Abstract
Purpose
Atypical femoral fracture (AFF), periprosthetic femoral fracture (PPFF) and femoral nonunion (FNU) are recalcitrant challenges for orthopedic surgeons. Teriparatide (TPTD) had been demonstrated to have anabolic effects on bone in various studies. We postulated that adjuvant TPTD after operation would enhance biologic stimulation for bone formation. We investigated (1) whether the adjuvant TPTD could achieve satisfactory union rate of surgically challenging cases such as displaced AFF, PPFF and FNU; (2) whether the adjuvant TPTD could promote development of abundant callus after surgical fixation; (3) whether the adjuvant TPTD had medically serious adverse effects.
Materials and Methods
Thirteen patients who agreed to off label use of TPTD in combination of operation were included in this retrospective case series. Median patients' age was 68.7 years, and there were three male and ten female patients. Their diagnoses were nonunion in six patients and acute fracture in seven. Medical records and radiographic images were reviewed.
Results
Twelve of thirteen fractures were united both clinically and radiologically within a year after adjuvant TPTD. Union completed radiologically median 5.4 months and clinically 5.7 months after the medication, respectively. Callus appeared abundantly showing median 1.4 of fracture healing response postoperatively. There was no serious adverse reaction of medication other than itching, muscle cramp, or nausea.
Conclusion
Even appropriate surgical treatment is a mainstay of treatment for AFF, PPFF, and FNU, the current report suggested that adjuvant TPTD combined with stable fixation results in satisfactory outcome for the challenging fractures of femur.
Keywords: Femur, Atypical femoral fracture, Femoral nonunion, Periprosthetic fractures, Terip
INTRODUCTION
Femoral fractures are mostly treated without causing significant long-term morbidity. However, some types of femoral fractures, such as an atypical femoral fracture (AFF), a periprosthetic femoral fracture (PPFF), and a subtrochanteric fractures are recalcitrant challenges for orthopedic surgeons1,2,3). Even after displaced AFF is treated with standard method, the results are not so satisfactory. Reportedly, up to 46% of AFFs treated with intramedullary nail (IMN) required reoperation for delayed union or nonunion4). High risk of complication has been reported with PPFF such as nonunion, aseptic loosening, infection, and death3). Femoral nonunion (FNU) is also surgically demanding since the failure rate after surgical treatment for FNU is reportedly high (0-53%)2,5) and their successful treatment may be prolonged and exploit numerous resources.
Moreover, those challenging problems are not so rare. AFF is reported to consist of around 30% of femoral diaphyseal fractures6). PPFF is expected to occur in 1.0% to 3.5% of primary total hip arthroplasty (THA)7), and the number of annual THA implantation was estimated around 1,000,000/year. FNU was reported to occur up to 20% of femoral fractures, depending on the type of fracture and on the technique used5).
Both AFF and FNU often have altered repair process of bone. The most well-known cause of AFF is long term use of bisphosphonate, which induces over-suppression of bone turnover. In normal bone, bone turnover repairs micro-damages which occur during normal daily activities, and micro-damage accumulates and leads to AFF without the repair process6). Nonunion is often a consequence of inadequate fixation or poor biology for bone forming cells after fracture treatment. For treating these problems, skeletal stabilization and provision of an environment conducive to bone healing are necessary.
Teriparatide (TPTD) is a synthetic/recombinant human parathyroid hormone consisting of a 1-34 N-terminal amino acid sequence of the intact parathyroid hormone molecule, which is approved for osteoporosis. Though accurate mechanism of TPTD's anabolic effect on bone is not completely understood yet, TPTD clearly have osteogenic effect8). Given anabolic action of TPTD and pathophysiology of AFF and FNU together, TPTD is expected to help treating FNU and AFF.
