Abstract
Background
Diabetes mellitus (DM) is associated with coronary artery disease (CAD) progression. Although previous studies have demonstrated the association of lipid and lipoprotein ratios with CAD, no data are currently available concerning the relationship between lipid and lipoprotein ratios and the severity of new on-set CAD in diabetics. Therefore, the aim of the present study was to investigate the usefulness of lipid and lipoprotein ratios in predicting the severity of CAD in patients with type 2 DM (T2DM).
Methods
A total of 380 consecutive T2DM patients with new on-set CAD were enrolled in the present study. Then, they were classified into the three groups according to Gensini score (GS) tertiles. The relationship between lipid and lipoprotein ratios currently used and the GS was investigated.
Results
Positive correlations of natural log-transformed GS (lnGS) with apolipoprotein B to apoA-I ratio (apoB/apoA-I), non-high-density lipoprotein cholesterol to apoA-I ratio (non-HDL-C/apoA-I), and low-density lipoprotein cholesterol to apoA-I ratio (LDL-C/apoA-I) were found (r = 0.18, 0.13, 0.12, respectively, all P < 0.05). Multivariate logistic analysis indicated apoB/apoA-I as the strongest predictor for high GS (OR = 5.67, 95% CI: 1.45–23.92, P = 0.003). Area under receivers operating characteristic curve of apoB/apoA-I was 0.63 (95% CI: 0.60–0.66, P = 0.001) for predicting high GS. The optimal cutoff value of apoB/apoA-I to predict high GS was 0.72 with the sensitivity of 61.2% and the specificity of 62.1%.
Conclusions
Lipid and lipoprotein ratios might be useful for predicting the severity of new on-set CAD in T2DM patients, and the apoB/apoA-I appeared as the most significant predictor in this population.
Keywords: Coronary artery disease, Lipid, Lipoprotein, Type 2 diabetes mellitus
1. Introduction
Dyslipidemia is well-documented as a major risk factor for coronary artery disease (CAD) to be closely associated with the progression of coronary atherosclerosis and the stability of coronary artery plaques. A lot of studies have demonstrated that elevated low-density lipoprotein cholesterol (LDL-C) level, in particular, plays a causal role in atherogenesis.[1] Recently, non-high density lipoprotein cholesterol (non-HDL-C) has also been emerged as a promising risk predictor particularly among hypertriglyceridemic individuals.[1] Moreover, apolipoproteins B (apoB), the major structural protein of non-HDL particles, is recognized as even a better risk predictor than LDL-C for CAD.[1] Interestingly, investigators also have paid attention to the usefulness of lipid and lipoprotein ratios in predicting the presence and the prognosis of CAD. Among them, total cholesterol to HDL-C ratio (TC/HDL-C), LDL-C/HDL-C and apoB/apoA-I are found to be the most commonly used ratios,[2]–[5] but whether they are stronger predictors for the severity of CAD compared with the traditional lipid and lipoprotein parameters remain under discussion.
Diabetes mellitus (DM) is an independent risk factor for the development and mortality of CAD.[6] The presence of DM portends an adverse cardiovascular prognosis with a 2- to 3-fold higher compared with nondiabetic people.[6] Of note, the lipid and lipoprotein profile in patients with type 2 DM is different from the general population, and the former is characterized by a high triglyceride (TG) level and a low HDL-C level.[7],[8] Although lipoprotein ratios can serve as markers of insulin resistance, whether a high TG or a low HDL-C relates to the relationship of DM to CAD progression is still controversial. To our best knowledge, little study has performed on the correlation between the lipid and lipoprotein ratios and the severity of CAD in type 2 DM (T2DM) patients. Much less data is available on the correlation using a strict assessment like Gensini score (GS) for CAD severity in DM patients, especially in patients with new on-set CAD and T2DM. Indeed, our previous study revealed that apoB/apoA-I ratio was associated with GS in stable CAD patients with T2DM and the association did not persist after adjustments for some CAD risk factors.[9] However, in our previous study, the participants were patients with stable CAD, but the ones with prior revascularization were included. The data are not available to date concerning the association between lipid and lipoprotein ratio and CAD severity in patients with T2DM and new on-set CAD.
The present study, therefore, aimed to explore the usefulness of the lipid and lipoprotein ratios of currently commonly used including TG/HDL-C, TC/HDL-C, LDL-C/HDL-C, TG/apoA-I, LDL-C/apoA-I, non-HDL-C/apoA-I and apoB/apoA-I in predicting the severity of new on-set CAD assessed by GS in patients with T2DM.
