.
Acknowledgments
The authors extend their sincere appreciation to Deanship of Scientific Research at King Saud University for funding this prolific research group (PRG-1437-38).
Appendix
List of Excluded Studies: Reason for Exclusion Is Shown in Parenthesis
Kâ K, Rousseau MC, Lambert M et al. Metabolic syndrome and gingival inflammation in Caucasian children with a family history of obesity. J Clin Periodontol. 2013; 40(11): 986–993 [cytokines studied in children].
Kâ K, M. C. Rousseau, Tran SD et al. Circulating undercarboxylated osteocalcin and gingival crevicular fluid tumour necrosis factor-α in children. J Clin Periodontol. 2014; 41(5): 467–472 [cytokines studied in children].
Buduneli N, Bıyıkoğlu B, Ilgenli T, Buduneli E, Nalbantsoy A, Saraç F, and Kinane DF. Is obesity a possible modifier of periodontal disease as a chronic inflammatory process? A case–control study. J Periodontal Res. 2014; 49(4): 465–471 [cytokines studied in serum].
Fell RA, Zee KY, and Arora M. The correlation of serum and gingival crevicular fluid cytokines in obese subjects. J Int Acad Periodontol. 2013; 15: 20–28 [no control group].
Lundin M, Yucel-Lindberg T, Dahllöf G, Marcus C, and Modéer T. Correlation between TNFa in gingival crevicular fluid and body mass index in obese subjects. Acta Odontol Scand. 2004; 62(5): 273–277 [no control group].
Mendoza-Azpur G, Castro C, Peña L, Guerrero ME, De La Rosa M, Mendes C et al. Adiponectin, leptin and TNF-α serum levels in obese and normal weight Peruvian adults with and without chronic periodontitis. J Clin Exp Dent. 2015; 7(3): e380 [cytokines studied in serum].
Nascimento GG, Leite FR, Correa MB, Peres MA, and Demarco FF. Does periodontal treatment have an effect on clinical and immunological parameters of periodontal disease in obese subjects? A systematic review and meta-analysis. Clin Oral Investig. 2015: 1–9 [review].
Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009; 80(2): 190–201 [focus question not answered].
Zhang L, Meng S, Tu Q, Yu L, Tang Y, Dard MM et al. Adiponectin ameliorates experimental periodontitis in diet-induced obesity mice. PloS One 2014; 9(5): e97824. doi: 10.1371/journal.pone.0097824 [experimental].
Zuza EP, Barroso EM, Carrareto AL, Pires JR, Carlos IZ, Theodoro LH et al. The role of obesity as a modifying factor in patients undergoing non-surgical periodontal therapy. J Periodontol. 2011; 82(5): 676–682 [cytokines studied in serum].
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Zohaib Akram initiated the idea, researched and authored the topic background, compiled the initial draft, revised late drafts, and edited the final draft. Fahim Vohra provided guidance throughout, reviewed the manuscript, extracted the data, edited the text, and helped with data analysis. Tariq Abduljabbar and Mohamed Ibrahim Abu Hassan contributed to the initial conception, were involved in the initial draft, revised late drafts, and edited the article. Fawad Javed provided guidance, contributed to the text, extracted the data, and revised and edited the article.
References
- 1.Heilbronn L. K., Campbell L. V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Current Pharmaceutical Design. 2008;14(12):1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 2.Bouloumié A., Curat C. A., Sengenès C., Lolmède K., Miranville A., Busse R. Role of macrophage tissue infiltration in metabolic diseases. Current Opinion in Clinical Nutrition & Metabolic Care. 2005;8(4):347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 3.Vecchia C. F. D., Susin C., Rösing C. K., Oppermann R. V., Albandar J. M. Overweight and obesity as risk indicators for periodontitis in adults. Journal of Periodontology. 2005;76(10):1721–1728. doi: 10.1902/jop.2005.76.10.1721. [DOI] [PubMed] [Google Scholar]
- 4.Perri R., Nares S., Zhang S., Barros S. P., Offenbacher S. MicroRNA modulation in obesity and periodontitis. Journal of Dental Research. 2012;91(1):33–38. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman A., Kaye E. K., Apovian C., Fung T. T., Nunn M., Garcia R. I. Overweight and obesity predict time to periodontal disease progression in men. Journal of Clinical Periodontology. 2012;39(2):107–114. doi: 10.1111/j.1600-051X.2011.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann G. S., Bastos M. F., Goncxalves T. E. D., Chambrone L., Duarte P. M. Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. Journal of Periodontology. 2013;84(5):624–633. doi: 10.1902/jop.2012.120254. [DOI] [PubMed] [Google Scholar]
- 7.Ylöstalo P., Suominen-Taipale L., Reunanen A., Knuuttila M. Association between body weight and periodontal infection. Journal of Clinical Periodontology. 2008;35(4):297–304. doi: 10.1111/j.1600-051X.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 8.Seymour G. J., Gemmell E. Cytokines in periodontal disease: where to from here? Acta Odontologica Scandinavica. 2001;59(3):167–173. doi: 10.1080/000163501750266765. [DOI] [PubMed] [Google Scholar]
