Abstract
Ham Yen orange (Citrus nobilis Lour) is the highly valuable commercial fruit of Vietnam. With the blooming of fruit production and farming area, this specialty crop is facing threats from several serious diseases; therefore the search for new effective biocontrollers is required to prevent the existing excessive use of fertilizers and plant protection chemicals. Endophytic actinomycetes are of great scientific interest due to their high potential of application in agriculture and pharmaceutical research. In this work, endophytic actinomycetes were isolated from a native orange species of Northeast mountainous province Tuyen Quang. Among 49 isolates obtained, the isolate TQR12-4 strongly inhibited test pathogens Colletotrichum truncatum, Geotrichum candidum, Fusarium oxysporum, and F. udum. This isolate gave comparatively high biomass yields on different substrates, for example, carboxy methyl cellulose, starch, protein, and chitin, within a wide range of temperature from 15 to 45°C and pH from 4 to 10. Sequence analysis of 16S rDNA gene showed that TQR12-4 shared 99% similarity to Streptomyces prasinopilosus; however, it slightly differed from the latter in spore morphology and hence was named as Streptomyces sp. TQR12-4. A thermostable antifungal substance of nonpeptide nature produced by Streptomyces sp. TQR12-4 had MIC against Fusarium udum of 100 μg/mL and 400 μg/mL respective to extract fractions X 4 and X 5.
1. Introduction
Ham Yen orange tree has been domesticated since 1890s by Muong minority people in Ham Yen district of mountainous province Tuyen Quang of Northeast Vietnam. This species produces the most nutritious and economically valuable fruit with current growing area of 4,900 hectares and yield of about 43,000 tons by 2015 and brings about the significant economic benefit for the local growers. Like other citrus species, Ham Yen orange plants are attacked by many diseases such as Tristeza, greening, citrus canker caused by various phytopathogens resulting in decreasing the yield and quality of the crop. Application of synthetic fertilizers and other chemicals to control the disease is not only very much effective but also hazardous to consumers and environment. Therefore, the search for effective disease biocontrollers from other organisms, especially microorganism, is necessary to reduce agrochemical inputs.
Endophytes are defined as the microorganisms that colonize in living plant tissues (roots, branches, and leaves) without causing visible harm to the host plant and can be isolated from surface disinfected plant tissues or extracted from inside the plant [1]. According to Kandpal et al. (2012), they can be found in almost all plant species studied to date and are recognised to be important for the development of host plant through microbe-host interaction [2]. While endophytes receive nutrients (mainly carbohydrates) from the host plant, the latter obtains compounds or secondary metabolites and antibiotics produced by endophytes to enhance the plant growth and to gain protection from pathogens [3–7].
Among studied endophytes, Streptomyces species, which are isolated from a diversity of plant species, are one particular group of interest because of their ability to produce antibiotics as well as other bioactive secondary metabolites [1, 8–16]. Of about 23,000 bioactive secondary metabolites discovered in endophytic microorganisms, approximately 10,000 substances were produced by endophytic actinomycetes and 7,600 substances were found in Streptomyces genus [17, 18].
Currently, there are only a limited number of reports on the presence of endophytes in citrus plants. According to Araújo et al. (2002), Physoderma citri was the first endophytic fungus found in healthy Citrus sinensis. After that, a number of bacteria including members of Achromobacter, Acinetobacter, Alcaligenes, Arthrobacter, Bacillus, Burkholderia, Citrobacter, Corynebacterium, Enterobacter, and Pseudomonas have been isolated from xylem of lemon roots [19]. Recently, Douanla-Meli et al. (2013) reported on the isolation of other endophytic fungi in citrus plants [20]. Shutsrirung et al. (2013) studied the biodiversity of endophytic actinomycetes in mandarin grown in Thailand and their production of growth regulating substances [21]. Kandpal et al. (2012) were interested in the antimicrobial activity of endophytic actinomycetes in Citrus aurantifolia grown in different regions of India [2].
In Vietnam, research on the endophytic microorganisms is at early stages. Nevertheless, the search for new substances from endophytic microorganisms is drawing a great attention. In this study, endophytic streptomycetes of Ham Yen orange were isolated from roots, branches, and leaves. Biological characteristics, identification, and substances production of the most active isolate TQR12-4 were extensively investigated. The results showed that TQR12-4 exhibited remarkably strong activity against four phytopathogenic fungi, Colletotrichum truncatum, Geotrichum candidum, Fusarium oxysporum, and Fusarium udum, and two Gram-positive bacteria, Bacillus subtilis and Staphylococcus aureus.
