Abstract
Background
Klotho proteins (α- and β) are membrane-based circulating proteins that regulate cell metabolism, as well as the lifespan modulating activity of Fibroblast Growth Factors (FGFs). Recent data has shown that higher plasma circulating Klotho levels reduce cardiovascular risk, suggesting Klotho has a protective role in cardiovascular diseases. However, although so far it has been identified in various organs, it is unknown whether cardiomyocytes express Klotho and FGFs, and whether high cardiovascular risk could affect cardiac expression of Klotho, FGFs and other molecules.
Methods
We selected 20 patients with an estimated 10-year high atherosclerotic cardiovascular disease and 10 age-matched control subjects with an estimated 10-year low risk undergone cardiac surgery for reasons other than coronary artery by-pass. In myocardial biopsies, we evaluated by immuno-histochemistry whether Klotho and FGFs were expressed in cardiomyocytes, and whether higher cardiovascular risk influenced the expression of other molecules involved in endoplasmic reticulum stress, oxidative stress, inflammation and fibrosis.
Results
Only cardiomyocytes of patients with a higher cardiovascular risk showed lower expression of Klotho, but higher expressions of FGFs. Furthermore, higher cardiovascular risk was associated with increased expression of oxidative and endoplasmic reticular stress, inflammation and fibrosis.
Conclusions
This study showed for the first time that Klotho proteins are expressed in human cardiomyocytes and that cardiac expression of Klotho is down-regulated in higher cardiovascular risk patients, while expression of stress-related molecules were significantly increased.
Keywords: Atherosclerotic disease, Cardiomyocytes, Cardiovascular risk, Human heart, Klotho
1. Introduction
Several conditions such as diabetes, dyslipidemia, hypertension and obesity are well-established risk factors for cardiovascular damage and coronary artery disease. However, the susceptibility, severity and progression of coronary disease are not fully explicable by these established risk factors and additional biological systems could potentially be involved in the genesis and progression of vascular atherosclerotic mineralization. Among these, the role of Klotho, fibroblast growth factors (FGFs) and related stress proteins has aroused considerable interest as mediators of coronary atherosclerosis and cardiomyocytes metabolic impairment.[1]
Klotho is a single-pass trans-membrane protein with a long extracellular domain and a short cytoplasmic tail. Two distinct forms have been currently identified: (1) α-Klotho (also referred to simply as Klotho), found in many organs including kidney, parathyroid gland, brain, adipose tissue and small intestine, and (2) β-Klotho, a protein that shares 41% amino acid identity with α-Klotho, and is expressed in adipose tissue, pancreas and liver. The extra-cellular domain of both Klotho may be cleaved and released into the blood, cerebrospinal fluid and urine as a soluble form.[2] It has been suggested that Klotho proteins (trans-membrane and circulating) influence the endocrine activity of FGFs, regulate mineral transport and cell metabolism preventing organ aging an death.[3]
Several experimental and clinical studies show that Klotho and FGFs are involved in the genesis and progression of various cardiovascular diseases,[3]–[5] preventing oxidative stress and so apoptosis and cell death.[6]–[9] However, it has not been clear whether Klotho is constitutionally expressed in human cardiomyocytes until now.
Considering the beneficial physiological functions of Klotho on cell metabolism and survival, we hypothesized that these molecules could be constitutionally expressed by human cardiomyocytes. We used immuno-histochemistry techniques, which penalizes protein quantification but locates these molecules more precisely in the different cell types of the myocardial tissue. We investigated whether α- and β-Klotho, FGFs (FGF21 and FGF23) were present in human cardiomyocytes and whether patients with higher cardiovascular risk (HCVR), estimated according to the 2013 ACC/AHA guidelines on the Assessment of Cardiovascular Risk, had altered Klotho, FGFs and any related stress molecules expression.
Recent experimental data has shown that Klotho attenuates endoplasmic reticulum (ER) stress and stress-induced apoptosis.[10]–[12] Indeed, many cardiovascular diseases affect the ER ability to synthesize, fold and sort proteins, increasing the expression of molecular chaperones that promote proper folding and cellular recovery.[13]–[16] We therefore evaluated the presence of chaperone GRP78, a marker of ER stress, in cardiomyocytes. The increase expression of GRP78 is induced by enhanced oxidative stress. Given that oxygen free radicals are scavenged by superoxide dismutase-1 (SOD1) that act as a molecular switch to activate the ER stress response and play an important role in cellular homeostasis,[17] we also examined SOD1 modulation.
Since oxidative stress is related to the inflammatory response, we also examined the presence of inducible-NOS (iNOS), endothelial-NOS (eNOS) and nuclear factor-kappa-B (NF-kB) proteins, which are molecules involved in the intracellular oxidative and inflammatory processes in cardiovascular diseases.[18]–[20] Interestingly, these molecules are regulated by Klotho.[4],[21]–[23]
Finally, it has been shown that Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling, and suppressed experimentally induced fibrosis.[24],[25] On the basis of these findings, we measured the degree of myocardial fibrosis by Sirius Red histochemistry and anti-TGF-β1 staining.
