Abstract
Disruption of blood-brain barrier (BBB) follows brain trauma or central nervous system (CNS) stress. However, the mechanisms leading to this process or the underlying neural plasticity are not clearly known. We hypothesized that ATP/P2X7R signaling regulates the integrity of BBB. Activation of P2X7 receptor (P2X7R) by ATP induces the release of interleukin-1β (IL-1β), which in turn enhances the activity of matrix metalloproteinase-9 (MMP-9). Degradation of tight junction proteins (TJPs) such as ZO-1 and occludin occurs, which finally contributes to disruption of BBB. A contact coculture system using human astrocytes and hCMEC/D3, an immortalized human brain endothelial cell line, was used to mimic BBB in vitro. Permeability was used to evaluate changes in the integrity of TJPs. ELISA, Western blot, and immunofluorescent staining procedures were used. Our data demonstrated that exposure to the photoreactive ATP analog, 3′-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate (BzATP), induced a significant decrease in ZO-1 and occludin expression. Meanwhile, the decrease of ZO-1 and occludin was significantly attenuated by P2X7R inhibitors, as well as IL-1R and MMP antagonists. Further, the induction of IL-1β and MMP-9 was closely linked to ATP/P2X7R-associated BBB leakage. In conclusion, our study explored the mechanism of ATP/P2X7R signaling in the disruption of BBB following brain trauma/stress injury, especially focusing on the relationship with IL-1β and MMP-9.
1. Introduction
Traumatic brain injury (TBI) often coexists with acute stress disorder (ASD) or post-traumatic stress disorder (PTSD) [1]. TBI is one of the major health problems that account for high mortality worldwide. According to the World Health Organization, TBI is expected to become the third leading cause of death and disability worldwide by 2020 [2]. TBI/PTSD or TBI/ASD are public health concerns warranting advanced investigation into the underlying mechanisms for appropriate therapeutic intervention [3]. Disruption of blood-brain barrier (BBB) is a common result following brain trauma/stress injury, and little is known about the changes in neural plasticity following brain injury and subsequent cellular responses. Currently, there is no effective neuroprotective agent that is available clinically, to prevent the damage to BBB following CNS trauma/stress injury [4, 5].
Extracellular ATP, which is dramatically increased after acute injury or stress to the central nervous system, is considered an initiator of secondary brain injury by activating a wide variety of purinergic receptors [6]. P2X7 is a unique purinergic receptor. In addition to the rapid opening of the cation channels, with prolonged exposure to high concentrations of ATP, it triggers a membrane pore facilitating the passage of large molecules, which suggests that P2X7 receptors may play a crucial role in the pathophysiology of brain trauma [7, 8]. It is reported that activation of P2X7 receptor resulted in the release of IL-1β [9, 10], which is an important proinflammatory cytokine. Furthermore, activation of IL-1β leads to the production of MMP-9 [11], which affects the tight junction proteins including ZO-1, and in turn, leads to disruption of blood-brain barrier [12, 13]. Overall, the ATP/P2X7R signaling pathway may be implicated in the process of BBB leakage following CNS trauma/stress injury. However, the underlying physiologic mechanisms following ATP-associated BBB damage and their relationship with IL-1β and MMP-9 are still unknown.
In this study, we used a coculture model in vitro comprising an immortal human cell line hCMEC/D3 expressing endothelial and BBB markers and human astrocytes to investigate the role of ATP/P2X7R signaling in the disruption of tight junction proteins [14]. We demonstrated that ATP/P2X7R signaling regulates the integrity of BBB. Activation of P2X7 receptor by ATP induces the release of IL-1β, which in turn enhances the production of MMP-9, leading to degradation of tight junction proteins, such as ZO-1 and occludin, resulting in the disruption of BBB.
2. Materials and Methods
2.1. Materials
Human astrocytes (HAs) were obtained from Jiamay Biolab (Beijing, China); hCMEC/D3 cell lines were purchased from Jiangyin Yuxi Biotechnology (Jiangsu, China); polyclonal anti-ZO-1 and anti-occludin antibodies were acquired from Biorbyt (Cambridge, UK, cat: orb11587, orb11181); Alexa Fluor 594-conjugated AffiniPure donkey anti-rabbit IgG was obtained from Jackson ImmunoResearch Inc. (West Grove, PA, USA, cat: 711-585-152); human interleukin-1β ELISA kit and human MMP-9 ELISA kit were purchased from Jiamay (Beijing, China, cat: FHK0016, FHK0144); high glucose Dulbecco's Modified Eagle's Medium (DMEM) basal medium was obtained from Hyclone (Logan, UT, United States); and EBM-2 basal medium was purchased from Lonza (Walkersville, MD, USA). Fetal bovine serum (FBS) was obtained from GIBCO (Rockville, MD, USA); phenylmethanesulfonyl fluoride (PMSF) was acquired from Amresco (Solon, OH, USA); the different cocktails were purchased from Yuanye Biotech (Shanghai, China); skimmed milk was obtained from Yili Industrial Group Co. Ltd. (Beijing, China); and anti-β-actin and other materials were purchased from Jiamay Biolab (Beijing, China).
2.2. In Vitro Model of Human BBB
The hCMEC/D3 cells and HAs were cultured as described in a previous study [15]. Briefly, hCMEC/D3 cells were cultured in EBM-2 basal medium supplemented with 5% FBS, growth factors, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. HAs were cultured in high glucose DMEM supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37°C in the presence of 5% CO2.
The contact coculture model of hCMEC/D3 and astrocytes was used as an in vitro model of human BBB [16]. Briefly, HAs (5 × 103 cells/cm2) were seeded externally on Transwell inserts (polyester membranes, 6.5 mm diameter, 0.4 μm pore size, Corning Costar) in an inverted position and allowed to adhere to the membrane overnight. After incubation, the hCMEC/D3 cells (2 × 105 cells/cm2) were added to the interior of the inserts and both the cell layers were grown to confluence.