2. Materials and Methods
2.1. Materials
Root, branch, and leaf samples were collected from seven-year-old orange trees grown in two mountainous villages, Deo-Ang and Hoc-Trai, of Ham Yen district, Tuyen Quang province. Test (indicator) microorganisms are used in this study including Colletotrichum truncatum VSVD 14 (causing anthracnose of citrus fruits), Geotrichum candidum VSVD 1 (sour rot of citrus fruits and vegetables), Fusarium oxysporum FO 5 (fusarium wilt), Fusarium udum VSVD 4 (wilt), and B. subtilis ATCC 6633 received from the Culture Collection of Soil Microbiology Laboratory, Inst. of Biology, Vietnam Academy of Science and Technology, and Staphylococcus aureus ATCC 25922 and Pseudomonas aeruginosa ATCC 10145 obtained from the National Institute of Drug Quality Control. Primers for amplification of 16S rDNA were purchased from Invitrogen (Hong Kong).
2.2. Isolation of Endophytic Actinomycetes
Plant samples were surface-sterilized as described by Shutsrirung et al. (2013) [21]. Then, the samples were homogenized in a mortar with about 2–5 mL of sterile water. The liquid was collected and spread on the screening medium containing 2.0 g/L K2HPO4, 0.02 g/L CaCO3, 2.0 g/L KNO3, 0.01 g/L FeSO4·7H2O, 2.0 g/L NaCl, 0.05 g/L MgSO4·H2O, 1.0 g/L humic acid, 10 mL of 0.2 N NaOH, and 18.0 g/L agar-agar, pH 7.0. To prevent the growth of bacteria and fungi, nystatin and nalidixic acid were added to the medium to the final concentrations of 100 mg/L and 10 mg/L, respectively. The inoculated plates were incubated at 28°C. Typical actinomycete colonies were selected from day 15 to day 60 and purified by consecutive streaking on ISP medium #2 containing 10 g/L glucose, 5 g/L meat extract, 4 g/L malt extract, and 18 g/L agar-agar, pH 7.0.
2.3. Morphological and Cultural Characterization
Morphological and cultural characterization of the isolates was studied according to the protocols of the International Streptomyces Project (ISP) [22–24]. The spores were examined under Scanning Electron Microscope FESEM S4800.
Qualitative evaluation of enzyme activity was done by plate assay method. Carboxy methyl cellulose, chitin, oat spelt xylan, casein, and starch were used to detect respective enzymes: cellulase, chitinase, xylanase, protease, and amylase [25]. The activity of an enzyme was exhibited as a clear zone (degraded substrate) around the colony. For detection of amylase and xylanase, before observation the plates were flooded with iodine and Congo Red solutions, respectively.
Suitable growth temperatures and pH and salinity tolerances of the isolates were determined on the ISP medium #2. The experiments were carried out at different temperatures from 15 to 50°C, pH from 3 to 9, and NaCl concentrations from 3 to 7%. The results were recorded after 14 days.
2.4. DNA Extraction and 16S rRNA Gene Amplification
Cultivation of the isolate was performed according to Ishikawa (2000) [26]. Genomic DNA was isolated using NucleoSpin® Tissue extraction kit (Macherey-Nagel, Germany) according to the manufacturer's instructions. The 16S rRNA genes were amplified using primers 27f (5′-TAACACATGCAAGTCGAACG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [27] and genomic DNA. The thermal cycle for PCR was described by Shutsrirung et al. (2013) [21]. The 16S rDNA nucleotide sequence of isolates was aligned with relevant known sequences from GenBank using http://www.ncbi.nlm.nih.gov/BLAST to obtain reference sequences. Phylogenetic analysis was done by CLC DNA workbench 6.6.
2.5. Screening for Antibacterial Activity
The screening was performed using agar diffusion method. Three test bacteria, B. subtilis ATCC 6633, S. aureus ATCC25922, and P. aeruginosa ATCC10145, were grown separately in LB broth for 24 hours. A 100 μL of a test bacterial culture was thoroughly spread on each of three LB agar plates. Agar blocks (Φ 5 mm) of actinomyces isolates previously grown for 7 days on ISP medium #2 were cut by a cork borer and placed on the LB plates inoculated with test bacterium. After overnight incubation at 37°C, growth inhibition was evaluated by measuring diameters of the halos surrounding the agar blocks.
