Abstract
Background. Treatment of chemotherapy-induced peripheral neuropathy (CIPN) remains a big challenge for oncologists. The aim of this study is to evaluate the effects of Radix Astragali- (RA-) based Chinese herbal medicine in the prevention and treatment of oxaliplatin-induced peripheral neuropathy, including the incidence and grading of neurotoxicity, effective percentage, and nerve conduction velocity. Methods. All randomized controlled trials (RCTs) were found using PubMed, Cochrane, Springer, China National Knowledge Infrastructure (CNKI), and Wanfang Database of China Science Periodical Database (CSPD) by keyword search. Meta-analysis was conducted using RevMan 5.0. Results. A total of 1552 participants were included in 24 trials. Meta-analysis showed the incidence of all-grade neurotoxicity was significantly lower in experimental groups and high-grade neurotoxicity was also significantly less. Effective percentage was significantly higher and sensory nerve conduction velocity was improved significantly, but changes in motor nerve conduction velocity were not statistically significant. No adverse events associated with RA-based intervention were reported. Conclusion. RA-based intervention may be beneficial in relieving oxaliplatin-induced peripheral neuropathy. However, more double-blind, multicenter, large-scale RCTs are needed to support this theory. Trial Registration. PROSPERO International prospective register of systematic reviews has registration number CRD42015019903.
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) results from toxic effects of chemotherapy drugs predominantly affecting the peripheral nervous system. The associated pain of CIPN can be extremely disabling, with a marked impact on quality of life (Qol), functions of daily living, and increases the risks of noncompliance with cancer treatment [1]. Oxaliplatin (OXAL), a third-generation platinum-based compound, has become pivotal for the therapy of metastatic colorectal cancer and other malignancies including lung, breast, and ovarian cancers [2, 3]. However, OXAL induced chronic neurotoxicity occurs in 63.6% or more of patients, which limited the dosing of OXAL [4].
Radix Astragali (the root of Astragalus mongholicus Bge. or Astragalus membranaceus Bge.) has been used as one of the primary tonic herbs in traditional Chinese and Japanese Kampo medicine. Recently, Radix Astragali (Huangqi, in Chinese) is being widely used, orally or topically, and alone or in combination with western conventional medicine to relieve CIPN. Multiple randomized clinical trials have suggested that Radix Astragali- (RA-) based intervention can reduce symptoms, improve Qol and immunologic function, increase plasma nerve growth factor (NGF) levels, and delay the progression of CIPN [5–28]. In vivo RA-based prescription (Huangqi Guizhi Wuwu Decoction) can effectively relieve pain and improve sciatic nerve conduction velocity and function in rats with CIPN [29, 30]. Its mechanism may be related to downregulating NR2B expression in L4–6 lumbar spinal segments and upregulating pNF-H protein levels in dorsal root ganglia [30]. However, no systematic review to date has reported effects of RA-based intervention on OXAL induced peripheral neuropathy. In this meta-analysis, the effectiveness and safety of RA-based intervention for preventing and treating OXAL induced peripheral neuropathy are evaluated for the first time.
2. Methods
Ethics data for this study were acquired through previously published work; no patient or hospital data were accessed. Therefore, written consent and institutional ethical review were not required for this research.
2.1. Database and Search Strategies
The electronic databases of MEDLINE (1982–2015), Cochrane Controlled Trials (2015, Issue 12), Springer (1997–2015), China National Knowledge Infrastructure (CNKI) database (1997–2015), and Wanfang Database of China Science Periodical Database (CSPD) (1998–2015) were searched by using keywords of “Neurotoxicity”, “Oxaliplatin” “Astragali”, or “Huangqi”, without language limitation. Reference lists from trials selected by electronic searching were hand searched. All of those searches ended before January 2016.
2.2. Inclusion Criteria
All randomized controlled trials (RCTs) investigating the effects of RA-based Chinese herbal medicine for preventing and treating OXAL induced peripheral neuropathy will be eligible for inclusion.
2.2.1. Types of Participants
All adult patients (18 years and older, no upper age limit) with a treatment of OXAL will be considered for this review. The participants had to conform to the following diagnostic criteria.
The patient was clearly diagnosed malignant by pathology or cytology.
The patient was treated by OXAL, FOLFOX (OXAL + 5-fluorouracil + calcium folinate), or XELOX (OXAL + capecitabine).
Age, gender, stages, and pathological types between the groups were balanced and comparable.
2.2.2. Types of Interventions
RA-based interventions included single herb (including extracts from RA) and a compound of several herbs, irrespective of dosage form (e.g., oral decoction or lotion). The mode of delivery (e.g., oral, topical administration or intravenous) was not restricted. Relative high dose RA (monarch drug) should be included in the prescription and regimen of herbs was not restricted.
The control interventions were placebo, no intervention, or conventional treatment such as mecobalamin, Ca/Mg infusions, or reduced glutathione. We also included trials of RA-based prescription plus conventional medicine versus the same conventional medicine alone.
2.3. Types of Outcome Measures
Grading of CIPN. Primary outcome was the grading of CIPN in at least 1 chemotherapy cycle, but preferably in 4 cycles of chemotherapy. We considered Levi's grade [31], World Health Organization (WHO) grade [32] or National Cancer Institute common terminology criteria for adverse events (NCI-CTCAE) for the clinical grading of CIPN [33] (Table 1).
Table 1.