2.3. ELISA for IL-1β and MMP-9
Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of IL-1β and MMP-9. The hCMEC/D3 cells and HAs grown on Transwell insert were treated with A438079 (a selective P2X7 receptor antagonist, 10 μM), or IL-1RA (an IL-1R antagonist, which competitively blocked the binding to IL-1 receptors, 100 μg/mL), or batimastat (a MMP antagonist, 5 μM) 30 min prior to the addition of BzATP (100 μg/mL). The cells were exposed to photoreactive ATP analog, 3′-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate (BzATP), for 2 h and their specific vehicles as control. After brief centrifugation, 100 μL of cell culture supernatant samples was analyzed using human IL-1β ELISA kit/human MMP-9 ELISA kit according to the manufacturer's instructions. Quantitative analysis was performed on a microplate reader (Thermo fisher, Multiskan MK3).
2.4. Permeability of the Coculture System
The hCMEC/D3 cells and HAs were cocultured on Transwell insert. Cells were pretreated with inhibitors (A438079 10 μM, or IL-1RA 100 μg/mL, or batimastat 5 μM) for 30 min followed by BzATP (100 μg/mL) for 2 h. To characterize the changes in structural integrity of BBB models in vitro, we measured the permeability of the coculture system to FITC-dextran, as reported in a previous study [17]. Fluorescein isothiocyanate-labeled dextran (FITC-dextran, 10 KDa, 10 μM) was added to the apical compartment. After 30 min, 100 μL of the medium was removed from the basal compartment. The fluorescence of the collected samples was measured at 485 nm/520 nm using a Fluorescence Microplate Reader (Thermo Fisher, Fluoroskan Ascent FL).
2.5. Immunofluorescence Staining of Tight Junction Proteins
After treatment with inhibitors (A438079 10 μM, or IL-1RA 100 μg/mL, or batimastat 5 μM) for 30 min prior to exposure to BzATP (100 μg/mL) for 2 h, cells were washed twice with PBS and fixed using 4% PFA at room temperature for 30 min. Excess PFA was removed with PBS three times. The cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min. After washing three times with PBS, the cells were incubated with 3% H2O2 for 10 min, followed by PBS three times. After blocking for 30 min with 10% FBS and three times with PBS, cells were incubated with polyclonal anti-occludin antibody (1 : 200) or polyclonal anti-ZO-1 antibody (1 : 200) at 4°C overnight. Unbound antibodies were removed using PBS. Alexa Fluor 594-conjugated AffiniPure donkey anti-rabbit IgG (1 : 500) was used as a secondary antibody and incubated with cells for 2 h at room temperature. Similarly, unbound antibodies were removed with PBS. Analysis and imaging were performed using a confocal laser-scanning microscope (TSC-SP2, Leica, Germany).
2.6. Western Blot of Tight Junction Proteins
Western blot was used to detect the expressions of occludin and ZO-1. The hCMEC/D3 cells were washed with PBS and harvested with 0.25% trypsin. The cells were suspended in 150 μL ice-cold RIPA lysis buffer, sonicated 10 times for 5 s with 10 s pauses in an ice-water bath, and centrifuged at 12,000 rpm for 5 min at 4°C. Protein quantity was detected using a BCA assay kit, and equal amounts of protein were used for Western blot analysis. Next, the protein was separated by SDS-PAGE and transferred to PVDF membranes using semidry methods. Membranes were blocked in TBST + 5% skimmed milk for 2 h at room temperature. Membranes were incubated overnight at 4°C with the primary antibodies: rabbit polyclonal anti-ZO-1 antibody (1 : 100) or polyclonal anti-occludin antibody (1 : 100). The membranes were washed for 10 min with TBST buffer three times. Immunoblots were processed with secondary antibodies (1 : 2000~1 : 5000) for 1 h at room temperature. β-Actin was blotted on the same membrane as the internal control. Blots were quantified using Image J software (NIH).
2.7. Statistical Analysis
All the experiments were repeated three times and similar results were obtained. Statistical analysis was performed by one-way ANOVA (multiple comparisons) using the SPSS 16.0 statistics software (SPSS, Chicago, IL). P value less than 0.05 was considered statistically significant.
3. Results
3.1. A438079 Attenuated BzATP-Induced Disruption of BBB In Vitro
3.1.1. Effect of BzATP/BzATP + A438079 on IL-1β and MMP-9 Induction
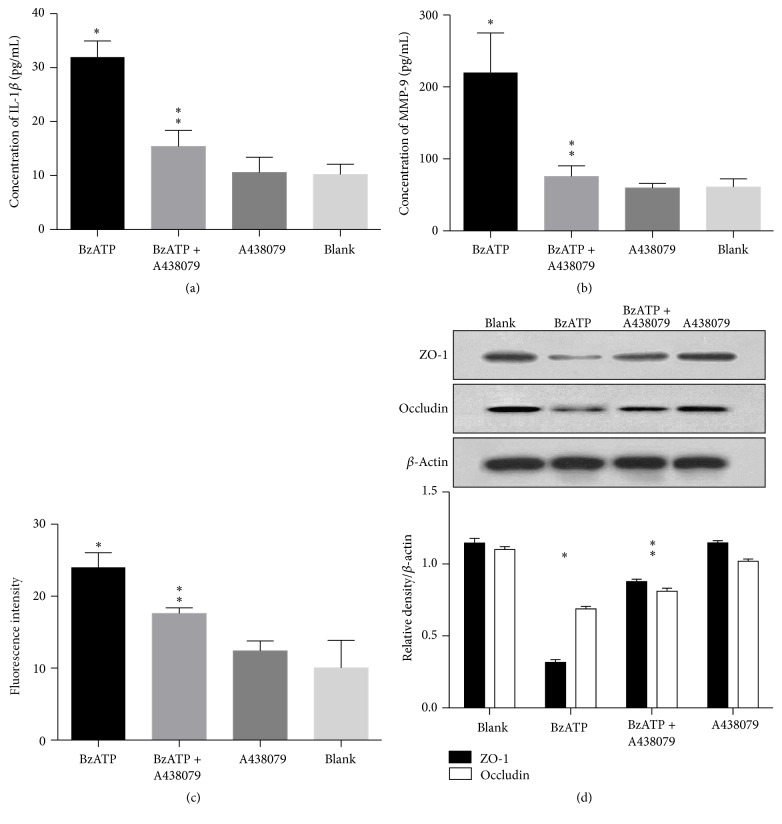
In this study, we investigated the effects of BzATP on the TJPs of blood-brain barrier and correlated the expression of IL-1β and MMP-9. First, we evaluated the role of BzATP in the induction of IL-1β and MMP-9. ELISA results showed that the levels of IL-1β and MMP-9 were significantly higher in the BzATP group than in the control group without BzATP (P < 0.05; Figures 1(a) and 1(b)). Second, A438079 treatment significantly attenuated the increase in IL-1β and MMP-9, compared with the vehicle group (P < 0.05, Figures 1(a) and 1(b)). Furthermore, administration of A438079 alone did not significantly affect the levels of IL-1β and MMP-9, compared with the blank control group (P < 0.05, Figures 1(a) and 1(b)).
