Abstract
Hemolytic anemia following a mitral valve repair is a very rare complication, with only 70 cases reported worldwide.
We present a case of an 8-year-old girl who presented with a very early onset hemolytic anemia following a mitral valve repair with ring annuloplasty, which necessitated a reoperation.
The report also discusses the various mechanisms of traumatic hemolysis associated with valve repair and probable lessons learnt and ways to avoid this complication.
Keywords: Hemolytic anemia, Valvular heart disease, Repair of valves, Rheumatic heart disease
1. Introduction
Hemolytic anemia following mitral valve replacement with either a mechanical or a bioprosthetic valve is a known complication. However, massive hemolysis following a mitral valve repair (MV repair) is an extremely rare complication, with only 70 cases reported in the worldwide literature.1 We present a very early onset hemolytic anemia occurring in an 8-year-old girl following MV repair with ring annuloplasty which necessitated a reoperation.
2. Case report
An 8-year-old girl presented with Cl III dyspnea and heart failure. On echocardiography, she had rheumatic heart disease, minor chordal rupture, prolapse of A1 and A2 segment of anterior mitral leaflet leading to severe mitral regurgitation (MR), mild aortic regurgitation, and severe pulmonary hypertension. She underwent a MV repair with creation of two neo-chordae using PTFE pledgeted sutures and annuloplasty was done with a 27 mm Annuloplasty ring (St Jude Medical Inc, MN, USA). Trans-esophageal echocardiography done intraoperatively suggested Grade 1 residual MR with no flow acceleration across the mitral valve, an essentially satisfactory result (Fig. 1, Fig. 2).
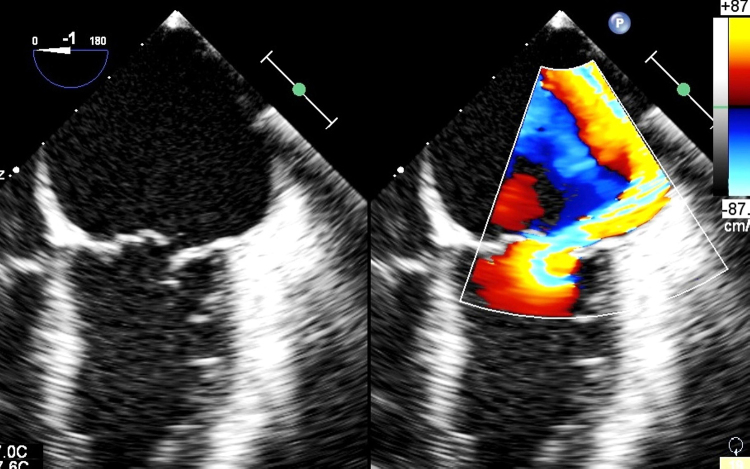
Fig. 1.
The preoperative TEE image showing preoperative MR jet.
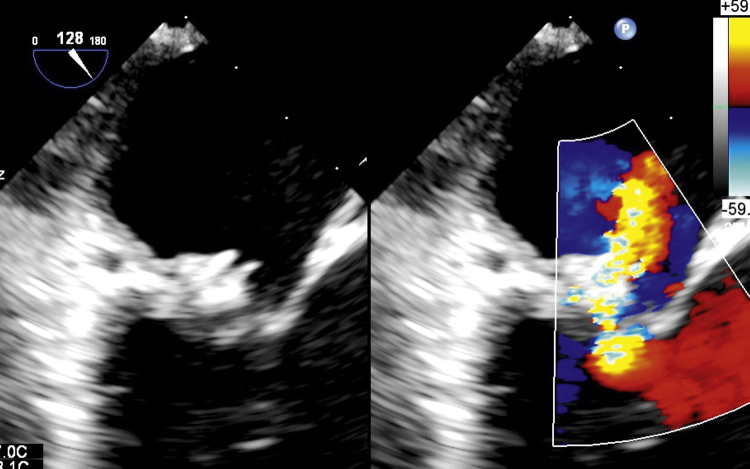
Fig. 2.
Postoperative TEE image of the repaired mitral valve showing the ring protruding into the cavity of LA.
The patient was shifted to the ICU with good hemodynamics.
Within 2–3 h of shifting to the ICU, the urine, which was clear after surgery, became dark brown colored and the hemoglobin (Hb) fell by 2 gm/dl, in absence of significant drainage, necessitating a transfusion. The urine continued to be dark colored. The patient was extubated after 12 h, the next morning and the lab reports revealed a further drop in Hb by 1 gm/dl to 9 gm/dl, requiring another transfusion.