Grading scales used to evaluate oxaliplatin-induced peripheral neuropathy.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Levi et al. [31] | Paresthesia or insensitive, complete relief in 1 week | Paresthesia or insensitive, complete relief in 14 days | Paresthesia or insensitive, complete relief in 21 days | Paresthesia or insensitive, combined with functional abnormality |
|
| ||||
| Miller et al. [32] | Paresthesias and/or decreased tendon reflex | Severe paresthesia and/or mild anergia | Intolerable paresthesia and/or marked motor loss | Paralysis |
|
| ||||
| CTCAE 4.03 [33] | Asymptomatic; loss of deep tendon reflexes or paresthesia | Moderate symptoms; limiting instrumental ADL | Severe symptoms; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated |
Clinical Effectiveness. Clinical effectiveness was assessed according to what is previously described [32, 34].
Complete Remission (CR). The patients felt completely free from all symptoms, with the grading of CIPN reduced to grade 0.
Partial Remission (PR). Symptoms abated obviously, and the grading of CIPN reduced ≥1 grade.
Nonperceptible (NP). Compared with before treatment symptoms have not abated, and the grading of CIPN did not reduce.
| (1) |
Nerve Conduction Velocity. Changes in values of sensory nerve conduction velocity (SNCV) or motor sensory nerve conduction velocity (MNCV) were measured by validated methods after 1 week of RA treatment or more.
Quality of Life (Qol) and Adverse Events. We extracted Qol, measured as Karnofsky (KPS) scale or Eastern Cooperative Oncology Group (ECOG) scale. Adverse events were also extracted.
2.4. Exclusion Criteria
We excluded studies with unclear diagnostic criteria and without the use of RA. Combinations of herbs and other forms of treatment (e.g., acupuncture or moxibustion) were excluded.
2.5. Data Extraction and Quality Assessment
Data were entered into an electronic database by two authors (Bo Deng and Liqun Jia) independently. Where differences in opinion existed, they were resolved by a third party. Improved Jadad scale was used to assess the quality of RCTs, including randomization, blinding of participants, personnel, and outcome assessors, incomplete outcome data, and other threats to validity [35]. High quality is 4–7 points. Low quality is 1–3 points.
2.6. Data Synthesis
Review Manager (RevMan) 5.0 software, provided by the Cochrane Collaboration (UK), was used to analyze the results of the trials. Dichotomous data were expressed as odds ratio (OR). Continuous data were expressed as mean difference (MD). Heterogeneity between results of different trials was tested, and heterogeneity was presented as significant when I 2 is over 50% or P < 0.1. Random effect model was used for the meta-analysis if there was significant heterogeneity and fixed effect model was used when the heterogeneity was not significant [35]. Publication bias was explored via a funnel plot analysis.
3. Results
3.1. Description of Studies and Methodological Quality (Figure 1 and Table 2)
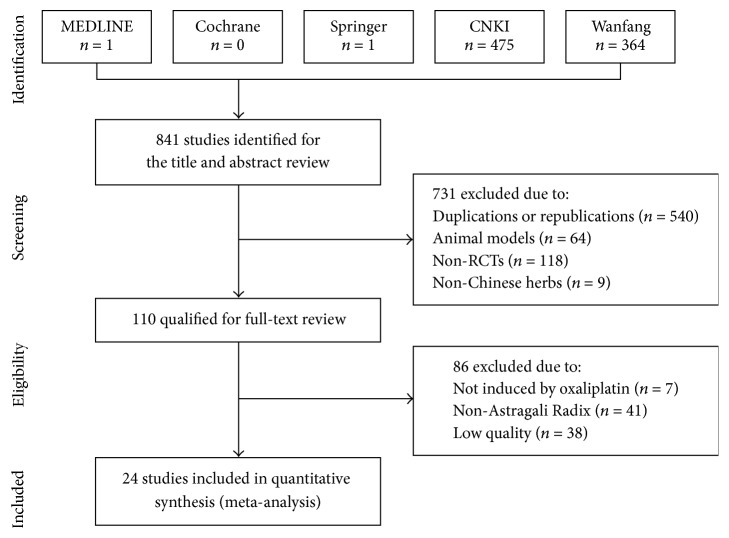
Figure 1.
Flow chart of included studies in this systematic review.
Table 2.
Characteristics of studies included in this systematic review.