2. Methods
2.1. Patient population
This research was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study was approved by the institutional ethics committees of the local health authority (ASL, Brescia, Italy, No.: 5/2008). Informed consent was obtained from all individual participants included in the study.
Ten year atherosclerotic cardiovascular disease (ASCVD) risk was estimated using the Risk Estimator application available online. This Risk Estimator is intended as a companion tool to the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk and the 2013 ACC/AHA Guideline on the Treatment of Blood Atherosclerotic Cardiovascular Risk in Adults. It is able to estimate 10-years cardiovascular disease. The information required to estimate ASCVD risk includes: age (from 40 to 79 years), sex, race, total cholesterol, high-density lipoprotein cholesterol (HDL), systolic blood pressure, blood pressure lowering medication use, diabetes status and smoking status.[26],[27]
Patients who have a high 10-year ASCVD of 7.5 risk, means that 7.5% of them would be expected to have artery diseases within the next 10 years. On the contrary, 10-year ASCVD risk estimated < 5 refers to low risk patients who have a probability to developed cardiovascular diseases in the next 10 years less than 5%.
Thirty strictly selected adult patients undergoing cardiac surgery were recruited between 2012 and 2015. They had undergone cardiac surgery for reasons other than coronary artery by-pass, such as aortic aneurism or atrial myxoma. Patients (n = 20) with an estimated high 10-year ASCVD risk were included in the HCVR group and age-matched patients (n = 10) with an estimated low 10-year ASCVD risk were included in Control group. All patients in both groups were statin-naïve and had normal coronary arteries (by left heart catheterization). Patient exclusion criteria included: reduced ejection fraction, enlargement or hypertrophy of cardiac chambers, previous myocardial infarct, arrhythmia, concomitant liver, inflammatory, autoimmune, endocrine, pulmonary or kidney diseases, myopathy and/or basal creatine phosphate kinase increase and chronic hypercholesterolemia that required statin administration. These very strict inclusion criteria were applied as we wanted to avoid any confounding patient-related factors. The demographic and clinical patients data are shown in Table 1.
Table 1. Patient demographics and clinical data.
| CTRL, n = 10 | HCVR, n = 20 | |
| Age, yrs | 55.3 ± 2.2 | 56.0 ± 1.5 |
| BMI, kg/m2 | 25.2 ± 1.5 | 30.3 ± 1.7* |
| Systolic blood pressure, mmHg | 124.9 ± 3.3 | 120.0 ± 2.5 |
| Diastolic blood pressure, mmHg | 82.3 ± 2.4 | 83.5 ± 3.2 |
| Total cholesterol, mg/dL | 200.3 ± 2.6 | 210.5 ± 1.5 |
| HDL, mg/dL | 73.2 ± 4.0 | 44.9 ± 3.2 |
| C-reactive protein, mg/dL | < 2 | < 2 |
| Glucose, mg/dL | 93.7 ± 5.3 | 90.2 ± 6.7 |
| Creatinine, mg/dL | 1.1 ± 0.3 | 1.0 ± 0.2 |
| AST, U/L | 19.2 ± 8.1 | 22.4 ± 5.3 |
| ALT, U/L | 16.5 ± 6.2 | 22.1 ± 7.4 |
| Gamma-GT, U/L | 32.3 ± 5.1 | 29.6 ± 10.3 |
| Alkaline phosphatase, mU/mL | 112 ± 34 | 89 ± 56 |
| Total bilirubin, mg/dL | 0.73 ± 0.1 | 0.69 ± 0.2 |
| Albumin, g/L | 3.9 ± 0.2 | 3.8 ± 0.1 |
| Treatment for hypertension | ||
| Ca2+ channel blockers | 0 | 12 |
| β-blockers | 0 | 10 |
| ACEI | 0 | 12 |
| Cigarette smokers | 0 | 0 |
| Alcohol drinking | 0 | 0 |
Values are mean ± SD. *P < 0.001. ACEI: angiotensin-converting enzyme inhibitors; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; HDL: high-density lipoprotein.
2.2. Surgical procedure
The anesthesia and cardioplegia we used are previously described.[28] The operations were all performed by the same surgeon.
Immediately after the start of cardio-pulmonary bypass before cardioplegia, a biopsy sample was obtained from the right atrium as previously described.[28] There were no clinical complications related to the sampling procedure. The samples were fixed in 4% formaldehyde and processed for paraffin embedding.
2.3. Immuno-histochemistry
Paraffin sections were incubated overnight with polyclonal primary anti-Klotho (sc-22220), anti-β-Klotho (sc-74343), anti-iNOS (sc-651), anti-eNOS (sc-654), anti-GRP78 (sc-1050), anti-TGF-b1 (sc-146), anti-SOD1 (FL-154) from Santa Cruz Biotechnology Inc; anti-FGF21 (ab171941) from Abcam; anti-FGF23 (bs-5768R) from BiossInc, diluted 1: 100 with PBS; and monoclonal anti-NF-kB (NB110-57266) from Novus Biologicals, diluted 1: 250. The sections were visualized with a rabbit ABC-peroxidase staining system kit (Santa Cruz Biotechnology Inc.). In order to exclude incorrect interpretation of immuno-staining due to endogenous biotin, we also carried out experiments using the peroxidase-anti-peroxidase detection system. We obtained similar results with both methods. The immuno-histochemistry control was performed by omitting the primary antibody.