Figure 1.
A438079 attenuates BzATP-induced disruption of blood-brain barrier in vitro. (a) The IL-1β level was significantly higher in BzATP groups than in control groups (∗ P < 0.05). A438079 treatment significantly reduced the levels of IL-1β compared with the vehicle group (∗∗ P < 0.05). (b) The level of MMP-9 was significantly higher in BzATP groups than in control groups (∗ P < 0.05). A438079 treatment significantly reduced the level of MMP-9 compared with the vehicle group (∗∗ P < 0.05). (c) BzATP induced hyperpermeability compared with the control groups (∗ P < 0.05), which was decreased by the P2X7 inhibitor A438079 in the coculture system (∗∗ P < 0.05). (d) Western blot analysis showed that the expression of ZO-1 and occludin in the BzATP groups was decreased compared with the controls (∗ P < 0.05), while A438079 treatment significantly attenuated the disruptions of ZO-1 and occludin (∗∗ P < 0.05).
3.1.2. Effect of A438079 on Permeability of Endothelial Coculture System
To determine the altered permeability of BBB model, we measured the fluorescence of FITC-dextran diffused through the coculture system. FITC-dextran (10 KDa, 10 μM) diffused across BBB from the apical to the basal compartment. The fluorescence intensity of samples collected basally was measured. As shown in Figure 1(c), exposure to BzATP significantly increased permeability to FITC-dextran compared with the control group (P < 0.05, Figure 1(c)). Inhibition of P2X7R abolished BzATP-induced paracellular permeability, while A438079 alone did not affect basal permeability levels (P < 0.05, Figure 1(c)).
3.1.3. Effect of A438079 on TJPs Degradation
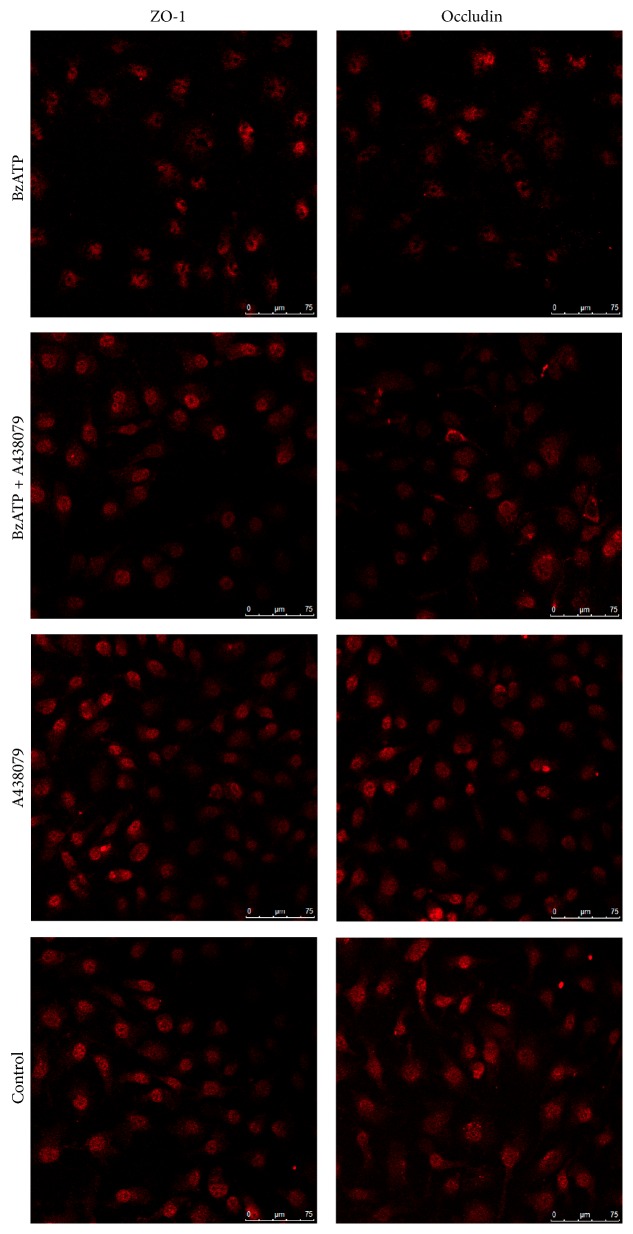
In an attempt to determine whether ATP-induced increase in BBB permeability was due to the altered levels of TJPs, the protein expression of occludin and ZO-1 was evaluated via immunofluorescence staining and Western blot. As shown in Figure 2, BzATP-treated cells showed significantly decreased staining of ZO-1 and occludin, which was significantly attenuated by A438079. Furthermore, Western blot analysis confirmed the decrease in the expression of ZO-1 and occludin in BzATP groups compared with the controls. Treatment with A438079 enhanced the levels of ZO-1 and occludin compared with BzATP alone (P < 0.05, Figure 1(d)). The results of Western blot were similar to immunofluorescence staining of occludin and ZO-1 in the following experiments.
Figure 2.
Confocal microscopy images of immunofluorescence staining of ZO-1 and occludin in hCMEC/D3 cells demonstrating the protective effect of A438079 against BzATP-induced tight junction disruption.