| Author | Year | Sample size |
Mean age (year) (median/range) |
% men | Chemotherapy | Radix Astragali intervention | Control |
|---|---|---|---|---|---|---|---|
| Cui et al. [5] | 2009 | 40 | 60 | 57.5 | FOLFOX | Single herb extract | No intervention |
| Feng [6] | 2011 | 40 | 28~55 | 67.5 | FOLFOX | Compound prescription | No intervention |
| Huang et al. [7] | 2013 | 61 | 62.3 | 62.3 | FOLFOX | Compound prescription | No intervention |
| Huang et al. [8] | 2010 | 60 | 46 | 71.7 | FOLFOX | Compound prescription | No intervention |
| Jin et al. [9] | 2015 | 77 | 47.2 | 48.1 | FOLFOX | Compound prescription | No intervention |
| Kong [10] | 2012 | 40 | 40~60 | 52.5 | OXAL | Compound prescription | No intervention |
| Li et al. [11] | 2013 | 60 | 50.1 | 31.7 | FOLFOX | Compound prescription | GSH |
| Liang et al. [12] | 2012 | 84 | 32~73 | 70.2 | FOLFOX | Compound prescription | No intervention |
| Liang et al. [13] | 2015 | 135 | 47.8 | 67.4 | FOLFOX | Compound prescription | No intervention |
| Lin and Luo [14] | 2011 | 90 | 51 | 54.4 | FOLFOX | Compound prescription | No intervention |
| Liu et al. [15] | 2011 | 60 | 61.5 | 64.3 | FOLFOX, OXAL | Compound prescription | Mecobalamin |
| Liu [16] | 2009 | 68 | 31~70 | 60.3 | FOLFOX | Compound prescription | No intervention |
| Lv [17] | 2015 | 90 | 53.0 | 53.3 | OXAL | Compound prescription | Mecobalamin |
| Qin and Sun [18] | 2015 | 42 | 55 | 61.9 | FOLFOX | Compound prescription | Mecobalamin |
| Qin et al. [19] | 2012 | 68 | 57.2 | 47.1 | OXAL, TAX | Compound prescription | Cobamamide |
| Shen et al. [20] | 2015 | 60 | 59.7 | 65.0 | OXAL | Compound prescription | Mecobalamin |
| Sun et al. [21] | 2010 | 60 | 55.3 | 63.3 | FOLFOX, XELOX, TAX + 5-Fu | Compound prescription | Mecobalamin |
| Tan and Qi [22] | 2015 | 63 | 31~70 | 60.3 | OXAL | Compound prescription | Mecobalamin |
| Wang [23] | 2015 | 60 | 52.3 | 50.0 | FOLFOX | Compound prescription | Ca/Mg infusions |
| Wu et al. [24] | 2015 | 89 | 49.2 | 70.8 | Platinum, TAX, | Compound prescription | No intervention |
| Wu et al. [25] | 2012 | 60 | 23~71 | 65.8 | FOLFOX | Compound prescription | No intervention |
| Wu et al. [26] | 2015 | 60 | 59.7 | 65.6 | Platinum, TAX, VCR | Compound prescription | No intervention |
| Xu et al. [27] | 2011 | 40 | ∗ | 45.0 | OXAL | Compound prescription | No intervention |
| Zhang and Lu [28] | 2013 | 70 | 31–77 | 65.7 | FOLFOX | Single herb extract + thioctic acid |
No intervention |
FOLFOX: oxaliplatin + 5-fluorouracil + calcium folinate.
5-Fu: 5-fluorouracil.
GSH: glutathione.
OXAL: oxaliplatin.
TAX: taxol.
VCR: vincristine.
XELOX: oxaliplatin + capecitabine.
Our primary searches identified 841 references from the above databases. After duplicates, animal studies, case reports, reviews, and obvious ineligibility were removed, we retrieved a total of 110 references for further assessment. After full-text reviews, 24 trials were included [5–28]. Included trials were published from 2009 to 2015, with the years 2011 to 2015 having a larger number of trials (20 trials, 85.70% patients) than other years. All trials were conducted in mainland China. Since all included trials were assessed to be of high quality (improved Jadad score of 4 or 5 points), the risk of bias in this systematic review was low. All 24 trials employed computer software or random number tables for randomization. Nine trials used conventional medicine as control, and only one trial performed double-blinding.
3.2. Participants
In total, 1552 participants with OXAL treatment were included in these 24 trials. The average size of the trials was 66 participants, ranging from 40 to 135 per trial. Eleven trials enrolled only inpatients (n = 689 patients, 44.39%). The remaining 13 trials did not specify the setting (n = 863 patients, 55.61%). All trials included both adult male and female patients, with 58.63% participants being male. Types of cancer in participants included colorectal cancer (n = 1033 patients), gastric cancer (n = 399 patients), and lung cancer/breast cancer/other cancers (n = 52 patients). The cancers of 68 patients were not specified. Accumulated OXAL dose varied from 130 mg/m2 to 800 mg/m2, with 260–600 mg/m2 (11 trials) being the most common. Eighteen trials used Levi's grading of CIPN, 3 used CTCAE criteria of CIPN, and 3 used WHO criteria of CIPN.
3.3. Intervention Comparisons (Tables 3 and 4)
Table 3.
Characteristics of Radix Astragali-based interventions.
| Author | Year | Radix Astragali prescription | Administration | Course of treatment (d) | Dose (g) | Jadad score | CIPN grade | Curative effect | NCV | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cui et al. [5] | 2009 | Huangqi Injection | i.v. | 7 | 30 mL | 4 | + | 5 | ||