Previous preclinical studies revealed TPTD improved fracture healing with or without surgery, and some clinical case series also introduced TPTD as potential agent which can promote union in incomplete AFF or FNU patients who was treated without surgery9). However, in clinical settings, question remains whether adjuvant TPTD expedites bone healing process of surgically challenging femoral fractures, such as AFF, PPFF, and FNU, or not. The present authors postulated that adjuvant TPTD could enhance surgical union rate and abundant callus formation without safety concerns even used at perioperative period. Therefore, we determined the following: (1) whether the adjuvant TPTD could achieve satisfactory union rate of surgically challenging cases such as displaced AFF, PPFF, and FNU; (2) whether the adjuvant TPTD could promote development of abundant callus after surgical fixation; (3) whether the adjuvant TPTD had medically serious adverse effects.
MATERIALS AND METHODS
From August 2010 to September 2014, all the patients who used TPTD after surgery for AFF, PPFF, and FNU (13 patients) were followed up more than a year after TPTD medication and included in this retrospective case series study, which was approved by the Institutional Review Board of Seoul National University College of Medicine (IRB no. 1401-046-547). Their medical records and radiographs were reviewed.
Since August 2010, postoperative adjuvant TPTD had been offered to all the patients who had gone through a surgery for osteosynthesis of AFF, PPFF, or FNU except for the patients with abnormality of serum laboratory test or history of bony malignancy. Relatively high prevalence of complication and treatment failure of AFF, PPFF, and FNU, the mechanism of TPTD, expected effects on fracture healing and the currently approved indications of TPTD were explained. TPTD was prescribed at the approved dose for the treatment of osteoporosis (20 µg/day) only to the patients who consented to an empirical, off-label therapy. In all cases except for one (Case 8), this was the first time to use TPTD.
Patients started injection median 13 days (interquartile range [IQR], 20.3 days) after the index surgery. The patients were instructed to inject 20 µg of TPTD once a day. The duration of injection was targeted not less than 12 weeks. Medication was stopped if the patient refused to continue the medication for any causes including intolerable adverse reaction and financial burden. The duration of injection was median 12 weeks (IQR, 6.0 weeks).
On radiologic evaluation, we classified fracture pattern (subtrochanteric or femur shaft fractures). The subtrochanteric fracture was defined as extending 5 cm below the lesser trochanter. The femur shaft fracture was defined as extending from below the subtrochanteric region to supracondylar metaphyseal flare.
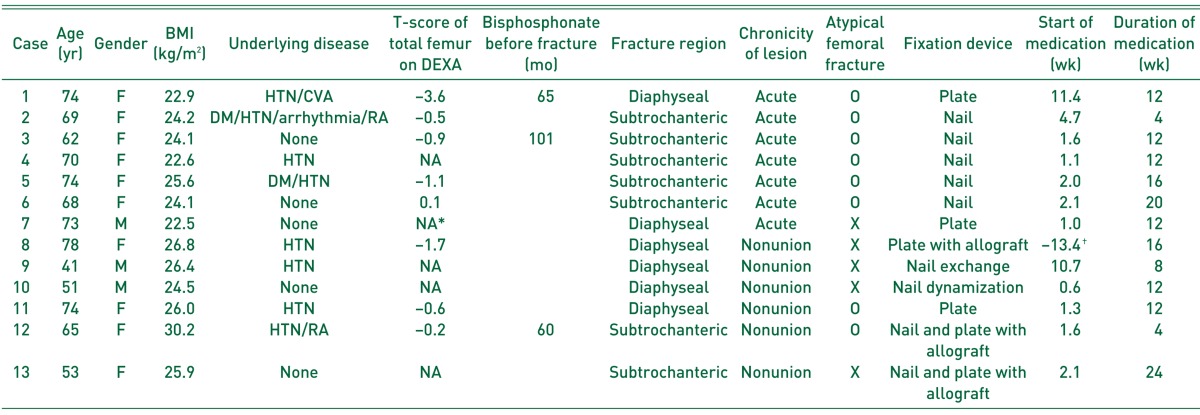
Three male patients and ten female patients were enrolled in the current study. Their median age was 68.7 years (IQR, 16.8 years). Six were acute AFFs, one was acute PPFF, and the other six were FNUs. Among 13 cases, none was smoker, three had been receiving nonsteroidal anti-inflammatory drugs as painkillers for less than one month, and two had diabetes mellitus10). The severity of osteoporosis was estimated on the basis of a bone mineral density measurement by dual-energy X-ray absorptiometry (DEXA) in eight patients. Body mass index was calculated as kg/m2. Six of the fractures affected the femoral shaft, and seven affected the subtrochanteric area (Table 1). Eight were AFF or FNU after AFF with all the major features of AFFs highlighted in the Task Force Report of the American Society for Bone and Mineral Research11). Three among eight patients who sustained AFF used bisphosphonate. After AFF, all the patients were counseled to stop bisphosphonate.