3.2. IL-1β Inhibition Attenuated BzATP-Induced Tight Junction Disruption and Endothelial Coculture Hyperpermeability
3.2.1. BzATP-Induced MMP-9 Induction Was Attenuated by IL-1RA
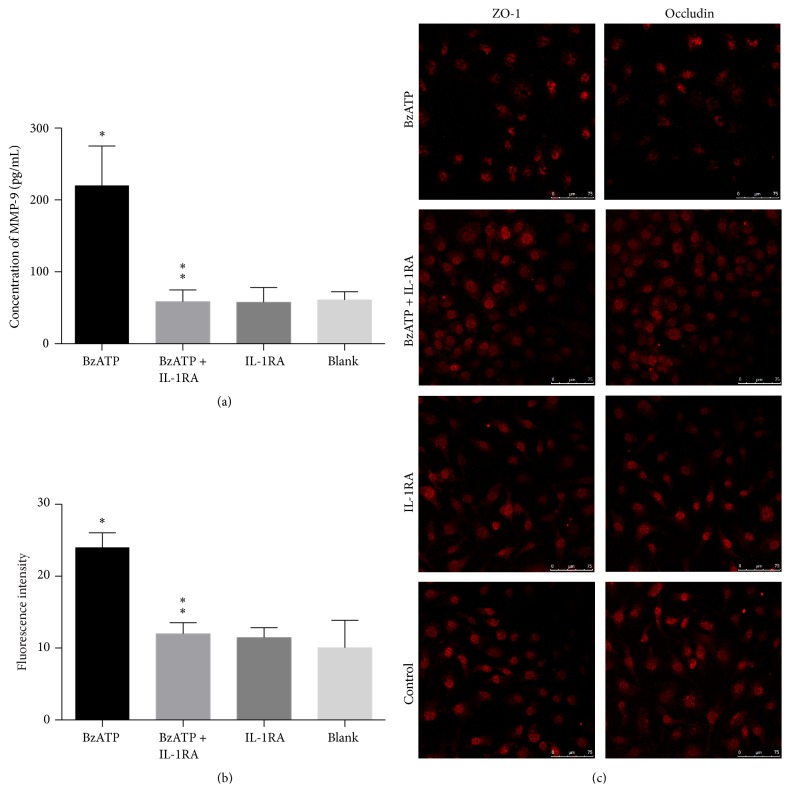
As discussed in Section 3.1.1, BzATP induced the release of IL-1β and MMP-9 in the BBB model. In order to correlate IL-1β and MMP-9, we designed two experiments (Sections 3.2 and 3.3), using their inhibitors, respectively. First, we investigated whether IL-1RA inhibited the BzATP-induced increase in MMP-9. As shown in Figure 3, IL-1RA treatment significantly attenuated the increase in MMP-9 levels triggered by BzATP compared with the vehicle group (P < 0.05, Figure 3(a)).
Figure 3.
BzATP-induced tight junction disruption and hyperpermeability of endothelial coculture system were attenuated by IL-1β inhibition. (a) The levels of MMP-9 were significantly higher in the BzATP groups than in the control groups (∗ P < 0.05). IL-1RA treatment significantly reduced the level of MMP-9 versus vehicle group (∗∗ P < 0.05). (b) BzATP induces hyperpermeability versus control groups (∗ P < 0.05), which was decreased by IL-1RA in the coculture system (∗∗ P < 0.05). (c) Confocal microscopy images of immunofluorescence staining of ZO-1 and occludin demonstrating the protective effect of IL-1RA against BzATP-induced tight junction disruption.
3.2.2. IL-1RA Attenuated Hyperpermeability of BzATP-Induced Endothelial Coculture System
Exposure to BzATP significantly increased the permeability to FITC-dextran compared with control group (P < 0.05, Figure 3(b)). IL-1RA attenuated BzATP-induced paracellular permeability. However, IL-1RA alone did not affect basal permeability levels (P < 0.05, Figure 3(b)).
3.2.3. BzATP-Induced TJP Disruption Was Attenuated by IL-1RA
Immunofluorescence staining showed that the expression of ZO-1 and occludin in BzATP groups was significantly decreased compared with controls, while treatment with IL-1RA and BzATP significantly increased the levels of the two tight junction proteins, indicating that the treatment reversed the degradation of TJPs (Figure 3(c)).
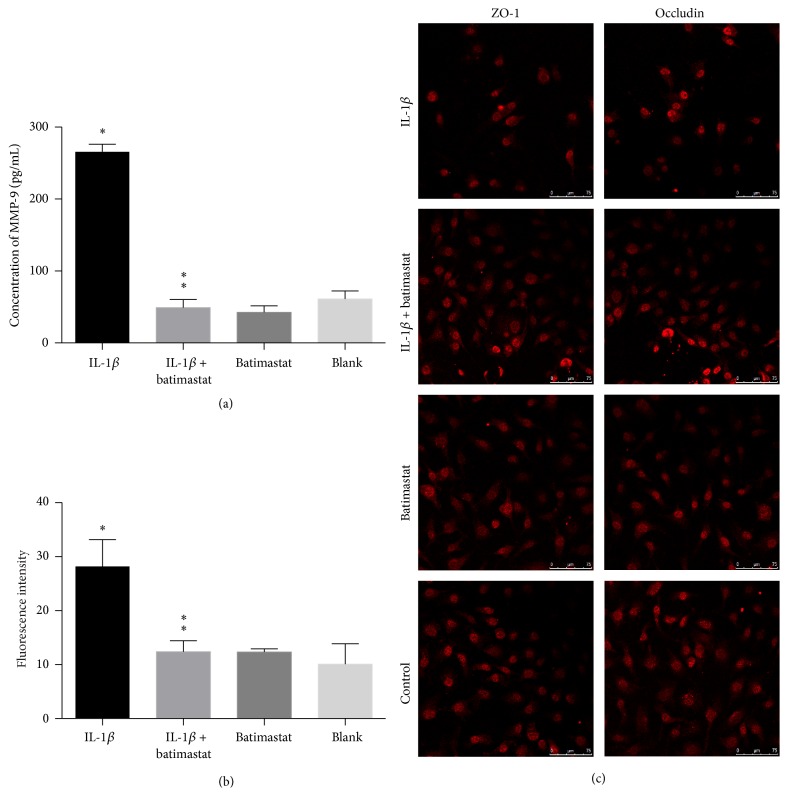
3.3. BzATP-Induced Tight Junction Disruption and Hyperpermeability of the Endothelial Coculture System Were Attenuated by MMP
3.3.1. BzATP-Induced IL-1β Induction Was Not Attenuated by MMP-9 Inhibition
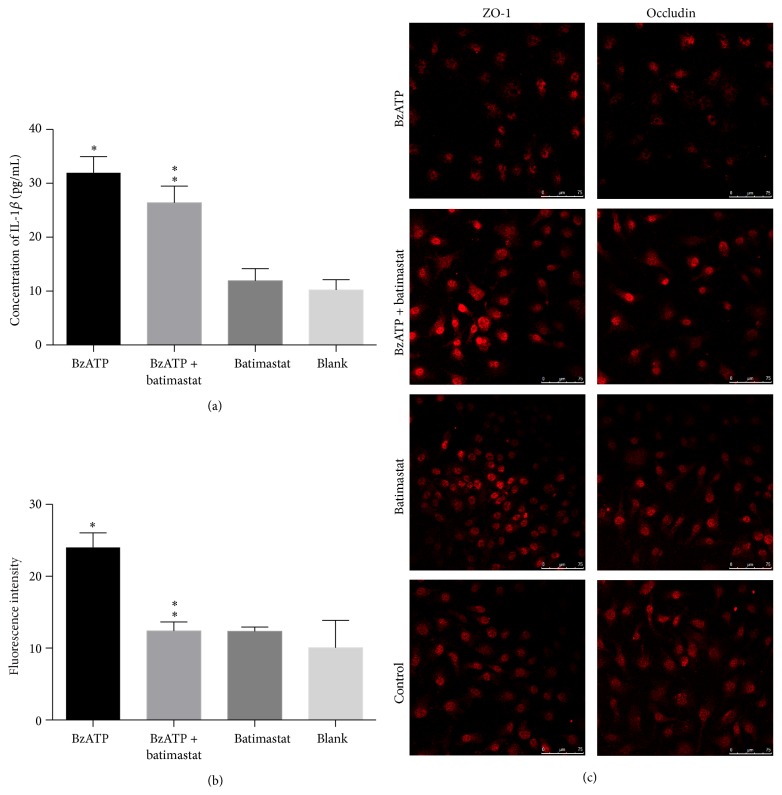
ELISA showed that the levels of IL-1β were significantly higher in the BzATP-treated groups than in the control group without BzATP. However, batimastat treatment did not alter the level of IL-1β (P > 0.05, Figure 4(a)).
Figure 4.
MMP-9-induced tight junction disruption and endothelial coculture system hyperpermeability were not attenuated by IL-1β inhibition. (a) The levels of IL-1β were significantly higher in BzATP groups than in control groups (∗ P < 0.05). However, batimastat + BzATP treatment did not alter the level of IL-1β versus BzATP group (∗∗ P > 0.05). (b) Exposure to BzATP significantly increased the permeability to FITC-dextran compared with the control groups (∗ P < 0.05). Batimastat attenuated BzATP-induced paracellular permeability versus BzATP group (∗∗ P < 0.05). (c) Confocal microscopy images of immunofluorescence staining of ZO-1 and occludin demonstrating the protective effect of batimastat against BzATP-induced tight junction disruption.