The patient was then investigated in detail for hemolysis and the results are tabulated below.
| Investigation | Preoperative values | Postoperative (after 48 h) |
|---|---|---|
| Hemoglobin | 12.4 | 9.1 |
| LDH | – | 4999 |
| Haptoglobin | – | <6.75 |
| Urine free Hemoglobin | – | Present |
| Reticulocyte count | – | 3 |
| Bilirubin (total/Indirect) | 0.8/0.3 | 5.2/3.2 |
| Peripheral smear | – | s/o Traumatic hemolytic anemia |
| Coombs test (direct and indirect) | – | Negative |
| G6PD | – | Negative |
| Creatinine | 0.4 | 0.6 |
| PCV transfusion | – | 3 |
The patient was treated with adequate hydration and forced diuresis to protect the kidneys (Table 1).
Table 1.
Preoperative and postoperative investigations.
| Preoperative investigations | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | 168 h | |
|---|---|---|---|---|---|---|---|---|
| Hb | 10.9 | 12.9 | 9.8 | 9.3 | 8.1 | 12.2 | 10.8 | 9.7 |
| Transfusion | 2 PCV (700 ml) | – | – | 1 PCV (360 ml) | – | – | – | |
| Haptoglobin | <6.75 (30–200) | |||||||
| LDH | 4999 | 7332 | 5756 | 7380 | ||||
| Retic count | 3 | 3.5 | 3.7 | |||||
| Bilirubin (total/direct) | 0.8/0.5/0.3 | 5.2/2/3.2 | 5.1/2/3.1 | 3.6/1.4/2.2 | ||||
| Urine free Hb | +ve (present) | |||||||
| Peripheral smear | Micro angiopathic hemolytic anaemia | |||||||
| Coombs test (direct) | Not done | Negative | ||||||
| Coombs test (indirect) | Not done | Negative | ||||||
| G6PD | Not done | Negative | ||||||
| Creatinine | 0.4 | 0.6 | 0.5 | 0.4 | 0.3 | 0.3 | ||
A repeat echocardiography done after 48 h revealed grade 1 + MR with the MR jet hitting the prosthetic ring, probably leading to hemolysis and causing damage by the mechanism of collision (Fig. 3, Fig. 4).2
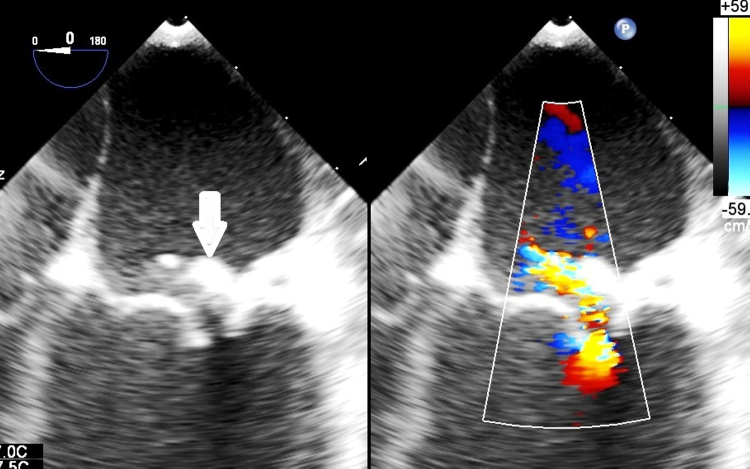
Fig. 3.
The regurgitant jet hitting the ring is seen. The ring is marked with a bold solid arrow.
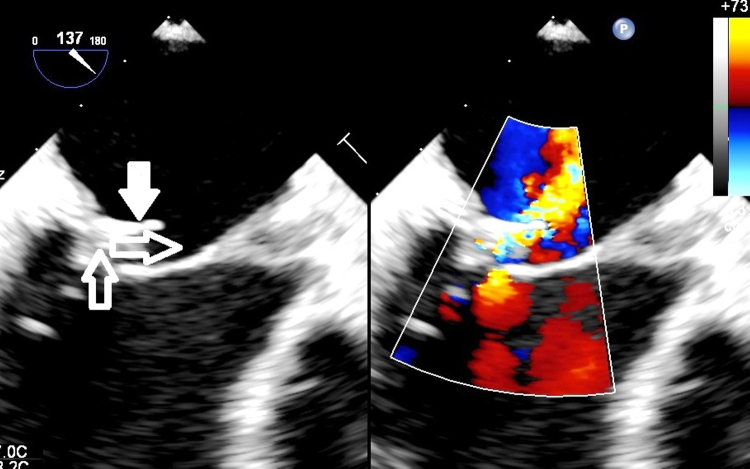
Fig. 4.