Table 1. Demographics and Results of Patients Treated with Adjuvant Teriparatide.
BMI: body mass index, DEXA: dual-energy X-ray absorptiometry, F: female, M: male, HTN: hypertension, CVA: cerebrovascular accident, DM: diabetes mellitus, RA: rheumatoid arthritis, NA: not available.
*T-score for total femur was not available for bilateral hip arthroplasty.
†Patient was medicated with TPTD before the index surgery.
With difference of potential impairment for bone healing, seven of acute fracture cases were designated as acute fracture group and six of FNU cases as FNU group.
Among all acute fractures, which were closed injury, six were AFFs, and the other was PPFF. Among six of AFFs, four fractures occurred without trauma and two with minor trauma such as slip down.
For six patients who sustained FNU, all the original injuries were closed femoral fractures, due in two cases to motor vehicle collisions and in four to slip downs including a case of Vancouver B1 periprosthetic fracture of bipolar hemi-arthroplasty12). The median time from initial injury to index operation with adjuvant TPTD was 8.4 months (IQR, 29.9 months), with a median of 1.5 operations before the index operation (IQR, 2.5 operations) in FNU group. IMN, plate or both were utilized for fixation depending on the surgeon's decision. Bone graft was done depending on the surgeon's decision as well.
In acute fracture group, two open reduction and internal fixation (ORIF) with plate, five IMNs to subtrochanteric AFFs were applied with adjuvant TPTD. Autogenic bone graft was performed in two cases (Case 1 and 2).
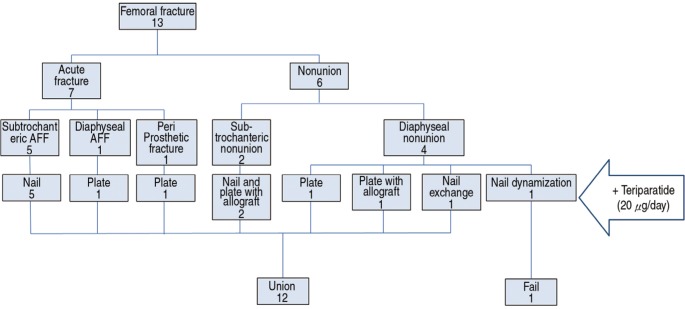
In FNU group, TPTD supplemented two IMNs (one exchange nailing and one dynamization with nail), four ORIFs (two with plate, two with plate and nail). Structural allogeneic bone graft was performed in three cases (Case 8, 12 and 13). At the time of surgery, all nonunions were aseptic according to intraoperative microbiology samples and the overall clinical profile of each case (Fig. 1).
Fig. 1. A concise diagram describes the preoperative diagnoses, fixation devices and outcomes of 13 cases. The 13 patients could be categorized into two groups as acute fracture group and nonunion group. They were treated with various fixation methods and adjuvant TPTD, 12 patients resulting in union.
During medication, patients were followed up every month to assess adverse response and necessity of TPTD continuation. After medication, visiting interval was individualized depending on progression to union, usually two to three months. After completion of union, patients were instructed to visit the clinic yearly.
Evidence of union was assessed with radiographs on every visit. Without consensus of the assessment of fracture union, several indicators were adopted for the assessment of union13). The pain-free ambulation was regarded as clinical union14). And cortical continuity of more than three cortices on anteroposterior and lateral plain radiographs was considered as complete radiologic union15). The intervals between medication and appearance of each sign of union were recorded.
