Abstract
Objective
This study aimed to investigate thalamic shape alterations and their relationships with various episodic memory impairments in subjects with amnestic mild cognitive impairment (aMCI).
Methods
We compared volumes and morphological alterations of the thalamus between aMCI subjects and healthy controls. In addition, we investigated the correlation between thalamic deformations and various memory impairments in aMCI subjects using a comprehensive neuropsychological battery.
Results
The normalized left thalamic volumes of the aMCI group were significantly smaller than those of the healthy control group (p<0.0001). aMCI subjects exhibited significant thalamic deformations in the left thalamic dorso-medial and antero-medial areas compared with healthy individuals. CERAD-K Word List Memory scores were significantly correlated with the left dorso-medial areas in aMCI subjects. There were no significant correlations between verbal fluency, Boston naming test, constructional praxis, Word List Recognition, and Visuospatial Recall scores and thalamic shape in aMCI subjects. Verbal delayed recall scores were also significantly correlated with the left dorso-medial areas in the aMCI group.
Conclusion
Structural alterations in the thalamic deformations in the left dorso-medial and antero-medial areas might be core underlying neurobiological mechanisms of thalamic dysfunction related to Word List Memory and delayed verbal recall in individuals with aMCI.
Keywords: Thalamic shape, Cognitive functions, Mild cognitive impairment
INTRODUCTION
Amnestic mild cognitive impairment (aMCI) is characterized by a selective decline in memory in the context of otherwise normal cognition and normal daily functioning.1 Patients with MCI progress to overt dementia at a rate of 10% to 15% per year, the majority being Alzheimer's disease (AD). Non-aMCI cases might be associated with various types of dementia (e.g., frontotemporal dementia, Lewy body dementia, vascular dementia, primary progressive aphasia, Parkinson's disease) apart from AD, and thus aMCI could be more closely related to AD.2
The thalamus functions as the principal relay station for information passed from the primary sensory brain regions, the basal ganglia, the cerebellum, and the limbic system, processing this information before passing it onto the cerebral cortex.3 A previous study reported atrophy in the thalami of subjects with MCI compared to healthy controls, and also that the thalamic areas are associated with cognitive function.4 Although a large number of studies on neurocognitive disorders have focused on medial temporal and cortical atrophy, structural alterations of thalamus are also considered to be one of the important biomarkers of aMCI.4 Furthermore, several investigators have reported that structural changes of the thalamus are associated with AD.5,6,7
Shape analysis of subcortical structures could provide useful information about the location and pattern of structural changes in aMCI. Indeed, several researchers employing this methodology have reported that patients with neurocognitive disorder exhibit shape changes in the hippocampus, corpus callosum, thalamus, and other subcortical structures.8,9,10,11 Several studies have demonstrated a close relationship between substructural deformations in the hippocampus and dysfunction of specific cognitive domains in aMCI.9,12,13,14 However, there is insufficient data regarding structural alterations of the thalamus in patients with aMCI.
Thalamic shape analysis might provide more accurate biomarkers of cognitive decline than simple volumetry. Specifically, while volumetric techniques may be useful for detecting thalamic volume loss, subtle morphological alterations of the thalamus may be analyzed through deformation-based morphometry. To date one study has analyzed shape abnormalities of subcortical and ventricular structures in MCI and AD, reporting that atrophy and expansion of the surfaces of each side of the thalamus in MCI and AD, respectively, compared with healthy controls.15 Although a few previous studies have carried out thalamic shape analysis of MCI patients, they have not performed correlation analysis with comprehensive neuropsychological tests. Therefore, the relationship between thalamic deformation and various forms of memory impairment remain unclear.
The aim of this study was to examine anatomical shape differences of the thalamus in patients with aMCI and investigate the relationship between various memory impairments and thalamic deformations. We hypothesized that patients with aMCI would have significant thalamic deformations compared with healthy subjects, and that these thalamic deformations would significantly correlate with various episodic memory impairments.