2.6. Screening for Antifungal Activity
This assay was conducted by agar diffusion or coculture method as described by Intra et al. (2011) [28], with some modifications. Initially, the test fungus was grown on Hansen agar for 10 days. The conidial suspension was prepared in saline solution to the density of 106 CFU/mL. Each Hansen agar plate was spread with 100 μL of conidial suspension. Six agar plugs (Φ 5 mm) were removed from every plate and replaced by six actinomycete plugs, each containing a single isolate colony previously grown on ISP medium #2. The results were recorded after 3 days.
Alternatively, the test fungus and actinomycete isolate were inoculated by parallel streaking on Hansen agar at the distance of 3 cm from each other (coculture). The plates were incubated at 28°C and inhibition of mycelial growth was measured after 7 days.
2.7. Thermal and pH Stability of the Crude Antifungal Substance
The antifungal substance of selected isolate TQR12-4 was produced in medium AH4 containing 10 g/L glucose, 10 g/L soybean meal, 5 g/L NaCl, and 1 g/L CaCO3, pH 7. The cultivation lasted for 120 hours at 28°C. The crude antifungal substance was extracted from the entire culture with ethyl acetate [28]. After evaporation of solvent in vacuum, the crude substance was dissolved in 5% methanol for evaluation of thermal and pH stability using agar well diffusion assay against test fungi, F. oxysporum, F. udum, and G. candidum.
The solution of crude substance was treated at temperatures of 28, 40, 50, 60, 70, 80, and 100°C during 30, 60, 90, and 120 minutes for each temperature.
The pH of crude antifungal substance was adjusted to values of 3, 4, 5, 6, 7, 8, and 9 and kept at 28°C for 60 minutes.
2.8. Recovery and Bioassay of Antifungal Substance
The antifungal substance was extracted from 120-hour submerged culture using ethyl acetate as solvent. The solvent was evaporated in vacuum, and the solid crude substance was dissolved in methanol for separation on silica gel column using gradient elution of CH2Cl2/CH3OH (50/1–1/1, v/v). Antifungal activity of recovered fractions was examined against F. udum using disk diffusion method. The dried fractions were redissolved in methanol to the concentration of 10 mg/mL. Sterile Whatman filter paper disks (Φ 5 mm) were loaded, each with 50 μL (0.5 mg) of a fraction solution, while the controls were loaded with only methanol. The loaded disks were left to dry at room temperature. Hansen agar plates were inoculated with the test fungus as described above. Triple test was performed, where 3 disks, each loaded with one fraction, and a control were placed on a plate. The diameters of inhibition zones were measured after incubation for 2 days at 28°C.
2.9. Determination of Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of fractions X 4 and X 5 was determined according to Narayana et al. (2007) [29] and Andrews (2001) [30], with some modifications. A commercial antifungal agent, carbendazim, was assayed in parallel for comparison. The test fungus, F. udum VSVD 4, was grown on Hansen agar medium for 10 days; then conidia were collected and suspended in saline solution to the density of 106 CFU/mL.
Fractions X 4 and X 5 and carbendazim were first dissolved in methanol solution of 5% and then diluted with Hansen broth to concentrations of 0.84 ÷ 200 μg/mL, 1.68 ÷ 400 μg/mL, and 3.125 ÷ 100 μg/mL, respectively.
In a test tube, equal volumes of 100 μL of the conidial suspension and X 4 or X 5 or carbendazim were mixed together. The control tube contained only Hansen broth and conidial suspension of F. udum. The tubes were incubated aerobically for 72 hrs at 25°C. MIC values were recorded with no visible fungal growth at the lowest concentration.
3. Results and Discussion
3.1. Isolation of Endophytic Actinomycetes
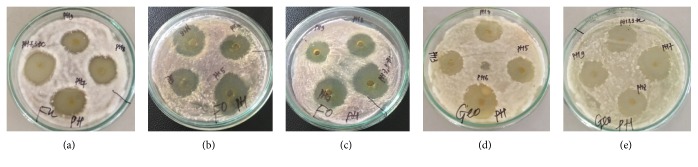
Forty-nine endophyte actinomycetes were isolated from Ham Yen orange plant samples. Of them, 18 isolates (36.7%) showed antimicrobial activity against test organisms. Particularly, 5 isolates inhibited growth of 6 to 7 test organisms, and 7 isolates inhibited 3 to 4 test organisms. The isolate TQR12-4 exhibited the highest activity against 4 test pathogenic fungi, Colletotrichum truncatum, Geotrichum candidum, Fusarium oxysporum, and Fusarium udum. The culture broth of this isolate inhibited the growth of Gram-positive S. aureus ATCC 25922 and B. subtilis ATCC 6633 but did not inhibit Gram-negative P. aeruginosa ATCC 25932 (Table 1 and Figure 1).