For evaluating the amount of callus, we evaluated fracture healing response, which was calculated by dividing largest diameter of the callus by the bone diameter at that level on the same radiograph16). The fracture healing response is one of the simple ways to assess healing mass. The healing mass is often irregular and eccentric, presenting geometrically complex shape. However, the largest diameter of even the most amorphous tube-like structure is substantially the most important determinant of volume in the absence of massive variations in length. A numerical value for fracture healing response was therefore calculated by dividing the largest diameter of callus by that of bone diameter at or adjacent to the fracture site on the same radiograph.
With variations of medication start after surgery and chronicity of lesion, fracture responses of both before TPTD start and after union were measured and ratio of both was calculated as a reflection of TPTD effect on callus formation. Adverse reaction was evaluated every visit with symptom evaluating questions and laboratory tests.
RESULTS
The median follow-up lasted 24.1 months (IQR, 21.7 months). Twelve of 13 patients attained both clinical and radiologic unions after TPTD use. All seven acute fractures were united and five of six FNUs were healed (Fig. 1, 2, Table 2). One patient (Case 10) who went through three operations before index operation did not progress to successful healing even after nail dynamization with three months of adjuvant TPTD.
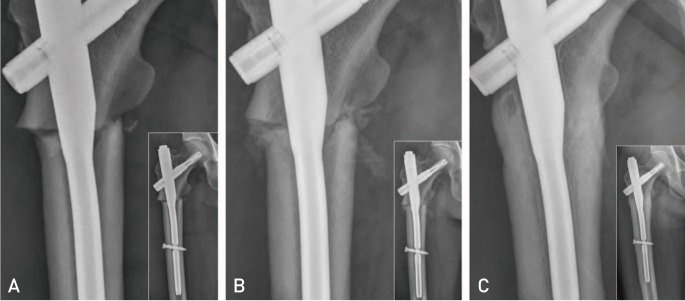
Fig. 2. The serial radiographs represent the course of atypical femoral fracture (Case 3) at subtrochanteric region with incomplete reduction. Each radiograph shows enlarged view of fracture and contains overview of proximal femur. The patient started to inject teriparatide (TPTD) after 11 days after surgery and kept using it for three months. Rapid growth of callus was observed between fracture fragments. (A) Immediate postoperative radiograph reveals slight varus reduction with a gap on lateral cortex and lack of contact on medial cortex. (B) Postoperative four-week radiograph reveals that abundant callus was formed with three weeks of TPTD injection. (C) Postoperative six month radiograph represents that the gap on both medial and lateral cortex was filled with abundant callus without trace of fracture line.
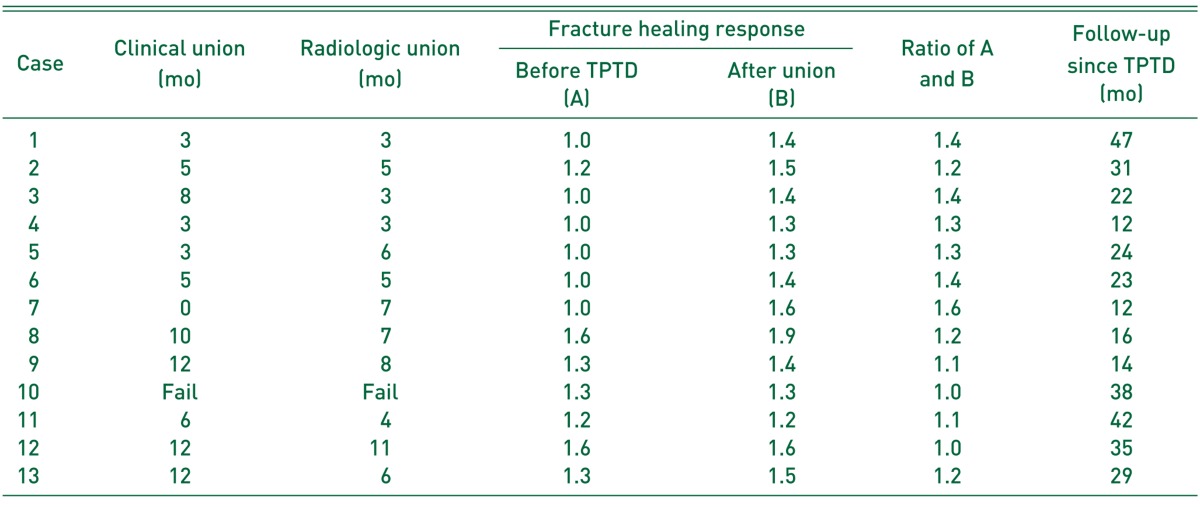
Table 2. Results of Patients Treated with Adjuvant Teriparatide (TPTD).