2. Methods
2.1. Study population
All patients in the study have been reviewed by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards as our previous studies.[10]–[12]
From a group of type 2 DM patients who were referred for elective coronary angiography due to angina-like chest pain and/or positive treadmill exercise test or clinically indicated coronary CT angiography in the Division of Dyslipidemia of Fu Wai Hospital (Beijing, China), 380 were newly angiography-proven onset CAD patients and referral for the present study. Patients with pre-percutaneous coronary intervention (Pre-PCI, n = 245), pre-coronary artery bypass grafting (pre-CABG, n = 21), post-myocardial infarction (MI) but did not underwent revascularization (n = 144), had an abnormal baseline cardiac troponin I (cTnI, n = 61) level and/or valvular heart disease (n = 7) were excluded. Patients with heart failure were not included in the present study, and all patients in this study were with a left ventricular ejection fraction (LVEF) > 50%. Clinical characteristics, lipid parameters and laboratory test information of the study patients were collected. Lipid ratios were calculated and recorded. The definitions of hypertension, DM, and dyslipidemia were reported in our previous studies.[10]–[12]
2.2. Laboratory tests
Baseline laboratory data measurements were conducted by the Clinical Chemistry Department of Fu Wai hospital. Blood samples were obtained for all patients from the cubital vein after a 12-h overnight fast before coronary angiography. TC and TG were measured by enzymatic method and HDL-C by a direct method (Roche Diagnostics, Basel, Switzerland). LDL-C was obtained by Friedewald's formula (if fasting triglycerides < 3.39 mmol/L) or by ultracentrifugation. ApoB and apoA-I were measured by an immunoturbidimetric method (Tina-quant, Roche Diagnostics) calibrated against the World Health Organization/International Federation of Clinical Chemistry reference standard SP3-07 as our previous study.[13] Lipid and lipoprotein ratios and non-HDL-C were calculated directly. The levels of high- sensitive C-reactive protein (hs-CRP) were measured using immunoturbidometry (Beckmann Assay 360, Bera, Calif, USA) as we previously reported.[14] All other including biomarkers were analyzed by standard hematological and biochemical tests. LVEF was accessed by biplane Simpson's method. The endocardial border was manually traced in the apical four-chamber and two-chamber views in end-diastole and end-systole. Left ventricular enddiastolic volume (LVEDV), left ventricular endsystolic volume (LVESV), and LVEF were assessed.
2.3. Angiographic examination
Coronary angiographic examination and the severity of CAD assessment using GS assessment system[15],[16] had been reported in our previous studies.[10]–[12]
Based on the tertiles of GS, the enrolled patients were classified into the three groups (low GS ≤ 24-point, n = 127; intermediate GS 24–47 points, n = 124; high GS ≥ 47-point, n = 129).
2.4. Statistical analysis
Data were showed as mean ± SD or median (Q1–Q3 quartiles) for continuous variables and number (percentage) for categorical variables. Independent sample t-test, Mann– Whitney U-test, and χ2-tests were performed where appropriate to examine the difference of variables between groups.
In order to identify the factors that were independently associated with high GS (over than 47-point), binary univariate and multiple logistic regression using forward stepwise selection process were performed. Variables for which in univariate logistic regression analysis with P < 0.05 were subsequently included in multivariate logistic regression analysis model. Correlations between two variables were examined using Spearman or Pearson's correlation analysis when appropriate. Receiver operating characteristic (ROC) curve analysis was constructed at the most discriminating cut-off value aimed to document the predictive power of the lipid ratio for high GS. A two-sided P < 0.05 was considered statistically significant.
The statistical analyses were performed using SPSS version19.0 software (Chicago, Illinois, USA) as our previous studies.[10]–[12]
3. Results
3.1. Baseline characteristics
The study population consisted of 258 men (67.9%) and 122 women (32.1%), aged 37–82 (mean: 59.9 ± 9.3) years. All patients were diagnosed with CAD accompanying with type 2 DM.
Patients were grouped according to the tertiles of GS (low GS group, n = 127, 33.4%; intermediate GS group, n = 124, 32.6%; high GS group, n = 129, 33.9%) and the baseline characteristics were summarized in Table 1. The GS ranged from 4-point to 194-point with median 32 point (18.8–56.5 point), In brief, patients with higher GS were often accompanied with higher levels of the atherogenic lipid and lipoprotein profile including LDL-C, non-HDL-C, lipoprotein (A), and apoB (all P < 0.05, Table 1), and higher lipid and lipoprotein ratios including LDL-C/HDL-C, LDL-C/apoA-I, non-HDL-C/apoA-I, and apoB/apoA-I (all P < 0.05, Table 1).