The staining intensity in both histochemical and immuno-histochemistry slides was evaluated using an optical microscope equipped with an image analysis program (ImageProPlus 4.5.1). The integrated optical density was calculated for arbitrary areas, by measuring 10 fields for each sample. On sections stained with anti-NF-kB, we also counted the percentage of immuno-stained nuclei.
Collagen density and fibrosis were evaluated using a Sirius red staining method,[29] using a light microscope under polarized light. The various thicknesses of collagen fibres showed a different gradient of colours. While the birefringent colour was more a measure of collagen fibre size than of collagen type, usually the thicker and denser type I collagen fibres ranged from orange to red, whereas the thinner type III collagen fibres appeared from yellow to green.[30]
Different researchers, blinded to the samples, independently analyzed all slides.
2.4. Statistical analysis
The statistical analysis was performed by one-way ANOVA followed by the Student-Newman-Keuls test or by a Student t-test, and by multivariate regression analysis. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Patient demographic and clinical data
Table 1 shows the demographic and clinical data of patients studied. Control and HCVR patients were the same age and had the same chemical clinical variables. HCVR had normal values of blood pressure but all of them were in therapy with anti-hypertensive drugs.
3.2. Higher ASCVD risk influenced the expression of Klotho proteins and FGFs
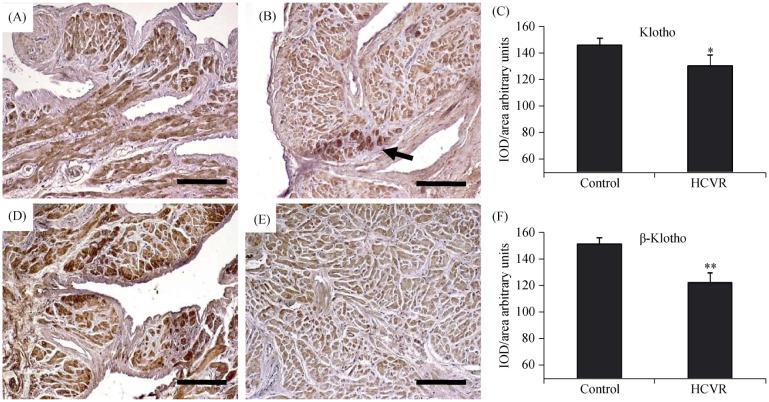
In the control patients, we observed a very intense cytoplasmic immunoreactivity of Klotho and β-Klotho proteins uniformly distributed in all cardiomyocytes (Figure1A and 1D, respectively). In HCVR patients, the Klotho immuno-staining intensity was lower. However, some clusters of cells show intense staining (Figure 1B). The β-Klotho immuno-staining of cells from HCVR patients was uniformly diffuse inside the cytoplasm of all cardiomyocytes, but was much lower than that seen in control patients (Figure 1E). The optical density value for Klotho and β-Klotho staining was resumed in Figure 1C and 1F, respectively.
Figure 1. Klotho (A-C) and β-Klotho (D-F) immuno-staining.
(A): Control cardiomyocytes show intense cytoplasm staining; (B): cardiomyocytes from HCVR patients show reduced staining; however, some clusters of cells show intense staining (arrow); (C): relative values of optical density according to groups; (D): control cardiomyocytes show intense cytoplasmic staining; (E): the staining is reduced in all cardiomyocytes from HCVR patients; and (F): relative values of optical density according to groups. Original magnification 100 ×; scale bar = 20 µm; *P < 0.05; **P < 0.01. HCVR: higher cardiovascular risk; IOD: integrated optical density.
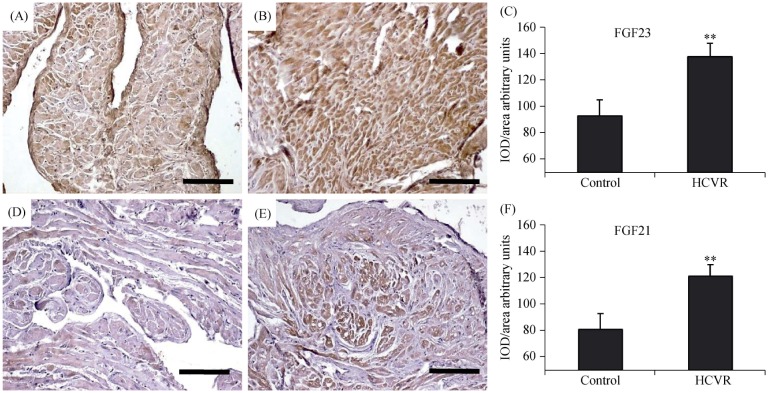
Since Klotho is the co-receptor for FGF23 and β-Klotho is the co-receptor for FGF21, we also tested the expression of these proteins in cardiomyocytes. In the cardiomyocytes from control patients, the FGF23 immuno-staining was seen to be faint although uniformly diffuse (Figure 2A). Cardiomyocytes from HCVR patients had higher FGF23 staining (Figure 2B). The mean optical density for FGF23 staining was resumed in Figure 2C. FGF21 immuno-staining from control patients showed diffuse but poor staining, which was not uniformly distributed inside the cytoplasm (Figure 2D). In contrast, cardiomyocytes from HCVR patients had more intense FGF21 staining, which was uniformly distributed inside the cytoplasm (Figure 2E). The optical density for FGF21 staining was resumed in Figure 2F.