The regurgitant jet changing direction after colliding with the protruding ring. The jet direction is marked with hollow arrows and the ring is marked with a solid arrow.
Decreasing the heart rate with B blockers and afterload reduction with ACE inhibitors did not decrease the hemolysis.3
After trying conservative management for a total of 7 days and needing a total of four transfusions of packed cells, it was decided to re-intervene.
The girl underwent a repeat median sternotomy and attempt at valve repair. The textile covering at the edge of the mitral ring was exposed and this was where the regurgitant jet appeared to be hitting. The ring was removed and the PTFE neo-chordae were removed and excised. The valve repair could not achieve a satisfactory result and hence mitral valve was replaced using 25 mm EPIC Stented valve (St Jude Medical Inc, MN, USA), with subvalvar preservation.
The intraoperative TEE revealed normally functioning prosthetic valve. The patient was shifted back to the ICU with minimal inotropes and extubated after 8 h. The hematuria completely subsided and urine was clear after the second surgery. There was no drop in Hb at 48 and 96 h after surgery.
3. Discussion
There are a few reports of severe hemolytic anemia following mitral valve repair.4, 5 However, none of the case reports or any studies indicate such an early presentation of the hemolysis and hematuria, especially in pediatric population. Also, as per Lam's study, the severity of hemolysis following MV repair does not depend on the echocardiographic variables, including the degree of MR.6 So, in spite of a good surgical result of MV repair, the collision of the RBCs with the prosthetic ring caused severe intravascular hemolysis requiring reintervention. The other mechanisms of traumatic hemolysis described by Garcia et al.2 are fragmentation, rapid acceleration, free jet, and slow deceleration.
So lessons learnt after a critical analysis of the outcome of the present patient were:
-
1)
A more critical TEE analysis, to define the regurgitant jet in detail, would have helped to decide about need for reintervention, so as to prevent the jet from hitting the prosthetic ring, which could have averted the outcome.
-
2)
Instead of a rigid prosthetic ring, if a treated autologous pericardial strip had been used, especially in a pediatric case with a growing annulus, causing a partial annuloplasty, we could have avoided this outcome and a valve replacement.
Conflicts of interest
The authors have none to declare.
References
- 1.Abourjaili G., Torbey E., Alsaghir T., Olkovski Y., Costantino T. Hemolytic anemia following mitral valve repair: a case presentation and literature review. Exp Clin Cardiol. 2012;17:248–250. [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia M.J., Vandervoort P., Stewart W.J. Mechanisms of hemolysis with mitral prosthetic regurgitation. Study using transesophageal echocardiography and fluid dynamic simulation. J Am Coll Cardiol. 1996;27:399–406. doi: 10.1016/0735-1097(95)00403-3. [DOI] [PubMed] [Google Scholar]
- 3.Cerfolio R.J., Orszulak* T.A., Daly R.C. Eur J Cardio-thoracic Surg. 1997;11:479–484. doi: 10.1016/s1010-7940(96)01091-3. (appendix A) [DOI] [PubMed] [Google Scholar]
- 4.Mok P., Lieberman E.H., Lilly L.S., Schafer A.I., Di Sesa V.J., Rutherford C.R. Severe haemolytic anaemia following mitral valve repair. Am Heart J. 1989;117:1171–1173. doi: 10.1016/0002-8703(89)90884-3. [DOI] [PubMed] [Google Scholar]
- 5.Wilson J.H., Rath R., Glaser R., Ranke T. Severe haemolysis after incomplete mitral valve repair. Ann Thoracic Surg. 1990;50:136–137. doi: 10.1016/0003-4975(90)90108-i. [DOI] [PubMed] [Google Scholar]
- 6.Lam B.K., Cosgrove D.M., Bhudia S.K., Gillinov A.M. Haemolysis after initial valve repair. Mechanisms and treatment. Ann Thoracic Surg. 2004;77:191–195. doi: 10.1016/s0003-4975(03)01455-3. [DOI] [PubMed] [Google Scholar]