Table 1. Clinical characteristics of the study population.
| Variables | Low GS | Intermediate GS | High GS | Pa | Pb |
| (≤ 24, n = 127) | (24–47, n = 124) | (≥ 47, n = 129) | |||
| Risk factors | |||||
| Age, yrs | 59.8 ± 8.8 | 59.2 ± 9.9 | 60.7 ± 9.3 | 0.554 | 0.312 |
| Male | 80 (63.0%) | 82 (66.1%) | 97 (75.2%) | 0.182 | 0.073 |
| Smoking | 58 (45.7%) | 57 (46.0%) | 61 (47.3%) | 0.951 | 0.775 |
| Family history of CAD | 18 (14.2%) | 20 (16.1%) | 23 (17.8%) | 0.632 | 0.365 |
| BMI, kg/m2 | 26.6 ± 3.8 | 25.9 ± 4.2 | 26.2 ± 3.3 | 0.223 | 0.550 |
| Hypertension | 97 (76.4%) | 94 (75.8%) | 91 (70.5%) | 0.584 | 0.300 |
| Dyslipidemia | 112 (88.2%) | 103 (83.1%) | 97 (75.2%) | 0.059 | 0.032 |
| PVD | 2 (1.6%) | 3 (2.4%) | 3 (2.3%) | 0.708 | 0.641 |
| Stroke | 4 (3.2%) | 3 (2.4%) | 4 (3.1%) | 0.969 | 0.896 |
| Duration of diabetes, yrs | 7.8 ± 6.2 | 8.1 ± 5.5 | 11.0 ± 6.3 | 0.022 | 0.006 |
| SBP, mmHg | 129.1 ± 18.2 | 123.5 ± 15.1 | 124.1 ± 13.9 | 0.197 | 0.486 |
| DBP, mmHg | 77.6 ± 9.8 | 74.9 ± 8.8 | 74.9 ± 9.9 | 0.299 | 0.465 |
| Lipid profile | |||||
| TG, mg/dL | 147.1 (109.9–225.0) | 140.0 (102.8–201.1) | 140.0 (104.5–187.8) | 0.385 | 0.413 |
| TC, mg/dL | 159.6 ± 41.3 | 157.5 ± 46.1 | 168.6 ± 46.1 | 0.182 | 0.069 |
| LDL-C, mg/dL | 91.2 ± 31.8 | 92.9 ± 35.4 | 104.0 ± 39.7 | 0.026 | 0.007 |
| HDL-C, mg/dL | 41.6 ± 11.0 | 41.5 ± 9.0 | 40.0 ± 8.6 | 0.451 | 0.206 |
| Non-HDL-C, mg/dL | 117.8 ± 38.2 | 115.6 ± 45.2 | 128.5 ± 43.9 | 0.079 | 0.026 |
| Lipoprotein (A), mg/dL | 13.4 (5.9–27.6) | 15.6 (4.9–27.4) | 19.9 (6.8–45.1) | 0.034 | 0.010 |
| ApoA-I, mg/dL | 136 (129–165) | 142.0 (129.8–155.3) | 138.0 (123.0–151.0) | 0.185 | 0.079 |
| ApoB, mg/dL | 95.9 ± 28.7 | 97.6 ± 32.5 | 107.0 ± 32.7 | 0.028 | 0.008 |
| Laboratory test | |||||
| Creatinine, µmol/L | 70.4 ± 15.5 | 73.1 ± 15.7 | 75.1 ± 15.0 | 0.096 | 0.075 |
| Uric acid, mmol/L | 325.6 ± 93.8 | 344.5 ± 83.3 | 350.9 ± 111.9 | 0.173 | 0.188 |
| AST, IU/L | 16.0 (12.0–20.0) | 16 (13–22) | 15.0 (12.0–18.0) | 0.058 | 0.019 |
| ALT, IU/L | 22.0 (16.5–31.5) | 23.0 (17.0–34.3) | 21.0 (15.0–29.0) | 0.158 | 0.055 |
| HbA1C, % | 7.1 (6.7–7.9) | 7.0 (6.4–7.9) | 7.4 (6.7–8.3) | 0.075 | 0.036 |
| NT-pro-BNP, fmol/mL | 516 (436.2–636.8) | 552.1 (428.0-668.2) | 600.2 (451.9–790.9) | 0.059 | 0.019 |
| LVEF, % | 65.9 ± 5.0 | 65.7 ± 5.1 | 64.4 ± 5.8 | 0.094 | 0.030 |
| Hs-CRP, mg/L | 1.9 (1.0–3.1) | 1.6 (0.6–3.3) | 2.0 (0.9–4.0) | 0.406 | 0.570 |
| Lipids and lipoprotein ratio | |||||
| TG/HDL-C | 3.86 (2.51–5.45) | 3.44 (2.41–5.27) | 3.58 (2.54–4.84) | 0.644 | 0.724 |
| TC/HDL-C | 4.0 ± 1.2 | 3.9 ± 1.3 | 4.3 ± 1.3 | 0.055 | 0.017 |
| LDL-C/HDL-C | 2.2 ± 0.9 | 2.3 ± 0.9 | 2.7 ± 1.0 | 0.005 | 0.001 |
| TG/ApoA-I | 1.08 (0.79–1.46) | 0.94 (0.73–1.39) | 1.00 (0.72–1.32) | 0.624 | 0.759 |
| Non-HDL-C/ApoA-I | 0.82 ± 0.28 | 0.81 ± 0.34 | 0.92 ± 0.31 | 0.004 | 0.006 |
| LDL-C/ApoA-I | 0.65 ± 0.22 | 0.64 ± 0.25 | 0.74 ± 0.27 | 0.021 | 0.001 |
| ApoB/ApoA-I | 0.66 ± 0.20 | 0.68 ± 0.23 | 0.77 ± 0.23 | 0.002 | 0.001 |