METHODS
Subjects
Sixty individuals took part in this study (30 with aMCI and 30 healthy controls). Patients with aMCI met Petersen's criteria: 1 1) memory complaint corroborated by an informant: 2) objective memory impairment for age, education, and sex; 3) essentially preserved general cognitive function; 4) largely intact functional activities; and 5) no dementia. All aMCI patients had an overall Clinical Dementia Rating of 0.5.16 Objective memory impairment was defined as a performance score of 1.5 SDs below the respective age-specific, education-specific, and sex-specific normative means for at least one of the four episodic memory tests included in the Korean version of the Consortium to Establish a Registry for Alzheimer's disease (CERAD-K) neuropsychological battery, namely, the Word List Memory, Word List Recall (WLR), Word List Recognition, and Constructional Recall (CR) tests.17 We excluded from the study those participants who had other neurological or psychiatric conditions. None of the participants of this study had a history of cerebro-vascular accident (CVA) and clinically meaningful white matter hyperintensities. All images were reviewed by a board-certified geriatric psychiatrist using the Fazekas scale,18 of which all subjects scored 0. The study was conducted in accordance with the ethical and safety guidelines set forth by the local Institutional Review Board of the Catholic University of Korea. Informed consent was obtained from all participants. All participants were right-handed.
MRI acquisition
All participants underwent MRI scans on a 3-Tesla whole body scanner equipped with an 8-channel phased-array head coil (Verio, Siemens, Erlangen, Germany). The scanning parameters of the T1-weighted three-dimensional magnetization-prepared rapid gradient-echo (3D-MPRAGE) sequences were as follows: TE=2.5 ms; TR=1900 ms; inversion time (TI)=900 ms; flip angle (FA)=9°; field of view (FOV)=250×250 mm; matrix=256×256; and voxel size=1.0×1.0×1.0 mm.
MRI preprocessing
The FIRST tool, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl/), was used for automatic registration and segmentation of the thalamus within the Bayesian Appearance Model framework as described previously.19 A two-stage affine registration to a standard space template (Montreal Neurological Institute space) with a 1 mm resolution using 121 degrees of freedom and a subcortical mask to exclude voxels outside the subcortical regions was performed for each participant's MPRAGE. Next, the bilateral thalami were segmented from the T1-weighted MR images using FSL-FIRST. Finally, a boundary correction was used to determine which boundary voxels belonged to the structure.
Shape analysis
FIRST creates a surface mesh for each subcortical structure using a deformable mesh model. The mesh is composed of a set of triangles, and the apex of adjoining triangles is called a vertex. The number of vertices for each structure is fixed such that the corresponding vertices can be compared across individuals and between groups. Vertex correspondence is crucial for the FIRST methodology, as it facilitates the investigation of localized shape differences through the examination of group differences in the spatial location of each vertex. Although the vertices retain correspondence, the surfaces reside in the native image space and thus have an arbitrary orientation and position. Therefore, the surfaces must all be aligned to a common space before investigating any group differences. The mean surface from the FIRST models can then be used as the target to which surfaces from the individual participants are aligned. Poses were removed by minimizing the sum-of-squares difference between the corresponding vertices of a participant's surface and the mean surface. Group comparisons of vertices were carried out using F-statistics.19 The effects of age, education, total intracranial volume, and sex were regressed out of the models. The statistical significance threshold was set at a p-value of less than 0.05 corrected for false discovery rate to resolve the problem of multiple comparisons. We referred to the Oxford Thalamic Atlas in determining the approximate anatomical locations of our statistical maps.
RESULTS
Demographic data
Table 1 shows the baseline demographic data for our different subject groups. No significant differences in age, sex, and education were observed between the aMCI group and the healthy control group. Compared with healthy controls, patients with aMCI showed significantly poorer performances in word list memory, word list recall, word list recognition and recall of constructional praxis on CERAD-K neuropsychological tests (p<0.05).
Table 1. Demographic, cognitive, and quantitative volumetric data.
CERAD-K: Korean version of the Consortium to Establish a Registry for Alzheimer's disease, F: female, M: male, MMSE-KC: Mini-Mental Status Examination in the Korean version of the CERAD Assessment Packet, SD: standard deviation, NS: non-specific
Quantitative volumetric data
The normalized left thalamic volumes of the aMCI group were significantly smaller than those of the healthy control group (p<0.0001) (Figure 1). However, correlation analysis showed that the left and right normalized thalamic volume did not correlate with cognitive performance in the aMCI group (p<0.0001, respectively). Likewise, the healthy control group did not exhibit a significant correlation between the normalized thalamic volume and cognitive performance.