Table 1.
Antimicrobial activity of the endophytic actinomycete isolate TQR12-4.
| Test organisms | Zone of inhibition (D, mm) | |
|---|---|---|
| Agar block | Well diffusion | |
| S. aureus ATCC 25922 | 26 ± 0,42 | 25 ± 1,00 |
| B. subtilis ATCC 6633 | 25 ± 0,50 | 25 ± 0,42 |
| P. aeruginosa ATCC 25932 | 0 | 0 |
| G. candidum VSVD 1 | 28 ± 0,81 | 19 ± 0,35 |
| F. oxysporum FO5 | 25 ± 0,50 | 17 ± 0,76 |
| F. udum VSVD 4 | 26 ± 0,32 | 18 ± 0,58 |
| C. truncatum VSVD 14 | 29 ± 0,81 | 20 ± 0,29 |
The results were recorded in 7-day culture on ISP medium #2.
Figure 1.
Screening of endophytic actinomycetes for antifungal and antibacterial activity. Test strains: C. truncatum VSVD 14 (a), S. aureus ATCC 25922 (b), F. oxysporum FO5 (c), and F. udum VSVD 4 (d).
3.2. Biological Characteristics of TQR12-4 Isolate
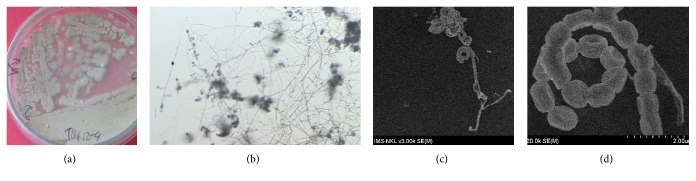
The colony surface was powdery and curled. Aerial and substrate mycelia were grey to light brown or light yellow to brownish yellow on media ISP2, ISP3, ISP4, ISP5, and ISP7. Soluble yellow pigment was observed on media ISP2 and ISP3. Its spiral spore chains bore from several to about 30 smooth spores/chain (Figure 2).
Figure 2.
Morphology of isolate TQR12-4 grown on ISP medium #4 (7-day culture): colonies (a); light microscope image (×40); mycelia and spore chains (b); scanning electron micrograph: spore chain (c); and spores (d). Bar = 2 μm.
In culture, isolate TQR12-4 can grow at temperature of 15 ÷ 45°C and pH of 4 ÷ 10. However, the most favorable temperature was 28°C and pH was 6.0 ÷ 7.0. Its salinity tolerance was 3% NaCl. The isolate degraded chitin, protein, starch, guaiacol, carboxymethyl cellulose, and assimilated carbon sources: D-glucose, D-xylose, D-mannitol, D-fructose, and D-cellulose. It showed week growth on L-arabinose, D-sucrose, and D-rhamnose and no growth on D-raffinose.
The obtained 16S rDNA nucleotide sequence (1361 bp) was registered at the National Center for Biotechnology Information (NCBI) GenBank database under accession number KX257804. It was compared with relative sequences available on GenBank database using BLAST tool on NCBI. It shared high similarity (99%) with the sequences of Streptomyces sp., for example, Streptomyces prasinopilosus N2 (KR703669) and Streptomyces hawaiiensis NBRC12784 (AB184143) (Figure 3). Despite such high similarity with Streptomyces prasinopilosus, there was still a slight difference in spore morphology between them. Therefore, our isolate was named as Streptomyces sp. TQR12-4.
Figure 3.
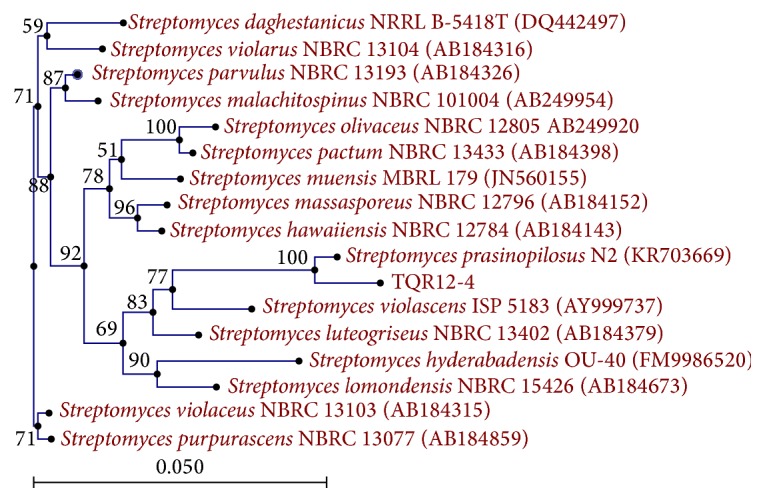
Neighbor-joining tree showing the phylogenetic relationships based on 16S rRNA gene sequence of the isolate TQR12-4 and closest species.