| Statin therapy | 69 (54.3) | 70 (56.5) | 67 (51.9) | 0.850 | 0.648 |
The data shown are the mean ± SD, median (Q1–Q3 quartiles) or n (%). aP value obtained from analysis of variance, Kruskal-Wallis test, or chi-squared test; bP value for high GS vs. non-high (low and intermediate), GS obtained from analysis of t-test, Mann-Whitney test, or Chi-squared test. ALP: alkaline phosphatase; ALT: alanine aminotransferase; ApoA-I: apolipoprotein A-I; Apo B: apolipoprotein B; AST: aspartate aminotransferase; BMI: body mass index; CAD: coronary artery disease; DBP: diastolic blood pressure; GS: Gensini score; HbA1c: glycosylated hemoglobinA1C; HDL-C: high density lipoprotein cholesterol; hs-CRP: high sensitivity C-reactive protein; LDL-C: low density lipoprotein cholesterol; LVFE: left ventricular ejection fraction; NT-pro-BNP: N-terminal pro-brain natriuretic peptide; PVD: peripheral vascular disease; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides.
3.2. Logistic regression analysis
Logistic regression analysis was used for detecting the independent predictors for high GS (> 47-point). According to the consequence of the univariate regression analysis, which was performed firstly (data not shown), The significant parameters with P < 0.05 including LDL-C, non-HDL-C, apoB, and lipoprotein (A), the ratios of TC/HDL-C, LDL-C/HDL-C, non-HDL-C/HDL-C, apoB/HDL-C, LDL-C/apoA-I, non-HDL-C/apoA-I, and apoB/apoA-I were subsequently included in the multivariate regression model (Table 2), while the rest of lipid and lipoprotein parameters including HDL-C, TG or TG/HDL-C and TG/apoA-I were not included in the model. In Addition, both LVEF and Glycosylated hemoglobinA1C (HbA1C) were with a P < 0.05 in the univariate regression analysis. When adjusted for gender, age, current smoking, hypertension, disperlipidemia and family history of CAD in model 1, parameters including apoB/apoA-I, non-HDL-C, LDL-C/apoA-I, non-HDL-C/apoA-I, and lipoprotein (A) showed relatively significant and strong associations with high GS (OR = 10.33, 8.40, 5.41, 3.35, 2.06, respectively, and all P < 0.05). After further adjustment for LVEF, HbA1C, and statin therapy (before hospitalization) in model 2, the lipoprotein (A), and the ratios including apoB/apoA-I, LDL-C/apoA-I, and non-HDL-C/apoA-I showed the much more powerful and independent predictive value for high GS compared with other lipid and lipoprotein parameters (OR = 5.67, 4.65, 3.42, 1.65, respectively, and all P < 0.05). As a result, the two multivariate logistic analysis models both demonstrated that the apoB/apoA-I was the most powerful predictor for high GS in type 2 DM-CAD patients. In addition, it's worth mentioning that the lipoprotein (A), the only one lipid measurement among the lipids and apos parameters, showed a significant association with high GS in each model, and it might be an important predictive factor for the severity of CAD. More interestingly, this predictive value was independent of the apo B/apo A-I (OR: 1.77, 95% CI: 1.04–3.01, P = 0.036) (data not shown).