The median time to clinical union was recorded as 5.7 months (IQR, 7.5 months), and the median time to radiological union was recorded as 5.4 months (IQR, 3.9 months). Median ratio of fracture healing responses before and after TPTD start was 1.2 (IQR, 0.3). With the difference of chronicity of lesion, acute fractures showed higher ratio of fracture healing response (median, 1.4; IQR, 0.1), whereas FNUs showed relatively lower ratios (median, 1.1; IQR, 0.2) (Table 2).
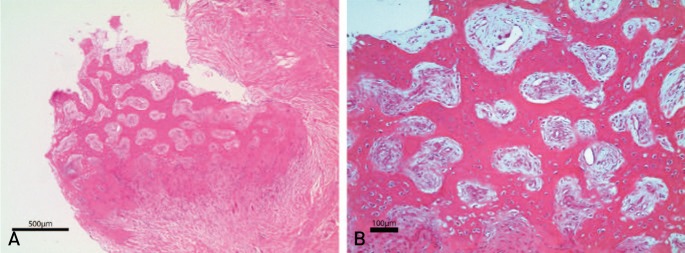
We harvested callus tissue for the microscopic examination from a patient who required revision surgery after two months of TPTD use (Case 8). The patient with bipolar hemiarthroplasty which was done four years before suffered from displaced periprosthetic fracture of Vancouver B1 type. Noncompliance to surgeon's instruction led to failure of ORIF after adjuvant TPTD of two months. While the patient had gone through revision with plate and structural allogeneic bone graft, callus tissue was harvested for histologic evaluation. Radiologic union was achieved 7.1 months after the revision. Abundant bone, cartilage, and fibrous tissue were observed in the harvested tissue under the microscopic examination (Fig. 3, Table 1).
Fig. 3. Under the microscopic examination, callus specimen harvested from the patient (Case 8) who underwent revision surgery after adjuvant teriparatide for two months shows abundant bone, cartilage, and fibrous tissue formation (A: ×40, B: ×100; hematoxylin and eosin staining).
Adverse reactions after medication occurred in two patients. One of two patients couldn't tolerate the adverse reaction and stopped medication. No one had history of allergic disease. One was 74-year old female (Case 1) with femoral shaft fracture who complained about nausea and leg cramps. Symptoms disappeared in two weeks without stopping medication. TPTD was used for 12 weeks as scheduled and union completed 12 weeks after medication without any other adverse reactions. The other was 69-year old female (Case 2) with subtrochanteric fracture who suffered from pruritus and rash around injection site. The discomfort occurred every time she injected for initial four weeks. She stopped medication after four-week use and union completed 48 weeks after medication without recurrence of discomfort. None had shown abnormal results on laboratory blood tests.
DISCUSSION
The current study reviewed 13 patients who were treated with adjuvant TPTD after surgical treatment for AFF, PPFF, or FNU. Abundant calluses were pronounced and successful union could be achieved in 12 patients without medically severe adverse reaction.