3.3.2. MMP Inhibition Attenuated Hyperpermeability of BzATP-Induced Endothelial Coculture System
Exposure to BzATP significantly increased the permeability to FITC-dextran compared with the control group (P < 0.05, Figure 4(b)). Batimastat attenuated BzATP-induced paracellular permeability, while batimastat alone did not affect the basal permeability levels (P < 0.05, Figure 4(b)).
3.3.3. BzATP-Induced TJP Disruption Was Attenuated by MMP Inhibition
Immunofluorescence staining showed that the expression of ZO-1 and occludin in the BzATP group was significantly decreased compared with that of the controls. In contrast, treatment with batimastat combined with BzATP significantly increased the levels of the two tight junction proteins, indicating that batimastat treatment reversed the degradation of TJPs (Figure 4(c)).
3.4. IL-1β-Induced Tight Junction Disruption and Hyperpermeability of Endothelial Coculture System Were Attenuated by MMP-9 Inhibition
3.4.1. IL-1β-Induced MMP-9 Induction Was Attenuated by MMP-9 Inhibition
We investigated the association between IL-1β and MMP-9 in the absence of BzATP. Our study showed that IL-1β treatment significantly increased the levels of MMP-9, compared with the vehicle group (P < 0.05, Figure 5(a)). Apparently, administration of batimastat significantly attenuated the levels of MMP-9 (P < 0.05, Figure 5(a)). However, MMP-9 did not significantly affect the level of IL-1β, as discussed in Section 3.5.1.
Figure 5.
IL-1β-induced tight junction disruption and endothelial coculture system hyperpermeability were attenuated by MMP-9 inhibition. (a) The levels of MMP-9 were significantly higher in IL-1β groups than in control groups (∗ P < 0.05). Batimastat treatment significantly reduced the level of MMP-9 versus vehicle group (∗∗ P < 0.05). (b) Exposure to IL-1β significantly increased the permeability to FITC-dextran compared with the control groups (∗ P < 0.05), which was decreased by batimastat in coculture system (∗∗ P < 0.05). (c) Confocal microscopy images of immunofluorescence staining of ZO-1 and occludin demonstrating the protective effect of batimastat against IL-1β-induced tight junction disruption.
3.4.2. MMP-9 Inhibition Attenuated Hyperpermeability of IL-1β-Induced Endothelial Coculture System
Exposure to IL-1β significantly increased the permeability to FITC-dextran compared with control group (P < 0.05, Figure 5(b)). Batimastat attenuated IL-1β-induced paracellular permeability, while batimastat alone did not affect the basal permeability levels (P < 0.05, Figure 5(b)).
3.4.3. IL-1β-Induced TJP Disruption Was Attenuated by MMP-9 Inhibition
Immunofluorescence staining showed that the expression of ZO-1 and occludin in the IL-1β groups was significantly decreased compared with the controls, while batimastat + IL-1β groups showed a significant increase in the levels of the two tight junction proteins, indicating that batimastat treatment reversed the degradation of TJPs (Figure 5(c)).