Figure 2. FGF23 (A-C) and FGF21 (D-F) immuno-staining.
(A): All control cardiomyocytes show moderate staining; (B): FGF23 staining increases intensely in cardiomyocytes from HCVR patients; (C): relative values of optical density according to groups; (D): the staining in cardiomyocytes from control patients is faint; (E): the staining increased in cells from HCVR patients; and (F): relative values of optical density according to groups. Original magnification 100 ×; scale bar = 20 µm; **P < 0.01. HCVR: higher cardiovascular risk; IOD: integrated optical density.
3.3. Higher ASCVD risk increases ER-stress (GRP78)
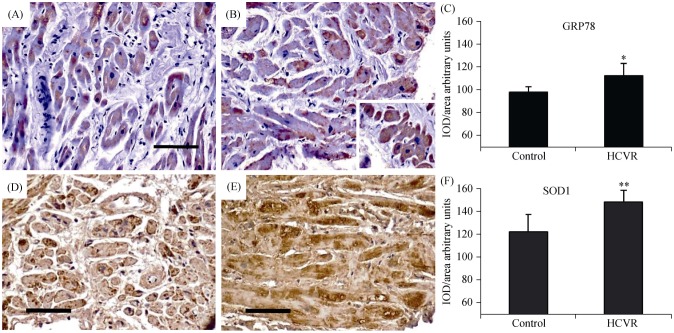
Since depletion of Klotho can promote cell stress, in order to evaluate ER stress and chronic oxidative alterations in cardiomyocytes from control and HCVR patients, we also detected GRP78 and SOD1. In cardiomyocytes from control patients, GRP78 staining intensity was weak to moderate and concentrated in cytoplasmic areas, sometimes near the nucleus (Figure 3A). In HCVR patients, GRP78 expression was higher and mostly concentrated in cytoplasmic areas (Figure 3B). Some cardiomyocytes were clustered and intensely stained in certain myocardial areas (Figure 3B, insert). The density for GRP78 staining was resumed in Figure 3C.
Figure 3. GRP78 (A-C) and SOD1 (D-F) immuno-staining.
(A): In cardiomyocytes from Control patients, the staining is weak to moderate and is concentrated in the cytoplasm, sometimes near the nucleus; (B): in cells from HCVR patients, GRP78 staining is more intense. Some cells are clustered and much intensely stained (insert); (C): relative values of optical density according to groups; (D): the staining is faint to moderate in all cells from Control patients; more intense staining is concentrated in some areas of the cytoplasm; (E): all cardiomyocytes from HCVR patients show intense staining uniformly distributed within the cytoplasm; (F): relative values of optical density according to groups. Original magnification 100 ×; scale bar = 20 µm; *P < 0.05; **P < 0.01. GRP78: glucose-regulated protein 78; HCVR: higher cardiovascular risk; IOD: integrated optical density; SOD1: superoxide dismutase-1.
SOD1 staining intensity was weakly to moderately in all cells from Control patients, and localized only in certain cytoplasmic areas (Figure 3D). In contrast, cardiomyocytes from HCVR patients showed intense immuno-staining, which was uniformly distributed throughout the cytoplasm (Figure 3E). The optical density for SOD1 staining was resumed in Figure 3F.
3.4. Higher ASCVD risk increases NF-kB immuno-reactivity
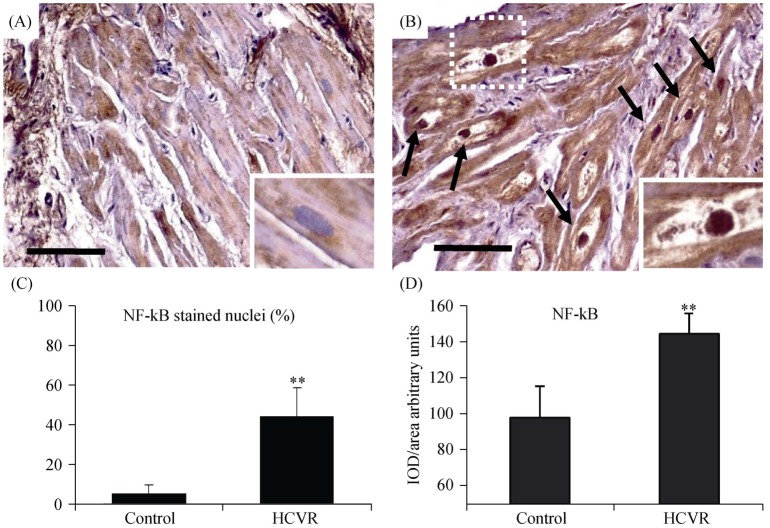
In order to evaluate the inflammatory alterations in cardiomyocytes from patients, we also detected NF-kB, which is a redox regulated transcription factor involved in inflammation, immune function, cellular growth and apoptosis. NF-kB was faintly expressed inside the cytoplasm of Control, and stained nuclei were observed only occasionally (Figure 4A). However, cardiomyocytes from HCVR patients had NF-kB immuno-staining which was intense and uniformly distributed not only inside the cytoplasm, but also strongly expressed in a large proportion of nuclei (Figure 4B). Large cardiomyocytes frequently containing debris and had large nuclei strongly immuno-stained (Figure 4B insert) that sometimes showed “bite-like” signs at pole extremities. The quantitative data of stained nuclei is shown in Figure 4C. The optical density for NF-kB staining is resumed in Figure 4D.