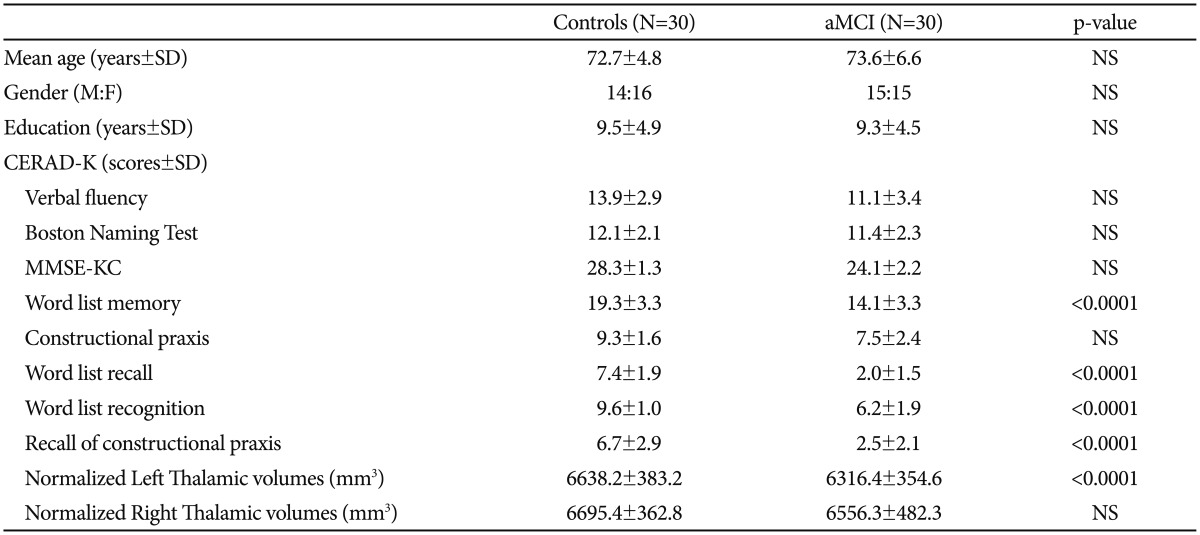
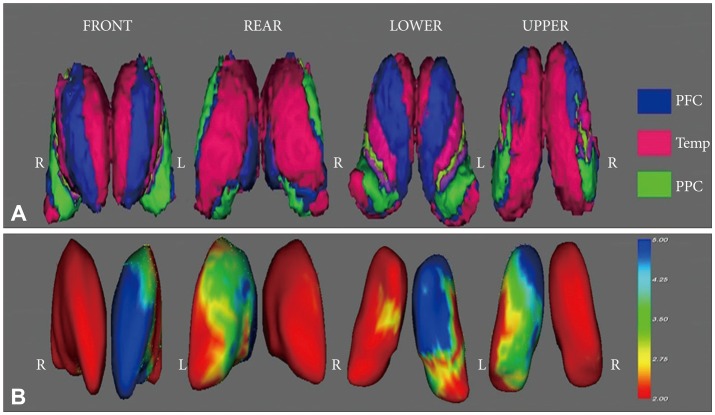
Figure 1. A: Oxford thalamic connectivity atlas. Blue region: thalamic area connected with the prefrontal area, Red region: thalamic area connected with the temporal lobe, Green region: thalamic area connected with the posterior parietal region. B: Statistical maps corrected for age, education, and sex showing thalamic shape deformation in patients with aMCI relative to the healthy controls. The results were color-coded by FDR-corrected F-statistic values. Cool color denotes regions of the thalamus with surface deformation. aMCI: amnestic mild cognitive impairment, FDR: false discovery rate, L: left, PFC: prefrontal area, PPC: posterior parietal region, R: right, Temp: temporal area.
Shape analysis using vertex-based comparisons
Patients with aMCI showed significant left thalamic atrophy compared with healthy controls. Furthermore, patients with aMCI exhibited significant thalamic deformations in the left dorso-medial and antero-medial areas compared with healthy individuals. These areas were mainly connected with the frontal and temporal areas.