Table 2. Multivariate logistic regression analysis of detecting independent factors for high GS ( > 47-point).
| Variables | Model 1 |
Model 2 |
|||||
| OR | 95% CI | P value | OR | 95% CI | P-value | ||
| LDL-C | 1.01 | 1.00–1.02 | 0.004 | 1.01 | 1.00–1.02 | 0.048 | |
| Non-HDL-C | 8.40 | 1.51–46.8 | 0.014 | 5.42 | 0.57–51.59 | 0.142 | |
| ApoB | 1.01 | 1.00–1.02 | 0.002 | 1.01 | 1.00–1.02 | 0.091 | |
| Lipoprotein (A) | 2.06 | 1.23–3.47 | 0.006 | 1.65 | 1.04–3.03 | 0.045 | |
| TC/HDL-C | 1.32 | 1.08–1.62 | 0.008 | 1.42 | 0.72–2.80 | 0.310 | |
| LDL-C/HDL-C | 1.57 | 1.19–2.06 | 0.001 | 1.43 | 1.03–1.98 | 0.033 | |
| ApoB/HDL-C | 1.62 | 1.21–2.15 | 0.001 | 1.36 | 0.98–1.91 | 0.069 | |
| Non-HDL-C/ApoA-1 | 3.35 | 1.49–7.51 | 0.003 | 3.42 | 1.21–9.67 | 0.021 | |
| LDL-C/ApoA-1 | 5.41 | 1.98–14.81 | 0.001 | 4.65 | 1.35–15.97 | 0.015 | |
| ApoB/ApoA-1 | 10.33 | 3.04–35.14 | 0.000 | 5.67 | 1.45–23.92 | 0.018 | |
Abnormal distribution data were analyzed after logarithmic transforming. Model 1: adjusted for gender, age, current smoking, hypertension, hyperlipidemia, family history of CAD; Model 2: as in model 1 further adjusted for LVEF, HbA1C, duration of diabetes, and statin therapy (before hospitalization). ApoA-1: apolipoprotein A-1; ApoB: apolipoprotein B; CAD: coronary artery disease; GS: Gensini score; HbA1C: glycosylated hemoglobinA1C; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; TC: total cholesterol.
3.3. Correlation analysis and receiver operating characteristic (ROC) curves analysis
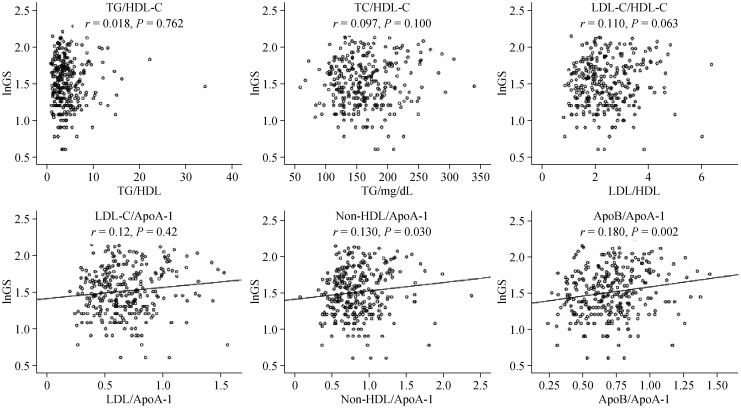
In correlation analysis, natural log-transformed GS (lnGS) was used. All of the lipid and lipoprotein ratios were normal distribution data. Pearson's correlation analysis was performed to investigate the correlation of the GS with the lipid and lipoprotein ratios. Positive and significant correlations of the ratios including apoB/apoA-I, non-HDL-C/apoA-I, and LDL-C/apoA-I with lnGS (r = 0.18, 0.13, 0.12, respectively, and all P < 0.05; Figure 1) were detected, but no significant correlation were observed between TG/HDL-C, TG/apoA-I, TC-C/HDL-C, or LDL-C/HDL-C and lnGS (Figure 1).
Figure 1. The correlation of the lipid and lipoprotein ratios with lnGS in type 2 DM.
Pearson's correlation analysis was performed. ApoA-I: apolipoprotein A-I; ApoB: apolipoprotein B; DM: diabetes mellitus; GS: Gensini score; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
In ROC curves analysis, area under the curve (AUC) indicated the well discriminatory power of apoB/apoA-I (AUC = 0.63, 95% CI: 0.60–0.66, and P = 0.001) for high GS (Figure 2). The optimal cutoff value of apoB/apoA-I to predict high GS was 0.72 (with a sensitivity of 61.2% and a specificity of 62.1%).
Figure 2. ROC curves analysis.