Figure 4. NF-kB immuno-staining.
(A): Cardiomyocytes from control patients showed a faint signal in the cytoplasm, while the nuclei are only stained occasionally (insert). (B): cardiomyocytes from HCVR patients have intense and uniformly distributed NF-kB staining also in many nuclei (arrows). Large cardiomyocytes with very pale cytoplasm containing a lot of cellular debris have large strongly stained nuclei (insert). (C): percentage of positively NF-kB stained nuclei according to groups. (D): relative values of optical density according to groups. Original magnification 200 ×; scale bar = 10 µm; **P < 0.01. HCVR: higher cardiovascular risk; IOD: integrated optical density; NF-KB: nuclear factor kappa B.
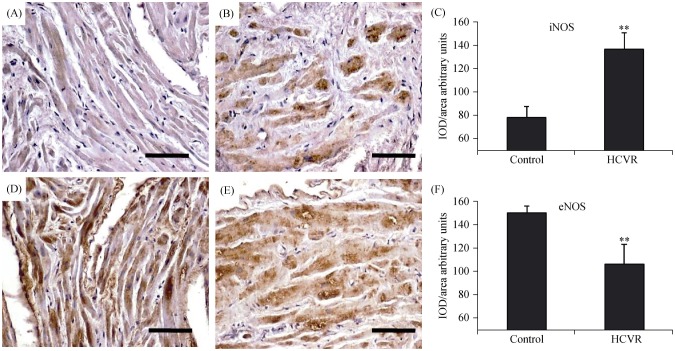
3.5. Higher ASCVD risk increases iNOS and decreases eNOS immuno-reactivity
The increased presence of ER-stress and oxidative stress markers, as well as inflammatory transcription factors in cardiomyocytes from HCVR patients, suggests a higher production of pro-inflammatory iNOS with reduced expression of eNOS. iNOS had very weak staining inside the cytoplasm of the control (Figure 5A), whereas essentially, all HCVR cardiomyocytes showed a moderate to intensely stained cytoplasm (Figure 5B). The mean optical density for iNOS staining was resumed in Figure 5C. The eNOS staining was intense in the cytoplasm of cells from control patients (Figure 5D), but lower in the cells from HCVR patients (Figure 5E). The optical density for eNOS staining was resumed in Figure 5F.
Figure 5. iNOS (A-C) and eNOS (D-F) immuno-staining.
(A): The staining is very weak in the cytoplasm of cells from Control patients; (B): cardiomyocytes from HCVR patients show intensely stained cytoplasm; (C): relative values of optical density according to groups; (D): the distribution of staining is uniform and intense in the cytoplasm of cells from Control patients; (E): cells from HCVR patients are faintly to moderately stained; and (F): relative values of optical density according to groups. Original magnification 200 ×; scale bar = 10 µm; **P < 0.01. eNOS: endothelial nitric oxide synthase; HCVR: higher cardiovascular risk; iNOS: inducible nitric oxide synthase; IOD: integrated optical density.
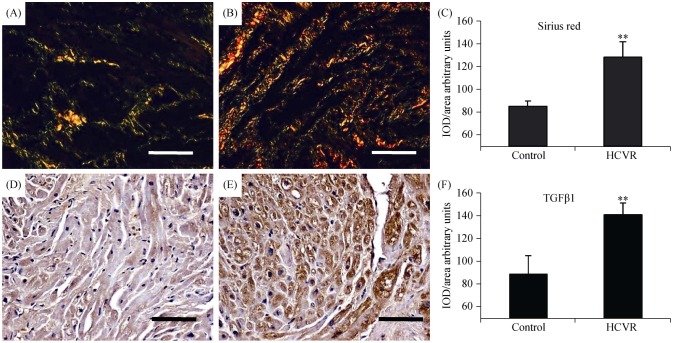
3.6. Higher ASCVD risk increases fibrosis and TGF-β1 immuno-reactivity
The degree of fibrosis was very poor in control patients, whereas it was more intense in HCVR cardiomyocytes, with a prevalence of thick orange-red fibres instead of thin green fibres (Figure 6A and 6B respectively). The density for Sirius-red staining in patient groups was resumed in Figure 6C. In line with histochemical data, the density of TGF-β1 staining, a fundamental protein for collagen synthesis, was more intense and uniformly distributed in the cytoplasm of cells from HCVR patients (Figure 6E), than in cells from control patients (Figure 6D). The optical density for TGF-β1 staining was resumed in Figure 6F.
