Abstract
We present a patient with asymptomatic apical hypertrophic cardiomyopathy (AHCM) who recently developed cardiac arrhythmias, and shortly discuss the diagnostic modalities, differential diagnosis, and treatment strategy for this condition. AHCM is a rare form of hypertrophic cardiomyopathy, which usually involves the apex of the left ventricle. AHCM can occur with varied presentations such as chest pain, palpitations, dyspnea, syncope, atrial fibrillation, myocardial infarction, embolic events, ventricular fibrillation, and congestive heart failure. The most peculiar electrocardiogram findings are giant T-waves inversion in the precordial leads with left ventricular (LV) hypertrophy. A transthoracic echocardiogram is the initial diagnostic modality in the evaluation of AHCM and shows hypertrophy of the LV apex. Other diagnostic modalities, including left ventriculography, multislice spiral computed tomography, and cardiac magnetic resonance imagings, are also valuable tools. Medications used to manage include verapamil, beta-blockers, and antiarrhythmic agents. An implantable cardioverter defibrillator (ICD) is recommended for high-risk patients.
Keywords: Apical hypertrophic cardiomyopathy, Electrocardiogram, Arrhythmia
1. Introduction
Apical hypertrophic cardiomyopathy (AHCM) is an uncommon type of hypertrophic cardiomyopathy (HCM), which usually involves the left ventricle apex and rarely involves the right ventricular apex or both.1 Initially, it was thought that AHCM is limited only to the Japanese population, but nowadays it is also found in other populations.
2. Case report
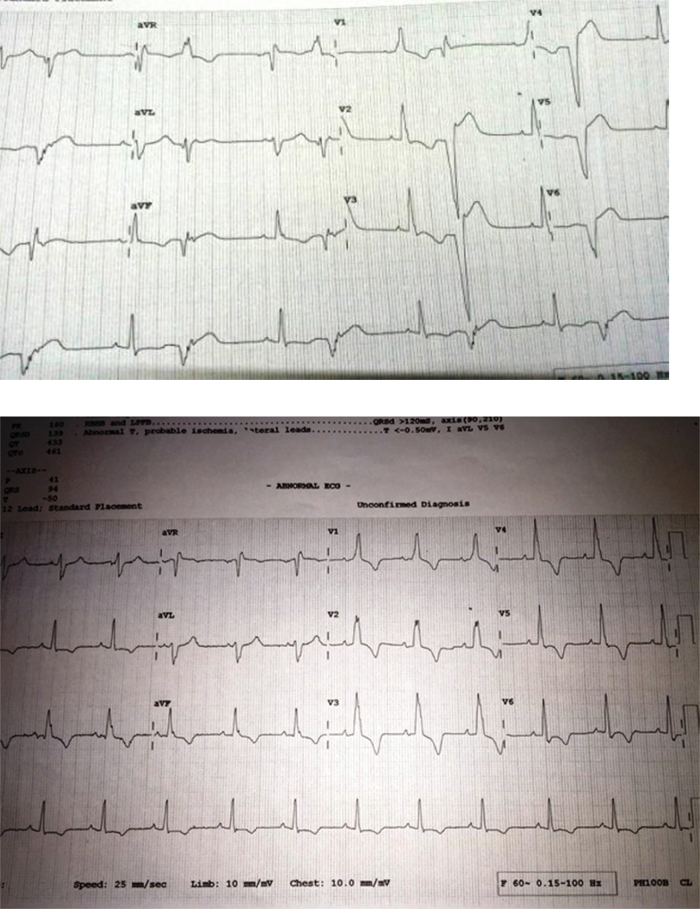
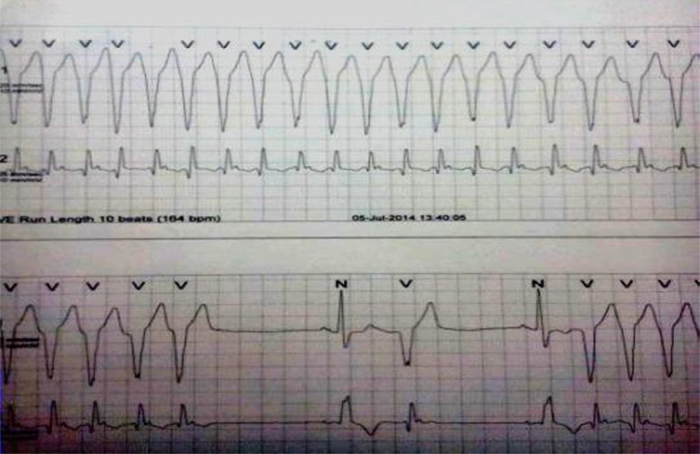
A 77-year-old Indian male with a history of hypertension and diabetes mellitus was asymptomatic before July 2014. He had complaints of two episodes of syncope in the last 1 month. There was no family history of sudden death, congestive heart failure, or cardiomyopathy. On examination, his blood pressure was 118/72 mmHg and heart rate 67 bpm (beats per minute), with no heart murmur or any signs of congestive heart failure. The rest of the examination was normal. A 12-lead electrocardiogram (ECG) showed right bundle branch block (RBBB) with generalized giant T wave inversion with frequent ventricular premature complexes (VPCs)/ventricular bigeminy (Fig. 1). The cardiac enzymes and chest X-ray were normal. Initial transthoracic echocardiogram (TTE) was reported as moderate concentric left ventricular (LV) hypertrophy (Inter ventricular septum thickness 1.39 cm) with Mild Aortic Regurgitation and normal LV systolic function LVEF (Left ventricular ejection fraction) 60%. Holter monitoring was done, which recorded frequent unifocal VPCs with episodes of ventricular runs (Maximum one lasted for 16 s) having all negative QRS concordance morphology (Fig. 2), which were managed with antiarrhythmic medication (Amiadarone).
Fig. 1.
Resting 12 lead electrocardiogram showing RBBB with generalized T wave inversion and ventricular bigeminy.
Fig. 2.
Holter strip showing ventricular run.
He was then referred to higher institute for electrophysiological study and Radio frequency ablation, where he had recurrent hemodynamically unstable VT (Ventricular tachycardia) with intermittent bradycardia (Heart rate 37 bpm) with blood pressure of 100/70 mmHg. He underwent Temporary pacemaker implantation in view of recurrent VT (Ventricular tachycardia) and underlying bradycardia. Thereafter, he was managed with antiarrhythmic (amiadarone infusion).
In view of underlying cardiac disease with recurrent VT (Ventricular tachycardia), an infiltrative disorder was suspected and CECT (Contrast enhanced Computerized tomography) chest was done, which did not reveal any significant findings.
He underwent Coronary angiography, which revealed single vessel disease (Non dominant Right Coronary Artery 100% mid occlusion) that did not explain the arrhythmia and syncopal episodes. Left ventriculography was done as a routine, which suggested gross apical hypertrophy.
Then again, meticulous transthoracic echocardiogram (TTE) was done which showed Apical hypertrophy of left ventricle; lateral wall hypertrophy more than apical septum (Apical IVS thickness 1.33 cm, Apex thickness 1.24 cm, Apical lateral wall thickness 1.98 cm, posterior wall thickness 1.18 cm) (Fig. 3 Classical “ace of spades sign”) typically of AHCM with thickened sclerotic Aortic valve, Mild AR, Normal LV systolic function; LVEF 60%. Lateral wall thickening was smooth, homogenous, and non-trabeculated (to differentiate with possibility of Non-compacted LV cardiomyopathy) and no wall motion abnormality was detected (to differentiate with possibility of LV clot). He was shifted for cardiac magnetic resonance imaging (MRI) but it could not be done due to hemodynamical instability and poor co-operation of patient.
Fig. 3.