- 9.Hanada T., Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine and Growth Factor Reviews. 2002;13(4-5):413–421. doi: 10.1016/S1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheim J. J., Neta R. Pathophysiological roles of cytokines in development, immunity, and inflammation. The FASEB Journal. 1994;8(2):158–162. doi: 10.1096/fasebj.8.2.8119486. [DOI] [PubMed] [Google Scholar]
- 11.Hasturk H., Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000. 2015;69(1):255–273. doi: 10.1111/prd.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh M. K., Jung M., Kim S. H., et al. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Experimental and Therapeutic Medicine. 2013;6(3):847–851. doi: 10.3892/etm.2013.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg A. H., Scherer P. E. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 14.Wellen K. E., Hotamisligil G. S. Obesity-induced inflammatory changes in adipose tissue. The Journal of Clinical Investigation. 2003;112(12):1785–1788. doi: 10.1172/jci200320514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suvan J. E., Petrie A., Nibali L., et al. Association between overweight/obesity and increased risk of periodontitis. Journal of Clinical Periodontology. 2015;42(8):733–739. doi: 10.1111/jcpe.12421. [DOI] [PubMed] [Google Scholar]
- 16.Modéer T., Blomberg C., Wondimu B., Lindberg T. Y., Marcus C. Association between obesity and periodontal risk indicators in adolescents. International Journal of Pediatric Obesity. 2011;6(3):e264–e270. doi: 10.3109/17477166.2010.495779. [DOI] [PubMed] [Google Scholar]
- 17.Duzagac E., Cifcibasi E., Erdem M. G., et al. Is obesity associated with healing after non-surgical periodontal therapy? A local vs. systemic evaluation. Journal of Periodontal Research. 2016;51(5):604–612. doi: 10.1111/jre.12340. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Gonçalves T. E. D., Zimmermann G. S., Figueiredo L. C., et al. Local and serum levels of adipokines in patients with obesity after periodontal therapy: one-year follow-up. Journal of Clinical Periodontology. 2015;42(5):431–439. doi: 10.1111/jcpe.12396. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep A. R., Nagpal K., Karvekar S., Patnaik K. Levels of lipocalin-2 in crevicular fluid and tear fluid in chronic periodontitis and obesity subjects. Journal of Investigative & Clinical Dentistry. 2015 doi: 10.1111/jicd.12165. [DOI] [PubMed] [Google Scholar]
- 21.Pradeep A. R., Karvekar S., Nagpal K., Patnaik K. Vaspin: a new adipokine correlating the levels of crevicular fluid and tear fluid in periodontitis and obesity. Journal of Investigative and Clinical Dentistry. 2016;7(3):232–238. doi: 10.1111/jicd.12149. [DOI] [PubMed] [Google Scholar]
- 22.Öngöz Dede F., Bozkurt Doğan Ş., Balli U., Avci B., Durmuşlar M. C. The effect of initial periodontal treatment on plasma, gingival crevicular fluid and salivary levels of 8-hydroxy-deoxyguanosine in obesity. Archives of Oral Biology. 2016;62:80–85. doi: 10.1016/j.archoralbio.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Patel S. P., Raju P. A. Gingival crevicular fluid and serum levels of resistin in obese and non-obese subjects with and without periodontitis and association with single nucleotide polymorphism at-420. Journal of Indian Society of Periodontology. 2014;18(5):555–559. doi: 10.4103/0972-124x.142438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadel H. T., Pliaki A., Gronowitz E., et al. Clinical and biological indicators of dental caries and periodontal disease in adolescents with or without obesity. Clinical Oral Investigations. 2014;18(2):359–368. doi: 10.1007/s00784-013-0972-9. [DOI] [PubMed] [Google Scholar]
- 25.Pradeep A. R., Kumari M., Kalra N., Priyanka N. Correlation of MCP-4 and high-sensitivity C-reactive protein as a marker of inflammation in obesity and chronic periodontitis. Cytokine. 2013;61(3):772–777. doi: 10.1016/j.cyto.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Pradeep A., Priyanka N., Prasad M., Kalra N., Kumari M. Association of progranulin and high sensitivity CRP concentrations in gingival crevicular fluid and serum in chronic periodontitis subjects with and without obesity. Disease Markers. 2012;33(4):207–213. doi: 10.3233/DMA-2012-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. The British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Graves D. Cytokines that promote periodontal tissue destruction. Journal of Periodontology. 2008;79(8):1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 31.Buduneli E., Vardar-Şengül S., Buduneli N., Atilla G., Wahlgren J., Sorsa T. Matrix metalloproteinases, tissue inhibitor of matrix metalloproteinase-1, and laminin-5 γ2 chain immunolocalization in gingival tissue of endotoxin-induced periodontitis in rats: effects of low-dose doxycycline and alendronate. Journal of Periodontology. 2007;78(1):127–134. doi: 10.1902/jop.2007.050451. [DOI] [PubMed] [Google Scholar]