Figure 6. Fibrosis (A-C) and TGF-β1 (D-F) immuno-staining.
(A): Control patients samples did not show any abnormal collagen fibres deposition. (B): HCVR patients had diffuse fibrosis. Type-I collagen fibres are shown as orange to red, whereas the thinner type-III collagen fibres appear yellow to green. (C): Relative values of optical density according to groups. (D): Cell staining from Control patients is faint. (E): Immuno-staining from HCVR patient cells is more intense. (F): Relative values of optical density according to groups. Original magnification 200 ×; scale bar = 10 µm; **P < 0.01. HCVR: higher cardiovascular risk; IOD: integrated optical density; TGF-β1: transforming growth factor-β1.
Multivariate regression analysis between klotho and the mean variation of other proteins is summarized in Table 2. The data shows that the variation of klotho was significantly related to the variation of NF-kB, iNOS, and TGF-β1.
Table 2. Multivariate regression analysis between klotho and mean variation of other proteins.
| Coefficients | Estimate SE | t-value | P | |
| Klotho (intercept) | −15.41429 | 0.60353 | −25.540 | 0.000 |
| β-Klotho | 0.11435 | 0.14003 | 0.817 | 0.431 |
| FGF-23 | 0.06996 | 0.05776 | 1.211 | 0.251 |
| FGF-21 | −0.02911 | 0.08540 | −0.341 | 0.740 |
| SOD1 | −0.02294 | 0.06257 | −0.367 | 0.721 |
| NF-kB | −0.29199 | 0.10764 | −2.713 | 0.020 |
| iNOS | 0.19065 | 0.07812 | 2.441 | 0.033 |
| eNOS | −0.04104 | 0.06767 | −0.607 | 0.556 |
| GRP78 | 0.12325 | 0.18054 | 0.683 | 0.509 |
| TGF-β1 | −0.19491 | 0.06192 | −3.148 | 0.009 |
eNOS: endothelial nitric oxide synthase; GRP78: glucose-regulated protein 78; FGF: fibroblast growth factors; HCVR: higher cardiovascular risk; iNOS: inducible nitric oxide synthase; NF-KB: nuclear factor kappa B; SOD1: superoxide dismutase-1; TGF-β1: transforming growth factor-β1.
4. Discussion
This is the first study to show that: (1) Klotho and FGF-21/FGF-23 proteins are expressed in human cardiomyocytes and (2) cardiomyocytes from patients with a higher cardiovascular risk disease had reduced expression of Klotho but increased expression of FGFs and expression of related stress molecules involved in cardiomyocyte damage.
Until now, Klotho had been shown to be expressed solely at the sinoatrial node in mouse hearts, where it is essential for the sinoatrial pacemaker function under stress conditions.[31] Here, we have shown expression of Klotho inside human cardiomyocytes. However, it should be noted that we examined human tissue samples of cardiac atria and hence we cannot translate our results to the whole heart, or to a peculiar role in the human sinoatrial node. Nonetheless, unlike the mouse study, the abundant cytoplasmic expression of Klotho proteins in human cardiomyocytes of low cardiovascular risk atria would strongly suggest the pivotal role played by such cardiomyocyte Klotho proteins in whole heart physiology. To the best of our knowledge, this is the first study that identifies Klotho proteins diffusely and intensely expressed inside the cytoplasm of human cardiomyocytes. The heart could therefore be regarded not only as a target of soluble Klotho produced by other organs, but also an organ that produces its own Klotho proteins. In particular, we have shown that Klotho protein expression is blunted in patients with HCVR. Our data agrees with previous studies showing a correlation between soluble Klotho concentration and myocardial diseases.[3],[7],[32],[33]
Recent studies show the association between circulating FGF23 levels and pathologic cardiovascular conditions, including left ventricular hypertrophy and vascular endothelial dysfunction.[34]–[37]
We did not measure serum concentrations of FGF23, but we did demonstrate that cardiomyocytes themselves may produce detectable amounts of FGF23 protein and that this was higher in HCVR patients. FGF23 is capable of binding FGF receptors, although with low affinity, also in the absence of membrane Klotho. It is therefore plausible that activation of FGF receptors also occurs in presence of high FGF23 levels in organs where Klotho is absent.[38] We therefore believe that cardiac pathophysiology is influenced not only by the circulating FGF23, but also by FGF23 produced by cardiomyocytes themselves and might play an important role in metabolic and cardiac functions, perhaps through an Klotho-independent way and/or with another unknown co-factor.