As some of biological factors unfavorable for fracture union are already known, such as AFF, nonunion, advanced age, medical comorbidities, smoking, nonsteroidal anti-inflammatory drug use etc.10), novel methods which can stimulate biology of bone healing process may be much help to the fracture patients with the unfavorable factors. Both AFF and FNU have poor biologic environment for fracture healing even after surgical treatment1,2). Autogenic bone graft is still gold standard for the purpose. However, considerable morbidity is associated with this established procedure, including blood loss, nerve and muscle injury, chronic pain at the donor site and local infection17). Various strategies had been attempted to overcome biologic impairment of bone healing18). Bone morphogenic protein also has been tried, but frequent adverse effects were reported with safety concerns19). Compared to other methods, adjuvant TPTD has certain advantages as a systemically administered agent which can stimulate bone healing process for a prolonged period without invasive procedure18).
TPTD was tried to reverse the unfavorable condition in several preclinical studies in conditions with, osteoporosis20), long term use of steroid21), diabetes22), stress fracture23), and surgically made nonunion20). The favorable results of the preclinical studies had been followed by clinical trials24,25,26). TPTD alone was tried for delayed union or nonunion and reported anecdotally24). In the most of reports, TPTD was applied to delayed union over 6 months after surgery. A preclinical study for unfavorable condition with surgical fixation27) showed positive results of adjuvant TPTD. While most of clinical reports for adjuvant TPTD were anecdotal25), Miyakoshi et al.26) reported 16 patients were treated with surgical fixation and adjuvant TPTD. They compared the results of AFF which was treated with or without TPTD. TPTD treatment significantly shortened the postoperative time for fracture healing and reduced rates of delayed healing or non-union after bisphosphonate-associated AFF. Clinical reports about adjuvant TPTD with surgery are still only one in AFF and none in nonunion. To our knowledge, the current study is the first case series which reports that adjuvant TPTD is effective to stimulate biology disrupted with surgical intervention. This case series will be the basis for further study.
Among 13 patients in the current study, one patient had failed to achieve union with adjuvant TPTD (Case 10). He had gone through three operations before the index operation, which was dynamization with three months of adjuvant TPTD. The dynamization 23 months after initial injury might be a risk factor for the failure. Dynamization is a simple method to stimulate osteogenesis by controlled axial instability28). Despite the theoretic basis, the success rate of dynamization for nonunion is reported around 50% and to be decreased when it is performed late after established nonunion28). Moreover, repeated operations around the fracture site may have aggravated the damage of local vascularity, which in turn diminishes biologic property of bone healing. As a result, TPTD failed to overcome these risks.
The current case series showed TPTD stimulate formation of abundant callus with median increase of 22% in diameter of callus. According to the difference of chronicity of lesion, the fracture healing responses increased in a various extent. Acute fractures had shown more increase in healing response than nonunions. However, taking it into consideration that the volume is proportional to the cube of its length, the increment of diameter does not fully reflect the increment of volume. As nonunions already had a certain amount of callus at the time of TPTD start, callus diameter of nonunions cannot be directly compared to those of acute fractures. Spencer16) reported femoral fracture healing response of control group up to 1.5. According to the study, more than half of patients were treated without internal fixation on the study. As lack of stability also leads to abundant formation of callus as well, the fracture healing responses cannot be compared directly to the results of the different studies and further prospective study with standard measurement of callus volume might reveal the comparison.
Clinical union was delayed more than radiographic union in five patients in the present study. We assumed that the major cause of the difference between clinical and radiographic union resulted from the retrospective nature of the present study. The retrospective review of medical records has certain limits on evaluating pain nature which determines whether the clinical union was achieved. To avoid a subjective interpretation of medical records, we evaluated clinical union in the most conservative way. As the method for determining union is still controversial, the further studies may have to adopt the prospective evaluation in the authorized methods29).
According to the report based on a telephone survey, intolerable adverse reaction was the major cause of TPTD discontinuation30). Therefore, adverse reactions of TPTD had been one of the major interests of previously published studies30). Previous reports about safety concerns of TPTD were studied in the postmenopausal women who were not in perioperative period. Around operative treatment, patients are exposed to various medical substances which cause or aggravate adverse effects which overlap to the ones of TPTD. Still none of reports were regarding the safety concerns of TPTD around perioperative period. Two adverse reactions of the current study were within the limits of the reports already published. The similar adverse reaction in patients of postoperative period provide us the impression that adverse reactions of adjuvant TPTD are not quite discordant with previously reported ones and encourage us to proceed to further study.