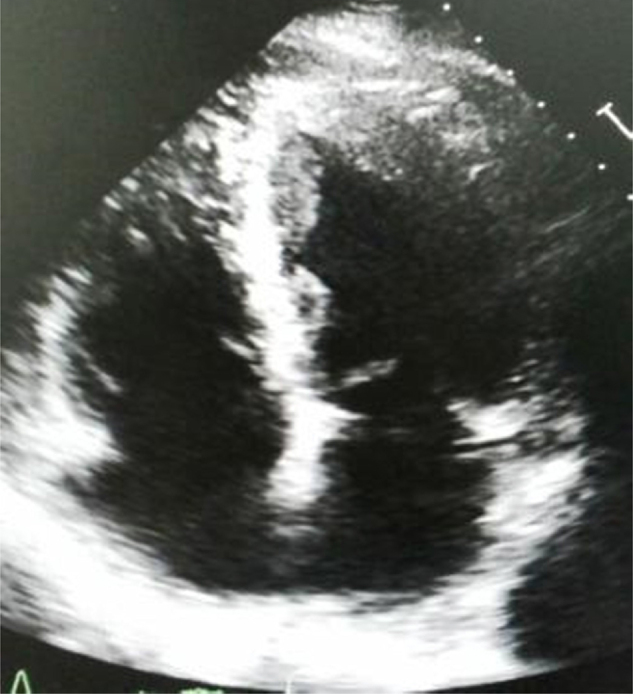
Transthoracic echocardiogram showed apical hypertrophy of left ventricle; lateral wall hypertrophy more than apical septum; Classical “ace of spades sign”
He then underwent dual chamber implantable cardioverter defibrillator (ICD) implantation for prevention of fatal ventricular arrhythmias. Thereafter, the patient is clinically and hemodynamically stable. On follow-up visits, his family was screened for familial association, which was negative.
3. Discussion
AHCM has been reported in few families with autosomal dominant inheritance.3 A mutation in sarcoma gene (E101K mutation in the alpha-cardiac actin gene) has been found in such families. AHCM is not common in compare of patients with asymmetric septal hypertrophy. Of all the HCM patients in Japan, the prevalence of AHCM was 15%, whereas in USA, the prevalence was only 3%.2 AHCM has been divided into three types as per morphology: pure focal, pure diffuse, and mixed, all of which pure focal is most common type.4 It can be divided into two groups based on whether they had isolated asymmetric apical hypertrophy (pure AHCM) or with co-existent hypertrophy of the interventricular septum (mixed AHCM).5 The diagnostic parameters for AHCM included presence of asymmetric LV hypertrophy, confined mainly to the LV apex, with an apical wall thickness ≥15 mm and a ratio of maximal apical to posterior wall thickness ≥1.5 mm, on the basis of trans thoracic echocardiogram or MRI.5
About 54% of patients with AHCM are symptomatic and the most common symptoms that patients present with are chest pain, followed by palpitations, dyspnea, and syncope.5 AHCM may also manifest as myocardial infarction, embolic events, atrial fibrillation, ventricular fibrillation, and congestive heart failure.5 Physical signs of an audible/palpable fourth heart sound (S4) and a new onset murmur are common.5 Our patient was with no family history and had no physical findings.
The most common ECG findings are negative T-waves in the precordial leads, which were seen in 93% of patients, followed by LV hypertrophy in 65% of patients.5 The ECG in our patient showed RBBB with generalized T wave inversion (>7 mm deep) and ventricular bigeminy. A contrast echocardiogram is useful in establishing the diagnosis when the baseline images are suboptimal.6 Multislice spiral computed tomography can also be useful to diagnose AHCM; apart from diagnosis it provides other information like cardiac anatomy, function, and coronary arteries.7 When diagnosing the patients with inconclusive echocardiography is the problem, then Cardiac MRI is also a valuable tool.8 Although transthoracic echocardiography is the most common initial diagnostic test for AHCM, still cardiac MRI is the best diagnostic tool to diagnose AHCM.