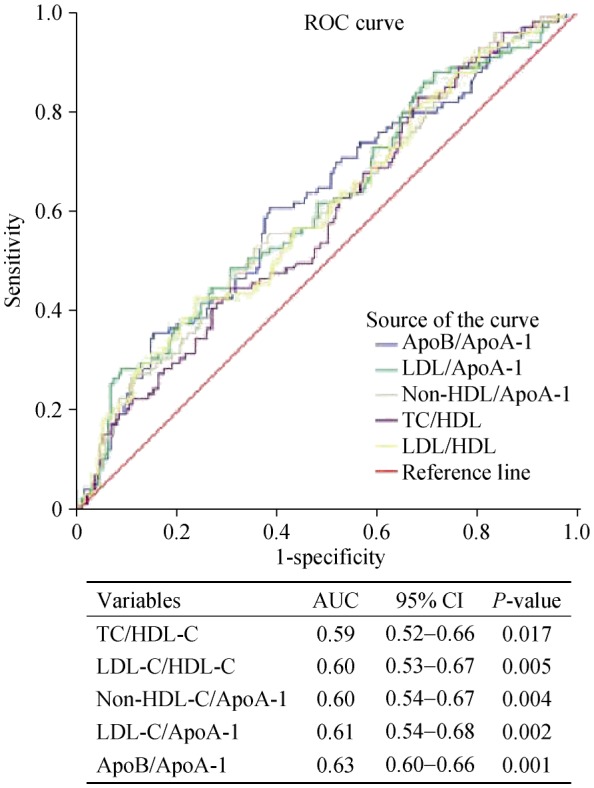
ROC curves showed discriminatory power of the lipid and lipoprotein ratios for high GS (≥ 47-point) in type 2 DM. ApoA-I: apolipoprotein A-I; ApoB: apolipoprotein B; AUC: area under the curve; DM: diabetes mellitus; GS: Gensini score; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; ROC: receiver-operating characteristic; TC: total cholesterol.
4. Discussion
The main findings of the present study were that: (1) the baseline apoB/apoA-I appeared to be superior to all traditional lipid and lipoprotein parameters in predicting high GS (≥ 47-point) in DM patients with newly diagnosed CAD; (2) there were significantly positive correlations of the ratios including apoB/apoA-I, non-HDL-C/apoA-I, and LDL-C/apoA-I with lnGS (r = 0.18, 0.13, 0.12, respectively; and all P < 0.05); (3) the optimal cutoff value of apoB/apoA-I in predicting for high GS in DM patients with CAD was 0.72 (with a sensitivity of 61.2% and a specificity of 62.1%).
As we know, GS assessment system has been world-widely used in cardiovascular field. It is a simple and useful tool in evaluating the severity of coronary stenosis and provides an accurate stratification of patients according to the functional significance of their disease.[16] In the current study, we chose the GS as an approach for evaluating the severity of the CAD in Chinese patients with T2DM and new on-set CAD.
Concerning the usefulness of the lipid and lipoprotein ratios in predicting CAD, considerable studies had already reported. For example, in a large sample, prospective study, the apoB/apoA-I and the TC/HDL-C was reported to be related to the first major adverse cardiovascular events.[17] Schmidt, et al.,[18] indicated that in initially healthy middle-aged men the apoB/apoA-I was the independent risk factor and the strongest predictor for MI. Sniderman, et al.,[19] suggested that the apoB/apoA-I was also an independent predictors of future MI in patients with insulin resistance and metabolic syndrome. In another prospective study of US population, Sierra-Johnson, et al.,[20] showed that both the apoB/apoA-I and the TC/HDL-C were related to CAD death. Furthermore, accumulating evidence had indicated that the apoB/apoA-I was a better marker of cardiovascular events than any of the cholesterol ratios,[4],[5] although dispute still existed.[21],[22] Interestingly, the apoB/apoA-I differed across ethnicities, among African Americans, but not in European-American subjects, elevated apoB/apoA-I independently associated with a greater risk of CAD.[2] Unfortunately, these studies did not investigate the relation between the lipid and lipoprotein ratios and the severity of coronary atherosclerosis.
Until recently, the close relation between the lipid and lipoprotein ratios and the severity of coronary artery stenosis was reported.[23],[24] Yang, et al.,[23] indicated in their study that the severity of coronary artery lesions assessed by GS was correlated with abnormal lipid metabolism. Meanwhile, the ratios of LDL-C/HDL-C and TC/HDL-C were better indicators than other lipid parameters. Following this study, Momiyama, et al.,[24] demonstrated that the LDL-C/HDL-C was associated with the severity of coronary atherosclerosis. Whereas, there were no more lipid and lipoprotein ratios, such as apoB/apoA-I and cholesterol to apoA-I ratios were compared.