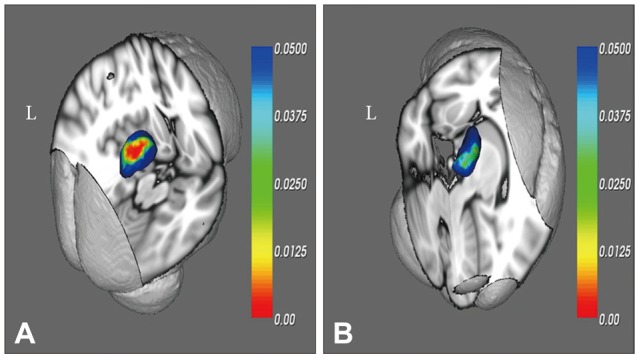
Significant correlations were observed between Word List Memory scores on CERAD-K and the left dorso-medial areas in aMCI (Figure 2). Conversely, there were no significant correlations between verbal fluency, Boston naming test, constructional praxis, Word List Recognition, and Visuospatial Recall scores and thalamic shape in aMCI subjects. Verbal delayed recall scores (CERAD-K Word List Recall) were also significantly correlated with the left dorso-medial areas in aMCI (Figure 2).
Figure 2. Statistical maps showing the regions of thalamic deformation correlated with CERAD-K Word List Memory scores (A) and CERAD-K Word List Recall scores (B) in the aMCI group. The results are color-coded by uncorrected p-values. aMCI: amnestic mild cognitive impairment, CERAD-K: the Korean version of Consortium to Establish a Registry for Alzheimer's Disease, L: left.
DISCUSSION
In the present study, we identified thalamic structures alterations in aMCI subjects compared with healthy subjects. Although the present study is not the first to perform thalamic shape analysis for MCI subjects, it is to the best of our knowledge the first study to simultaneously evaluate structural changes of the thalamus in aMCI and correlate these deformations with cognitive performance. Because all the participants in the present study had no vascular risk factors (no CVA history and 0 score on the Fazekas scale), we were able to control the effects of vascular risk factors. In addition, the effects of medication on thalamic changes were not investigated because all participants were drug-naive.
Four key findings emerged from our study: 1) significant thalamic deformations in the left dorso-medial and antero-medial areas of the thalamus were observed in the aMCI group compared to healthy control subjects; 2) there was a significant correlation between the left dorso-medial areas of the thalamus and verbal immediate recall scores (Word List Memory scores on CERAD-K) confined to the aMCI group compared to healthy control subjects; 3) the left dorso-medial areas of the thalamus were significantly correlated with verbal delayed recall scores (Word List Recall on CERAD-K) in the aMCI group; and 4) the aMCI group exhibited significant left thalamic atrophy compared to healthy control subjects.
The mediodorsal nucleus is described as the thalamic relay nucleus for association areas in the frontal lobe. The dorso-medial area of the thalamus includes the medio-dorsal nucleus, whose major inputs are from the basal ganglia, the amygdala, the hypothalamus, the nucleus accumbens, and olfactory system. On the other hand, the major outputs of the medio-dorsal nucleus comprise the prefrontal cortex, cingulate gyrus, and nucleus basalis.3 According to previous studies, the mediodorsal nucleus is closely related with learning, emotion, cognition and memory functions. Indeed, Edelstyn and colleagues reported a patient with a unilateral left thalamic lesion centered on the mediodorsal thalamic nucleus who exhibited severe impairment of verbal memory and symptoms of executive dysfunction.20 Likewise, Van der Werf et al.21 reported that the mediodorsal thalamic nucleus affects executive processes connected with declarative memory. Pergola et al.22 demonstrated that the parvocellular subnucleus of mediodorsal nucleus has a role in recall and recollection. Our findings were highly consistent with the results of previous studies with respect to the association between the mediodorsal thalamic nucleus and memory. Furthermore, our findings suggested that structural deformations in the dorso-medial area of the thalamus might appear in the early stages of neurocognitive disorders. In addition, our findings suggested a pivotal role of the dorso-medical area of thalamus in immediate recall and delayed recall impairment of aMCI patients. To the best of our knowledge, this is the first study to report a correlation between the left dorso-medial areas of the thalamus and verbal immediate recall scores (Word List Memory scores on CERAD-K) and verbal delayed recall scores (Word List Recall on CERAD-K) in aMCI subjects. Several investigators reported mediodorsal thalamus was closely related to verbal memory.20,23 These findings of the studies appear to be consistent with the present study. Dos Santos et al.24 showed that deficits in constructional praxis and constructional praxis recall referred to sites in the left thalamus and cerebellum, and the temporal cortices. Therefore more research is needed to determine the association between thalamic subdivisions and various cognitive functions including verbal and constructional memory.