AHCM may simulate other conditions, including apical cardiac tumors,9 LV apical thrombus,10 isolated ventricular non-compaction,11 endomyocardial fibrosis (EMF),12 and coronary artery disease.13 Patients with AHCM having chest pain can be mistaken for ischemia from coronary artery disease.13 Mostly these patients undergo a nuclear scan or coronary angiography for abnormal ECG.15 Most of the patients with AHCM with myocardial infarction have an apical infarct and in these patients echocardiography imaging showed that wall motion abnormalities vary from apical aneurysm to apical hyopokinesis.5 Some patients may have asymptomatic apical infarction.5
Cardiac MRI differentiates Isolated ventricular non-compaction from AHCM by high resolution images.11 Contrast echocardiogram can be used to differentiate AHCM from a LV apical mass (thrombus or tumor).10 Verapamil, beta-blockers, and antiarrhythmic agents are used in symptomatic patients with AHCM.5 Verapamil and beta-blockers are proved to be beneficial in ameliorates the symptoms in AHCM patients.14, 16 Amiodarone is used in the treatment of atrial fibrillation and ventricular arrhythmias.17 ICD has been used in AHCM patients with cardiac arrest or frequent non-sustained ventricular tachycardia on holter monitoring.18
The prognosis of AHCM is usually benign. AHCM patients had overall mortality rate of 10.5% and mortality due to cardiovascular disease was 1.9% after a follow-up of 13.6 ± 8.3 years.5 Patients with asymmetric septal hypertrophy have more common sudden death and cardiovascular events than in those with AHCM.19
Conflicts of interest
The authors have none to declare.
References
- 1.Albanesi Filho F.M., Castier M.B., Lopes A.S., Ginefra P. Is the apical hypertrophic cardiomyopathy seen in one population in Rio de Janeiro city similar to that found in the East? Arq Bras Cardiol. 1997;69:117–123. [PubMed: 9567334] [PubMed] [Google Scholar]
- 2.Kitaoka H., Doi Y., Casey S.A., Hitomi N., Furuno T., Maron B.J. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol. 2003;92:1183–1186. doi: 10.1016/j.amjcard.2003.07.027. [PubMed: 14609593] [DOI] [PubMed] [Google Scholar]
- 3.Arad M., Penas-Lado M., Monserrat L. Gene mutations in apical hypertrophic cardiomyopathy. Circulation. 2005;112:2805–2811. doi: 10.1161/CIRCULATIONAHA.105.547448. [PubMed: 16267253] [DOI] [PubMed] [Google Scholar]
- 4.Choi E.Y., Rim S.J., Ha J.W. Phenotypic spectrum and clinical characteristics of apical hypertrophic cardiomyopathy: multicenter echo-Doppler study. Cardiology. 2008;110:53–61. doi: 10.1159/000109407. [PubMed: 17934270] [DOI] [PubMed] [Google Scholar]
- 5.Eriksson M.J., Sonnenberg B., Woo A. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:638–645. doi: 10.1016/s0735-1097(01)01778-8. [PubMed: 11849863] [DOI] [PubMed] [Google Scholar]
- 6.Patel J., Michaels J., Mieres J., Kort S., Mangion J.R. Echocardiographic diagnosis of apical hypertrophic cardiomyopathy with optison contrast. Echocardiography. 2002;19:521–524. doi: 10.1046/j.1540-8175.2002.00521.x. [PubMed: 12356350] [DOI] [PubMed] [Google Scholar]
- 7.Zhou X.H., Peng Z.P., Peng Q. Clinical application of 64-slice spiral CT for apical hypertrophic cardiomyopathy. Chin J Radiol. 2008;42:911–915. [Google Scholar]