| Feng [6] | 2011 | Buyang Huanwu Decoction | ad us. ext | 5 ∗ 6 chemotherapeutic cycles | 120 | 4 | + | 6 | ||
| Huang et al. [7] | 2013 | Huangqi Guizhi Wuwu Decoction | p.o. | 5 ∗ 4 chemotherapeutic cycles | 30 | 4 | + | 7 | ||
| Huang et al. [8] | 2010 | Huangqi Guizhi Wuwu Decoction | ad us. ext | 5 ∗ 4 chemotherapeutic cycles | 100 | 4 | + | + | 8 | |
| Jin et al. [9] | 2015 | Self-made prescription | ad us. ext | 10 ∗ 4 chemotherapeutic cycles | 15 | 4 | + | 9 | ||
| Kong [10] | 2012 | Self-made prescription | ad us. ext | 6 ∗ 3 chemotherapeutic cycles | 30 | 4 | + | 10 | ||
| Li et al. [11] | 2013 | Self-made prescription | p.o. | 5 ∗ 6 chemotherapeutic cycles | 30 | 4 | + | 11 | ||
| Liang et al. [12] | 2012 | Buyang Huanwu Decoction | p.o. | 28 | 40 | 4 | + | 12 | ||
| Liang et al. [13] | 2015 | Buyang Huanwu Decoction | p.o | 28 | 30 | 4 | + | 13 | ||
| Lin and Luo [14] | 2011 | Huangqi Guizhi Wuwu Decoction | ad us. ext | 84 | ≥15 | 4 | + | 14 | ||
| Liu et al. [15] | 2011 | Huangqi Guizhi Wuwu Decoction | p.o. | 42 | 30 | 4 | + | + | 15 | |
| Liu [16] | 2009 | Self-made prescription | ad us. ext | 14 | ≥15 | 4 | + | 16 | ||
| Lv [17] | 2015 | Self-made prescription | ad us. ext | 7 | 50 | 5 | + | + | 17 | |
| Qin and Sun [18] | 2015 | Self-made prescription | p.o.+ ad us. ext | 14 ∗ 5 chemotherapeutic cycles | 30 | 4 | + | 18 | ||
| Qin et al. [19] | 2012 | Self-made prescription | ad us. ext | 14 | 20 | 4 | + | 19 | ||
| Shen et al. [20] | 2015 | Huangqi Guizhi Wuwu Decoction | ad us. ext | 14 | 50 | 4 | + | 20 | ||
| Sun et al. [21] | 2010 | Buyang Huanwu Decoction | ad us. ext | 14 | 180 | 4 | + | + | 21 | |
| Tan and Qi [22] | 2015 | Self-made prescription | p.o. | 10 ∗ 2 chemotherapeutic cycles | 45 | 4 | + | 22 | ||
| Wang [23] | 2015 | Huangqi Guizhi Wuwu Decoction | ad us. ext | 3 ∗ 8 chemotherapeutic cycles | 45 | 4 | + | 23 | ||
| Wu et al. [24] | 2015 | Huangqi Guizhi Wuwu Decoction | p.o. | 5 ∗ 4 chemotherapeutic cycles | 30 | 4 | + | 23 | ||
| Wu et al. [25] | 2012 | Buyang Huanwu Decoction | p.o. | 112 | 60 | 4 | + | + | 25 | |
| Wu et al. [26] | 2015 | Self-made prescription | p.o. | 7 ∗ 2 chemotherapeutic cycles | ≥15 | 4 | + | 26 | ||
| Xu et al. [27] | 2011 | Self-made prescription | ad us. ext | 5 ∗ 2 chemotherapeutic cycles | 30 | 4 | + | 27 | ||
| Zhang and Lu [28] | 2013 | Huang qijing oral liquid | p.o. | 5 ∗ 8 chemotherapeutic cycles | 20 | 4 | + | + | 28 |
Table 4.
Chinese herbs combination in Radix Astragali-based prescriptions.
| Latin name | English name | Chinese name | Counts | Frequency (%) |
|---|---|---|---|---|
| Angelica sinensis (Oliv.) Diels | Radix Angelicae sinensis | Danggui | 19 | 86.36 |
| Cinnamomum cassia Presl | Ramulus Cinnamomi | Guizhi | 17 | 77.27 |
| Paeonia lactiflora Pall. | Radix Paeoniae Alba | Baishao | 16 | 72.73 |
| Spatholobus suberectus Dunn | Caulis spatholobi | Jixueteng | 13 | 59.09 |
| Ligusticum chuanxiong Hort. | Rhizoma Chuanxiong | Chuanxiong | 12 | 54.55 |
| Carthamus tinctorius L. | Flos Carthami | Honghua | 10 | 50.00 |
| Prunus persica (L.) Batsch | Semen persicae | Taoren | 9 | 40.91 |
| Ziziphus jujuba Mill. | Fructus Jujubae | Dazao | 8 | 36.36 |
| Salvia miltiorrhiza Bge. | Radix Salviae Miltiorrhizae | Danshen | 7 | 31.82 |
| Zingiber officinale Rosc. | Rhizoma Zingiberis (recens) | Jiang | 7 | 31.82 |
| Pheretima aspergillum (E. Perrier) | Pheretima | Dilong | 7 | 31.82 |
Sixteen trials (n = 1060 patients) compared RA-based intervention with no intervention. Three trials (n = 159 patients) tested RA-based prescriptions against mecobalamin. Another 5 trials (n = 333 patients) tested RA-based prescriptions in combination treatment remedies compared to the same western medications for CIPN management. Three types of administration methods were employed in these 24 trials, including oral administration (10 trials), topical administration (12 trials), and intravenous drip (1 trial). One trial employed oral administration combined with topical administration. The most popular prescriptions were modified Huangqi Guizhi Wuwu Decoction (7 trials) and modified Buyang Huanwu Decoction (5 trials). Prescriptions composed by the investigators themselves were combined and modified from these 2 prescriptions (10 trials). More than 50% of RA-based prescriptions included Danggui, Guizhi, Baishao, Jixueteng, Chuanxiong, and Honghua. These herbs may augment the effects of RA intervention on CIPN. Doses of RA ranged from 15 g to 180 g but most fell in the range of 30 to 50 g (12 trials). The duration of treatment varied mostly from 2 weeks to 8 chemotherapy cycles. Regarding topical administration, the temperature of decoction ranged from 35°C to 42°C, but most were in the range of 38–42°C (6 trials).