3.3. Thermal and pH Stability of the Antifungal Substance
The crude extract of antifungal substance from Streptomyces sp. TQR12-4 showed activity against all three test fungi over a wide range of pH values from 3 to 9. The most suitable pH was 6 ÷ 7; otherwise, the activity slightly declined towards acidic or basic pH. Compared with the highest activity at pH 7.0, after 1-hour treatment at extreme pH of 3 and 9, the activity was reduced by 36 and 26%, respectively (Figures 5 and 6).
Figure 5.
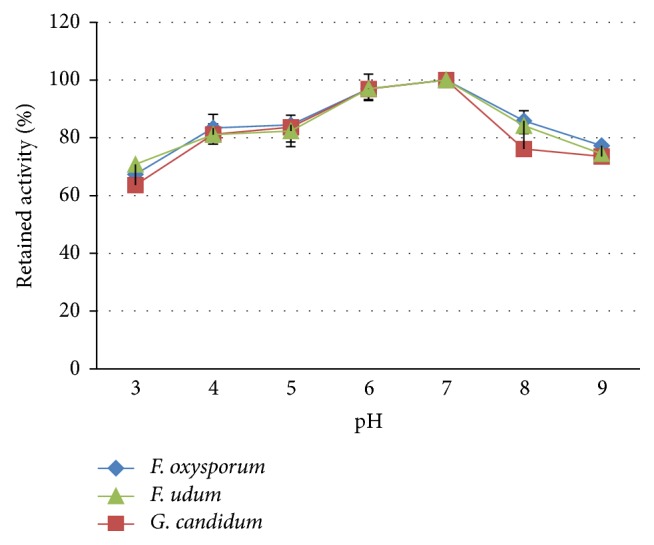
Stability to pH of the antifungal substance.
Figure 6.
Well diffusion assay for stability to pH of the antifungal substance. (a) F. udum, (b, c) F. oxysporum, and (d, e) G. candidum.
Treatment at different temperatures showed relatively high thermal stability of the crude substance. Assuming that the antifungal activity at 28°C was 100%, incubation for 120 minutes at 60 and 100°C resulted in its decrease to 77% and 60%, respectively. Because of high similarity in responses of all three test fungi, only data for F. oxysporum were presented in Figure 4.
Figure 4.
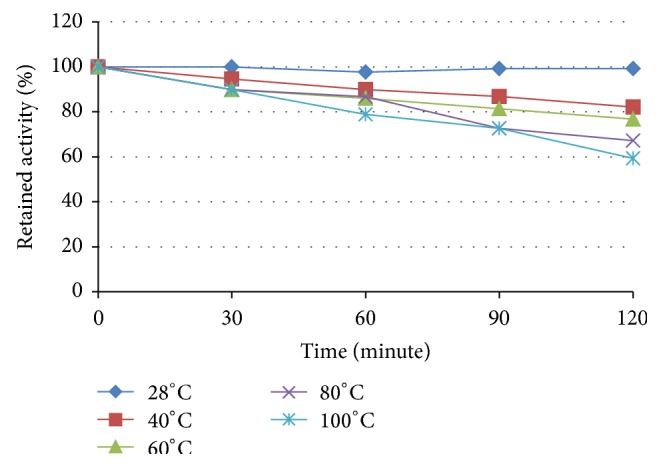
Thermal stability of the antifungal substance against F. oxysporum.
In the study by Prapagdee et al. (2008), the culture filtrates of Streptomyces hygroscopicus were treated at 100°C for 45 minutes and then tested against Colletotrichum gloeosporioides and Sclerotium rolfsii. The findings showed that the antifungal activity of the exponential culture was mainly assigned to heat labile proteins such as extracellular hydrolytic enzymes, while the activity of stationary culture was exhibited by more stable compounds of another nature [31]. Carvalho and Van Der Sand (2016) reported that the centrifuged culture broth of an endophytic actinomycete R18(6) might be exposed to 80°C for 30 minutes without losing much of antibacterial activity. However, that extract did not stand treatment at 100°C, as its activity started to drop after 3 minutes and was completely lost after 30 minutes. The author suggested the presence of peptide bonds in the molecule of that substance [32]. Since the substance produced by our strain Streptomyces sp. TQR12-4 was extracted with ethyl acetate and then subjected to treatment at 100°C for 120 minutes but still retained 60% of its activity, it may be of nonpeptide nature.