The anterior area of thalamus involves the anterodorsal, the anteroventral, and the anteromedial nuclei. The anterior thalamic nuclei receive input from the mammillary body by way of the mammillothalamic tract and projects to the cingulate gyrus.3 The mammillothalamic tract including the anterior thalamic nuclei is a component of Papez circuit, which is related to emotion and memory. In addition, the anterior thalamic nuclei are assumed to be one of the neural substrates involved in learning and memory.25 Several studies have reported a relationship between the anteromedial thalamus and memory. Kishiyama et al.26 examined explicit memory performance in a patient with bilateral anterior and medial thalamic lesions, and suggested that the anteromedial thalamus is important for both recollection- and familiarity-based recognition memory. Stenset et al.27 presented a patient with a left anteromedial thalamic stroke and subsequent amnesic syndrome. The authors suggested the importance of a network that included the anterior and dorsomedian nuclei, which influences the activity in areas of the cortex responsible for memory processes. Edelstyn et al.23 reported two patients with unilateral right and left mediodorsal thalamic pathology plus probable correspondingly lateralized damage of the mammillo-thalamic tract. In their study, the authors provided evidence that material-specific lateralization of long-term memory extends to the anteromedial thalamus. Van der Werf et al.21 reported that the anterior nuclei have a key role in the selection of material to be remembered. Thus, the findings of the present study appear to be consistent with other studies that collectively suggest a key role of the anteromedial thalamus in memory processes.
Our findings showed that patients with aMCI have significant left thalamic atrophy compared with healthy controls (Table 1). While this result is consistent with a previous study demonstrating thalamic volume reduction in patients with aMCI,4 it is inconsistent with respect to previous studies concerning thalamic volume reductions in patients with MCI.28 Neuropsychological findings suggest material-specific lateralization of the medial temporal lobe's role in long-term memory, with greater left-sided involvement in verbal memory, and greater right-sided involvement in visual memory.23 Therefore, the results of our study indicate that early neurocognitive disorder with memory decline may be associated with disease-specific deformities in the left side of the thalamus. In this way, left-side lateralization of thalamus deformation may serve as a biomarker of aMCI, allowing it to be distinguished from other types of MCI. However, Pedro et al.4 reported volume reductions of both sides of the thalamus in patients with aMCI. Thus, more studies are necessary to determine the lateralization in thalamus deformities in aMCI pathology. Furthermore, the results of our study showed that the left and right normalized thalamic volume did not correlate with cognitive performance in the aMCI group. Thalamic shape deformation than thalamic volume reduction might have more sensitive correlation with various forms of cognitive impairment in MCI, the early stages of neurocognitive disorder. De Jong et al.29 reported that volume reduction in thalamus correlated linearly with impaired global cognitive performance in Alzheimer's disease, the advanced stage of neurocognitive disorder.
Since our findings showed volume reduction and morphological deformities of the left thalamus in aMCI patients, volume reduction and morphological deformity in the thalamus may be a useful biomarker of memory impairment in patients with aMCI. To date, structural deformities of brain structures including the thalamus (especially the left side of thalamus, according to the results of our study) have been shown to serve as specific pathologies of neurocognitive disorders. Indeed, structural degeneration of the left medial lobe (including the parahippocampus, the amygdala, and the hippocampus)30 has been proposed as a predictive factor of conversion from MCI to AD. Predictive factors of conversion from MCI to AD and biomarkers of early neurocognitive disorders may vary between studies, including those described above. Thus, structural deformation of the thalamus in early neurocognitive disorders may be a biomarker of cognitive decline, but not a disease-specific biomarker. Qui et al.31 reported that regionally-specific thalamic shape compression is associated with poor performance in executive functioning and spatial working memory in first-episode schizophrenia. In addition, Palm et al.32 reported that persons with subjective memory complaints have relevant differences in the shape of the ventricular surface adjacent to the thalamus and corona radiata compared with control subjects. Importantly, these studies highlight the relevance of morphological deformities with various diseases, especially changes in the thalamus, suggesting that thalamic deformation may be a biomarker of memory impairment. Using a meta-analysis approach, Ferreira et al.33 reported that volume reduction in the left medial temporal lobe including the left hippocampus and parahippocampal gyrus is the most consistent neurostructural biomarker to predict conversion from aMCI to AD. Therefore, all patients with aMCI who eventually convert to dementia as well as those who do not may have common deformities in the thalamus (especially in the left dorso-medial and antero-medial areas in the thalamus), which could be a neuropathology related to memory impairment. In other words, thalamic deformities caused by benign neuropathologies, but not those related to dementia, may serve as biomarkers for identifying pre-dementia patients with aMCI who are unlikely to convert to dementia. However, since there are few longitudinal studies regarding morphological changes in the thalamus, it will be necessary to continue to investigate thalamic deformities to obtain a clearer sense of the role of the thalamus in aMCI.