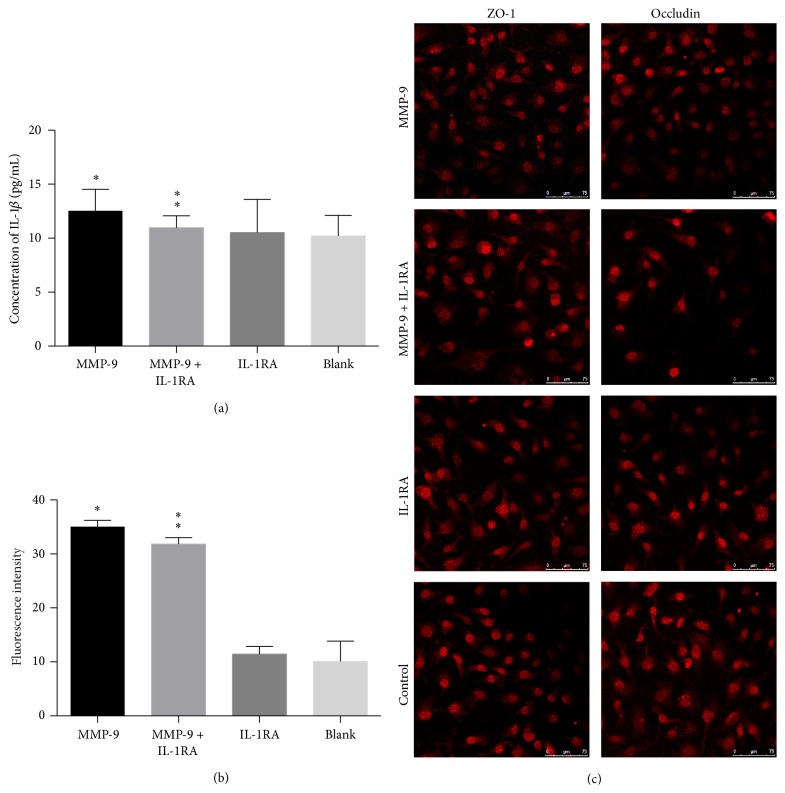
3.5. MMP-9-Induced Tight Junction Disruption and Hyperpermeability of Endothelial Coculture System Were Not Attenuated by IL-1β Inhibition
3.5.1. MMP-9 Has No Effect on IL-1β Induction
To further confirm the relationship between IL-1β and MMP-9, we investigated whether MMP-9 elevated the levels of IL-1β. ELISA analysis showed that the levels of IL-1β were not altered in MMP-9-treated groups compared with controls. In addition, there were no significant differences in the levels of IL-1β between IL-1RA + MMP-9 and MMP-9 groups (P > 0.05, Figure 6(a)).
Figure 6.
BzATP-induced tight junction disruption and endothelial coculture system hyperpermeability were attenuated by MMP inhibition. (a) The levels of IL-1β were not significantly altered in MMP-9-treated groups versus control groups (∗ P > 0.05). No statistically significant differences were found between IL-1RA + MMP-9 groups and MMP-9 groups in the levels of IL-1β (∗∗ P > 0.05). (b) Exposure to MMP-9 significantly increased the permeability to FITC-dextran compared with the control group (∗ P < 0.05). However, IL-1RA failed to attenuate MMP-9-induced paracellular hyperpermeability (∗∗ P > 0.05). (c) Immunofluorescence staining showed that the expression of ZO-1 and occludin in MMP-9 groups was significantly decreased compared with controls, while IL-1RA + MMP-9 groups showed no improvement in the levels of the two tight junction proteins.
3.5.2. Hyperpermeability of MMP-9-Induced Endothelial Coculture System Was Not Attenuated by IL-1β Inhibition
Exposure to MMP-9 significantly increased the permeability to FITC-dextran compared with the control group (P < 0.05, Figure 6(b)). However, IL-1RA failed to attenuate MMP-9-induced paracellular hyperpermeability (P < 0.05, Figure 6(b)).
3.5.3. MMP-9-Induced TJP Disruption Was Not Attenuated by IL-1β Inhibition
Immunofluorescence staining showed that the expression of ZO-1 and occludin in the MMP-9 groups was significantly decreased compared with that of the controls. However, the IL-1RA + MMP-9 groups showed no improvement in the levels of the two tight junction proteins, indicating that IL-1RA treatment failed to reverse the degradation of TJPs (Figure 6(c)).
4. Discussion
Disruption of BBB is a hallmark of brain trauma/stress injury. It is associated with an increase in the permeability of damaged endothelium leading to cerebral edema [4]. The structural integrity of BBB is determined by tight junction proteins (TJPs). In other words, the decreased expression of TJPs in the brain microvessels is strongly correlated with disruption of the BBB [18, 19]. In addition, evidence strongly suggests that P2X7 receptors are activated by extracellular ATP following CNS injury/stress, triggering secondary brain damage [6], and deficits in the structural integrity of the tight junction proteins, leading to BBB dysfunction and brain edema [4, 20]. Therefore, elucidation of the cellular mechanisms of ATP-associated BBB damage may facilitate the development of effective therapeutics to improve patient outcomes after brain injury.
The increasingly important role of P2X7R in the pathophysiology of CNS trauma/stress suggests that targeted therapies hold the key to the treatment of cerebral edema following CNS stress/trauma. Previous reports showed that administration of BBG, an antagonist of P2X7 receptor, improved neurological function and exerted neuroprotective effect in spinal cord and traumatic brain injuries in murine models [21–23]. However, activation of P2X7R-induced disruption of tight junction proteins in human BBB models has not been reported. Therefore, we utilized an in vitro coculture BBB model consisting of an immortal human cell line hCMEC/D3 with human astrocytes to investigate the role of ATP/P2X7R signaling in the disruption of tight junction proteins. Our model extends previous work in determining the translational potential of human cells. In the present study, we demonstrated that A438079, a more selective antagonist of P2X7 receptors, attenuated hyperpermeability in a human BBB model as well as disruption of ZO-1 and occludin, which is closely associated with IL-1β and MMP-9 expression.