3.4. Effects of Interventions
3.4.1. Incidence of All-Grade CIPN (Figure 2)
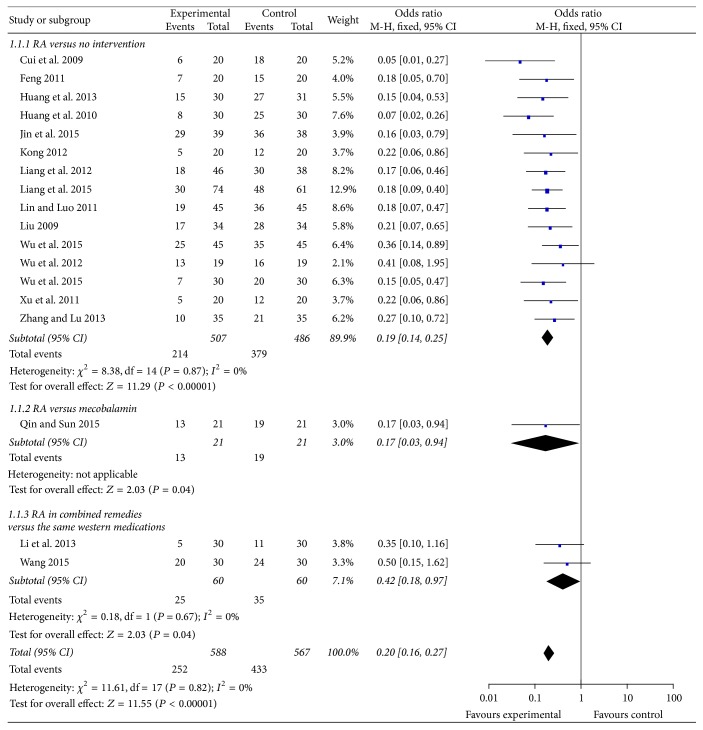
Figure 2.
Forest plot of comparison: incidence of all-grade oxaliplatin-induced peripheral neuropathy.
Eighteen trials reported incidence of all-grade (grades 1–4) CIPN. Five trials included CIPN patients and reported curative effects of RA-based prescriptions. And 1 trial only reported incidence of high-grade CIPN. Fifteen trials compared RA-based intervention to no intervention. RA-based intervention significantly reduced CIPN occurrence (n = 993 patients; OR, 0.19, 95% CI, 0.14 to 0.25, P < 0.01). One trial compared RA-based prescription to mecobalamin. RA-based prescription significantly reduced CIPN occurrence (n = 42 patients; OR, 0.17, 95% CI, 0.03 to 0.94, P < 0.05). Two trials compared RA-based prescriptions plus reduced glutathione or Ca/Mg infusions with the same conventional medications. RA-based prescriptions in combined remedies significantly reduced CIPN occurrence (n = 120 patients; OR, 0.42, 95% CI, 0.18 to 0.97, P < 0.05).
3.4.2. Incidence of High-Grade CIPN (Figure 3)
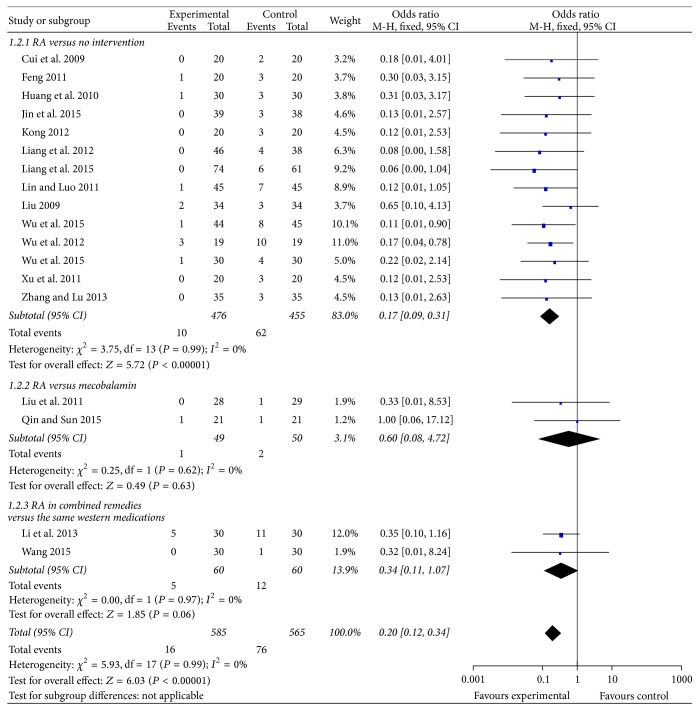
Figure 3.
Forest plot of comparison: incidence of high-grade oxaliplatin-induced peripheral neuropathy.
Nineteen trials reported incidence of high-grade (grades 3-4) CIPN. No patients develop high-grade CIPN in 1 trial. Therefore 18 trials were included in a forest plot. Fourteen trials compared RA-based intervention to no intervention, mostly by using Levi's grading (11 trials). RA-based intervention significantly reduced high-grade CIPN (n = 931 patients; OR, 0.17, 95% CI, 0.09 to 0.31, P < 0.01). However, 2 trials compared modified RA-based prescriptions to mecobalamin, and 2 trials compared RA-based prescriptions plus reduced glutathione or Ca/Mg infusions with the same conventional medications. In these trials, there was no statistical difference between groups.
3.4.3. Curative Effect of RA-Based Prescriptions (Figure 4)
Figure 4.
Forest plot of comparison: curative effects of Radix Astragali-based prescriptions on oxaliplatin-induced peripheral neuropathy.
Five trials included 341 patients that had already developed CIPN and reported curative effects of RA-based prescriptions. The total effective rate of RA-based prescriptions was 79.07%, compared with 54.44% in the control group. Three trials compared curative effects of RA-based prescriptions plus mecobalamin to mecobalamin alone, where RA-based prescriptions were significantly more effective in relieving CIPN (n = 213 patients; OR, 4.84, 95% CI, 2.38 to 9.83, P < 0.01). However, 1 trial compared RA-based prescription to mecobalamin, and 1 trial compared RA-based prescription to no treatment. In these trials, there was no statistical difference between groups.