The connection between the medial temporal lobe, the medial thalamus, and the prefrontal cortex suggests the presence of a brain network that shares the crucial role in memory function. Although our study evaluated the structural alteration in thalamus, our findings may suggest the importance to explore network pathology in aMCI, since the thalamus functions as a principal relay station and source of diffuse projections. Importantly, we identified structural deformation in the anteromedial thalamus, which is part of the Papez circuit. Thus, previous studies about neural circuits including the thalamus may be another methodology useful for evaluating aMCI neuropathology.
The current study has some limitations. First, the sample size might not have been large enough to generalize structural deformation of the thalamus in aMCI. A second limitation of this study was its cross-sectional design. Specifically, we did not consider the possibility that some patients with aMCI would be in a transition stage of dementia while other patients with aMCI will not develop dementia. Thus, it will be necessary to perform follow-up longitudinal studies to confirm our suggestions regarding the clinical value of thalamus morphology in aMCI.
In this study, we identified thalamic deformations in the left dorso-medial and antero-medial areas of the thalamus and left thalamic atrophy in the aMCI group as compared to that of healthy controls. In addition, we reported significant correlations between the left dorso-medial areas of the thalamus and verbal immediate recall scores and verbal delayed recall scores. These morphological changes in the thalamus might be key to understanding the underlying neurobiological mechanisms of aMCI.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A02036578).
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 3.Clark DL, Boutros NN, Mendez MF. The Brain and Behavior: An Introduction to Behavioral Neuroanatomy. New York: Cambridge University Press; 2010. [Google Scholar]
- 4.Pedro T, Weiler M, Yasuda CL, D'Abreu A, Damasceno BP, Cendes F, et al. Volumetric brain changes in thalamus, corpus callosum and medial temporal structures: mild Alzheimer's disease compared with amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2012;34:149–155. doi: 10.1159/000342118. [DOI] [PubMed] [Google Scholar]
- 5.Stepan-Buksakowska I, Szabo N, Horinek D, Toth E, Hort J, Warner J, et al. Cortical and subcortical atrophy in Alzheimer disease: parallel atrophy of thalamus and hippocampus. Alzheimer Dis Assoc Disord. 2014;28:65–72. doi: 10.1097/WAD.0b013e318299d3d6. [DOI] [PubMed] [Google Scholar]
- 6.Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, et al. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain. 2013;136:1399–1414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol. 1991;81:261–268. doi: 10.1007/BF00305867. [DOI] [PubMed] [Google Scholar]
- 8.Lim HK, Jung WS, Ahn KJ, Won WY, Hahn C, Lee SY, et al. Relationships between hippocampal shape and cognitive performances in drug-naive patients with Alzheimer's disease. Neurosci Lett. 2012;516:124–129. doi: 10.1016/j.neulet.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 9.Lim HK, Hong SC, Jung WS, Ahn KJ, Won WY, Hahn C, et al. Hippocampal shape and cognitive performance in amnestic mild cognitive impairment. Neuroreport. 2012;23:364–368. doi: 10.1097/WNR.0b013e328351ddc9. [DOI] [PubMed] [Google Scholar]
- 10.Bachman AH, Lee SH, Sidtis JJ, Ardekani BA. Corpus callosum shape and size changes in early alzheimer's disease: a longitudinal MRI study using the OASIS brain database. J Alzheimers Dis. 2014;39:71–78. doi: 10.3233/JAD-131526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H, Kim JH, Kim C, Ye BS, Kim HJ, Yoon CW, et al. Shape changes of the basal ganglia and thalamus in alzheimer's disease: a three-year longitudinal study. J Alzheimers Dis. 2014;40:285–295. doi: 10.3233/JAD-132072. [DOI] [PubMed] [Google Scholar]