It is well acknowledged that type 2 DM is often accompanied with CAD, the long-time survival is significantly reduced in DM-CAD patients when compared with CAD patients without DM.[25] Therefore, intensive attention should be paid to the control of CAD risk factors in type 2 DM patients. However, the intensive glycemic control seems not to bring about an improved survival. Of note, patients with type 2 DM have an increased prevalence of lipid and lipoprotein abnormalities contributing to their high risk of CAD.[26] As mentioned before, lipid profile in type 2 DM is characterized by an increased TG, apo B level, and a lower HDL-C level.[8] Moreover, hypertriglyceridemia is frequently associated with premature cardiovascular disease.[27] Accordingly, detecting those lipid-related risk predictors of the severity of CAD in type 2 DM might have a crucial significance.
To our knowledge, no study has explored the relationship between the lipid and lipoprotein ratios and the severity of new on-set CAD in type 2 DM until now. Actually, our previous study has found that apoB/apoA-I ratio was more significantly associated with the extent of coronary artery lesions in T2DM patients with stable CAD by univariate logistic regression analysis and the association disappeared by multivariate analysis.[9] Despite this, the participants in our previous study were patients with stable CAD, of whom the ones under pre-percutaneous coronary intervention, pre-coronary artery bypass grafting or post-MI were not excluded. Hence, we further evaluated the association between apoB/apoA-I ratio and the severity of CAD in patients with T2DM and new on-set CAD. In the present study, we found that the apoB/apoA-I was still the best indicator in predicting for high GS of CAD in type 2 DM patients after adjustments for some traditional risk factors of cardiovascular disease. Although some patients were on statins, no significant difference in usage rate of statins was found among the three groups of low, middle and high GS. Furthermore, after adjustment for statins therapy, apoB/apoA-1 was still associated with GS. Moreover, the ratios concerning apoA-I seemed to be superior to all traditional lipid and lipoprotein parameters and other ratios. In the present study, there were positive and significant correlations of the ratios including apoB/apoA-I, LDL-C/apoA-I, and non-HDL-C/apoA-I with lnGS. Regarding to the reasons of the apoB/apoA-I as a superior predictor for the CAD risk, previous studies has reported extensively.[2],[5],[17],[28] Firstly, the pathophysiologic bases for the superiority of apoB over LDL-C should be taken into consideration. Secondly, measurement of Apos has methodological advantages over lipids, and the former has international standardization with no need for fasting. Thirdly, apoB level can be measured directly and accurately and the measurement is still reliable for patients with high TG levels (such as that in type 2 DM).[29] Therefore, it is noteworthy that the apoB/apoA-I has the powerful predictive value for the severity of new on-set CAD in type 2 DM. Nevertheless, we must to point out that although the apo B/apo A-I appears to predict GS better than other ratios, the additional predictive value over lipid and lipoprotein ratios, e.g., LDL-C/HDL-C was modest. The underlying explanations are still under discussion.
In conclusion, the present study explored the relation between the lipid and lipoprotein ratios, and CAD risk in DM patients and confirmed their values for predicting the severity of CAD. Importantly, our study indicated that the apoB/apoA-I might be the most useful predictor for the severity of CAD in DM patients. Additionally, the values of other lipid and lipoprotein ratios including LDL-C/HDL-C, non HDL-C/apo A1, and LDL-C/apo A1 were also of some clinical importance. These findings might be meaningful to confirm the importance of the lipid and lipoprotein indicators of atherosclerosis and disease progression, and contribute a better predictive outcome or provide an important proxy for treatment protocols in DM patients with CAD.