We acknowledge that our study has several limitations. First, this study is limited by its retrospective nature. As the purpose of the current case series was to review all the patients with adjuvant TPTD in our center and to plan the further study based on this review, further prospective study would be possible after more encouraging results are published including the current study. Second, the small sample size in our study gives little power to detect any relationships. As high cost and application to unapproved indication of TPTD make patients hesitate to use it, the reports for adjuvant TPTD for surgically treated patients are still anecdotal. Further studies are expected to reveal whether adjuvant TPTD is effective for the surgically challenging cases. Third, this study involved a single cohort without control group. Comparing the results of this study with the ones of literature can lead to a hasty conclusion because of the small sample size and inconsistent methods to measure outcomes. We thought it is reasonable to understand the high union rate and the tendency of abundant callus formation in the current study as the potential effect of adjuvant TPTD. Forth, the current study included the patients with heterogeneous problems. Six were acute AFF, and the others were nonunion with various operation history. One case was suffering from nonunion of periprosthetic fracture. Surgical intervention was individualized according to the problem which each patient was bearing. The various treatments for complex problems made hard for us to control variables. As the purpose of the present study was to review all the patients with adjuvant TPTD in our center and to find a positive role of adjuvant TPTD, the current case series fulfilled the purpose with suggesting the potential effect of adjuvant TPTD. Fifth, application of TPTD was not homogeneous. Three patients started TPTD more than a month after index surgery, and three patients stopped the medication before 12 weeks. Although this heterogeneity attenuates the strength of the evidence for adjuvant TPTD, 92% of the present case series with challenging fracture achieved union, revealing the clue for the positive role of adjuvant TPTD.
CONCLUSION
Adjuvant TPTD was tried to 13 challenging cases including AFF, PPFF, and FNU. The results suggested adjuvant TPTD expedites bone-healing process in surgically treated patients. Abundant calluses were pronounced in all the patients and successful union could be achieved in 12 patients. No medically serious adverse reaction was observed in our series. The present review of 13 cases warrants further studies for the positive role of adjuvant TPTD on the bone healing of patients with defective bone healing potential.
ACKNOWLEDGEMENTS
We received funding from the Seoul National University Hospital Research Fund (Grant no. 06-03-063).
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article.
References
- 1.Kang JS, Won YY, Kim JO, et al. Atypical femoral fractures after anti-osteoporotic medication: a Korean multicenter study. Int Orthop. 2014;38:1247–1253. doi: 10.1007/s00264-013-2259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkemeier CG, Chapman MW. Treatment of femoral diaphyseal nonunions. Clin Orthop Relat Res. 2002;(398):223–234. doi: 10.1097/00003086-200205000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57:209–213. doi: 10.1503/cjs.014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weil YA, Rivkin G, Safran O, Liebergall M, Foldes AJ. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J Trauma. 2011;71:186–190. doi: 10.1097/TA.0b013e31821957e3. [DOI] [PubMed] [Google Scholar]
- 5.Gelalis ID, Politis AN, Arnaoutoglou CM, et al. Diagnostic and treatment modalities in nonunions of the femoral shaft: a review. Injury. 2012;43:980–988. doi: 10.1016/j.injury.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Saita Y, Ishijima M, Kaneko K. Atypical femoral fractures and bisphosphonate use: current evidence and clinical implications. Ther Adv Chronic Dis. 2015;6:185–193. doi: 10.1177/2040622315584114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frenzel S, Vécsei V, Negrin L. Periprosthetic femoral fractures--incidence, classification problems and the proposal of a modified classification scheme. Int Orthop. 2015;39:1909–1920. doi: 10.1007/s00264-015-2967-4. [DOI] [PubMed] [Google Scholar]