3.4. Bioassay of the Antifungal Substance
The crude antifungal substance was separated on silica gel column giving five fractions labelled as X 1 to X 5. The dry weights of recovered fractions were 300, 45, 36, 78, and 46 mg, respectively.
The bioassay of all five fractions which was performed by well diffusion method revealed that fractions X 1, X 2, and X 3 did not inhibit test fungal growth. Meanwhile, fractions X 4 and X 5 gave clear inhibition zones of 17 and 15 mm in diameter, respectively (Figure 7). The MICs against F. udum of fractions X 4 and X 5 and carbendazim were 100, 400, and 50 μg/mL, respectively.
Figure 7.
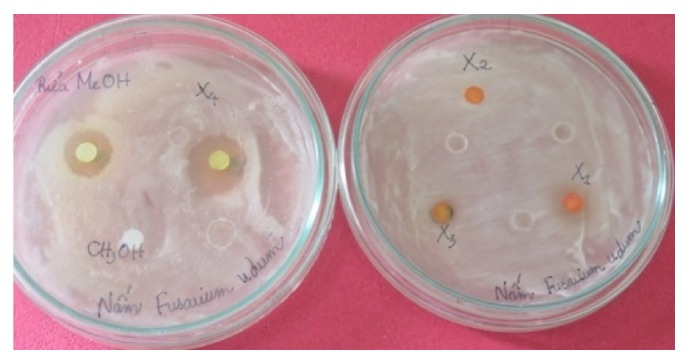
Activity against F. udum of recovered fractions. Faction X 5 is to the left of X 4 on the left plate.
The antimicrobial activity of a certain substance depends on the strain-producer and test microorganisms. In a study by Kim et al. (2013), antifungal niphimycin from Streptomyces sp. KP6107 inhibited strains of genera Alternaria, Aspergillus, Colletotrichum, Cercospora, Cylindrocarpon, Fusarium, and Rhizoctonia with MIC of 8 ÷ 64 μg/mL [33]. In assays with vegetative mycelia, the MICs of a bioactive compound, 3-phenylpropionic acid (3-PPA), from Streptomyces albidoflavus ANU 6277 against Aspergillus flavus, A. niger, Fusarium oxysporum, F. udum, Penicillium citrinum, and Candida albicans were of 10 ÷ 100 μg/mL [29]. For spores of F. udum, the MICs of carbendazim, 3-PPA, and tricyclazole were 50, 100, and 500 μg/mL, respectively. Our substance, produced by Streptomyces sp. TQR12-4, showed similar activity against F. udum, that is, MIC of 100 μg/mL, and MIC of carbendazim was of 50 μg/mL. However, further study must be carried out to understand the chemical nature of this substance and more of its properties.
As mentioned above, endophytic actinomycetes are reported to play an important role in the growth, physiology, and health of the host plant via their metabolic activity and mechanisms such as antibiosis, niche competition, and induction of host systemic resistance. However, the relationship between the endophytic microorganism and the host remains insufficiently explained.
Taechowisan et al. (2005) [34] studied antifungal substances produced by endophytic Streptomyces aureofaciens CMUAc130 isolated from the root tissue of Zingiber officinale. The substances were identified as 5,7-dimethoxy-4-p-methoxylphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin, which inhibited the phytopathogenic fungi Colletotrichum musae and Fusarium oxysporum. Interestingly, as summarized by the authors, 5,7-dimethoxy-4-p-methoxylphenylcoumarin can be extracted from a number of plants but not from the members of Zingiberaceae, which in turn host the streptomycete producing this substance. In our opinion, this fact may be a suggestion to study on the origin of certain antimicrobial compounds found in plants.