Acknowledgments
This work was partly supported by National Natural Scientific Foundation (81070171, 81241121), Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), Capital Special Foundation of Clinical Application Research (Z121107001012015), Capital Health Development Fund (2011400302), and Beijing Natural Science Foundation (7131014) awarded to Dr. Jian-Jun LI.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Enkhmaa B, Anuurad E, Zhang Z, et al. Usefulness of apolipoprotein B/apolipoprotein A-I ratio to predict coronary artery disease independent of the metabolic syndrome in African Americans. Am J Cardiol. 2010;106:1264–1269. doi: 10.1016/j.amjcard.2010.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsenault BJ, Boekholdt SM, Kastelein JJ. Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol. 2011;8:197–206. doi: 10.1038/nrcardio.2010.223. [DOI] [PubMed] [Google Scholar]
- 4.Sniderman AD, Kiss RS. The strengths and limitations of the apoB/apoA-I ratio to predict the risk of vascular disease: a Hegelian analysis. Curr Atheroscler Rep. 2007;9:261–265. doi: 10.1007/s11883-007-0031-6. [DOI] [PubMed] [Google Scholar]
- 5.Goswami B, Rajappa M, Mallika V, et al. Apo-B/apo-AI ratio: a better discriminator of coronary artery disease risk than other conventional lipid ratios in Indian patients with acute myocardial infarction. Acta Cardiol. 2008;63:749–755. doi: 10.2143/AC.63.6.2033393. [DOI] [PubMed] [Google Scholar]
- 6.Srikanth S, Deedwania P. Management of coronary artery disease in patients with type 2 diabetes mellitus. Curr Cardiol Rep. 2007;9:264–271. doi: 10.1007/BF02938374. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls SJ, Tuzcu EM, Wolski K, et al. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol. 2011;57:153–159. doi: 10.1016/j.jacc.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Resnick HE, Foster GL, Bardsley J, et al. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 9.Hong LF, Yan XN, Fan Y, et al. Is the ratio of apoB/apoA-1 the best predictor for the severity of coronary artery lesions in Chinese diabetics with stable angina pectoris? An assessment based on Gensini scores. J Geriatr Cardiol. 2015;12:402–409. doi: 10.11909/j.issn.1671-5411.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zhang Y, Liu J, et al. Role of lipoprotein (A) in predicting the severity of new on-set coronary artery disease in type 2 diabetics: A Gensini score evaluation. Diab Vasc Dis Res. 2015;12:258–264. doi: 10.1177/1479164115579004. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Chen MH, Li S, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of coronary artery disease: a Gensini score assessment. J Atheroscler Thromb. 2014;21:1271–1282. doi: 10.5551/jat.25940. [DOI] [PubMed] [Google Scholar]
- 12.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: A report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Li XL, Hong LF, Luo SH, et al. Impact of admission triglyceride for early outcome in diabetic patients with stable coronary artery disease. Lipids Health Dis. 2014;13:73. doi: 10.1186/1476-511X-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong LF, Li XL, Luo SH, et al. Association of fibrinogen with severity of stable coronary artery disease in patients with type 2 diabetic mellitus. Dis Markers. 2014;2014:485687. doi: 10.1155/2014/485687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 16.Sinning C, Lillpopp L, Appelbaum S, et al. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol. 2013;102:495–503. doi: 10.1007/s00392-013-0555-4. [DOI] [PubMed] [Google Scholar]
- 17.Kappelle PJ, Gansevoort RT, Hillege HJ, et al. Common variation in cholesteryl ester transfer protein: relationship of first major adverse cardiovascular events with the apolipoprotein B/apolipoprotein A-I ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. J Clin Lipidol. 2013;7:56–64. doi: 10.1016/j.jacl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C, Bergstrom G. Apolipoprotein B/Apolipoprotein A-I Ratio and Apolipoprotein B: long-term predictors of myocardial infarction in initially healthy middle-aged men: a 13-year follow-up. Angiology. 2014;65:901–905. doi: 10.1177/0003319713511849. [DOI] [PubMed] [Google Scholar]
- 19.Sniderman AD, Faraj M. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr Opin Lipidol. 2007;18:633–637. doi: 10.1097/MOL.0b013e3282f0dd33. [DOI] [PubMed] [Google Scholar]
- 20.Sierra-Johnson J, Fisher RM, Romero-Corral A, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J. 2009;30:710–717. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 22.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Liu X, Xiang M. The correlation between lipids ratio and degree of coronary artery stenosis. High Blood Press Cardiovasc Prev. 2011;18:53–56. doi: 10.2165/11593480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Momiyama Y, Ohmori R, Fayad ZA, et al. The LDL-cholesterol to HDL-cholesterol ratio and the severity of coronary and aortic atherosclerosis. Atherosclerosis. 2012;222:577–580. doi: 10.1016/j.atherosclerosis.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MH. Cardiovascular risk factors in a patient with diabetes mellitus and coronary artery disease: therapeutic approaches to improve outcomes: perspectives of a preventive cardiologist. Am J Cardiol. 2012;110:43B–49B. doi: 10.1016/j.amjcard.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl. 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 27.Iwanishi M, Ebihara K, Kusakabe T, et al. Premature atherosclerosis in a Japanese diabetic patient with atypical familial partial lipodystrophy and hypertriglyceridemia. Intern Med. 2012;51:2573–2579. doi: 10.2169/internalmedicine.51.7461. [DOI] [PubMed] [Google Scholar]
- 28.Kastelein JJ, van der Steeg WA, Holme I, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
- 29.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–446. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]