- 8.Tashjian AH, Jr, Gagel RF. Teriparatide [human PTH (1-34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res. 2006;21:354–365. doi: 10.1359/JBMR.051023. [DOI] [PubMed] [Google Scholar]
- 9.Lee YK, Ha YC, Koo KH. Teriparatide, a nonsurgical solution for femoral nonunion? A report of three cases. Osteoporos Int. 2012;23:2897–2900. doi: 10.1007/s00198-012-2172-x. [DOI] [PubMed] [Google Scholar]
- 10.Hak DJ, Fitzpatrick D, Bishop JA, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 12.Masri BA, Meek RM, Duncan CP. Periprosthetic fractures evaluation and treatment. Clin Orthop Relat Res. 2004;(420):80–95. doi: 10.1097/00003086-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Corrales LA, Morshed S, Bhandari M, Miclau T., 3rd Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008;90:1862–1868. doi: 10.2106/JBJS.G.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tower SS, Beals RK, Duwelius PJ. Resonant frequency analysis of the tibia as a measure of fracture healing. J Orthop Trauma. 1993;7:552–557. doi: 10.1097/00005131-199312000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Spencer RF. The effect of head injury on fracture healing. A quantitative assessment. J Bone Joint Surg Br. 1987;69:525–528. doi: 10.1302/0301-620X.69B4.3611151. [DOI] [PubMed] [Google Scholar]
- 17.Kanakaris NK, Lasanianos N, Calori GM, et al. Application of bone morphogenetic proteins to femoral non-unions: a 4-year multicentre experience. Injury. 2009;40(Suppl 3):S54–S61. doi: 10.1016/S0020-1383(09)70013-0. [DOI] [PubMed] [Google Scholar]
- 18.Virk MS, Lieberman JR. Biologic adjuvants for fracture healing. Arthritis Res Ther. 2012;14:225. doi: 10.1186/ar4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone. 2008;42:90–97. doi: 10.1016/j.bone.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Bostrom MP, Gamradt SC, Asnis P, et al. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone. 2000;26:437–442. doi: 10.1016/S8756-3282(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 22.Hamann C, Picke AK, Campbell GM, et al. Effects of parathyroid hormone on bone mass, bone strength, and bone regeneration in male rats with type 2 diabetes mellitus. Endocrinology. 2014;155:1197–1206. doi: 10.1210/en.2013-1960. [DOI] [PubMed] [Google Scholar]
- 23.Sloan AV, Martin JR, Li S, Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2010;47:235–240. doi: 10.1016/j.bone.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Uemura T, Okada M, Yokoi T, Shintani K, Nakamura H. Successful bone healing of nonunion after ulnar shortening osteotomy for smokers treated with teriparatide. Orthopedics. 2015;38:e733–e737. doi: 10.3928/01477447-20150804-90. [DOI] [PubMed] [Google Scholar]
- 25.Tarazona-Santabalbina FJ, Aguilella-Fernández L. Bisphosphonate long-term treatment related bilateral subtrochanteric femoral fracture. Can teriparatide be useful? Aging Clin Exp Res. 2013;25:605–609. doi: 10.1007/s40520-013-0137-3. [DOI] [PubMed] [Google Scholar]
- 26.Miyakoshi N, Aizawa T, Sasaki S, et al. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2015;33:553–559. doi: 10.1007/s00774-014-0617-3. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Z, Wei L, Liu J, et al. Effect of intermittent PTH (1-34) on posterolateral spinal fusion with iliac crest bone graft in an ovariectomized rat model. Osteoporos Int. 2013;24:2693–2700. doi: 10.1007/s00198-013-2385-7. [DOI] [PubMed] [Google Scholar]
- 28.Lynch JR, Taitsman LA, Barei DP, Nork SE. Femoral nonunion: risk factors and treatment options. J Am Acad Orthop Surg. 2008;16:88–97. doi: 10.5435/00124635-200802000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Axelrad TW, Einhorn TA. Use of clinical assessment tools in the evaluation of fracture healing. Injury. 2011;42:301–305. doi: 10.1016/j.injury.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Briot K, Ravaud P, Dargent-Molina P, Zylberman M, Liu-Leage S, Roux C. Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int. 2009;20:625–630. doi: 10.1007/s00198-008-0698-8. [DOI] [PubMed] [Google Scholar]