- 32.Theoleyre S., Wittrant Y., Tat S. K., Fortun Y., Redini F., Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine & Growth Factor Reviews. 2004;15(6):457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Gregor M. F., Hotamisligil G. S. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 34.Holmlund A., Hänström L., Lerner U. H. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. Journal of Clinical Periodontology. 2004;31(6):475–482. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 35.Baqui A. A. M. A., Meiller T. F., Jabra-Rizk M. A., Zhang M., Kelley J. I., Falkler W. A., Jr. Enhanced interleukin 1β, interleukin 6 and tumor necrosis factor α in gingival crevicular fluid from periodontal pockets of patients infected with human immunodeficiency virus 1. Oral Microbiology and Immunology. 2000;15(2):67–73. doi: 10.1034/j.1399-302x.2000.150201.x. [DOI] [PubMed] [Google Scholar]
- 36.Javed F., Al-Askar M., Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: a literature review. Journal of Periodontology. 2012;83(2):156–161. doi: 10.1902/jop.2011.110207. [DOI] [PubMed] [Google Scholar]
- 37.Engebretson S. P., Hey-Hadavi J., Ehrhardt F. J., et al. Gingival crevicular fluid levels of interleukin-1β and glycemic control in patients with chronic periodontitis and type 2 diabetes. Journal of Periodontology. 2004;75(9):1203–1208. doi: 10.1902/jop.2004.75.9.1203. [DOI] [PubMed] [Google Scholar]
- 38.Honda T., Domon H., Okui T., Kajita K., Amanuma R., Yamazaki K. Balance of inflammatory response in stable gingivitis and progressive periodontitis lesions. Clinical & Experimental Immunology. 2006;144(1):35–40. doi: 10.1111/j.1365-2249.2006.03028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin S. S., Qasim A., Reilly M. P. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. Journal of the American College of Cardiology. 2008;52(15):1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karthikeyan B. V., Pradeep A. R. Leptin levels in gingival crevicular fluid in periodontal health and disease. Journal of Periodontal Research. 2007;42(4):300–304. doi: 10.1111/j.1600-0765.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 41.Yeşim Bozkurt F., Yetkin Ay Z., Sütçü R., Delibaş N., Demirel R. Gingival crevicular fluid leptin levels in periodontitis patients with long-term and heavy smoking. Journal of Periodontology. 2006;77(4):634–640. doi: 10.1902/jop.2006.050277. [DOI] [PubMed] [Google Scholar]
- 42.Finck B. N., Johnson R. W. Tumor necrosis factor (TNF)-α induces leptin production through the p55 TNF receptor. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2000;278(2):R537–R543. doi: 10.1152/ajpregu.2000.278.2.R537. [DOI] [PubMed] [Google Scholar]
- 43.Bergström J., Eliasson S., Dock J. A 10-year prospective study of tobacco smoking and periodontal health. Journal of Periodontology. 2000;71(8):1338–1347. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 44.Machuca G., Rosales I., Lacalle J. R., Machuca C., Bullón P. Effect of cigarette smoking on periodontal status of healthy young adults. Journal of Periodontology. 2000;71(1):73–78. doi: 10.1902/jop.2000.71.1.73. [DOI] [PubMed] [Google Scholar]
- 45.Tymkiw K. D., Thunell D. H., Johnson G. K., et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. Journal of Clinical Periodontology. 2011;38(3):219–228. doi: 10.1111/j.1600-051X.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akram Z., Rahim Z. H. A., Taiyeb-Ali T. B., et al. Resistin as potential biomarker for chronic periodontitis: a systematic review and meta-analysis. Archives of Oral Biology. 2016 doi: 10.1016/j.archoralbio.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K., Pontillo A., Di Palo C., et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. The Journal of the American Medical Association. 2003;289(14):1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 48.Kopp H. P., Kopp C. W., Festa A., et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(6):1042–1047. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 49.Park H.-S., Nam H.-S., Seo H.-S., Hwang S.-J. Change of periodontal inflammatory indicators through a 4-week weight control intervention including caloric restriction and exercise training in young Koreans: a pilot study. BMC Oral Health. 2015;15(1, article 109) doi: 10.1186/s12903-015-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akram Z., Safii S. H., Vaithilingam R. D., Baharuddin N. A., Javed F., Vohra F. Efficacy of non-surgical periodontal therapy in the management of chronic periodontitis among obese and non-obese patients: a systematic review and meta-analysis. Clinical Oral Investigations. 2016;20(5):903–914. doi: 10.1007/s00784-016-1793-4. [DOI] [PubMed] [Google Scholar]
- 51.Akram Z., Al-Shareef S. A., Daood U., et al. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomedicine and Laser Surgery. 2016;34(4):137–149. doi: 10.1089/pho.2015.4076. [DOI] [PubMed] [Google Scholar]
- 52.Vohra F., Akram Z., Safii S. H., et al. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagnosis and Photodynamic Therapy. 2016;13:139–147. doi: 10.1016/j.pdpdt.2015.06.010. [DOI] [PubMed] [Google Scholar]