- 8.Alpendurada F., Prasad S.K. The missing spade: apical hypertrophic cardiomyopathy investigation. Int J Cardiovasc Imaging. 2008;24:687–689. doi: 10.1007/s10554-008-9335-z. [PubMed: 18595001] [DOI] [PubMed] [Google Scholar]
- 9.Veinot J.P., O’Murchu B., Tazelaar H.D., Orszulak T.A., Seward J.B. Cardiac fibroma mimicking apical hypertrophic cardiomyopathy: a case report and differential diagnosis. J Am Soc Echocardiogr. 2008;9:94–99. doi: 10.1016/s0894-7317(96)90110-8. [PubMed: 8679244] [DOI] [PubMed] [Google Scholar]
- 10.Thanigaraj S., Pérez J.E. Apical hypertrophic cardiomyopathy: echocardiographic diagnosis with the use of intravenous contrast image enhancement. J Am Soc Echocardiogr. 2000;13:146–149. doi: 10.1016/s0894-7317(00)90026-9. [PubMed: 10668018] [DOI] [PubMed] [Google Scholar]
- 11.Spirito P., Autore C. Apical hypertrophic cardiomyopathy or left ventricular non-compaction? A difficult differential diagnosis. Eur Heart J. 2007;28:1923–1924. doi: 10.1093/eurheartj/ehm266. [PubMed: 17623677] [DOI] [PubMed] [Google Scholar]
- 12.Hassan W.M., Fawzy M.E., Al Helaly S., Hegazy H., Malik S. Pitfalls in diagnosis and clinical, echocardiographic, and hemodynamic findings in endomyocardial fibrosis: a 25-year experience. Chest. 2005;128:3985–3992. doi: 10.1378/chest.128.6.3985. [PubMed: 16354870] [DOI] [PubMed] [Google Scholar]
- 13.Duygu H., Zoghi M., Nalbantgil S. Apical hypertrophic cardiomyopathy might lead to misdiagnosis of ischaemic heart disease. Int J Cardiovasc Imaging. 2008;24:675–681. doi: 10.1007/s10554-008-9311-7. [PubMed: 18373279] [DOI] [PubMed] [Google Scholar]
- 14.Chen S.C., Wang K.T., Hou C.J.Y., Chou Y.S., Tsai C.H. Apical hypertrophic cardiomyopathy with severe myocardial bridging in a syncopal patient. Acta Cardiol Sin. 2003;19:179–184. [Google Scholar]
- 15.Cianciulli T.F., Saccheri M.C., Masoli O.H. Myocardial perfusion SPECT in the diagnosis of apical hypertrophic cardiomyopathy. J Nucl Cardiol. 2009;16:391–395. doi: 10.1007/s12350-008-9045-x. [PubMed: 19130165] [DOI] [PubMed] [Google Scholar]
- 16.Ridjab D., Koch M., Zabel M., Schultheiss H.P., Morguet A.J. Cardiac arrest and ventricular tachycardia in Japanese-type apical hypertrophic cardiomyopathy. Cardiology. 2007;107:81–86. doi: 10.1159/000094147. [PubMed: 16804296] [DOI] [PubMed] [Google Scholar]
- 17.Okishige K., Sasano T., Yano K., Azegami K., Suzuki K., Itoh K. Serious arrhythmias in patients with apical hypertrophic cardiomyopathy. Intern Med. 2001;40:396–402. doi: 10.2169/internalmedicine.40.396. [PubMed: 11393409] [DOI] [PubMed] [Google Scholar]
- 18.Maron B.J. Risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Cardiol Rev. 2002;10:173–181. doi: 10.1097/00045415-200205000-00006. [PubMed: 12047795] [DOI] [PubMed] [Google Scholar]
- 19.Yang H.S., Song J.K., Song J.M. Comparison of the clinical features of apical hypertrophic cardiomyopathy versus asymmetric septal hypertrophy in Korea. Korean J Intern Med. 2005;20:111–115. doi: 10.3904/kjim.2005.20.2.111. [PMC free article: 3891378] [PubMed: 16134764] [DOI] [PMC free article] [PubMed] [Google Scholar]