Klotho also works as a receptor for FGF21. FGF21, initially identified as a liver-derived endocrine factor and subsequently found in adipose tissue and muscle,[38] stimulates glucose uptake in adipocytes (independently from insulin), and has subsequently been related to extension of animal life-span.[39],[40] Experimental studies have shown that in response to ischemia-reperfusion injury to the myocardium, FGF21 is up-regulated and released from hepatic cells and adipocytes into the circulation. It then interacts with FGFR1 on cardiomyocytes with β-Klotho, inducing phosphorylation of FGFR1. In turn, this reduces apoptosis, cell death and myocardial infarction, improving myocardial function.[39],[41] Serum FGF21 has been found to be higher in patients with chronic metabolic and heart diseases. In addition, FGF21 levels correlate positively with triglyceride, fasting blood glucose, apolipoprotein-B100 and insulin resistance, but negatively with HDL and apolipoprotein-A1.These findings indicate a positive correlation between FGF21 and adverse lipid profile in chronic heart disease patients and a possible compensatory response or resistance to FGF21.[42] Here, we show that FGF21 is over-expressed by cardiomyocytes. This would however suggest a possible adaptation or compensatory elevation of FGF21 in order to overcome cardiovascular metabolic disorders.[43]
The intracellular distribution of Klotho mostly overlaps with that of the ER and Golgi apparatus,[44] whose expression has been linked to the ability of cells to withstand stress conditions. Indeed, Klotho decreases the GRP78 expression and related apoptosis in mice.[10] Our data agree with these observations. Indeed, cardiomyocytes from HCVR patients had reduced Klotho expression and higher increases of GRP78 expression. GRP78 recognizes misfolded proteins inside endoplasmic reticulum and dissociates its binding from its molecular partners. This in turn triggers the unfolded protein response by regulating gene responses aimed at restoring endoplasmic reticulum proteome stability.[13] Failure to promote cell survival by decreasing misfolded proteins and oxidative stresses may promote apoptotic cell death.[45]
GRP78 is induced by increased oxidative stress related to the inflammatory response, and inflammatory cytokines are known to down-regulate Klotho through NF-kB.[21],[22] We therefore examined the presence and modulation of SOD1, iNOS and NF-kB proteins. Our results demonstrated an increase of SOD1, iNOS and NF-kB inside the cardiomyocytes from HCVR patients, suggesting a chronic pro-oxidative, pro-inflammatory and pro-apoptotic status. This is in line with previous research and shows that there is a correlation between obesity, diabetes, decrease in Klotho and increase in NF-kB and oxidative stress. In addition, we observed reduced immuno-staining of eNOS in HCVR cardiomyocytes. Notably, cytoplasmic eNOS improves energetic cellular metabolisms and stimulates mitochondrial biogenesis.[46] So, there also exists the possibility of a disruption of energetic metabolism and altered mitochondrial biogenesis in HCVR patients cardiomyocytes.
Not surprisingly, we detected fibrosis and higher TGF-β1 expression in cardiomyocytes of HCVR patients. We believe that the reduced Klotho proteins expression found in cardiomyocytes from HCVR patients, could be related to the modulation of TGF-β1 synthesis and subsequent fibrosis. Indeed, Klotho is able to interfere with TGF-β1 signaling.[24] Furthermore, enhanced synthesis of Klotho improves the arrangement of cardiomyocytes and myocardial fibres in animal models.[10] Although these studies show an association between both Klotho deficiency and metabolic and cardiac diseases, the cause-effect relationship remains to be clarified.
4.1. Clinical implications
It is accepted that diabetes, obesity and hypertension are risk factors for both coronary calcification and cardiomyocyte pathology. However, the susceptibility, severity and progression of cardiovascular diseases are not completely explained by these risk factors. Therefore, identification of additional molecules involved in the pathophysiology and the evolution of cardiovascular diseases is fundamental to achieve important objectives such as the development of specific new therapies, identification of biomarkers for grading cardiovascular risk and monitoring the effects of therapy including physical rehabilitation.
4.2. Limitations
Certain limitations of this study need to be recognized. Firstly, we collected atrial biopsies but atrial tissues were considered myocardium and have the same metabolism and cellular signaling as the cardiomyocytes. In addition, sampling aimed to provide an appropriate amount of tissue without compromising the left ventricular function, and consequently the patient's survival.
Secondly, only a limited number of patients were enrolled in this study. The patients were however very highly selected to avoid any confounding co-morbidity and, in addition, the sample was adequate for us to identify the major relationship between Klotho and related protein presence and cardiovascular risk.
Thirdly, we only used immuno-histochemistry to support our results. This was dictated by the rules in force in our operating theatre, which excluded the possibility of using liquid nitrogen and then immediately freeze the biopsies for molecular biology evaluations. However, although the immuno-histochemistry does not quantify molecules as accurately as molecular biology, we believe that this technique has certain advantages. Indeed, the standard fixation maintains the architecture of the tissue, allowing adequate morphological assessments. In addition, immuno-histochemistry allows us to localize the analyzed molecules both between different cells types and inside the cells. In contrast, molecular biology requires immediate freezing and homogenization of the sample. As a consequence, it does not take into account the specificities of the location of immuno-staining, and so the morphology of the tissue.