4. Conclusions
A streptomycete TQR12-4 isolated from Ham Yen orange specialty of Northeast Vietnam showed strong activity against 2 Gram-positive bacteria, B. subtilis and S. aureus, and 4 phytopathogenic fungi, C. truncatum, G. candidum, F. oxysporum, and F. udum. Biological and taxonomical characteristics and sequence analysis of 16S rDNA gene indicated that this isolate belongs to genus Streptomyces. It shares 99% similarity with Streptomyces prasinopilosus but has a slight difference in spore morphology and hence was named as Streptomyces sp. TQR12-4. The crude antimicrobial substance from this strain was comparatively stable over wide ranges of pH (3 to 9) and temperatures (40 to 100°C). Separation on silica gel gave five fractions, two of which (X 4 and X 5) inhibited the growth of F. udum at MIC of 100 and 400 μg/mL, respectively. Studied properties of this substance indicate its remarkable advantages in production by microbiological fermentation and application in agricultural practice. To achieve these goals, further research is required to understand the chemical nature of this substance and more of its antimicrobial spectrum and production.
Acknowledgments
This work was funded by Vietnam Academy of Science and Technology through VAST.DLT.12/15-16 project. The National Key Laboratory of Gene Technology, Institute of Biotechnology, provides facilities for this study.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Shimizu M. Endophytic actinomycetes: biocontrol agents and growth promoters. In: Maheshwari D. K., editor. Bacteria in Agrobiology: Plant Growth Responses. San Diego, Calif, USA: Elsevier Academic Press; 2011. pp. 201–220. [Google Scholar]
- 2.Kandpal K. C., Jain D. A., Kumar U., et al. Isolation and screening of endophytic actinomycetes producing antibacterial compound from Citrus aurantifolia Fruit. European Journal of Experimental Biology. 2012;2(5):1733–1737. [Google Scholar]
- 3.Mercado-Blanco J., Lugtenberg B. Biotechnological applications of bacterial endophytes. Current Biotechnology. 2014;3(1):60–75. doi: 10.2174/22115501113026660038. [DOI] [Google Scholar]
- 4.Rosenblueth M., Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Molecular Plant-Microbe Interactions. 2006;19(8):827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 5.Ryan R. P., Germaine K., Franks A., Ryan D. J., Dowling D. N. Bacterial endophytes: recent developments and applications. FEMS Microbiology Letters. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 6.Mei C., Flinn B. S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Patents on Biotechnology. 2010;4(1):81–95. doi: 10.2174/187220810790069523. [DOI] [PubMed] [Google Scholar]
- 7.Strobel G. A. Endophytes as sources of bioactive products. Microbes and Infection. 2003;5(6):535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z., Gao D., Song X., Xu Y. A reiew of endophyte and its use and function. Proceedings of the International Conference on Environmental Engineering and Technology. Advances in Biomedical Engineering (ICEET '12); 2012; Zhangjiaji, China. Environmental Engineering and Technology; pp. 124–130. [Google Scholar]
- 9.Gangwar M., Rani S., Sharma N. Diversity of endophytic actinomycetes from wheat and its potential as plant growth promoting and biocontrol agent. Advanced Laboratory Research in Biology. 2012;3(1):15–23. [Google Scholar]
- 10.Gangwar M., Dogra S., Gupta U. P., Kharwar R. N. Diversity and biopotential of endophytic actinomycetes from three medicinal plants in India. African Journal of Microbiology Research. 2014;8(2):184–191. [Google Scholar]
- 11.Joseph B., Sankarganesh P., Edwin B. T., Raj S. J. Endophytic streptomycetes from plants with novel green chemistry: review. International Journal of Biological Chemistry. 2012;6(2):42–52. doi: 10.3923/ijbc.2012.42.52. [DOI] [Google Scholar]
- 12.Shiomi K., Hatae K., Hatano H., et al. A new antibiotic, antimycin a9, produced by Streptomyces sp. K01-0031. Journal of Antibiotics. 2005;58(1):74–78. doi: 10.1038/ja.2005.10. [DOI] [PubMed] [Google Scholar]
- 13.Taechowisan T., Chanaphat S., Ruensamran W., Phutdhawong W. S. Antibacterial activity of 1-methyl ester-nigericin from Streptomyces hygroscopicus BRM10; an endophyte in Alpinia galanga . Journal of Applied Pharmaceutical Science. 2013;3(5):104–109. doi: 10.7324/japs.2013.3520. [DOI] [Google Scholar]
- 14.Vollmar D., Thorn A., Schuberth I., Grond S. A comprehensive view on 4-methyl-2-quinazolinamine, a new microbial alkaloid from Streptomyces of TCM plant origin. Journal of Antibiotics. 2009;62(8):439–444. doi: 10.1038/ja.2009.68. [DOI] [PubMed] [Google Scholar]