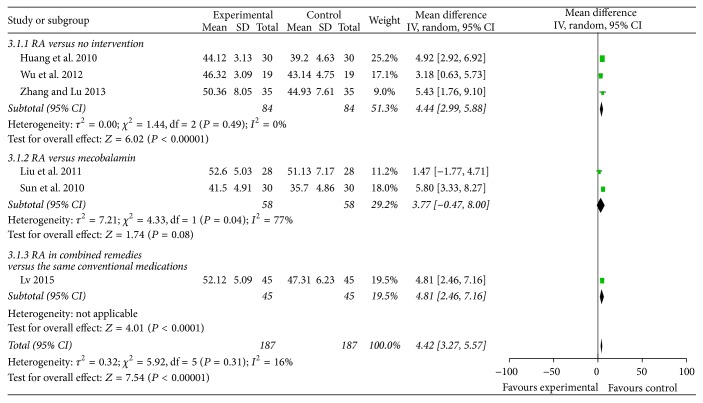
3.4.4. SNCV and MNCV (Figures 5 and 6)
Figure 5.
Forest plot of comparison: sensory nerve conduction velocity.
Figure 6.
Forest plot of comparison: motor nerve conduction velocity.
Six trials reported RA-based interventions significantly improved SNCV (MD 4.42 m/s, 95% CI 3.27 to 5.57, P < 0.01). However, regarding MNCV, there was no statistical difference between groups.
3.4.5. Safety, Quality of Life, and Publication Bias
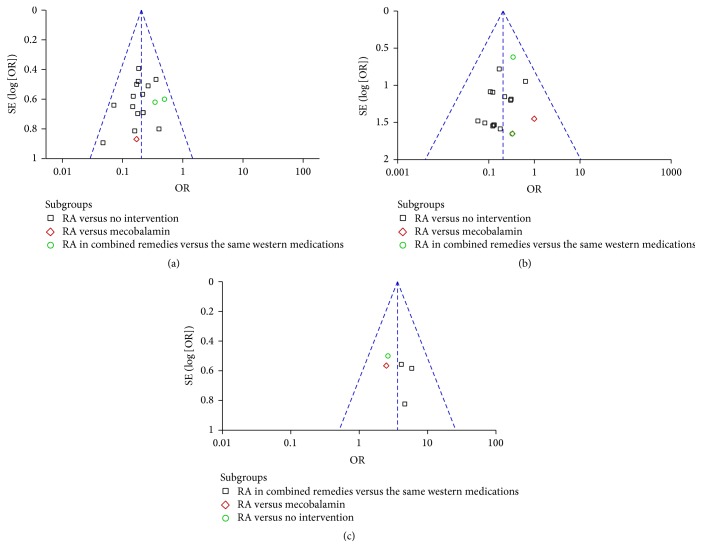
Among the 24 articles incorporated in the meta-analysis, no adverse events associated with RA-based interventions were reported. Nineteen trials reported Qol (KPS score > 60 or ECOG score ≤ 2) before RA intervention, and 2 trials reported Qol improvement. One trial reported the percentage of patients with Qol improvement while the other reported the increased level of KPS score. Therefore, the results of these 2 trials could not be combined in the meta-analysis. Exploration of the funnel plots (Figure 7) for all-grade CIPN, high-grade CIPN, and curative effects between RA-based interventions and control suggested near symmetry. No significant publication bias was found.
Figure 7.
Funnel plot analysis of risk of bias. (a) Funnel plot analysis of incidence of all-grade chemotherapy-induced peripheral neuropathy (CIPN). (b) Funnel plot analysis of incidence high-grade CIPN (grades 3-4). (c) Funnel plot analysis of curative effects of Radix Astragali-based prescriptions on CIPN.
4. Discussion
CIPN is not recorded in classic TCM books, so it remains a big challenge for TCM oncologists. Based on syndrome differentiation and treatment, TCM oncologists believe that CIPN falls under the category of Bi syndrome in TCM. The pathogenesis of CIPN is believed to be asthenia of qi and blood, qi stagnation and blood stasis. These lead to tendon and vessel malnutrition and stasis in collaterals. The treatment includes benefiting qi and nourishing blood, regulating ying and wei, and promoting blood circulation to remove meridian obstruction.
RA is one of the most commonly used herbs tonifying qi. In vitro and in vivo studies suggest RA extract can be a potential nerve growth-promoting factor, being salutary in encouraging the growth of axons in peripheral nerves [36]. Astragaloside IV, an active ingredient in RA, contributed to sciatic nerve regeneration and functional recovery in mice. The mechanism underlying this effect may be associated with the upregulation of growth-associated protein-43 expression [37]. RA extract promoted neural-directed differentiation of mesenchymal stem cells into nerve cells in vitro and also had neuroprotective effects on the central nervous system [38, 39].
This review identified a relatively large amount of evidence on the effectiveness of RA-based interventions, either tested alone or tested in combined remedies, for the prevention and treatment of OXAL induced peripheral neuropathy. Compared with no intervention or conventional western medicine, RA-based interventions have the potential of being more effective in relieving CIPN. RA-based interventions also lead to improvement of SNCV. No adverse event was reported and 2 trials reported Qol improvement after RA-based interventions. In China, there is a general perception that it could improve Qol for various conditions. However, clinical trials need to monitor and report Qol improvement.
Most of RA-based prescriptions included Danggui, Guizhi, Baishao, Jixueteng, Chuanxiong, and Honghua. These herbs may improve the effects of RA intervention on CIPN. Individualized treatment in TCM requires the modification of herbs with various symptoms in different patients. So the herbs included in RA-based prescriptions were heterogeneous. There were variations in the formulation, dosage, administration, and duration of treatment in the included trials. Even for herbal intervention of the same name, there were still differences in the specific composition or dose of included Chinese herbal medicine. Information about quality control was lacking on the development of the herbal preparations or the manufacture of herbal products. Future trials should provide information about standardization, including composition, quality control, and detailed regimens. The majority of trials compared RA-based intervention with no intervention; others used western conventional medicine as controls. Only 1 trial used a formal placebo control, so the positive effect should be interpreted conservatively.