P2X7R is the receptor mediating ATP-dependent maturation and release of IL-1β. Activation of caspase-1 plays a key role in P2X7R-dependent IL-1β release. ATP/P2X7R signaling leads to the opening of large membrane pores, triggering changes in the intracellular ionic homeostasis to activate the caspase-1 cascade resulting in the transformation of pro-IL-1β to IL-1β [9]. Exposure to Ac-YVAD-CMK, a caspase-1 antagonist, attenuated the maturation of IL-1β induction and inhibited MMP-9 activation and conservation of ZO-1 in mice [24]. Using a human BBB model in vitro, we confirmed that the extracellular ATP-induced activation of P2X7R led to the release of IL-1β, which was inhibited by the administration of A438079. Consistent with pharmacological inhibition of P2X7R, a significant reduction in IL-1β expression following experimental brain trauma/stress was observed in the cortex of P2X7 knockout mice, when compared with wild-type mice [22]. In addition, treatment with an IL-1 receptor antagonist followed by BzATP significantly attenuated the disruption of ZO-1 and occludin, indicating that ATP/P2X7R signal-induced degradation of tight junction proteins was strongly associated with IL-1 receptors. Overall, our study suggested that secretion of IL-1β induced by ATP-stimulated P2X7R activation played a crucial role in triggering endothelial hyperpermeability and dysfunction of BBB.
In the present study, we investigated the relationship between IL-1β and MMP-9. IL-1β, one of the proinflammatory cytokines, is an important mediator of brain injuries/stress, and its role in the induction of MMP-9 has been well documented [24–26]. Ranaivo et al. reported that IL-1β enhanced the release of MMP-9 via activation of the ERK pathway [27]. However, Wu and his colleagues argued that IL-1β-induced MMP-9 expression depends on JNK1/2 signaling in astrocytes, involving CaMKII and phosphorylation of c-Jun, which are the upstream and downstream regulators of JNK, respectively [28, 29]. In our study, we found that both IL-1β and MMP-9 were induced after ATP/P2X7R activation. Induction of IL-1β increased the level of MMP-9. However, the levels of IL-1β were not altered in MMP-9-treated groups. Therefore, no obvious interaction exists between MMP-9 and IL-1β following disruption of BBB secondary to brain trauma/stress.
MMPs comprise a number of zinc-dependent endopeptidases that mediate the degradation of extracellular matrix. MMP-9 has a significant role in CNS injury/stress following activation by IL-1β and close association with tight junction protein degradation [30]. ATP-treated glial cultures derived from neonatal C57BL/6 mice showed an increase in MMP-9 activity via activation of the P2X7 receptor [31]. In addition, increased MMP-9 activity promoted the disruption of tight junctions in cerebral endothelial cells, such as occludin and ZO-1, which in turn disrupt the structural integrity and hyperpermeability of BBB [12, 32, 33]. Pharmacological inhibitors of MMP-9 significantly attenuated the disruption of BBB in rats [34]. Similarly, MMP-9 knockout abrogated the increased vascular permeability and restored neurological function [35, 36]. Our study showed that inhibitors of P2X7R and IL-1β prevented the increase in MMP-9 expression and consequently suppressed the degradation of ZO-1 and occludin in human BBB model in vitro. Furthermore, inhibition by batimastat decreased the disruption of tight junction proteins, indicating that MMP-9 was essential for ATP-induced hyperpermeability of BBB. These findings strongly supported the hypothesis that MMP-9 was regulated upstream by IL-1β and modulated BBB integrity downstream following CNS trauma/stress, suggesting that MMP-9 may also represent a therapeutic target as well as IL-1β inhibition in the ATP-induced BBB leakage.
Our study has several limitations. First, BBB model in vitro does not simulate the in vivo conditions, totally. For example, shear stress played a role in BBB physiology in vivo [37]. Second, studies suggest that microglia are the main cell type in the brain, which is responsible for IL-1β and IL-18 secretion [38]. Therefore, analysis of the relationship between microglia and other cell types such as astrocytes is critical to fully understand the mechanism of ATP/P2X7R signaling. Third, BBB disruption is a complex and multistep process involving several interrelated factors. As a result, agents such as P2X7R antagonists that selectively target specific steps in the process are likely to be more effective when deployed in combination with agents that target other processes [39].
5. Conclusions
Our study demonstrated the potential mechanism of ATP/P2X7R signaling that may be targeted to attenuate BBB disruption and brain edema following brain trauma/stress. Concurrently, we tested P2X7R antagonist in a human BBB model, indicating its translational potential. However, a more detailed understanding of ATP/P2X7R pathway is needed. Future studies will focus on elucidating the mechanisms of cytokine-mediated neural plasticity and their implications for the pathophysiology and treatment of brain trauma/stress.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant no. 81271365).
Abbreviations
- BzATP:
3′-O-(4-Benzoyl)benzoyl adenosine 5′-triphosphate
- BBB:
Blood-brain barrier
- CNS:
Central nervous system
- P2X7R:
P2X7 receptor
- IL-1β:
Interleukin-1β
- MMP-9:
Matrix metalloproteinase-9
- TJP:
Tight junction protein
- ELISA:
Enzyme-linked immunosorbent assay
- IL-1R:
Interleukin-1 receptor
- IL-1RA:
Interleukin-1 receptor antagonist
- TBI:
Traumatic brain injury
- ASD:
Acute stress disorder
- PTSD:
Post-traumatic stress disorder
- HA:
Human astrocyte
- DMEM:
High glucose Dulbecco's Modified Eagle's Medium
- FBS:
Fetal bovine serum
- PMSF:
Phenylmethanesulfonyl fluoride
- FITC:
Fluorescein isothiocyanate.
Competing Interests
The authors declare that they have no competing interests regarding the publication of this paper.