- 15.Wang F., Xu M., Li Q., Sattler I., Lin W. P-aminoacetophenonic acids produced by a mangrove endophyte Streptomyces sp. (strain HK10552) Molecules. 2010;15(4):2782–2790. doi: 10.3390/molecules15042782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan L.-L., Han N.-N., Zhang Y.-Q., et al. Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. The Journal of Antibiotics. 2010;63(5):259–261. doi: 10.1038/ja.2010.21. [DOI] [PubMed] [Google Scholar]
- 17.Bérdy J. Bioactive microbial metabolites: a personal view. Journal of Antibiotics. 2005;58(1):1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M. Actinomycetes: source, identification, and their applications. International Journal of Current Microbiology and Applied Sciences. 2014;3(2):801–832. [Google Scholar]
- 19.Araújo W. L., Marcon J., Maccheroni W., Jr., Van Elsas J. D., Van Vuurde J. W. L., Azevedo J. L. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Applied and Environmental Microbiology. 2002;68(10):4906–4914. doi: 10.1128/aem.68.10.4906-4914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douanla-Meli C., Langer E., Mouafo F. T. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon . Fungal Ecology. 2013;6(3):212–222. doi: 10.1016/j.funeco.2013.01.004. [DOI] [Google Scholar]
- 21.Shutsrirung A., Chromkaew Y., Pathom-Aree W., Choonluchanon S., Boonkerd N. Diversity of endophytic actinomycetes in mandarin grown in northern Thailand, their phytohormone production potential and plant growth promoting activity. Soil Science and Plant Nutrition. 2013;59(3):322–330. doi: 10.1080/00380768.2013.776935. [DOI] [Google Scholar]
- 22.Shirling E. B., Gottlieb D. Cooperative description of type culture of Streptomyces species . International Journal of Systematic Bacteriology. 1966;19(4):391–512. [Google Scholar]
- 23.Shirling E. B., Gottlieb D. Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology. 1966;16(3):313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- 24.Nomomura H. Key for classification and identification of 458 species of the Streptomyces included in ISP. Journal of Fermentation Technology. 1974;52(2):78–92. [Google Scholar]
- 25.Johnsen H. R., Krause K. Cellulase activity screening using pure carboxymethylcellulose: application to soluble cellulolytic samples and to plant tissue prints. International Journal of Molecular Sciences. 2014;15(1):830–838. doi: 10.3390/ijms15010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa J., Tsuchizaki N., Yoshida M., Ishiyama D., Hotta K. Colony PCR for detection of specific DNA sequences in actinomycetes. Actinomycetologica. 2000;14(1):1–5. doi: 10.3209/saj.14_1. [DOI] [Google Scholar]
- 27.Lane D. J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic Acid Techniques in Bacterial Systematics. Chichester, UK: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 28.Intra B., Mungsuntisuk I., Nihira T., Igarashi Y., Panbangred W. Identification of actinomycetes from plant rhizospheric soils with inhibitory activity against Colletotrichum spp., the causative agent of anthracnose disease. BMC Research Notes. 2011;4, article 98 doi: 10.1186/1756-0500-4-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayana K. J. P., Prabhakar P., Vijayalakshmi M., Venkateswarlu Y., Krishna P. S. J. Biological activity of phenylpropionic acid isolated from a terrestrial Streptomycetes . Polish Journal of Microbiology. 2007;56(3):191–197. [PubMed] [Google Scholar]
- 30.Andrews J. M. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy. 2001;48(supplement 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 31.Prapagdee B., Kuekulvong C., Mongkolsuk S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. International Journal of Biological Sciences. 2008;4(5):330–337. doi: 10.7150/ijbs.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho T., Van Der Sand S. Evaluation of antimicrobial activity of the endophytic actinomycete R18(6) against multiresistant Gram-negative bacteria. Anais da Academia Brasileira de Ciências. 2016;88(1):155–163. doi: 10.1590/0001-3765201620140655. [DOI] [PubMed] [Google Scholar]
- 33.Kim H. Y., Kim J. D., Hong J. S., Ham J. H., Kim B. S. Identification of antifungal niphimycin from Streptomyces sp. KP6107 by screening based on adenylate kinase assay. Journal of Basic Microbiology. 2013;53(7):581–589. doi: 10.1002/jobm.201200045. [DOI] [PubMed] [Google Scholar]
- 34.Taechowisan T., Lu C., Shen Y., Lumyong S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology. 2005;151(5):1691–1695. doi: 10.1099/mic.0.27758-0. [DOI] [PubMed] [Google Scholar]