This review has its limitations. We only included studies published in journals. Dissertations and conference papers were not included. Only high quality (improved Jadad score ≥ 4 points) trials were included. We excluded 38 trials with low quality or insufficient information for assessing risk of bias. Therefore, it may not be possible to achieve a complete summary of all existent evidence. Quantitative subgroup analysis exploring the effects of age, disease history, and duration could not be performed due to insufficient data. No multicenter, large-scale RCTs were identified. Most trials focused on short-term rather than long-term outcomes. Future trials should assure adequate concealment of allocation and blinding of outcome assessors.
5. Conclusions
From our study, we found that RA-based intervention may have clinical effectiveness for relieving OXAL induced peripheral neuropathy and lead to improvement of SNCV. However, the evidence is not sufficient. In the future, results from double-blind, multicenter, large-scale RCTs are needed to draw more definitive conclusions.
Acknowledgments
This work was supported by Beijing Municipal Science & Technology Commission no. Z151100003815019 and National Fund of Natural Sciences no. 81173421 of China. The authors thank Dr. Wei Sun for translating Latin names of herbal medicine.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this manuscript.
References
- 1.Wilson R. H., Lehky T., Thomas R. R., Quinn M. G., Floeter M. K., Grem J. L. Acute oxaliplatin-induced peripheral nerve hyperexcitability. Journal of Clinical Oncology. 2002;20(7):1767–1774. doi: 10.1200/JCO.2002.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Kelly H., Goldberg R. M. Systemic therapy for metastatic colorectal cancer: current options, current evidence. Journal of Clinical Oncology. 2005;23(20):4553–4560. doi: 10.1200/jco.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 3.André T., Boni C., Mounedji-Boudiaf L., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. The New England Journal of Medicine. 2004;350(23):2343–2351. doi: 10.1056/nejmoa032709. [DOI] [PubMed] [Google Scholar]
- 4.Ventzel L., Jensen A. B., Jensen A. R., Jensen T. S., Finnerup N. B. Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 2016;157(3):560–568. doi: 10.1097/j.pain.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 5.Cui H., Li O., Tan H., Li Y. Clinical observation of efficacy of Huangqi injection in prevention and treatment of neurotoxicity induced by oxaliplatin-containing chemotherapy regimen. Adverse Drug Reactions Journal. 2009;11(4):249–252. [Google Scholar]
- 6.Feng Y. Nursing observation of prevention and treatment of oxaliplatin-induced peripheral neuropathy. Strait Pharmaceutical Journal. 2011;23(12):203–204. [Google Scholar]
- 7.Huang J., Lin Q., Qiu Z., et al. Clinical study of protective effect of Yiqi Wenjing Yangxue Huoxue recipe combined with reduced glutathione on oxaliplatin-induced chronic neurotoxicity. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(4):312–315. [Google Scholar]
- 8.Huang Z., Huang Z., Chen G., et al. Clinical study on external bath of ‘Huangqi Guizhi Decoction’ in relieving oxaliplatin-induced peripheral neurotoxicity. Shanghai Journal of Traditional Chinese Medicine. 2010;44(5):40–42. [Google Scholar]
- 9.Jin Y., Su Q., Chen X., Pang D., He G. Effects of TCM fumigation combined with twelve meridians flapping exercises on oxaliplatin-induced peripheral neurotoxicity. Chinese Journal of Modern Nursing. 2015;21(11):1316–1318. [Google Scholar]
- 10.Kong M. Administration of Huoxue Huayu Herbs in treating patients with oxaliplatin-induced peripheral neurotoxicity. Journal of Qilu Nursing. 2012;18(34):p. 141. [Google Scholar]
- 11.Li Z., Dai A., Yang H., Li S., Wan Y., Yang W. The random parallel control study of protective effect of Rongjin Fang on oxaliplatin-induced chronic neurotoxicity in treating colorectal cancer. Journal of Practical Traditional Chinese Internal Medicine. 2013;27(10):46–49. [Google Scholar]
- 12.Liang X., Chen M., Chen D., Lin Q. Liqi Yufeng Decoction relieve oxaliplatin-induced chronic neurotoxicity: a report of 46 cases. Chinese Journal of Traditional Medical Science and Technology. 2012;19(1):p. 94. [Google Scholar]
- 13.Liang X., Chen M., Lin Q. Lixue Yufeng Tang relieve oxaliplatin-induced chronic neurotoxicity in patients of post-operational colorectal cancer: a report of 74 cases. Zhejiang Journal of Traditional Chinese Medicine. 2015;50(7):522–523. [Google Scholar]
- 14.Lin H., Luo K. Nursing observation of modified Huangqi Guizhi Wuwu Tang in treating peripheral neurotoxicity induced by oxaliplatin. Chinese General Nursing. 2011;9(2):484–485. [Google Scholar]
- 15.Liu H., Zhou Z., Wu L., et al. Clinical research on the effects of ‘Huangqi Guizhi Wuwu Decoction’ on peripheral neurotoxicity induced by oxaliplatin. Shanghai Journal of Traditional Chinese Medicine. 2011;45(3):44–47. [Google Scholar]
- 16.Liu S. Nursing observation of Huoxue Tongluo Formula preventing oxaliplatin-induced peripheral neurotoxicity. Beijing Journal of Traditional Chinese Medicine. 2009;28(1):45–46. [Google Scholar]