References
- 1.Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues in Clinical Neuroscience. 2011;13(3):251–262. doi: 10.31887/DCNS.2011.13.2/rbryant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The changing landscape of traumatic brain injury research. Lancet Neurology. 2012;11(8):p. 651. doi: 10.1016/s1474-4422(12)70166-7. [DOI] [PubMed] [Google Scholar]
- 3.Corps K. N., Roth T. L., McGavern D. B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurology. 2015;72(3):355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature Reviews Neurology. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConeghy K. W., Hatton J., Hughes L., Cook A. M. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26(7):613–636. doi: 10.2165/11634020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Fiebich B. L., Akter S., Akundi R. S. The two-hit hypothesis for neuroinflammation: role of exogenous ATP in modulating inflammation in the brain. Frontiers in Cellular Neuroscience. 2014;8, article 260 doi: 10.3389/fncel.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Current Medicinal Chemistry. 2015;22(7):819–844. doi: 10.2174/0929867321666140706130415. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Purine and pyrimidine receptors. Cellular and Molecular Life Sciences. 2007;64(12):1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari D., Pizzirani C., Adinolfi E., et al. The P2X7 receptor: a key player in IL-1 processing and release. Journal of Immunology. 2006;176(7):3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 10.Takenouchi T., Sugama S., Iwamaru Y., Hashimoto M., Kitani H. Modulation of the ATP-lnduced release and processing of IL-1β in microglial cells. Critical Reviews in Immunology. 2009;29(4):335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- 11.Gu B. J., Wiley J. S. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107(12):4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 12.Harkness K. A. C., Adamson P., Sussman J. D., Davies-Jones G. A. B., Greenwood J., Woodroofe M. N. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123(4):698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 13.Mori T., Wang X., Aoki T., Lo E. H. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. Journal of Neurotrauma. 2002;19(11):1411–1419. doi: 10.1089/089771502320914642. [DOI] [PubMed] [Google Scholar]
- 14.Weksler B. B., Subileau E. A., Perrière N., et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB Journal. 2005;19(13):1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 15.Sajja R. K., Prasad S., Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids and Barriers of the CNS. 2014;11(1, article 8) doi: 10.1186/2045-8118-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatherell K., Couraud P.-O., Romero I. A., Weksler B., Pilkington G. J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. Journal of Neuroscience Methods. 2011;199(2):223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Sahagun G., Moore S. A., Hart M. N. Permeability of neutral vs. anionic dextrans in cultured brain microvascular endothelium. American Journal of Physiology—Heart and Circulatory Physiology. 1990;259(1, part 2):H162–H166. doi: 10.1152/ajpheart.1990.259.1.H162. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Muneer P. M., Schuetz H., Wang F., et al. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radical Biology and Medicine. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Zhao Z., Chai Y., Luo L., Jiang R., Zhang J. The incidence of critical-illness-related-corticosteroid-insufficiency is associated with severity of traumatic brain injury in adult rats. Journal of the Neurological Sciences. 2014;342(1-2):93–100. doi: 10.1016/j.jns.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Ng I., Yap E., Tan W. L., Kong N. Y. Blood-brain barrier disruption following traumatic brain injury: roles of tight junction proteins. Annals of the Academy of Medicine, Singapore. 2003;32(5):S63–S66. [PubMed] [Google Scholar]
- 21.Peng W., Cotrina M. L., Han X., et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimbler D. E., Shields J., Yanasak N., Vender J. R., Dhandapani K. M. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041229.e41229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y. C., Cui Y., Cui J. Z., et al. Neuroprotective effects of brilliant blue G on the brain following traumatic brain injury in rats. Molecular Medicine Reports. 2015;12(2):2149–2154. doi: 10.3892/mmr.2015.3607. [DOI] [PubMed] [Google Scholar]
- 24.Sozen T., Tsuchiyama R., Hasegawa Y., et al. Role of interleukin-1β in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40(7):2519–2525. doi: 10.1161/strokeaha.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecil G. G., Larsen P. H., Corley S. M., et al. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. Journal of Neuroscience Research. 2000;61(2):212–224. doi: 10.1002/1097-4547(20000715)61:2<212::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Ruhul Amin A. R. M., Senga T., Oo M. L., Thant A. A., Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1β: s role for the dual signalling pathways, Akt and Erk. Genes to Cells. 2003;8(6):515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 27.Ranaivo H. R., Zunich S. M., Choi N., Hodge J. N., Wainwright M. S. Mild stretch-induced injury increases susceptibility to interleukin-1β-induced release of matrix metalloproteinase-9 from astrocytes. Journal of Neurotrauma. 2011;28(9):1757–1766. doi: 10.1089/neu.2011.1799. [DOI] [PubMed] [Google Scholar]
- 28.Wu C.-Y., Hsieh H.-L., Jou M.-J., Yang C.-M. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-κB in interleukin-1β-induced matrix metalloproteinase-9 expression in rat brain astrocytes. Journal of Neurochemistry. 2004;90(6):1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu C.-Y., Hsieh H.-L., Sun C.-C., Yang C.-M. IL-1β induces MMP-9 expression via a Ca2+-dependent CaMKII/JNK/c-Jun cascade in rat brain astrocytes. GLIA. 2009;57(16):1775–1789. doi: 10.1002/glia.20890. [DOI] [PubMed] [Google Scholar]
- 30.Abdul-Muneer P. M., Pfister B. J., Haorah J., Chandra N. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Molecular Neurobiology. 2015 doi: 10.1007/s12035-015-9520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy N., Lynch M. A. Activation of the P2X7 receptor induces migration of glial cells by inducing cathepsin B degradation of tissue inhibitor of metalloproteinase 1. Journal of Neurochemistry. 2012;123(5):761–770. doi: 10.1111/jnc.12031. [DOI] [PubMed] [Google Scholar]
- 32.Chen F., Ohashi N., Li W., Eckman C., Nguyen J. H. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology. 2009;50(6):1914–1923. doi: 10.1002/hep.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubio-Araiz A., Perez-Hernandez M., Urrutia A., et al. 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) disrupts blood-brain barrier integrity through a mechanism involving P2X7 receptors. International Journal of Neuropsychopharmacology. 2014;17(8):1243–1255. doi: 10.1017/s1461145714000145. [DOI] [PubMed] [Google Scholar]
- 34.Yong V. W., Power C., Forsyth P., Edwards D. R. Metalloproteinases in biology and pathology of the nervous system. Nature Reviews Neuroscience. 2001;2(7):502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Jung J., Asahi M., et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. The Journal of Neuroscience. 2000;20(18):7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muradashvili N., Benton R. L., Saatman K. E., Tyagi S. C., Lominadze D. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metabolic Brain Disease. 2015;30(2):411–426. doi: 10.1007/s11011-014-9550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cucullo L., Hossain M., Puvenna V., Marchi N., Janigro D. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neuroscience. 2011;12, article 40 doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustin A., Kirchmeyer M., Koncina E., et al. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0130624.e0130624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walcott B. P., Kahle K. T., Simard J. M. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9(1):65–72. doi: 10.1007/s13311-011-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]