4.3. Conclusions
In conclusion, we have reported for the first time the presence of Klotho proteins in human hearts in vivo, and that HCVR cardiomyocytes have deranged expressions of Klotho, FGF23, FGF21 and related stress molecules involved in cellular injury. We believe that modulation of these molecules could play a pivotal role in heart pathophysiology. We therefore suggest that preventing the Klotho decrease or promoting its increase, and/or FGFs modulation inside cardiomyocytes, could prevent and/or reduce cardiovascular diseases in high risk patients.
Acknowledgments
We would like to thank Prof. Robert Coates (Centro Lingusitico, Bocconi University, Milan, Italy) for his linguistic revision and Prof. Stefano Calza (Department of Molecular and Translational Medicine, University of Brescia, Italy) for his statistical advice. The authors declare that they have no conflict of interests. This study was funded by grants from the University of Brescia (to Corsetti G).
References
- 1.Evrard S, Delanaye P, Kamel S, et al. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–414. doi: 10.1016/j.cca.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Gonzalez JF, Donate-Correa J, Muros de Fuentes M, et al. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40. doi: 10.1136/heartjnl-2013-304746. [DOI] [PubMed] [Google Scholar]
- 4.Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzi O, Veyrat-Durebex C, Wollheim CB, et al. Evidence against a direct role of Klotho in insulin resistance. Pflugers Arch. 2010;459:465–473. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semba RD, Cappola AR, Sun K, et al. Plasma Klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar V, Christopher R. Association of exonic variants of Klotho with metabolic syndrome in Asian Indians. Clin Chim Acta. 2011;412:1116–1121. doi: 10.1016/j.cca.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Moe SM. Klotho: a master regulator of cardiovascular disease? Circulation. 2012;125:2181–2183. doi: 10.1161/CIRCULATIONAHA.112.104828. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Gao P, Xiao H, et al. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8:e82968. doi: 10.1371/journal.pone.0082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabadkai G, Rizzuto R. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 2004;567:111–115. doi: 10.1016/j.febslet.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada K, Minamino T, Tsukamoto Y, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 15.Martindale JJ, Fernandez R, Thuerauf D, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 16.Hamada H, Suzuki M, Yuasa S, et al. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homma K, Fujisawa T, Tsuburaya N, et al. SOD1 as a molecular switch for initiating the homeostatic ER stress response under zinc deficiency. Mol Cell. 2013;52:75–86. doi: 10.1016/j.molcel.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CK, Barnard RJ, Sindhu RK, et al. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol. 2005;98:203–210. doi: 10.1152/japplphysiol.00463.2004. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Wang WQ, Zhang H, et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 20.Toda N, Tanabe S, Nakanishi S. Nitric oxide-mediated coronary flow regulation in patients with coronary artery disease: recent advances. Int J Angiol. 2011;20:121–134. doi: 10.1055/s-0031-1283220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno JA, Izquierdo MC, Sanchez-Nino MD, et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston RD, Larmonier CB, Majewski PM, et al. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology. 2010;138:1384–1394. doi: 10.1053/j.gastro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teocchi MA, Ferreira AE, da Luz de Oliveira EP, et al. Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J Neuroinflammation. 2013;10:53. doi: 10.1186/1742-2094-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kararigas G, Dworatzek E, Petrov G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160–1167. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 26.Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2886. doi: 10.1016/j.jacc.2014.02.606. [DOI] [PubMed] [Google Scholar]
- 27.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Lalu MM, Pasini E, Schulze CJ, et al. Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J. 2005;26:27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]
- 29.Dayan D, Hiss Y, Hirshberg A, et al. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–29. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 30.Koren R, Yaniv E, Kristt D, et al. Capsular collagen staining of follicular thyroid neoplasms by picrosirius red: role in differential diagnosis. Acta Histochem. 2001;103:151–157. doi: 10.1078/0065-1281-00587. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 32.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelin B, Larsson TE, Rudling M. Circulating fibroblast growth factors as metabolic regulators−a critical appraisal. Cell Metab. 2012;16:693–705. doi: 10.1016/j.cmet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirza MA, Larsson A, Lind L, et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Shibata K, Fujita S, Morita H, et al. Association between circulating fibroblast growth factor 23, alpha-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS One. 2013;8:e73184. doi: 10.1371/journal.pone.0073184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin A, David V, Quarles LD. Regulation and function of the FGF23/Klotho Endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenyon C. Could a hormone point the way to life extension? eLife. 2012;1:e00286. doi: 10.7554/eLife.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu SQ, Roberts D, Kharitonenkov A, et al. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Z, Wu Z, Yin X, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5:e15534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unger RH. Klotho-induced insulin resistance: a blessing in disguise? NatMed. 2006;12:56–57. doi: 10.1038/nm0106-56. [DOI] [PubMed] [Google Scholar]
- 44.Li SA, Watanabe M, Yamada H, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 45.Niforou K, Cheimonidou C, Trougakos IP. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014;2:323–332. doi: 10.1016/j.redox.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]