- 12.Costafreda SG, Dinov ID, Tu Z, Shi Y, Liu CY, Kloszewska I, et al. Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage. 2011;56:212–219. doi: 10.1016/j.neuroimage.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- 14.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Holland D, Dale AM, Younes L, Miller MI; Shape abnormalities of subcortical and ventricular structures in mild cognitive impairment and Alzheimer's disease: detecting, quantifying, and predicting. Hum Brain Mapp. 2014;35:3701–3725. doi: 10.1002/hbm.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57:P47–P53. doi: 10.1093/geronb/57.1.p47. [DOI] [PubMed] [Google Scholar]
- 18.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 19.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelstyn NM, Ellis SJ, Jenkinson P, Sawyer A. Contribution of the left dorsomedial thalamus to recognition memory: a neuropsychological case study. Neurocase. 2002;8:442–452. doi: 10.1076/neur.8.5.442.16180. [DOI] [PubMed] [Google Scholar]
- 21.Van der Werf YD, Jolles J, Witter M, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- 22.Pergola G, Gunturkun O, Koch B, Schwarz M, Daum I, Suchan B. Recall deficits in stroke patients with thalamic lesions covary with damage to the parvocellular mediodorsal nucleus of the thalamus. Neuropsychologia. 2012;50:2477–2491. doi: 10.1016/j.neuropsychologia.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Edelstyn NM, Mayes AR, Denby C, Ellis SJ. Impairment in material-specific long-term memory following unilateral mediodorsal thalamic damage and presumed partial disconnection of the mammillo-thalamic tract. J Neuropsychol. 2012;6:119–140. doi: 10.1111/j.1748-6653.2011.02019.x. [DOI] [PubMed] [Google Scholar]
- 24.Dos Santos V, Thomann P, Wüstenberg T, Seidl U, Essig M, Schröder J. Morphological cerebral correlates of CERAD test performance in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011;23:411–420. doi: 10.3233/JAD-2010-100156. [DOI] [PubMed] [Google Scholar]
- 25.Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci. 2013;7:45. doi: 10.3389/fnsys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishiyama MM, Yonelinas AP, Kroll NE, Lazzara MM, Nolan EC, Jones EG, et al. Bilateral thalamic lesions affect recollection- and familiarity-based recognition memory judgments. Cortex. 2005;41:778–788. doi: 10.1016/s0010-9452(08)70296-x. [DOI] [PubMed] [Google Scholar]
- 27.Stenset V, Grambaite R, Reinvang I, Hessen E, Cappelen T, Bjornerud A, et al. Diaschisis after thalamic stroke: a comparison of metabolic and structural changes in a patient with amnesic syndrome. Acta Neurol Scand Suppl. 2007;187:68–71. doi: 10.1111/j.1600-0404.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Schuff N, Camacho M, Chao L, Fletcher T, Yaffe K, et al. MRI markers for mild cognitive impairment: comparisons between white matter integrity and gray matter volume measurements. PLoS One. 2013;8:e66367. doi: 10.1371/journal.pone.0066367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Pan P, Song W, Huang R, Li J, Chen K, et al. Voxelwise meta-analysis of gray matter anomalies in Alzheimer's disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci. 2012;316:21–29. doi: 10.1016/j.jns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Qiu A, Zhong J, Graham S, Chia MY, Sim K. Combined analyses of thalamic volume, shape and white matter integrity in first-episode schizophrenia. Neuroimage. 2009;47:1163–1171. doi: 10.1016/j.neuroimage.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 32.Palm WM, Ferrarini L, van der Flier WM, Westendorp RG, Bollen EL, Middelkoop HA, et al. Cerebral atrophy in elderly with subjective memory complaints. J Magn Reson Imaging. 2013;38:358–364. doi: 10.1002/jmri.23977. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira L, Diniz B, Forlenza O, Busatto G, Zanetti M. Neurostructural predictors of Alzheimer's disease: a meta-analysis of VBM studies. Neurobiol Aging. 2011;32:1733–1741. doi: 10.1016/j.neurobiolaging.2009.11.008. [DOI] [PubMed] [Google Scholar]