- 17.Lv P. Clinical observation of integrated Chinese and Western medicine in the treatment of peripheral neuropathy caused by oxaliplatin. China Medical Herald. 2015;12(21):131–134. [Google Scholar]
- 18.Qin B., Sun C. Protective effect of Yiqi Huoxue Formula on oxaliplatin-induced neurotoxicity in colorectal cancer. Shandong Journal of Traditional Chinese Medicine. 2015;34(3):176–178. [Google Scholar]
- 19.Qin Y., Lin H., Hua B. Clinical observation of network vessel-freeing formula as topical wash combined for 34 cases of chemotherapy-induced peripheral neuropathy. Journal of Traditional Chinese Medicine. 2012;53(23):2014–2016. [Google Scholar]
- 20.Shen J., He S., Sun X., Hu N., Cai Y. Clinical study on external bath of modified Huangqi Guizhi Wuwu Decoction for peripheral neurotoxicity induced by oxaliplatin. Chinese Journal of Information on TCM. 2015;22(11):13–15. [Google Scholar]
- 21.Sun W., Li X., Zhang X., Liu Y., Li Y., Li J. Buyang Huanwu Tang immersion for treating 34 cases of chemotherapy-induced peripheral neuropathy. Journal of Fujian University of TCM. 2010;20(5):8–9. [Google Scholar]
- 22.Tan Z., Qi Y. Clinical study on gastric cancer patients with chemotherapy-induced neurotoxicity treated with Yiqi Wenyang Huoxue Method. Shandong Journal of Traditional Chinese Medicine. 2015;34(2):101–103. [Google Scholar]
- 23.Wang Q. Huangqi Guizhi Wuwu Tang hand-foot bath combined with Ca/Mg infusions in relieving oxaliplatin-induced peripheral neurotoxicity: a clinical observsion. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2015;24(3):318–320. [Google Scholar]
- 24.Wu G., Yao X., Wu X., Liu F. Curative effect observsion of modified Huangqi Guizhi Wuwu Tang in relieving postoperative chemotherapy induced peripheral neurotoxicity. Journal of Sichuan of Traditional Chinese Medicine. 2015;33(12):132–133. [Google Scholar]
- 25.Wu M., Chen X., Zhou Y., Ying X., Wang Y. Clinic research of Yiqihuoxue and Jianpibushen therapy on prevention of oxaliplatin-induced neurotoxicity. Chinese Archives of Traditional Chinese Medicine. 2012;30(1):111–113. [Google Scholar]
- 26.Wu T., Jin Y., Zhong Y., et al. Efficacy of Huangqi Guizhi Wuwu decoction combined with needle warming moxibustion on peripheral neurotoxicity and immunologic function of patients with malignant cancer after chemotherapy. Shandong Medical Journal. 2015;55(33):1–4. [Google Scholar]
- 27.Xu M., Wu X., Fang N., Zhu W., Zhu S. Clinical observation of external administion of Huoxue Huayu Formula in reliving oxaliplatin-induced peripheral neurotoxicity. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2011;17(12):32–33. [Google Scholar]
- 28.Zhang Y., Lu X. Clinical study of the protective effect of thioctic acid combined with Huangqi Oral Liquid on oxaliplatin-induced neurotoxicity. China Journal of Chinese Medicine. 2013;28(186):1617–1618. [Google Scholar]
- 29.Ma Y., Zhou R., Ye W., Liu H. Influence of ‘Huangqi Guizhi Wuwu Decoction’ on nerve conduction velocity in oxaliplatin-induced peripheral neurotoxicity rats. Shanghai Journal of Traditional Chinese Medicine. 2011;45(1):75–78. [Google Scholar]
- 30.Huo J., Hu Y., Yang J., et al. Effect of Huangqi Guizhi Wuwu Decoction on chemotherapy-induced peripheral never injury in rats. Journal of Traditional Chinese Medicine. 2012;53(23):2013–2034. [Google Scholar]
- 31.Levi F., Misset J.-L., Brienza S., et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992;69(4):893–900. doi: 10.1002/1097-0142(19920215)69:460;893::aid-cncr282069041062;3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.03. National Cancer Institute; 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]
- 34.Shi Y., Sun Y. Manual of Clinical Oncology. 6th. Shelton, Conn, USA: People's Medical Publishing House; 2015. [Google Scholar]
- 35.Higgins J. P. T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2012. http://handbook.cochrane.org. [Google Scholar]
- 36.Lu M.-C., Yao C.-H., Wang S.-H., Lai Y.-L., Tsai C.-C., Chen Y.-S. Effect of Astragalus membranaceus in rats on peripheral nerve regeneration: in vitro and in vivo studies. Journal of Trauma and Acute Care Surgery. 2010;68(2):434–440. doi: 10.1097/ta.0b013e31819adb38. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Chen J. The mechanism of astragaloside IV promoting sciatic nerve regeneration. Neural Regeneration Research. 2013;8(24):2256–2265. doi: 10.3969/j.issn.1673-5374.2013.24.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Si Y.-C., Li Q., Xie C.-E., Niu X., Xia X.-H., Yu C.-Y. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chinese Medicine. 2014;9(1, article 13) doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X.-P., Ding H., Lu J.-D., Tang Y.-H., Deng B.-X., Deng C.-Q. Effects of the combination of the main active components of Astragalus and Panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. American Journal of Chinese Medicine. 2015;43(7):1419–1438. doi: 10.1142/s0192415x15500809. [DOI] [PubMed] [Google Scholar]